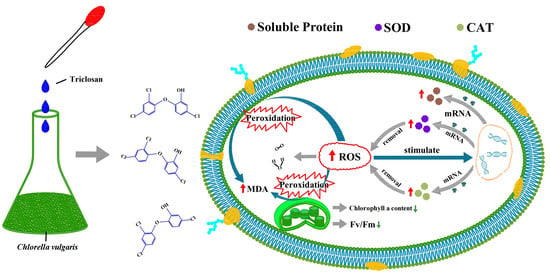
The Effects of Triclosan on Physiological and Photosynthetic Characteristics of Chlorella vulgaris
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagent
2.2. Microalgae Culture
2.3. Experimental Design and Test Methods
3. Results and Discussion
3.1. Effects of Triclosan Concentration on the Growth of Chlorella vulgaris
3.2. Effects of Triclosan Exposure Concentration on Chlorophyll a Content
3.3. Effects of Triclosan on the Photochemical Efficiency of Photosystem II
3.4. Effects of Exposure to Various Triclosan Concentrations on Soluble Protein Content
3.5. Effects of Triclosan on the Activity of Antioxidant Enzymes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sabaliunas, D.; Webb, S.F.; Hauk, A.; Jacob, M.; Eckhoff, W.S. Environmental fate of Triclosan in the River Aire Basin, UK. Water Res. 2003, 37, 3145–3154. [Google Scholar] [CrossRef]
- Schweizer, H.P. Triclosan: A widely used biocide and its link to antibiotics. FEMS Microbiol. Lett. 2001, 202, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Singer, H.; Müller, S.; Tixier, C.; Pillonel, L. Triclosan: Occurrence and fate of a widely used biocide in the aquatic environment: Field Measurements in wastewater treatment plants, surface waters, and lake sediments. Environ. Sci. Technol. 2002, 36, 4998–5004. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, Y.; Wang, C.; Zhao, X.; Mahadeva, G.D.; Wu, Y.; Ma, J.; Zhao, F. Anoxic biodegradation of triclosan and the removal of its antimicrobial effect in microbial fuel cells. J. Hazard. Mater. 2018, 344, 669–678. [Google Scholar] [CrossRef]
- Kookana, R.S.; Ying, G.-G.; Waller, N.J. Triclosan: Its occurrence, fate and effects in the Australian environment. Water Sci. Technol. 2011, 63, 598–604. [Google Scholar] [CrossRef]
- Villaverde-de-Sáa, E.; González-Mariño, I.; Quintana, J.B.; Rodil, R.; Rodríguez, I.; Cela, R. In-sample acetylation-non-porous membrane-assisted liquid–liquid extraction for the determination of parabens and triclosan in water samples. Anal. Bioanal. Chem. 2010, 397, 2559–2568. [Google Scholar] [CrossRef]
- Robert, L.; Jan, W.; Tania, H.; Georg, H. Polar herbicides, pharmaceutical products, perfluorooctanesulfonate (PFOS), perfluorooctanoate (PFOA), and nonylphenol and its carboxylates and ethoxylates in surface and tap waters around lake maggiore in northern Italy. Anal. Bioanal. Chem. 2007, 387, 1469–1478. [Google Scholar]
- Kantiani, L.; Farré, M.; Asperger, D.; Rubio, F.; González, S.; López-de-Alda, M.J. Triclosan and methyl-triclosan monitoring study in the northeast of Spain using a magnetic particle enzyme immunoassay and confirmatory analysis by gas chromatography–mass spectrometry. J. Hydrol. 2008, 361, 1–9. [Google Scholar] [CrossRef]
- Sorensen, J.; Lapworth, D.; Nkhuwa, D.; Stuart, M.; Gooddy, D.; Bell, R.; Chirwa, M.; Kabika, J.; Liemisa, M.; Chibesa, M.; et al. Emerging contaminants in urban groundwater sources in Africa. Water Res. 2015, 72, 51–63. [Google Scholar] [CrossRef]
- Zhao, J.-L.; Ying, G.-G.; Liu, Y.-S.; Chen, F.; Yang, J.-F.; Wang, L. Occurrence and risks of triclosan and triclocarban in the Pearl River system, South China: From source to the receiving environment. J. Hazard. Mater. 2010, 179, 215–222. [Google Scholar] [CrossRef]
- Agüera, A.; Fernández-Alba, A.R.; Piedra, L.; Piedra, L.; Mézcua, M.; Gómez, M.J. Evaluation of triclosan and biphenylol in marine sediments and urban wastewaters by pressurized liquid extraction and solid phase extraction followed by gas chromatography mass spectrometry and liquid chromatography mass spectrometry. Anal. Chim. Acta 2003, 480, 193–205. [Google Scholar] [CrossRef]
- Wilson, B.; Zhu, J.; Cantwell, M.; Olsen, C.R. Short-term dynamics and retention of Triclosan in the lower Hudson River Estuary. Mar. Pollut. Bull. 2008, 56, 1230–1233. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ying, G.-G.; Su, H.-C.; Yang, X.-B.; Wang, L. Simultaneous determination and assessment of 4-nonylphenol, bisphenol A and triclosan in tap water, bottled water and baby bottles. Environ. Int. 2010, 36, 557–562. [Google Scholar] [CrossRef] [PubMed]
- Benotti, M.J.; Trenholm, R.A.; Vanderford, B.J.; Holad, J.C.; Stanford, B.D.; Snyder, S.A. Pharmaceuticals and endocrine disrupting compounds in U.S. drinking wate. Environ. Sci. Technol. 2009, 43, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Quan, B.; Li, X.; Zhang, H.; Zhang, C.; Ming, Y.; Huang, Y.; Xi, Y.; Weihua, X.; Yunguo, L.; Tang, Y. Technology and principle of removing triclosan from aqueous media: A review. Chem. Eng. J. 2019, 378, 122185. [Google Scholar] [CrossRef]
- Bedoux, G.; Roig, B.; Thomas, O.; Dupont, V.; Le Bot, B. Occurrence and toxicity of antimicrobial triclosan and by-products in the environment. Environ. Sci. Pollut. Res. 2012, 19, 1044–1065. [Google Scholar] [CrossRef] [PubMed]
- Coogan, M.A.; Edziyie, R.E.; Point, T.W.L.; Venables, B.J. Algal bioaccumulation of triclocarban, triclosan, and methyl-triclosan in a North Texas wastewater treatment plant receiving stream. Chemosphere 2007, 67, 1911–1918. [Google Scholar] [CrossRef]
- Jiang, Y.-J.; He, W.; Liu, W.-X.; Qin, N.; Ouyang, H.-L.; Wang, Q.-M.; Kong, X.-Z.; He, Q.-S.; Yang, C.; Yang, B.; et al. The seasonal and spatial variations of phytoplankton community and their correlation with environmental factors in a large eutrophic Chinese lake (Lake Chaohu). Ecol. Indic. 2014, 40, 58–67. [Google Scholar] [CrossRef]
- Ouyang, H.L.; Kong, X.Z.; Lavoie, M.; He, W.; Qin, N.; He, Q.S.; Yang, B.; Wang, R.; Xu, F.L. Photosynthetic and cellular toxicity of cadmium in Chlorella vulgaris. Environ. Toxicol. Chem. 2013, 32, 2762–2770. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Ouyang, H.-L.; Jiang, Y.-J.; Kong, X.-Z.; He, W.; Liu, W.-X.; Yang, B.; Xu, F.-L. Effects of phosphorus stress on the photosynthetic and physiological characteristics of Chlorella vulgaris based on chlorophyll fluorescence and flow cytometric analysis. Ecol. Indic. 2017, 78, 131–141. [Google Scholar] [CrossRef]
- Govindje, E. Sixty-Three Years Since Kautsky: Chlorophyll a Fluorescence. Funct. Plant Biol. 1995, 22, 131–160. [Google Scholar] [CrossRef]
- Krause, G.H. Photoinhibition of photosynthesis. An evaluation of damaging and protective mechanisms. Physiol. Plant. 1988, 74, 566–574. [Google Scholar] [CrossRef]
- Lazar, D. Chlorophyll a fluorescence induction. Biochim. Biophys. Acta (BBA) Bioenerg. 1999, 1412, 1–28. [Google Scholar]
- Davis, E.F.; Klosterhaus, S.L.; Stapleton, H.M. Measurement of flame retardants and triclosan in municipal sewage sludge and biosolids. Environ. Int. 2012, 40, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Poole, A.C.; Pischel, L.; Ley, C.; Suh, G.; Goodrich, J.K.; Haggerty, T.D.; Ley, R.E.; Parsonnet, J. Crossover Control Study of the Effect of Personal Care Products Containing Triclosan on the Microbiome. mSphere 2016, 1, 1–15. [Google Scholar] [CrossRef]
- Yoon, J.H.; Sang, J.S.; Kim, M.S.; Tai, H.P. High cell density culture of Anabaena variabilis using repeated injections of carbon dioxide for the production of hydrogen. Int. J. Hydrogen Energy 2002, 27, 1265–1270. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, W.; Hu, B.; Min, M.; Chen, P.; Ruan, R.R. Integration of algae cultivation as biodiesel production feedstock with municipal wastewater treatment: Strains screening and significance evaluation of environmental factors. Bioresour. Technol. 2011, 102, 10861–10867. [Google Scholar] [CrossRef]
- Xiong, J.Q.; Kurade, M.B.; Abou-Shanab, R.A.I.; Ji, M.K.; Choi, J.; Kim, J.O.; Jeon, B.H. Biodegradation of carbamazepine using freshwater microalgae chlamydomonas mexicana and scenedesmus obliquus and the determination of its metabolic fate. Bioresour. Technol. 2016, 205, 183–190. [Google Scholar] [CrossRef]
- Henriques, F.S. Leaf Chlorophyll Fluorescence: Background and Fundamentals for Plant Biologists. Bot. Rev. 2009, 75, 249–270. [Google Scholar] [CrossRef]
- Campbell, D.; Hurry, V.; Clarke, A.K.; Gustafsson, P.; Oquist, G. Chlorophyll fluorescence analysis of cyanobacterial photosynthesis and acclimation. Microbiol. Mol. Biol. Rev. 1998, 62, 667–683. [Google Scholar] [CrossRef]
- Wang, N.; Li, H.; Wang, L.; Zhang, L.; Xu, Y. Effects of chitosan-RE3+-bentonite on growth of Chlorella vulgaris. J. Rare Earths 2010, 28, 149–153. [Google Scholar] [CrossRef]
- Ding, T.; Yang, M.; Zhang, J.; Yang, B.; Lin, K.; Li, J.; Gan, J. Toxicity, degradation and metabolic fate of ibuprofen on freshwater diatom Navicula sp. J. Hazard. Mater. 2017, 330, 127–134. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Pandey, B.; Suthar, S. Phytotoxicity and degradation of antibiotic ofloxacin in duckweed (Spirodela polyrhiza) system. Ecotoxicol. Environ. Saf. 2019, 179, 88–95. [Google Scholar] [CrossRef]
- Bai, X.; Acharya, K. Removal of seven endocrine disrupting chemicals (EDCs) from municipal wastewater effluents by a freshwater green alga. Environ. Pollut. 2019, 247, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.L.; Ji, F.; Zhang, H.; Hu, C.; Wong, M.H.; Hu, D.; Cai, Z. Formation of dioxins from triclosan with active chlorine: A potential risk assessment. J. Hazard. Mater. 2019, 367, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.; Price, O.R.; Bettles, N.; Rendal, C.; Van Egmond, R. Accounting for dissociation and photolysis: A review of the algal toxicity of triclosan. Environ. Toxicol. Chem. 2014, 33, 2551–2559. [Google Scholar] [CrossRef]
- Piddington, D.L.; Fang, F.C.; Laessig, T.; Cooper, A.M.; Orme, I.M.; Buchmeier, N.A. Cu, Zn Superoxide Dismutase of Mycobacterium tuberculosis Contributes to Survival in Activated Macrophages That Are Generating an Oxidative Burst. Infect. Immun. 2001, 69, 4980–4987. [Google Scholar] [CrossRef]
- Winston, G.W.; Gioliu, R.T.D. Prooxidant and antioxidant mechanisms in aquatic organisms. Aquat. Toxicol. 1991, 19, 137–161. [Google Scholar] [CrossRef]
- Lu, Y.; Jin, H.; Shao, B.; Xu, H.; Xu, X. Physiological and biochemical effects of triclocarban stress on freshwater algae. SN Appl. Sci. 2019, 1, 1685. [Google Scholar] [CrossRef]
- Rocha, S.; Gomes, D.; Lima, M.; Da Rocha, E.B.; Santos-Silva, A. Peroxiredoxin 2, glutathione peroxidase, and catalase in the cytosol and membrane of erythrocytes under H2O2-induced oxidative stress. Free Radic. Res. 2015, 49, 990–1003. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Lü, K.; Minter, E.J.; Chen, Y.; Yang, Z.; Montagnes, D.J. Combined effects of ammonia and microcystin on survival, growth, antioxidant responses, and lipid peroxidation of bighead carp Hypophthalmythys nobilis larvae. J. Hazard. Mater. 2012, 221–222, 213–219. [Google Scholar] [CrossRef] [PubMed]






| Triclosan Concentration (mg/L) | MDA Content (nmol/mg Protein) | SOD Activity (U/mg Protein) | CAT Activity (U/mg Protein) |
|---|---|---|---|
| 0 | 3.935 ± 0.405 | 0.283 ± 0.080 | 6.318 ± 0.774 |
| 0.05 | 4.116 ± 0.363 | 0.451 ± 0.066 | 7.635 ± 0.940 |
| 0.15 | 4.028 ± 0.710 | 0.467 ± 0.038 | 9.887 ± 1.795 |
| 0.45 | 4.111 ± 0.340 | 0.448 ± 0.050 | 12.473 ± 1.663 |
| 0.75 | 4.234 ± 0.301 | 0.442 ± 0.012 | 13.953 ± 0.530 |
| 1.05 | 12.865 ± 0.424 | 0.526 ± 0.083 | 9.800 ± 0.439 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, Z.; Luo, X.; Yang, A.; Wang, J.; Fu, H.; Wu, Y. The Effects of Triclosan on Physiological and Photosynthetic Characteristics of Chlorella vulgaris. Water 2021, 13, 1355. https://doi.org/10.3390/w13101355
Dai Z, Luo X, Yang A, Wang J, Fu H, Wu Y. The Effects of Triclosan on Physiological and Photosynthetic Characteristics of Chlorella vulgaris. Water. 2021; 13(10):1355. https://doi.org/10.3390/w13101355
Chicago/Turabian StyleDai, Zhineng, Xing Luo, Aili Yang, Jinsong Wang, Haiyan Fu, and Yicheng Wu. 2021. "The Effects of Triclosan on Physiological and Photosynthetic Characteristics of Chlorella vulgaris" Water 13, no. 10: 1355. https://doi.org/10.3390/w13101355
APA StyleDai, Z., Luo, X., Yang, A., Wang, J., Fu, H., & Wu, Y. (2021). The Effects of Triclosan on Physiological and Photosynthetic Characteristics of Chlorella vulgaris. Water, 13(10), 1355. https://doi.org/10.3390/w13101355