Adsorption of Azo-Anionic Dyes in a Solution Using Modified Coconut (Cocos nucifera) Mesocarp: Kinetic and Equilibrium Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Experiment Design
2.3. Experimental Methodology
2.3.1. Mechanical Treatment of Biomass
2.3.2. Cellulose Extraction
2.3.3. Cellulose Quaternization
2.3.4. Characterization of Bioadsorbents
2.3.5. Adsorption Tests
2.4. Adsorption Isotherms
2.5. Adsorption Kinetics
3. Results and Discussion
3.1. Characterization of Biomaterials
3.1.1. TGA and DSC Analysis
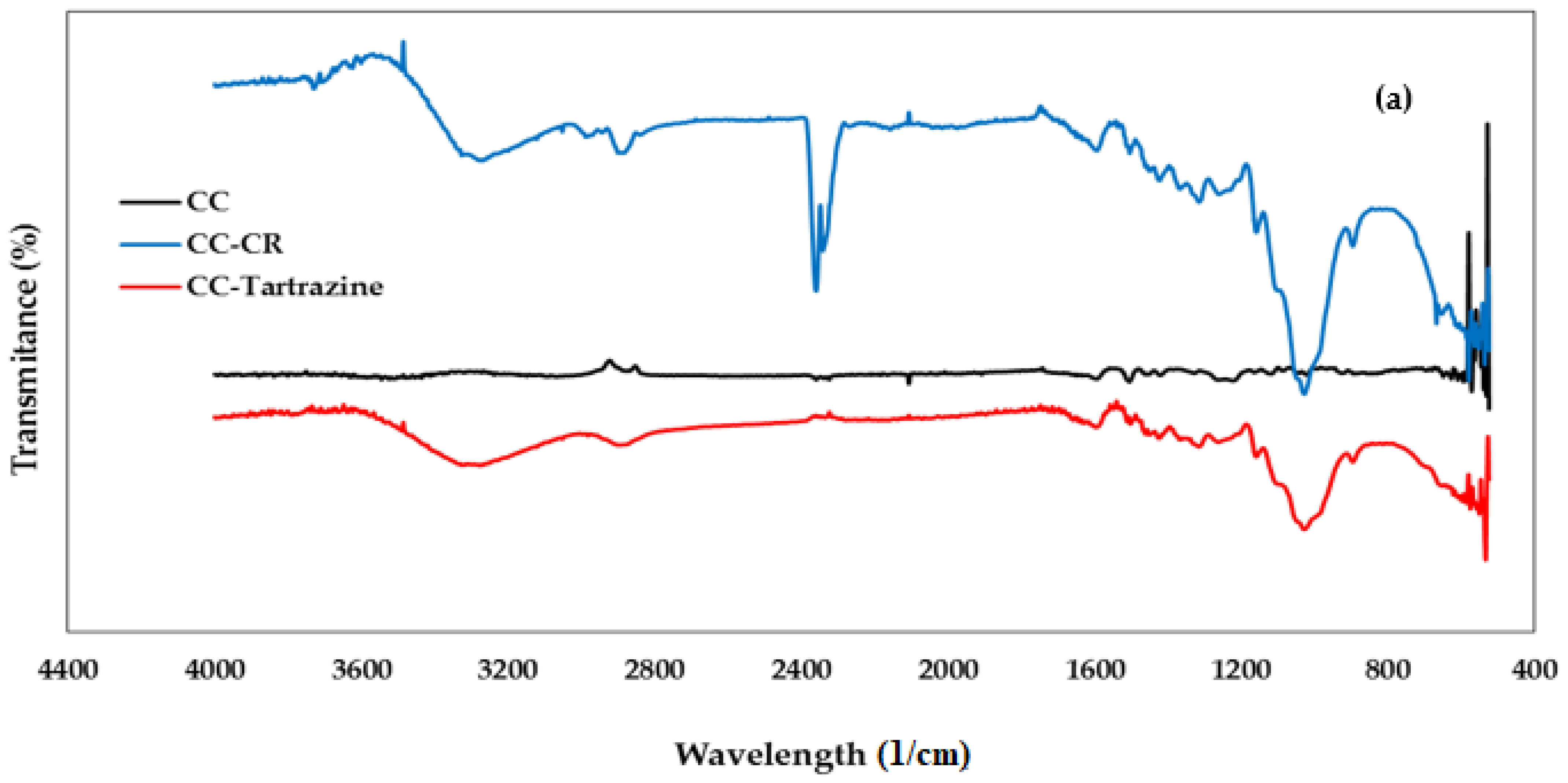
3.1.2. FTIR Spectroscopy Analysis
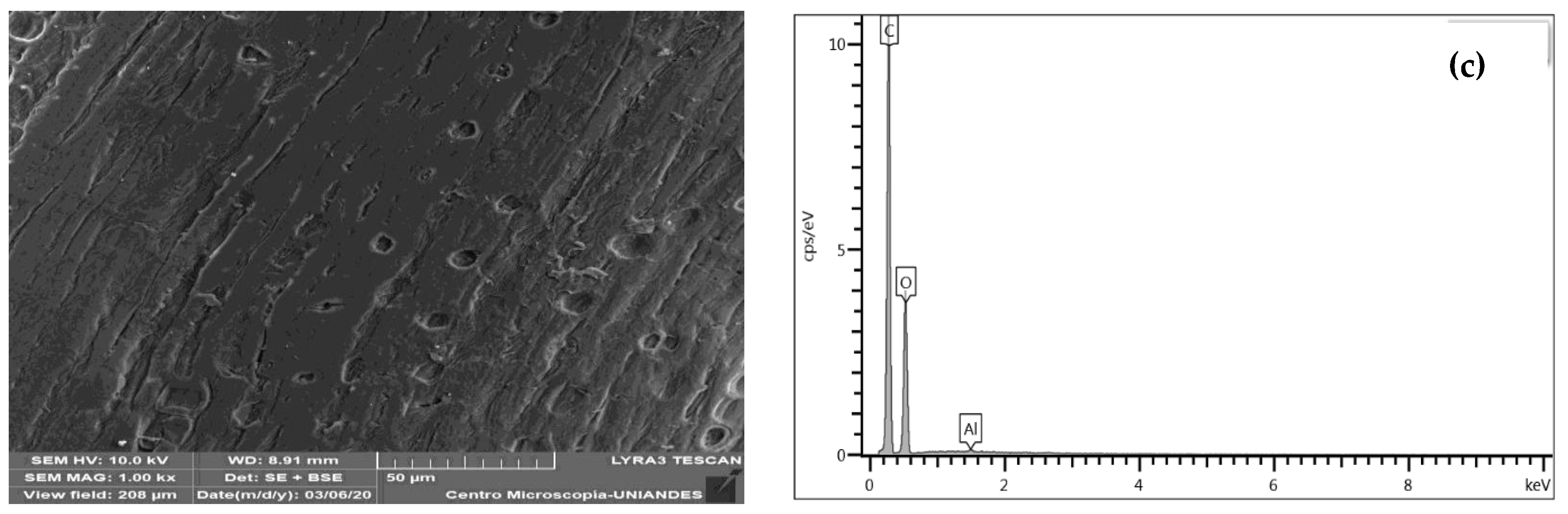
3.1.3. SEM-EDS Structural Analysis
3.1.4. Zero Load Point pH
3.2. Effect of Adsorbent Dosage and Initial Concentration
3.3. Adsorption Equilibrium
3.4. Adsorption Kinetics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gautam, R.K.; Gautam, P.K.; Banerjee, S.; Rawat, V.; Soni, S.; Sharma, S.K.; Chattopadhyaya, M.C. Removal of tartrazine by activated carbon biosorbents of Lantana camara: Kinetics, equilibrium modeling and spectroscopic analysis. J. Environ. Chem. Eng. 2015, 3, 79–88. [Google Scholar] [CrossRef]
- Adegoke, K.A.; Bello, O.S. Dye sequestration using agricultural wastes as adsorbents. Water Resour. Ind. 2015, 12, 8–24. [Google Scholar] [CrossRef] [Green Version]
- Shalla, A.H.; Bhat, M.A.; Yaseen, Z. Hydrogels for removal of recalcitrant organic dyes: A conceptual overview. J. Environ. Chem. Eng. 2018, 6, 5938–5949. [Google Scholar] [CrossRef]
- Mani, S.; Bharagava, R.N.; Chowdhary, P. Textile Wastewater Dyes: Toxicity Profile and Treatment Approaches. In Emerging and Eco-Friendly Approaches for Waste Management; Springer: Singapore, 2018; Volume 1, pp. 219–244. ISBN 9789811086694. [Google Scholar]
- Hussain, I.; Li, Y.; Qi, J.; Li, J.; Wang, L. Nitrogen-enriched carbon sheet for Methyl blue dye adsorption. J. Environ. Manag. 2018, 215, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Angelika, T.; Kamila, M.; Andrzej, P. Synthetic organic dyes as contaminants of the aquatic environment and their implications for ecosystems: A review. Sci. Total Environ. 2020, 717, 137222. [Google Scholar]
- Javaid, R.; Qazi, U.Y. Catalytic oxidation process for the degradation of synthetic dyes: An overview. Int. J. Environ. Res. Public Health 2019, 16, 2066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Food, E.; Authority, S.; Baert, K.; Levorato, S.; Binaglia, M. Dyes in aquaculture and reference points for action. EFSA J. 2017, 15, 4920. [Google Scholar]
- Lellis, B.; Fávaro-Polonio, C.Z.; Pamphile, J.A.; Polonio, J.C. Effects of textile dyes on health and the environment and bioremediation potential of living organisms. Biotechnol. Res. Innov. 2019, 3, 275–290. [Google Scholar] [CrossRef]
- Kaykhaii, M.; Sasani, M.; Marghzari, S. Removal of Dyes from the Environment by Adsorption Process. Chem. Mater. Eng. 2018, 6, 31–35. [Google Scholar] [CrossRef] [Green Version]
- Kooh, M.R.R.; Lim, L.B.L.; Lim, L.H.; Malik, O.A. Phytoextraction potential of water fern (Azolla pinnata) in the removal of a hazardous dye, methyl violet 2B: Artificial neural network modelling. Int. J. Phytoremediat. 2018, 20, 424–431. [Google Scholar] [CrossRef]
- Rodrigues de Almeida, E.J.; Christofoletti Mazzeo, D.E.; Deroldo Sommaggio, L.R.; Marin-Morales, M.A.; Rodrigues de Andrade, A.; Corso, C.R. Azo dyes degradation and mutagenicity evaluation with a combination of microbiological and oxidative discoloration treatments. Ecotoxicol. Environ. Saf. 2019, 183, 109484. [Google Scholar] [CrossRef]
- Olusegun, O.A.; Martincigh, B.S. Allergic contact dermatitis: A significant environmental and occupational skin disease. Int. J. Dermatol. 2021, in press. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Sader, K.; García-Padilla, A.; Realpe, A.; Acevedo-Morantes, M.; Soares, J. Removal of Heavy Metal Water Pollutants (Co2+ and Ni2+) Using Polyacrylamide/Sodium Montmorillonite (PAM/Na-MMT) Nanocomposites. ACS Omega 2019, 4, 10834–10844. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Song, X.; Xu, Y.; Shen, H.; Kong, X.; Xu, H. Utilization of wheat bran for producing activated carbon with high specific surface area via NaOH activation using industrial furnace. J. Clean. Prod. 2019, 210, 366–375. [Google Scholar] [CrossRef]
- Tan, C.H.C.; Sabar, S.; Hussin, M.H. Development of immobilized microcrystalline cellulose as an effective adsorbent for methylene blue dye removal. South Afr. J. Chem. Eng. 2018, 26, 11–24. [Google Scholar] [CrossRef]
- Khurana, I.; Saxena, A.; Khurana, J.M.; Rai, P.K. Removal of Dyes Using Graphene-Based Composites: A Review. Water Air Soil Pollut. 2017, 228, 180. [Google Scholar] [CrossRef]
- Kono, H.; Ogasawara, K.; Kusumoto, R.; Oshima, K.; Hashimoto, H.; Shimizu, Y. Cationic cellulose hydrogels cross-linked by poly(ethylene glycol): Preparation, molecular dynamics, and adsorption of anionic dyes. Carbohydr. Polym. 2016, 152, 170–180. [Google Scholar] [CrossRef]
- Jiang, Z.; Hu, D. Molecular mechanism of anionic dyes adsorption on cationized rice husk cellulose from agricultural wastes. J. Mol. Liq. 2019, 276, 105–114. [Google Scholar] [CrossRef]
- Safari, M.; Khataee, A.; Darvishi Cheshmeh Soltani, R.; Rezaee, R. Ultrasonically facilitated adsorption of an azo dye onto nanostructures obtained from cellulosic wastes of broom and cooler straw. J. Colloid Interface Sci. 2018, 522, 228–241. [Google Scholar] [CrossRef]
- Igwegbe, C.A.; Onyechi, P.C.; Onukwuli, O.D.; Nwokedi, I.C. Adsorptive Treatment of Textile Wastewater Using Activated Carbon Produced from Mucuna pruriens Seed Shells. World J. Eng. Technol. 2016, 4, 21–37. [Google Scholar] [CrossRef] [Green Version]
- Madan, S.; Shaw, R.; Tiwari, S.; Tiwari, S.K. Adsorption dynamics of Congo red dye removal using ZnO functionalized high silica zeolitic particles. Appl. Surf. Sci. 2019, 487, 907–917. [Google Scholar] [CrossRef]
- Otavo-Loaiza, R.A.; Sanabria-González, N.R.; Giraldo-Gómez, G.I. Tartrazine Removal from Aqueous Solution by HDTMA-Br-Modified Colombian Bentonite. Sci. World J. 2019, 2019, 2042563. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Hanafy, H.; Zhang, L.; Sellaoui, L.; Schadeck Netto, M.; Oliveira, M.L.S.; Seliem, M.K.; Luiz Dotto, G.; Bonilla-Petriciolet, A.; Li, Q. Adsorption of congo red and methylene blue dyes on an ashitaba waste and a walnut shell-based activated carbon from aqueous solutions: Experiments, characterization and physical interpretations. Chem. Eng. J. 2020, 388, 124263. [Google Scholar] [CrossRef]
- Reck, I.M.; Paixão, R.M.; Bergamasco, R.; Vieira, M.F.; Vieira, A.M.S. Removal of tartrazine from aqueous solutions using adsorbents based on activated carbon and Moringa oleifera seeds. J. Clean. Prod. 2018, 171, 85–97. [Google Scholar] [CrossRef]
- Mashkoor, F.; Nasar, A.; Asiri, A.M. Exploring the Reusability of Synthetically Contaminated Wastewater Containing Crystal Violet Dye using Tectona grandis Sawdust as a Very Low-Cost Adsorbent. Sci. Rep. 2018, 8, 8314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dogar, S.; Nayab, S.; Farooq, M.Q.; Said, A.; Kamran, R.; Duran, H.; Yameen, B. Utilization of biomass fly ash for improving quality of organic dye-contaminated water. ACS Omega 2020, 5, 15850–15864. [Google Scholar] [CrossRef]
- Magdy, Y.H.; Altaher, H. Kinetic analysis of the adsorption of dyes from high strength wastewater on cement kiln dust. J. Environ. Chem. Eng. 2018, 6, 834–841. [Google Scholar] [CrossRef]
- Trujillo, A.F.; Arias, L.S. The coconut, a renewable resource for thedesign of green materials. Entre Cienc. Ing. 2013, 7, 93–100. [Google Scholar]
- Andrade, S.N.; Veloso, C.M.; Fontan, R.C.I.; Bonomo, R.C.F.; Santos, L.S.; Brito, M.J.P.; Diniz, G.A. Chemical-activated carbon from coconut (Cocos nucifera) endocarp waste and its application in the adsorption of ß-lactoglobulin protein. Rev. Mex. Ing. Química 2018, 17, 436–475. [Google Scholar]
- Inseemeesak, B.; Areeprasert, C. Fiber extraction and energy recovery from Cocos nucifera Linn mesocarp residues employing steam explosion and anaerobic digestion. Ind. Crop. Prod. 2020, 147, 112180. [Google Scholar] [CrossRef]
- Collazo-Bigliardi, S.; Ortega-Toro, R.; Chiralt Boix, A. Isolation and characterisation of microcrystalline cellulose and cellulose nanocrystals from coffee husk and comparative study with rice husk. Carbohydr. Polym. 2018, 191, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Krietemeyer, E.F.; Boddu, V.M.; Liu, S.X.; Liu, W.C. Production and characterization of cellulose nanofibril (CNF) from agricultural waste corn stover. Carbohydr. Polym. 2018, 192, 202–207. [Google Scholar] [CrossRef]
- Herrera-Barros, A.; Tejada-Tovar, C.; Villabona-Ortíz, A.; Gonzalez-Delgado, A.D.; Benitez-Monroy, J. Cd (II) and Ni (II) uptake by novel biosorbent prepared from oil palm residual biomass and Al2O3 nanoparticles. Sustain. Chem. Pharm. 2020, 15, 100216. [Google Scholar] [CrossRef]
- Al-Ghouti, M.A.; Da’ana, D.A. Guidelines for the use and interpretation of adsorption isotherm models: A review. J. Hazard. Mater. 2020, 393, 122383. [Google Scholar] [CrossRef]
- Afroze, S.; Sen, T.K. A review on heavy metal ions and dye adsorption from water by agricultural solid waste adsorbents. Water Air Soil Pollut. 2018, 229, 225. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Adsorption kinetic models: Physical meanings, applications, and solving methods. J. Hazard. Mater. 2020, 390, 122156. [Google Scholar] [CrossRef] [PubMed]
- Herlina Sari, N.; Wardana, I.N.G.; Irawan, Y.S.; Siswanto, E. Characterization of the Chemical, Physical, and Mechanical Properties of NaOH-treated Natural Cellulosic Fibers from Corn Husks. J. Nat. Fibers 2018, 15, 545–558. [Google Scholar] [CrossRef]
- Fan, C.; Zhang, Y. Adsorption isotherms, kinetics and thermodynamics of nitrate and phosphate in binary systems on a novel adsorbent derived from corn stalks. J. Geochem. Explor. 2018, 188, 95–100. [Google Scholar] [CrossRef]
- Bryś, A.; Bryś, J.; Ostrowska-Ligęza, E.; Kaleta, A.; Górnicki, K.; Głowacki, S.; Koczoń, P. Wood biomass characterization by DSC or FT-IR spectroscopy. J. Therm. Anal. Calorim. 2016, 126, 27–35. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Zhang, P.; Pei, Z.; Wang, D. Investigation on characteristics of corn stover and sorghum stalk processed by ultrasonic vibration-assisted pelleting. Renew. Energy 2017, 101, 1075–1086. [Google Scholar] [CrossRef] [Green Version]
- Nagarajan, K.J.; Balaji, A.N.; Ramanujam, N.R. Extraction of cellulose nanofibers from cocos nucifera var aurantiaca peduncle by ball milling combined with chemical treatment. Carbohydr. Polym. 2012, 212, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Ilyas, R.A.; Sapuan, S.M.; Ishak, M.R. Isolation and characterization of nanocrystalline cellulose from sugar palm fibres (Arenga Pinnata). Carbohydr. Polym. 2018, 181, 1038–1051. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; He, S.; Zhao, Z.; Liu, X. Efficient preparation of cetyltrimethylammonium bromide-graphene oxide composite and its adsorption of Congo red from aqueous solutions. Colloids Surf. A 2018, 554, 227–236. [Google Scholar] [CrossRef]
- Uma Maheswari, C.; Obi Reddy, K.; Muzenda, E.; Guduri, B.R.; Varada Rajulu, A. Extraction and characterization of cellulose microfibrils from agricultural residue-Cocos nucifera L. Biomass Bioenergy 2012, 46, 555–563. [Google Scholar] [CrossRef]
- Ouassif, H.; Moujahid, E.M.; Lahkale, R.; Sadik, R.; Bouragba, F.Z.; Sabbar, E.M.; Diouri, M. Zinc-Aluminum layered double hydroxide: High efficient removal by adsorption of tartrazine dye from aqueous solution. Surf. Interfaces 2020, 18, 100401. [Google Scholar] [CrossRef]
- Singh, S.; Perween, S.; Ranjan, A. Dramatic enhancement in adsorption of congo red dye in polymer-nanoparticle composite of polyaniline-zinc titanate. J. Environ. Chem. Eng. 2021, 9, 105149. [Google Scholar] [CrossRef]
- Kumar, R.; Ansari, M.O.; Parveen, N.; Barakat, M.A.; Cho, M.H. Simple route for the generation of differently functionalized PVC@graphene-polyaniline fiber bundles for the removal of Congo red from wastewater. RSC Adv. 2015, 5, 61486–61494. [Google Scholar] [CrossRef]
- Aiyesanmi, A.F.; Adebayo, M.A.; Arowojobe, Y. Biosorption of Lead and Cadmium from Aqueous Solution in Single and Binary Systems Using Avocado Pear Exocarp: Effects of Competing Ions. Anal. Lett. 2020, 53, 2868–2885. [Google Scholar] [CrossRef]
- Kumar, A.; Negi, Y.S.; Choudhary, V.; Bhardwaj, N.K. Characterization of Cellulose Nanocrystals Produced by Acid-Hydrolysis from Sugarcane Bagasse as Agro-Waste. J. Mater. Phys. Chem. 2014, 2, 1–8. [Google Scholar] [CrossRef]
- Mohamed Pauzan, A.S.; Ahad, N. Biomass Modification Using Cationic Surfactant Cetyltrimethylammonium Bromide (CTAB) to Remove Palm-Based Cooking Oil. J. Chem. 2018, 2018, 5059791. [Google Scholar]
- Bakar, A.H.B.A.; Koay, Y.S.; Ching, Y.C.; Abdullah, L.C.; Choong, T.S.Y.; Alkhatib, M.; Mobarekeh, M.N.; Zahri, N.A.M. Removal of fluoride using quaternized palm kernel shell as adsorbents: Equilibrium isotherms and kinetics studies. BioResources 2016, 11, 4485–4511. [Google Scholar]
- Wang, H.; Wang, S.; Gao, Y. Cetyl trimethyl ammonium bromide modified magnetic biochar from pine nut shells for efficient removal of acid chrome blue K. Bioresour. Technol. 2020, 312, 123564. [Google Scholar] [CrossRef]
- Hu, Q.; Chen, N.; Feng, C.; Hu, W.; Liu, H. Kinetic and isotherm studies of nitrate adsorption on granular Fe-Zr-chitosan complex and electrochemical reduction of nitrate from the spent regenerant solution. RSC Adv. 2016, 6, 61944–61954. [Google Scholar] [CrossRef]
- Bhattacharya, A.K.; Venkobachar, C. Removal of Cadmium (II) by Low Cost Adsorbents. J. Environ. Eng. 1984, 110, 110–122. [Google Scholar] [CrossRef]
- Santana, S.A.A.; Vieira, A.P.; da Silva Filho, E.C.; Melo, J.C.P.; Airoldi, C. Immobilization of ethylenesulfide on babassu coconut epicarp and mesocarp for divalent cation sorption. J. Hazard. Mater. 2010, 174, 714–719. [Google Scholar] [CrossRef]
- Vieira, A.P.; Santana, S.A.A.; Bezerra, C.W.B.; Silva, H.A.S.; Chaves, J.A.P.; de Melo, J.C.P.; da Silva Filho, E.C.; Airoldi, C. Kinetics and thermodynamics of textile dye adsorption from aqueous solutions using babassu coconut mesocarp. J. Hazard. Mater. 2009, 166, 1272–1278. [Google Scholar] [CrossRef]
- Vieira, A.P.; Santana, S.A.A.; Bezerra, C.W.B.; Silva, H.A.S.; Chaves, J.A.P.; Melo, J.C.P.; Filho, E.C.S.; Airoldi, C. Removal of textile dyes from aqueous solution by babassu coconut epicarp (Orbignya speciosa). Chem. Eng. J. 2011, 173, 334–340. [Google Scholar] [CrossRef]
- Sahnoun, S.; Boutahala, M. Adsorption removal of tartrazine by chitosan/polyaniline composite: Kinetics and equilibrium studies. Int. J. Biol. Macromol. 2018, 114, 1345–1353. [Google Scholar] [CrossRef] [PubMed]
- Dawood, S.; Sen, T.K.; Phan, C. Synthesis and characterisation of novel-activated carbon from waste biomass pine cone and its application in the removal of congo red dye from aqueous solution by adsorption. Water Air Soil Pollut. 2014, 225, 1818. [Google Scholar] [CrossRef]
- Mondal, N.K.; Kar, S. Potentiality of banana peel for removal of Congo red dye from aqueous solution: Isotherm, kinetics and thermodynamics studies. Appl. Water Sci. 2018, 8, 157. [Google Scholar] [CrossRef] [Green Version]
- Kırbıyık, Ç.; Pütün, A.E.; Pütün, E. Equilibrium, kinetic, and thermodynamic studies of the adsorption of Fe(III) metal ions and 2,4-dichlorophenoxyacetic acid onto biomass-based activated carbon by ZnCl2 activation. Surf. Interfaces 2017, 8, 182–192. [Google Scholar] [CrossRef]
- Chukwuemeka-Okorie, H.O.; Ekuma, F.K.; Akpomie, K.G.; Nnaji, J.C.; Okereafor, A.G. Adsorption of tartrazine and sunset yellow anionic dyes onto activated carbon derived from cassava sievate biomass. Appl. Water Sci. 2021, 11, 27. [Google Scholar] [CrossRef]
- Ezekoye, O.M.; Akpomie, K.G.; Eze, S.I.; Chukwujindu, C.N.; Ani, J.U.; Ujam, O.T. Biosorptive interaction of alkaline modified Dialium guineense seed powders with ciprofloxacin in contaminated solution: Central composite, kinetics, isotherm, thermodynamics, and desorption. Int. J. Phytoremediat. 2020, 22, 1028–1037. [Google Scholar] [CrossRef]
- Kumari, S.; Mankotia, D.; Chauhan, G.S. Crosslinked cellulose dialdehyde for Congo red removal from its aqueous solutions. J. Environ. Chem. Eng. 2016, 4, 1126–1136. [Google Scholar] [CrossRef]
- Shen, Z.; Zhang, Y.; McMillan, O.; Jin, F.; Al-Tabbaa, A. Characteristics and mechanisms of nickel adsorption on biochars produced from wheat straw pellets and rice husk. Environ. Sci. Pollut. Res. 2017, 24, 12809–12819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elmoubarki, R.; Mahjoubi, F.Z.; Tounsadi, H.; Moustadraf, J.; Abdennouri, M.; Zouhri, A.; El Albani, A.; Barka, N. Adsorption of textile dyes on raw and decanted Moroccan clays: Kinetics, equilibrium and thermodynamics. Water Resour. Ind. 2015, 9, 16–29. [Google Scholar] [CrossRef]
- Parvin, S.; Biswas, B.K.; Rahman, M.A.; Rahman, M.H.; Anik, M.S.; Uddin, M.R. Study on adsorption of Congo red onto chemically modified egg shell membrane. Chemosphere 2019, 236, 124326. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, P.; Zhang, S.; Zhou, Q. Comparative Study on the Adsorption of Tartrazine and Indigo Carmine onto Maize Cob Carbon. Sep. Sci. Technol. 2014, 49, 877–886. [Google Scholar] [CrossRef]
- Gupta, V.K.; Jain, R.; Shrivastava, M.; Nayak, A. Equilibrium and Thermodynamic Studies on the Adsorption of the Dye Tartrazine onto Waste “Coconut Husks” Carbon and Activated Carbon. J. Chem. Eng. Data 2010, 55, 5083–5090. [Google Scholar] [CrossRef]
- Banerjee, S.; Chattopadhyaya, M.C. Adsorption characteristics for the removal of a toxic dye, tartrazine from aqueous solutions by a low cost agricultural by-product. Arab. J. Chem. 2017, 10, S1629–S1638. [Google Scholar] [CrossRef] [Green Version]
- Mittal, A.; Kurup, L.; Mittal, J. Freundlich and Langmuir adsorption isotherms and kinetics for the removal of Tartrazine from aqueous solutions using hen feathers. J. Hazard. Mater. 2007, 146, 243–248. [Google Scholar] [CrossRef] [Green Version]
- Rani, K.C.; Naik, A.; Chaurasiya, R.S.; Raghavarao, K.S.M.S. Removal of toxic Congo red dye from water employing low-cost coconut residual fiber. Water Sci. Technol. 2017, 75, 2225–2236. [Google Scholar] [CrossRef]
- Manirethan, V.; Gupta, N.; Balakrishnan, R.M.; Raval, K. Batch and continuous studies on the removal of heavy metals from aqueous solution using biosynthesised melanin-coated PVDF membranes. Environ. Sci. Pollut. Res. 2019, 27, 24723–24737. [Google Scholar] [CrossRef]
- Litefti, K.; Freire, M.S.; Stitou, M.; González-Álvarez, J. Adsorption of an anionic dye (Congo red) from aqueous solutions by pine bark. Sci. Rep. 2019, 9, 16530. [Google Scholar] [CrossRef] [Green Version]
- Raymundo, A.S.; Zanarotto, R.; Belisário, M.; de Pereira, M.G.; Ribeiro, J.N.; Nardy Ribeiro, A.V.F. Evaluation of sugar-cane bagasse as bioadsorbent in the textile wastewater treatment contaminated with Carcinogenic congo red dye. Braz. Arch. Biol. Technol. 2010, 53, 931–938. [Google Scholar] [CrossRef]
- Ahmad, R.; Kumar, R. Adsorptive removal of congo red dye from aqueous solution using bael shell carbon. Appl. Surf. Sci. 2010, 257, 1628–1633. [Google Scholar] [CrossRef]
- Kim, U.J.; Kimura, S.; Wada, M. Highly enhanced adsorption of Congo red onto dialdehyde cellulose-crosslinked cellulose-chitosan foam. Carbohydr. Polym. 2019, 214, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Siddiqui, Z.M.; Dhar, S.; Mehta, P.; Pathania, D. Adsorptive removal of congo red dye (CR) from aqueous solution by Cornulaca monacantha stem and biomass-based activated carbon: Isotherm, kinetics and thermodynamics. Sep. Sci. Technol. 2019, 54, 916–929. [Google Scholar] [CrossRef]
- Marques, B.S.; Frantz, T.S.; Sant’Anna Cadaval Junior, T.R.; de Almeida Pinto, L.A.; Dotto, G.L. Adsorption of a textile dye onto piaçava fibers: Kinetic, equilibrium, thermodynamics, and application in simulated effluents. Environ. Sci. Pollut. Res. 2019, 26, 28584–28592. [Google Scholar] [CrossRef]
- Tran, H.N.; You, S.J.; Hosseini-Bandegharaei, A.; Chao, H.P. Mistakes and inconsistencies regarding adsorption of contaminants from aqueous solutions: A critical review. Water Res. 2017, 120, 88–116. [Google Scholar] [CrossRef] [PubMed]
- Mohebali, S.; Bastani, D.; Shayesteh, H. Equilibrium, kinetic and thermodynamic studies of a low-cost biosorbent for the removal of Congo red dye: Acid and CTAB-acid modified celery (Apium graveolens). J. Mol. Struct. 2019, 1176, 181–193. [Google Scholar] [CrossRef]
- Xu, C.; Wang, H.; Yang, W.; Ma, L.; Lin, A. Expanded Graphite Modified by CTAB-KBr/H3PO4 for Highly Efficient Adsorption of Dyes. J. Polym. Environ. 2018, 26, 1206–1217. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, Y.; Guo, Z.; Ding, H. Stabilization/solidification of hexavalent chromium containing tailings using low-carbon binders for cemented paste backfill. J. Environ. Chem. Eng. 2021, 9, 104738. [Google Scholar]
- Shamsollahi, Z.; Partovinia, A. Recent advances on pollutants removal by rice husk as a bio-based adsorbent: A critical review. J. Environ. Manag. 2019, 246, 314–323. [Google Scholar]






| Model. | Equation | Parameters |
|---|---|---|
| Langmuir | qmax (mg/g): maximum amount of analyte removed per unit weight of biomass b (L/mg): constant related to the affinity of the binding sites with the contaminant Ce (mg/L): concentration of the remaining contaminant in solution | |
| Freundlich | kF (mg/g (L/mg)1/n): adsorption capacity indicator n: indicates the effect of concentration on adsorption capacity and represents the adsorption intensity. | |
| Dabunin–Radushkevich | ε2: l Polanyi’s potential which is based on temperature KDR (mol2/kJ2): Dubinin–Radushkevich constant related to adsorption energy E (kJ/mol): average adsorption energy per molecule of adsorbate required to transfer one mole of the ion from the solution to the adsorbent surface |
| Model | Equation | Parameters |
|---|---|---|
| Pseudo-first-order | k1 (min−1): Lagergren’s kinetic constant qe1 (mg/g): adsorption capacity at equilibrium. qt (mg/g): adsorption capacity at time t t (min): time | |
| Pseudo-secon -order | (9) | k2 (g/ mg·min): pseudo-second order kinetic constant qe2 (mg/g): adsorption capacity at equilibrium. qt (mg/g): adsorption capacity at time t t (min): time |
| Elovich | (10) | qt (mg/g): adsorption capacity at time t α (mg/g.min−1): initial adsorption rate β (g/mg): desorption constant related to the surface area of the biomaterial |
| Intraparticle diffusion | qt (mg/g): adsorption capacity at time t t (min): time k3 (mg/g.min1/2): kinetic constant of intra-particle diffusion |
| Peak (Wavelength cm−1) | Functional Group | Chemical Component | |
|---|---|---|---|
| CC | MCC | ||
| 3450 | 3390 | Stretching -OH | Cellulose |
| 2937 | 2937 | Stretching -CH | Cellulose |
| 2275 | 2337 | Stretching -C=O | Cellulose |
| 1720 | 1691 | Stretching -C=C | Hemicellulose |
| 1640 | 1640 | Vibration N=N | Lignin |
| 1455 | 1455 | Bending vibration -OH | Cellulose |
| -- | 1472 | Symmetric deformation of C6H6-Cl | CTAC |
| 1100 | 1100 | Stretching C-O-C | Polysaccharides |
| 900 | 890 | Vibration -CH | Cellulose |
| Model | Parameters | Tartrazine | Congo Red | ||
|---|---|---|---|---|---|
| CC | MCC | CC | MCC | ||
| Langmuir | qmax (mg/g) | 5.222 | 18.412 | 10.890 | 19.372 |
| b (L/mg) | 2.0967 × 10−5 | 1.220 × 10−4 | 0.215 | 0.353 | |
| R2 | 0.933 | 0.914 | 0.899 | 0.722 | |
| SS | 12.120 | 0.022 | 1.219 | 5.632 | |
| Freundlich | kF (mg/g (L/mg)1/n) | 0.033 | 0.956 | 3.516 | 5.598 |
| n | 0.937 | 0.973 | 3.527 | 2.224 | |
| R2 | 0.936 | 0.915 | 0.732 | 0.629 | |
| SS | 0.177 | 0.646 | 2.236 | 2.219 | |
| Dubinin–Radushkevich | qDR (mg/g) | 5.214 | 16.512 | 8.544 | 15.072 |
| KDR (mol2/kJ2) | 2.441 × 10−4 | 5.181 × 10−6 | 7.106 × 10−7 | 4.3683 × 10−7 | |
| E (KJ/mol) | 45.229 | 310.65 | 838.83 | 1069.86 | |
| R2 | 0.933 | 0.834 | 0.683 | 0.879 | |
| SS | 0.464 | 2.129 | 0.914 | 1.506 | |
| Contaminant | Adsorbent | qmax (mg/g) | Reference |
|---|---|---|---|
| Tartrazine | Organobentonite | 40.79 | [23] |
| Activated carbon from corn cob modified with H2PO4 | 89.75 | [69] | |
| Coconut shell | 4.452 | [70] | |
| Commercial activated carbon | 24.22 | ||
| Chitosan/polyaniline compound | 617.8 | [59] | |
| Chitosan | 46.4 | ||
| Polyaniline | 434.5 | ||
| Sawdust | 7.71 | [71] | |
| Chicken feathers | 0.097 | [72] | |
| Activated carbon from moringa seeds | 91.27 | [25] | |
| Activated carbon from babassu bone | 11.99 | ||
| Activated carbon from babassu coconut | 19.20 | ||
| Coconut cellulose | 5.222 | Present study | |
| CTAC modified coconut mesocarp cellulose | 18.412 | ||
| Congo Red | Residual coconut fiber modified with hexane and HCl | 181.82 | [73] |
| Mucuna pruriens activated carbon modified with orthophosphoric acid | 55.56 | [21] | |
| Activated carbon from Cornulaca modified with NaOH and NaClO | 78.19 | [74] | |
| Cornulaca monacantha | 43.42 | ||
| Pine bark | 3.92 | [75] | |
| Sugar cane bagasse | 4.43 | [76] | |
| Banana peel | 1.721 | [61] | |
| Activated carbon from pine cones | 434.78 | [60] | |
| Activated carbon from bael shell | 65.039 | [77] | |
| Zeolitic particles with high silica content functionalized with ZnO | 161.3 | [22] | |
| Eggshell membrane chemically modified with HCl | 117.65 | [68] | |
| Coconut cellulose | 5.222 | Present study | |
| Coconut mesocarp cellulose modified with CTAC | 18.412 |
| Kinetic Model | Parameters | Tartrazine | Congo Red | ||
|---|---|---|---|---|---|
| CC | MCC | CC | MCC | ||
| Pseudo-first-order | qe1 | 4.131 | 11.390 | 7.884 | 11.793 |
| k1 | 0.014 | 0.419 | 0.393 | 0.904 | |
| SS | 0.297 | 0.151 | 5.3278 | 0.005 | |
| R2 | 0.454 | 0.640 | 0.995 | 0.999 | |
| Pseudo-second-order | k2 | 0.818 | 11.561 | 7.122 | 8.396 |
| qe2 | 0.033 | 0.116 | 6.775 | 11.787 | |
| SS | 0.227 | 0.088 | 5.971 | 0.005 | |
| R2 | 0.533 | 0.999 | 0.997 | 0.999 | |
| Elovich | β | 0.131 | 1.860 | 3.497 | 11.743 |
| α | 8.560 | 19.642 | 2.626 | 12.828 | |
| SS | 0.082 | 0.122 | 0.603 | 0.036 | |
| R2 | 0.756 | 0.987 | 0.985 | 0.998 | |
| Intraparticle diffusion | k3 | 0.066 | 2.048 | 1.138 | 9.3 |
| SS | 0.134 | 0.163 | 27.665 | 0.007 | |
| R2 | 0.456 | 0.867 | 0.632 | 0.999 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tejada-Tovar, C.; Villabona-Ortíz, Á.; Gonzalez-Delgado, Á.D. Adsorption of Azo-Anionic Dyes in a Solution Using Modified Coconut (Cocos nucifera) Mesocarp: Kinetic and Equilibrium Study. Water 2021, 13, 1382. https://doi.org/10.3390/w13101382
Tejada-Tovar C, Villabona-Ortíz Á, Gonzalez-Delgado ÁD. Adsorption of Azo-Anionic Dyes in a Solution Using Modified Coconut (Cocos nucifera) Mesocarp: Kinetic and Equilibrium Study. Water. 2021; 13(10):1382. https://doi.org/10.3390/w13101382
Chicago/Turabian StyleTejada-Tovar, Candelaria, Ángel Villabona-Ortíz, and Ángel Darío Gonzalez-Delgado. 2021. "Adsorption of Azo-Anionic Dyes in a Solution Using Modified Coconut (Cocos nucifera) Mesocarp: Kinetic and Equilibrium Study" Water 13, no. 10: 1382. https://doi.org/10.3390/w13101382
APA StyleTejada-Tovar, C., Villabona-Ortíz, Á., & Gonzalez-Delgado, Á. D. (2021). Adsorption of Azo-Anionic Dyes in a Solution Using Modified Coconut (Cocos nucifera) Mesocarp: Kinetic and Equilibrium Study. Water, 13(10), 1382. https://doi.org/10.3390/w13101382








