Sorption and Desorption Analysis of Nitrobenzene on Differently Functionalized Multiwalled Carbon Nanotubes and Implications on the Stability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sorption−Desorption Experiments
2.3. Stabilization Experiments
2.4. Data Analysis
2.4.1. Sorption Isotherm Fitting
2.4.2. Thermodynamic Index of Irreversibility
3. Results
3.1. Characterization of Carbon Nanotubes
3.2. Sorption Isotherms Modeling
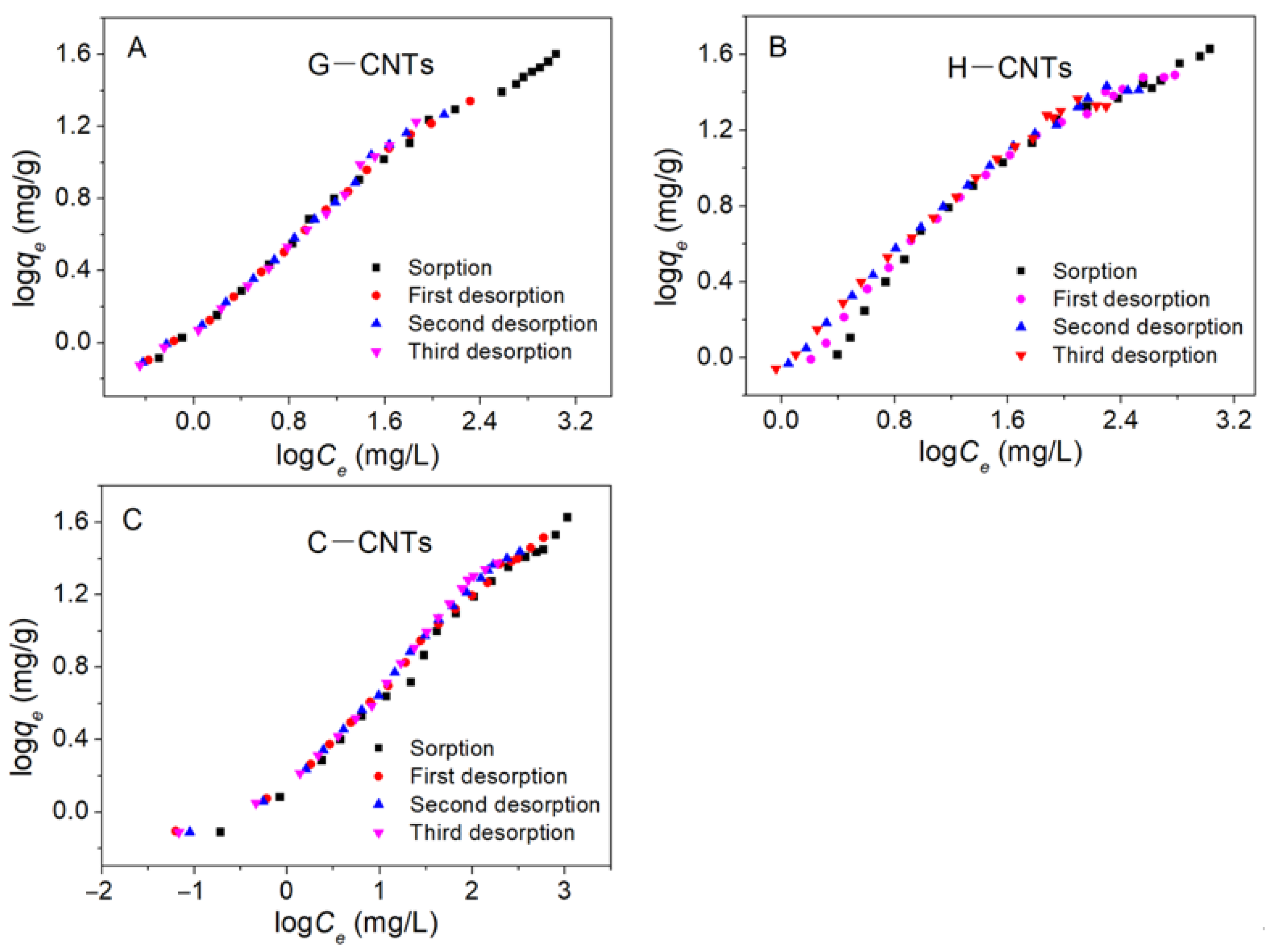
3.3. Sorption Hysteresis
3.4. Stabilization
3.5. Possible Adsorption−Desorption Mechanisms of Nitrobenzene on MWCNTs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y. Materials for electrochemical capacitors. Nat. Mater. 2008, 7, 845–854. [Google Scholar] [CrossRef] [Green Version]
- De Volder, M.F.L.; Tawfick, S.H.; Baughman, R.H.; Hart, A.J. Carbon nanotubes: Present and future commercial applications. Science 2013, 339, 535–539. [Google Scholar] [CrossRef] [Green Version]
- Humoud, M.S.; Roy, S.; Mitra, S. Scaling reduction in carbon nanotube-immobilized membrane during membrane distillation. Water 2019, 11, 2588. [Google Scholar] [CrossRef] [Green Version]
- Kukkar, D.; Rani, A.; Kumar, V.; Younis, S.A.; Zhang, M.; Lee, S.S.; Tsang, D.C.W.; Kim, K.H. Recent advances in carbon nanotube sponge-based sorption technologies for mitigation of marine oil spills. J. Colloid Interf. Sci. 2020, 570, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, J.; Naeem, S.; Ahmad, M.; Usman, A.R.A.; Al-Wabel, M.I. A critical review on organic micropollutants contamination in wastewater and removal through carbon nanotubes. J. Environ. Manag. 2019, 246, 214–228. [Google Scholar] [CrossRef]
- Zhao, J.; Luo, W.; Xu, Y.; Ling, J.; Deng, L. Potential reproductive toxicity of multi-walled carbon nanotubes and their chronic exposure effects on the growth and development of Xenopus tropicalis. Sci. Total Environ. 2021, 766, 142652. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Xing, B. Phytotoxicity of nanoparticles: Inhibition of seed germination and root growth. Environ. Pollut. 2007, 150, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Klaine, S.J.; Alvarez, P.J.J.; Batley, G.E.; Fernandes, T.F.; Handy, R.D.; Lyon, D.Y.; Mahendra, S.; McLaughlin, M.J.; Lead, J.R. Nanomaterials in the environment: Behavior, fate, bioavailability, and effects. Environ. Toxicol. Chem. 2008, 27, 1825–1851. [Google Scholar] [CrossRef]
- Glomstad, B.; Zindler, F.; Jenssen, B.M.; Booth, A.M. Dispersibility and dispersion stability of carbon nanotubes in synthetic aquatic growth media and natural freshwater. Chemosphere 2018, 201, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Xing, B.S. Adsorption of organic compounds by carbon nanomaterial in aqueous phase: Polanyi theory and its application. Chem. Rev. 2010, 110, 5989–6008. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Zhu, L.Z.; Xing, B.S. Adsorption of polycyclic aromatic hydrocarbons by carbon nanomaterials. Environ. Sci. Technol. 2006, 40, 1855–1861. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Y.; Yu, X.D.; Pan, B.; Xing, B.S. Norfloxacin sorption and its thermodynamics on surface-modified carbon nanotubes. Environ. Sci. Technol. 2010, 44, 978–984. [Google Scholar] [CrossRef] [PubMed]
- Pan, B.; Lin, D.H.; Mashayekhi, H.; Xing, B.S. Adsorption and hysteresis of bisphenol A and 17α-ethinyl estradiol on carbon nano-materials. Environ. Sci. Technol. 2008, 42, 5480–5485. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.; Yang, J.; Bitter, J.L.; Ball, W.P.; Fairbrother, D.H. Influence of Surface Oxygen on the interactions of carbon nanotubes with natural organic matter. Environ. Sci. Technol. 2012, 46, 12839–12847. [Google Scholar] [CrossRef]
- Li, Y.H.; Wang, S.G.; Wei, J.Q.; Zhang, X.F.; Xu, C.L.; Luan, Z.K.; Wu, D.H.; Wei, B.Q. Lead adsorption on carbon nanotubes. Chem. Phys. Lett. 2002, 357, 263–266. [Google Scholar] [CrossRef]
- Zhao, X.C.; Liu, R.T.; Chi, Z.X.; Teng, Y.; Qin, P.F. New insights into the behavior of bovine serum albumin adsorption adsorbed onto carbon nanotubes: Comprehensive spectroscopic studies. J. Phys. Chem. B 2010, 114, 5625–5631. [Google Scholar] [CrossRef]
- Pignatello, J.J.; Xing, B.S. Mechanisms of slow sorption of organic chemicals to natural particles. Environ. Sci. Technol. 1996, 30, 1–11. [Google Scholar] [CrossRef]
- Sander, M.; Pignatello, J.J. On the reversibility of sorption to black carbon: Distinguishing true hysteresis from artificial hysteresis caused by dilution of a competing adsorbate. Environ. Sci. Technol. 2007, 41, 843–849. [Google Scholar] [CrossRef]
- Sander, M.; Pignatello, J.J. Sorption irreversibility of 1,4-dichlorobenzene in two natural organic matter-rich geosorbents. Environ. Toxicol. Chem. 2009, 28, 447–457. [Google Scholar] [CrossRef]
- Huang, W.L.; Yu, H.; Weber, W.J. Hysteresis in the sorption and desorption of hydrophobic organic contaminants by soils and sediments: 1. A comparative analysis of experimental protocols. J. Contam. Hydrol. 1998, 31, 129–148. [Google Scholar] [CrossRef]
- Weber, W.J.; Huang, W.L.; Yu, H. Hysteresis in the sorption and desorption of hydrophobic organic contaminants by soils and sediments: 2. Effects of soil organic matter heterogeneity. J. Contam. Hydrol. 1998, 31, 149–165. [Google Scholar] [CrossRef]
- Lu, Y.F.; Pignatello, J.J. History-dependent sorption in humic acids and a lignite in the context of a polymer model for natural organic matter. Environ. Sci. Technol. 2004, 38, 5853–5862. [Google Scholar] [CrossRef]
- Lu, Y.F.; Pignatello, J.J. Sorption of apolar aromatic compounds to soil humic acid particles affected by aluminum(III) ion crosslinking. J. Environ. Qual. 2004, 33, 1314–1321. [Google Scholar] [CrossRef] [PubMed]
- Sander, M.; Lu, Y.F.; Pignatello, J.J. A thermodynamically based method to quantify true sorption hysteresis. J. Environ. Qual. 2005, 34, 1063–1072. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.F.; Pignatello, J.J. Demonstration of the “conditioning effect” in soil organic matter in support of a pore deformation mechanism for sorption hysteresis. Environ. Sci. Technol. 2002, 36, 4553–4561. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Xing, B.S. Desorption of polycyclic aromatic hydrocarbons from carbon nanomaterials in water. Environ. Pollut. 2007, 145, 529–537. [Google Scholar] [CrossRef]
- Zhao, J.J.; Buldum, A.; Han, J.; Lu, J.P. Gas molecule adsorption in carbon nanotubes and nanotube bundles. Nanotechnology 2002, 13, 195–200. [Google Scholar] [CrossRef]
- Deline, A.R.; Frank, B.P.; Smith, C.L.; Sigmon, L.R.; Wallace, A.N.; Gallagher, M.J.; Goodwin, D.G., Jr.; Durkin, D.P.; Fairbrother, D.H. Influence of oxygen-containing functional groups on the environmental properties, transformations, and toxicity of carbon nanotubes. Chem. Rev. 2020, 120, 11651–11697. [Google Scholar] [CrossRef]
- Cho, H.H.; Smith, B.A.; Wnuk, J.D.; Fairbrother, D.H.; Ball, W.P. Influence of surface oxides on the adsorption of naphthalene onto multiwalled carbon nanotubes. Environ. Sci. Technol. 2008, 42, 2899–2905. [Google Scholar] [CrossRef]
- Hevia, L.G.; Fanarraga, M.L. Microtubule cytoskeleton-disrupting activity of MWCNTs: Applications in cancer treatment. J. Nanobiotechnol. 2020, 18, 11. [Google Scholar] [CrossRef]
- Fan, B.; Wang, X.; Xie, Z.; Li, J.; Gao, X.; Cui, L.; Gao, S.; Liu, Z. Aquatic life criteria & human health ambient water quality criteria derivations and probabilistic risk assessments of 7 benzenes in China. Chemosphere 2021, 274, 129784. [Google Scholar]
- Nematollahzadeh, A.; Babapoor, A.; Mousavi, S.M.; Nuri, A. Nitrobenzene adsorption from aqueous solution onto polythiophene-modified magnetite nanoparticles. Mater. Chem. Phys. 2021, 262, 124266. [Google Scholar] [CrossRef]
- Zhang, D.; Pan, B.; Zhang, H.; Ning, P.; Xing, B. Contribution of Different Sulfamethoxazole Species to Their Overall Adsorption on Functionalized Carbon Nanotubes. Environ. Sci. Technol. 2010, 44, 3806–3811. [Google Scholar] [CrossRef]
- Li, H.; Zhang, D.; Han, X.; Xing, B. Adsorption of antibiotic ciprofloxacin on carbon nanotubes: pH dependence and thermodynamics. Chemosphere 2014, 95, 150–155. [Google Scholar] [CrossRef]
- Lin, D.H.; Xing, B.S. Tannic acid adsorption and its role for stabilizing carbon nanotube suspensions. Environ. Sci. Technol. 2008, 42, 5917–5923. [Google Scholar] [CrossRef]
- Lin, D.H.; Liu, N.; Yang, K.; Xing, B.S.; Wu, F.C. Different stabilities of multiwalled carbon nanotubes in fresh surface water samples. Environ. Pollut. 2010, 158, 1270–1274. [Google Scholar] [CrossRef]
- Xing, B.S.; Pignatello, J.J. Dual-mode sorption of low-polarity compounds in glassy poly(vinyl chloride) and soil organic matter. Environ. Sci. Technol. 1997, 31, 792–799. [Google Scholar] [CrossRef]
- Pan, B.; Zhang, D.; Li, H.; Wu, M.; Wang, Z.Y.; Xing, B.S. Increased adsorption of sulfamethoxazole on suspended carbon nanotubes by dissolved humic acid. Environ. Sci. Technol. 2013, 47, 7722–7728. [Google Scholar] [CrossRef]
- Zhao, Q.; Li, P.J.; Stagnitti, F.; Ye, J.; Dong, D.B.; Zhang, Y.Q.; Li, P. Effects of aging and freeze-thawing on extractability of pyrene in soil. Chemosphere 2009, 76, 447–452. [Google Scholar] [CrossRef]
- Matarredona, Q.; Rhoads, H.; Li, Z.R.; Harwell, J.H.; Balzano, L.; Resasco, D.E. Dispersion of single-walled carbon nanotubes in aqueous solutions of the anionic surfactant NaDDBS. J. Phys. Chem. B 2003, 107, 13357–13367. [Google Scholar] [CrossRef]
- Liu, C.H.; Li, J.J.; Zhang, H.L. Structure dependent interaction between organic dys and carbon nanotubes. Colloid Surface A 2008, 313–314, 9–12. [Google Scholar] [CrossRef]
- Kettler, K.; Veltman, K.; van de Meent, D.; van Wezel, A.; Hendriks, A.J. Cellular uptake of nanoparticles as determined by particle properties, experimental conditions, and cell type. Environ. Toxicol. Chem. 2014, 33, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.J.; Zhang, Z.M.; Zhang, S.Y.; Xing, W.W.; Wang, J.; Li, H.B.; Zhao, Q.; Xing, B.S. Size effect on the cytotoxicity of layered black phosphorus and underlying mechanisms. Small 2017, 13, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Nel, A.; Xia, T.; Madler, L.; Li, N. Toxic potential of materials at the nanolevel. Science 2006, 311, 622–627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azari, M.R.; Mohammadian, Y. Comparing in vitro cytotoxicity of graphite, short multi-walled carbon nanotubes, and long multi-walled carbon nanotubes. Environ. Sci. Pollut. Res. 2020, 27, 15401–15406. [Google Scholar] [CrossRef]
- Magrez, A.; Kasas, S.; Salicio, V.; Pasquier, N.; Seo, J.W.; Celio, M.; Catsicas, S.; Schwaller, B.; Forro, L. Cellular toxicity of carbon-based nanomaterials. Nano Lett. 2006, 6, 1121–1125. [Google Scholar] [CrossRef]
- Zhao, Q.; Yang, K.; Zhang, S.Y.; Chefetz, B.; Zhao, J.; Mashayekhi, H.; Xing, B.S. Dispersant selection for nanomaterials: Insight into dispersing functionalized carbon nanotubes by small polar aromatic organic molecules. Carbon 2015, 91, 494–505. [Google Scholar] [CrossRef]


| Sorption Isotherms Parameters Fitted by Four Models 1 | |||||||
|---|---|---|---|---|---|---|---|
| Langmuir model (LM) | |||||||
| CNTs | Q0 | P of Q0 | b | P of b | - | - | radj2 |
| G−CNTs | 38.9 ± 1.7 | <0.0001 | 0.0078 ± 0.0013 | <0.0001 | - | - | 0.971 |
| H−CNTs | 42.6 ± 2.3 | <0.0001 | 0.0070 ± 0.0013 | <0.0001 | - | - | 0.964 |
| C−CNTs | 42.6 ± 2.9 | <0.0001 | 0.0052 ± 0.0010 | <0.0001 | - | - | 0.961 |
| Freundlich model (FM) | |||||||
| CNTs | Kf | P of Kf | n | P of n | - | - | radj2 |
| G−CNTs | 2.20 ± 0.20 | <0.0001 | 0.413 ± 0.014 | <0.0001 | - | - | 0.992 |
| H−CNTs | 2.16 ± 0.27 | <0.0001 | 0.430 ± 0.020 | <0.0001 | - | - | 0.983 |
| C−CNTs | 1.73 ± 0.19 | <0.0001 | 0.454 ± 0.018 | <0.0001 | - | - | 0.989 |
| Dual-mode model (DMM) | |||||||
| CNTs | Q0 | P of Q0 | b | P of b | Kd | P of Kd | radj2 |
| G−CNTs | 19.5 ± 0.8 | <0.0001 | 0.0299 ± 0.0032 | <0.0001 | 0.020 ± 0.001 | <0.0001 | 0.998 |
| H−CNTs | 22.5 ± 1.1 | <0.0001 | 0.0237 ± 0.0030 | <0.0001 | 0.020 ± 0.001 | <0.0001 | 0.996 |
| C−CNTs | 18.9 ± 1.5 | <0.0001 | 0.0221 ± 0.0043 | <0.0001 | 0.022 ± 0.004 | <0.0001 | 0.993 |
| Polanyi-Manes model (PMM) | |||||||
| CNTs | Q0 | P of Q0 | b | P of b | a | P of a | radj2 |
| G−CNTs | 44.2 ± 2.2 | <0.0001 | 1.1704 ± 0.0977 | <0.0001 | −10.669 ± 2.201 | <0.0001 | 0.993 |
| H−CNTs | 46.4 ± 2.6 | <0.0001 | 1.2851 ± 0.1217 | <0.0001 | −14.243 ± 3.807 | <0.0001 | 0.988 |
| C−CNTs | 50.5 ± 4.1 | <0.0001 | 1.0443 ± 0.1128 | <0.0001 | −9.130 ± 2.184 | <0.0001 | 0.989 |
| Fitting Parameters under Every C0 (mg/L) by Freundlich Model 1 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| G−CNTs | H−CNTs | C−CNTs | |||||||||
| C0 | Kf | n | radj2 | C0 | Kf | n | radj2 | C0 | Kf | n | radj2 |
| 5.68 | 0.987 ± 0.046 | 0.253 ± 0.053 | 0.919 | 8.98 | 0.902 ± 0.010 | 0.167 ± 0.020 | 0.973 | 5.06 | 1.258 ± 0.022 | 0.144 ± 0.032 | 0.912 |
| 7.48 | 1.163 ± 0.027 | 0.347 ± 0.054 | 0.953 | 11.06 | 1.008 ± 0.022 | 0.221 ± 0.029 | 0.966 | 14.48 | 1.493 ± 0.042 | 0.316 ± 0.046 | 0.959 |
| 10.47 | 1.130 ± 0.018 | 0.538 ± 0.052 | 0.982 | 14.93 | 1.220 ± 0.039 | 0.284 ± 0.032 | 0.976 | 19.54 | 1.570 ± 0.078 | 0.370 ± 0.047 | 0.969 |
| 14.66 | 1.152 ± 0.038 | 0.581 ± 0.044 | 0.983 | 21.13 | 1.380 ± 0.068 | 0.361 ± 0.035 | 0.981 | 27.57 | 1.527 ± 0.115 | 0.441 ± 0.047 | 0.977 |
| 21.30 | 1.049 ± 0.041 | 0.658 ± 0.031 | 0.996 | 28.06 | 1.513 ± 0.035 | 0.392 ± 0.014 | 0.998 | 39.08 | 1.888 ± 0.296 | 0.349 ± 0.074 | 0.915 |
| 28.98 | 0.987 ± 0.083 | 0.676 ± 0.049 | 0.990 | 38.83 | 1.275 ± 0.163 | 0.572 ± 0.062 | 0.977 | 54.18 | 2.352 ± 0.585 | 0.269 ± 0.096 | 0.795 |
| 39.68 | 0.774 ± 0.227 | 0.814 ± 0.141 | 0.945 | 54.01 | 1.265 ± 0.174 | 0.583 ± 0.055 | 0.982 | 75.65 | 1.734 ± 1.187 | 0.464 ± 0.215 | 0.742 |
| 54.37 | 0.829 ± 0.128 | 0.749 ± 0.061 | 0.987 | 73.34 | 1.326 ± 0.166 | 0.581 ± 0.044 | 0.989 | 103.5 | 1.975 ± 0.353 | 0.441 ± 0.053 | 0.971 |
| 74.82 | 0.912 ± 0.095 | 0.685 ± 0.035 | 0.995 | 103.7 | 1.533 ± 0.179 | 0.542 ± 0.035 | 0.992 | 144.3 | 2.266 ± 0.311 | 0.411 ± 0.036 | 0.985 |
| 104.4 | 1.206 ± 0.260 | 0.595 ± 0.064 | 0.977 | 143.8 | 2.177 ± 0.239 | 0.452 ± 0.029 | 0.992 | 199.9 | 2.772 ± 0.269 | 0.373 ± 0.017 | 0.992 |
| 144.5 | 4.197 ± 0.732 | 0.274 ± 0.047 | 0.945 | 200.3 | 2.077 ± 0.285 | 0.483 ± 0.033 | 0.991 | 277.1 | 3.236 ± 0.258 | 0.347 ± 0.017 | 0.995 |
| 200.8 | 2.323 ± 0.342 | 0.442 ± 0.035 | 0.987 | 384.2 | 3.537 ± 0.306 | 0.345 ± 0.017 | 0.995 | 381.9 | 3.906 ± 0.428 | 0.318 ± 0.022 | 0.991 |
| 276.8 | 3.356 ± 0.395 | 0.352 ± 0.026 | 0.989 | 531.6 | 6.334 ± 1.149 | 0.255 ± 0.034 | 0.965 | 531.6 | 5.631 ± 1.096 | 0.261 ± 0.037 | 0.963 |
| 531.6 | 5.792 ± 0.656 | 0.246 ± 0.021 | 0.985 | 578.6 | 7.483 ± 1.134 | 0.213 ± 0.028 | 0.966 | 658.7 | 7.555 ± 0.345 | 0.209 ± 0.008 | 0.997 |
| 664.3 | 4.736 ± 0.212 | 0.283 ± 0.008 | 0.999 | 659.2 | 7.663 ± 1.003 | 0.219 ± 0.024 | 0.977 | 757.0 | 8.640 ± 0.913 | 0.187 ± 0.019 | 0.981 |
| 757.9 | 4.490 ± 1.420 | 0.298 ± 0.053 | 0.968 | 872.2 | 6.899 ± 0.753 | 0.254 ± 0.019 | 0.989 | 997.3 | 6.217 ± 0.331 | 0.255 ± 0.009 | 0.998 |
| 873.5 | 0.357 ± 0.056 | 0.357 ± 0.056 | 0.975 | 1143.1 | 3.305 ± 0.547 | 0.362 ± 0.026 | 0.990 | 1314.1 | 3.573 ± 0.736 | 0.354 ± 0.032 | 0.984 |
| - | - | - | - | 1324.5 | 2.105 ± 0.604 | 0.430 ± 0.044 | 0.980 | - | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, Z.; Li, D. Sorption and Desorption Analysis of Nitrobenzene on Differently Functionalized Multiwalled Carbon Nanotubes and Implications on the Stability. Water 2021, 13, 1426. https://doi.org/10.3390/w13101426
Ji Z, Li D. Sorption and Desorption Analysis of Nitrobenzene on Differently Functionalized Multiwalled Carbon Nanotubes and Implications on the Stability. Water. 2021; 13(10):1426. https://doi.org/10.3390/w13101426
Chicago/Turabian StyleJi, Zhanhua, and Dengyu Li. 2021. "Sorption and Desorption Analysis of Nitrobenzene on Differently Functionalized Multiwalled Carbon Nanotubes and Implications on the Stability" Water 13, no. 10: 1426. https://doi.org/10.3390/w13101426
APA StyleJi, Z., & Li, D. (2021). Sorption and Desorption Analysis of Nitrobenzene on Differently Functionalized Multiwalled Carbon Nanotubes and Implications on the Stability. Water, 13(10), 1426. https://doi.org/10.3390/w13101426





