Antimicrobial Effect of Plasma-Activated Tap Water on Staphylococcus aureus, Escherichia coli, and Candida albicans
Abstract
:1. Introduction
2. Materials and Methods
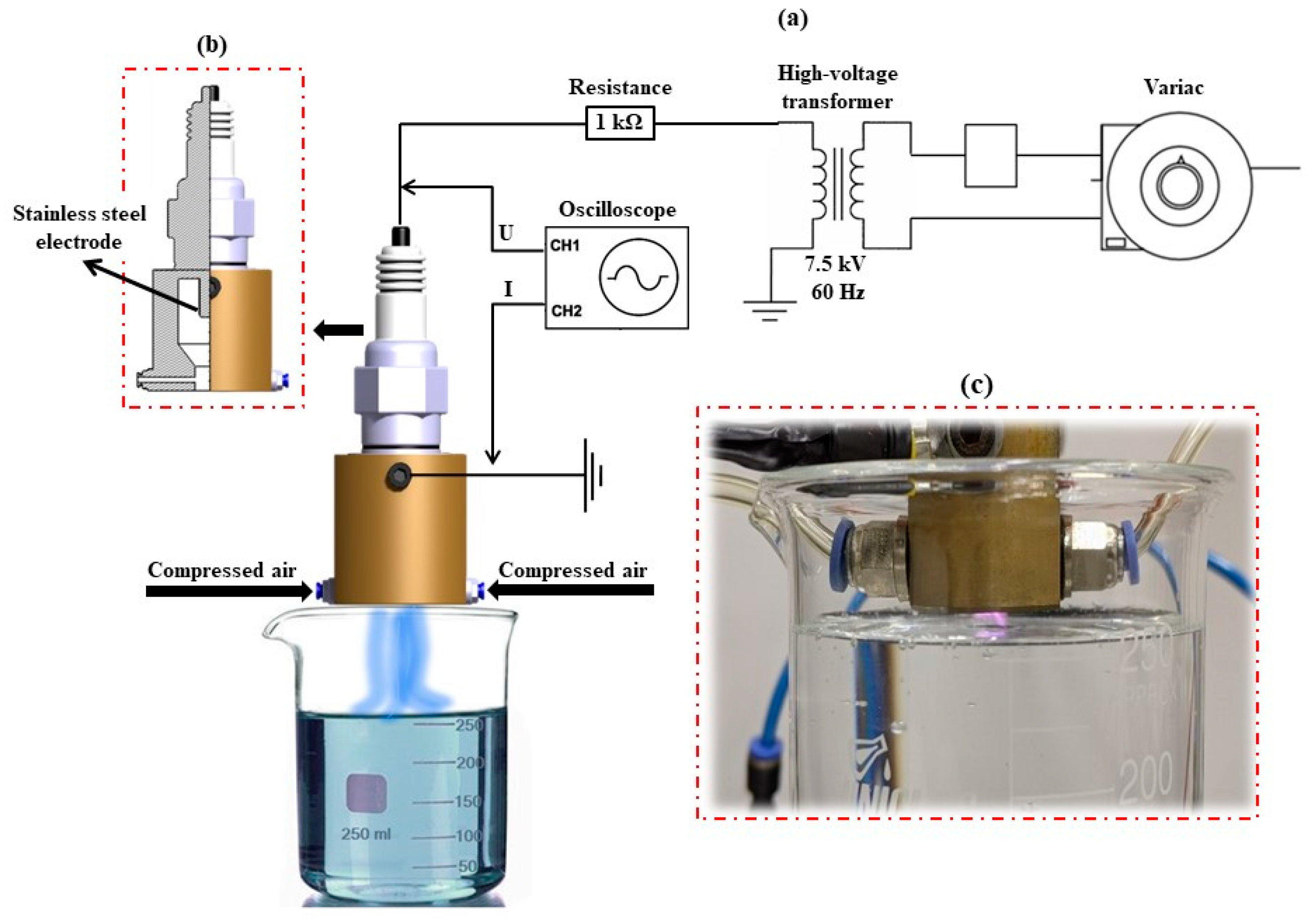
2.1. Experimental Configuration for Obtaining PAW
2.2. Water Physicochemical Properties Measurements
2.3. Microbiological Assays
2.3.1. Strains and Inocula Preparation
2.3.2. Antimicrobial Activity
2.3.3. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Measurements and Thermal Analysis of Water
3.1.1. Effect of Treatment Time
3.1.2. Effect of Water Dynamic Plasma Activation
3.1.3. Thermal Analysis of tap Water during Plasma Treatment
3.2. Microbiological Analyses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Subramanian, P.S.G.; Jain, A.; Shivapuji, A.M.; Sundaresan, N.R.; Dasappa, S.; Rao, L. Plasma-activated water from a dielectric barrier discharge plasma source for the selective treatment of cancer cells. Plasma Process. Polym. 2020, 17, 201900260. [Google Scholar] [CrossRef]
- Li, Y.; Pan, J.; Ye, G.; Zhang, Q.; Wang, J.; Zhang, J.; Fang, J. In vitro studies of the antimicrobial effect of non-thermal plasma-activated water as a novel mouthwash. Eur. J. Oral Sci. 2017, 125, 463. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Wang, S.; Li, B.; Qi, M.; Feng, R.; Li, Q.; Zhang, H.; Chen, H.; Kong, M.G. Effects of plasma-activated water on skin wound healing in mice. Microorganisms 2020, 8, 1091. [Google Scholar] [CrossRef] [PubMed]
- Bruggeman, P.; Leys, C. Non-thermal plasmas in and in contact with liquids. J. Phys. D Appl. Phys. 2009, 42, 053001. [Google Scholar] [CrossRef]
- Jablonowski, H.; von Woedtke, T. Research on plasma medicine-relevant plasma–liquid interaction: What happened in the past five years? Clin. Plasma Med. 2015, 3, 42–52. [Google Scholar] [CrossRef]
- Ramli, N.A.H.; Zaaba, S.K.; Mustaffa, M.T.; Zakaria, A.; Ab, S. Review on the development of plasma discharge in liquid solution. AIP Conf. Proc. 2017, 1824, 030015. [Google Scholar]
- Gamaleev, V.; Iwata, N.; Hori, M.; Hiramatsu, M.; Ito, M. Direct treatment of liquids using low-current arc in ambient air for biomedical applications. Appl. Sci. 2019, 9, 3505. [Google Scholar] [CrossRef] [Green Version]
- Zhou, R.; Zhou, R.; Wang, P.; Xian, Y.; Mai-Prochnow, A.; Lu, X.; Cullen, P.J.; Ostrikov, K.; Bazaka, K. Plasma-activated water: Generation, origin of reactive species and biological applications. J. Phys. D Appl. Phys. 2020, 53, 303001. [Google Scholar] [CrossRef]
- Yoon, S.-Y.; Jeon, H.; Yi, C.; Park, S.; Ryu, S.; Kim, S.B. Mutual interaction between plasma characteristics and liquid properties in AC-driven pin-to-liquid discharge. Sci. Rep. 2018, 8, 12037. [Google Scholar] [CrossRef]
- Zhang, J.J.; Kwon, T.; Kim, S.B.; Jeong, D.K. Plasma farming: Non-thermal dielectric barrier discharge plasma technology for improving the growth of soybean sprouts and chickens. Plasma 2018, 1, 285–296. [Google Scholar] [CrossRef] [Green Version]
- Ito, M.; Oh, J.-S.; Ohta, T.; Shiratani, M.; Hori, M. Current status and future prospects of agricultural applications using atmospheric-pressure plasma technologies. Plasma Proc. Polym. 2017, 15, e1700073. [Google Scholar] [CrossRef]
- Judée, F.; Simon, S.; Bailly, C.; Dufour, T. Plasma-activation of tap water using DBD for agronomy applications: Identification and quantification of long lifetime chemical species and production/consumption mechanisms. Water Res. 2018, 133, 47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Zhou, R.; Bazaka, K.; Liu, Y.; Zhou, R.; Chen, G.; Chen, Z.; Liu, Q.; Yang, S.; Ostrikov, K. Quantification of plasma produced OH radical density for water sterilization. Plasma Proc. Polym. 2018, 15, e1700241. [Google Scholar] [CrossRef]
- Zhang, Z.; Shen, J.; Cheng, C.; Xu, Z.; Xia, W. Generation of reactive species in atmospheric pressure dielectric barrier discharge with liquid water. Plasma Sci. Tech. 2018, 20, 044009. [Google Scholar] [CrossRef] [Green Version]
- Nascimento, L.; Gasi, F.; Landers, R.; da Silva Sobrinho, A.; Aragão, E.; Fraga, M.; Petraconi, G.; Chiappim, W.; Pessoa, R. Physicochemical studies on the surface of polyamide 6.6 fabrics functionalized by DBD plasmas operated at atmospheric and sub-atmospheric pressures. Polymers 2020, 12, 2128. [Google Scholar] [CrossRef] [PubMed]
- Miranda, F.S.; Rabelo, S.C.; Pradella, J.G.C.; Di Carli, C.; Petraconi, G.; Maciel, H.S.; Pessoa, R.S.; Vieira, L. Plasma in-liquid using non-contact electrodes: A method of pretreatment to enhance the enzymatic hydrolysis of biomass. Waste Biomass. Valor. 2020, 11, 4921–4931. [Google Scholar] [CrossRef]
- Figueira, F.R.; Doria, A.C.O.C.; Khouri, S.; Maciel, H.S.; Pessoa, R.S.; Ramos, M.A.R. Effect of storage temperature on pH and conductivity of reverse osmosis water treated with atmospheric plasma. Plasma Med. 2018, 8, 237–244. [Google Scholar] [CrossRef]
- Kolb, J.F.; Joshi, R.P.; Xiao, S.; Schoenbach, K.H. Streamers in water and other dielectric liquids. J. Phys. D Appl. Phys. 2008, 41, 234007. [Google Scholar] [CrossRef]
- Bruggeman, P.; Ribezl, E.; Maslani, A.; Degroote, J.; Malesevic, A.; Rego, R.; Vierendeels, J.; Leys, C. Characteristics of atmospheric pressure air discharges with a liquid cathode and a metal anode. Plasma Sources Sci. Technol. 2008, 17, 025012. [Google Scholar] [CrossRef]
- Chen, C.W.; Lee, H.-M.; Chang, M.B. Inactivation of aquatic microorganisms by low-frequency AC discharges. IEEE Trans. Plasma Sci. 2008, 36, 215. [Google Scholar] [CrossRef]
- Kovacevic, V.V.; Dojcinovic, B.P.; Jovic, M.; Roglic, G.M.; Obradovic, B.M.; Kuraica, M.M. Measurement of reactive species generated by dielectric barrier discharge in direct contact with water in different atmospheres. J. Phys. D Appl. Phys. 2017, 50, 155205. [Google Scholar] [CrossRef]
- Oehmigen, K.; Hähnel, M.; Brandenburg, R.; Wilke, C.; Weltmann, K.-D.; von Woedtke, T. The role of acidification for antimicrobial activity of atmospheric pressure plasma in liquids. Plasma Process. Polym. 2010, 7, 250. [Google Scholar] [CrossRef]
- Burlica, R.; Kirkpatrick, M.J.; Locke, B.R. Formation of reactive species in gliding arc discharges with liquid water. J. Electrostat. 2006, 64, 35. [Google Scholar] [CrossRef]
- Schmidt, M.; Hahn, V.; Altrock, B.; Gerling, T.; Gerber, I.C.; Weltmann, K.-D.; von Woedtke, T. Plasma-activation of larger liquid volumes by an inductively-limited discharge for antimicrobial purposes. Appl. Sci. 2019, 9, 2150. [Google Scholar] [CrossRef] [Green Version]
- Han, S.H.; Suh, H.J.; Hong, K.B.; Kim, S.Y.; Min, S.C. Oral Toxicity of Cold Plasma-Treated Edible Films for Food Coating. J. Food Sci. 2016, 81, T3052–T3057. [Google Scholar] [CrossRef]
- Borges, A.C.; Lima, G.M.G.; Nishime, T.M.C.; Gontijo, A.V.L.; Kostov, K.G.; Koga-Ito, C.Y. Amplitude-modulated cold atmospheric pressure plasma jet for treatment of oral candidiasis: In vivo study. PLoS ONE 2018, 13, e0199832. [Google Scholar] [CrossRef]
- İbiş, F.; Ercan, U.K. Inactivation of biofilms in endotracheal tube by cold atmospheric plasma treatment for control and prevention of ventilator-associated pneumonia. Plasma Proc. Polym. 2020, 17, 2000065. [Google Scholar] [CrossRef]
- Tang, Q.; Jiang, W.; Cheng, Y.; Lin, S.; Lim, T.M.; Xiong, J. Generation of reactive species by gas-phase dielectric barrier discharges. Ind. Eng. Chem. Res. 2011, 50, 9839. [Google Scholar] [CrossRef]
- Xu, H.; Liu, D.; Wang, W.; Liu, Z.; Guo, L.; Rong, M.; Kong, M.G. Investigation on the RONS and bactericidal effects induced by He + O2 cold plasma jets: In open air and in an airtight chamber. Phys. Plasmas 2018, 25, 113506. [Google Scholar] [CrossRef] [Green Version]
- Zhou, R.; Zhou, R.; Prasad, K.; Fang, Z.; Speight, R.; Bazaka, K.; Ostrikov, K. Cold atmospheric plasma activated water as a prospective disinfectant: The crucial role of peroxynitrite. Green Chem. 2018, 20, 5276. [Google Scholar] [CrossRef]
- Oh, J.-S.; Kakuta, M.; Furuta, H.; Akatsuka, H.; Hatta, A. Effect of plasma jet diameter on the efficiency of reactive oxygen and nitrogen species generation in water. Jpn. J. Appl. Phys. 2016, 55, 06HD01. [Google Scholar] [CrossRef]
- Chauvin, J.; Judée, F.; Yousfi, M.; Vicendo, P.; Merbahi, N. Analysis of reactive oxygen and nitrogen species generated in three liquid media by low temperature helium plasma jet. Sci. Rep. 2017, 7, 4562. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, K.; Oh, J.-S.; Gaur, N.; Hong, S.-H.; Kurita, H.; Mizuno, A.; Hatta, A.; Short, R.D.; Ito, M.; Szili, E.J. Modulating the concentrations of reactive oxygen and nitrogen species and oxygen in water with helium and argon gas and plasma jets. Jpn. J. Appl. Phys. 2019, 58, SAAB01. [Google Scholar] [CrossRef]
- Iwata, N.; Gamaleev, V.; Hashizume, H.; Oh, J.-S.; Ohta, T.; Ishikawa, K.; Hori, M.; Ito, M. Simultaneous achievement of antimicrobial property and plant growth promotion using plasma-activated benzoic compound solution. Plasma Proc. Polym. 2019, 16, e1900023. [Google Scholar] [CrossRef]
- Kim, D.Y.; Kim, S.J.; Joh, H.M.; Chung, T.H. Characterization of an atmospheric pressure plasma jet array and its application to cancer cell treatment using plasma activated medium. Phys. Plasma 2018, 25, 073505. [Google Scholar] [CrossRef]
- Zhou, R.; Zhou, R.; Zhuang, J.; Zong, Z.; Zhang, X.; Liu, D.; Bazaka, K.; Ostrikov, K. Interaction of atmospheric-pressure air microplasmas with amino acids as fundamental processes in aqueous solution. PLoS ONE 2016, 11, e0155584. [Google Scholar] [CrossRef] [Green Version]
- Gharagozalian, M.; Dorranian, D.; Ghoranneviss, M. Water treatment by the AC gliding arc air plasma. J. Theor. Appl. Phys. 2017, 11, 171. [Google Scholar] [CrossRef] [Green Version]
- Guofeng, X.; Xinwei, D. Electrical characterization of a reverse vortex gliding arc reactor in atmosphere. IEEE Trans. Plasma Sci. 2012, 40, 3458. [Google Scholar] [CrossRef]
- Doria, A.C.O.C.; Figueira, F.R.; de Lima, J.S.B.; Figueira, J.A.N.; Castro, A.H.R.; Sismanoglu, B.N.; Petraconi, G.; Maciel, H.S.; Khouri, S.; Pessoa, R.S. Inactivation of Candida albicans biofilms by atmospheric gliding arc plasma jet: Effect of gas chemistry/flow and plasma pulsing. Plasma Res. Express 2019, 1, 015001. [Google Scholar] [CrossRef] [Green Version]
- Hänsch, M.A.C.; Mann, M.; Weltmann, K.-D.; von Woedtke, T. Analysis of antibacterial efficacy of plasma-treated sodium chloride solutions. J. Phys. D Appl. Phys. 2015, 48, 454001. [Google Scholar] [CrossRef]
- Oehmigen, K.; Winter, J.; Hähnel, M.; Wilke, C.; Brandenburg, R.; Weltmann, K.-D.; von Woedtke, T. Estimation of possible mechanisms of Escherichia coli inactivation by plasma treated sodium chloride solution. Plasma Process. Polym. 2011, 8, 904. [Google Scholar] [CrossRef]
- Traylor, M.J.; Pavlovich, M.J.; Karim, S.; Hait, P.; Sakiyama, Y.; Clark, D.S.; Graves, D.B. Long-term antibacterial efficacy of air plasma-activated water. J. Phys. D Appl. Phys. 2011, 44, 472001. [Google Scholar] [CrossRef] [Green Version]
- Joslin, J.M.; McCall, J.R.; Bzdek, J.P.; Johnson, D.C.; Hybertson, B.M. Aqueous plasma pharmacy: Preparation methods, chemistry, and therapeutic applications. Plasma Med. 2016, 6, 135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inmetro. Available online: http://www.inmetro.gov.br/laboratorios/rble/docs/CRL0168.pdf (accessed on 28 January 2021).
- Miles, A.A.; Misra, S.S.; Irwin, J.O. The estimation of the bactericidal power of the blood. Blood J. Hyg. 1938, 38, 732–749. [Google Scholar] [CrossRef] [Green Version]
- Xiang, Q.; Liu, X.; Liu, S.; Ma, Y.; Xu, C.; Bai, Y. Effect of plasma-activated water on microbial quality and physicochemical characteristics of mung bean sprouts. Innov. Food Sci. Emer. Technol. 2019, 58, 49–56. [Google Scholar] [CrossRef]
- Xiang, Q.; Zhang, R.; Fan, L.; Ma, Y.; Wu, D.; Li, K.; Bai, Y. Microbial inactivation and quality of grapes treated by plasma-activated water combined with mild heat. LWT 2020, 126, 109336. [Google Scholar] [CrossRef]
- Zhao, Y.-M.; Ojha, S.; Burgess, C.M.; Sun, D.-W.; Tiwari, B.K. Inactivation efficacy and mechanisms of plasma activated water on bacteria in planktonic state. J. Appl. Microbiol. 2020, 129, 14677. [Google Scholar] [CrossRef]
- Chen, D.; Chen, P.; Cheng, Y.; Peng, P.; Liu, J.; Ma, Y.; Liu, Y.; Ruan, R. Deoxynivalenol decontamination in raw and germinating barley treated by plasma-activated water and intense pulsed light. Food Bioproc. Tech. 2019, 12, 246–254. [Google Scholar] [CrossRef]
- Lu, P.; Boehm, D.; Bourke, P.; Cullen, P.J. Achieving reactive species specificity within plasma-activated water through selective generation using air spark and glow discharges. Plasma Process. Polym. 2017, 14, e1600207. [Google Scholar] [CrossRef] [Green Version]
- Patange, A.; Lu, P.; Boehm, D.; Cullen, P.J.; Bourke, P. Efficacy of cold plasma functionalised water for improving microbiological safety of fresh produce and wash water recycling. Food Microbiol. 2019, 84, 103226. [Google Scholar] [CrossRef]
- Liao, L.B.; Chen, W.M.; Xiao, X.M. The generation and inactivation mechanism of oxidation–reduction potential of electrolyzed oxidizing water. J. Food Eng. 2007, 78, 1326–1332. [Google Scholar] [CrossRef]
- Lukes, P.; Clupek, M.; Babicky, V.; Sunka, P. Ultraviolet radiation from the pulsed corona discharge in water. Plasma Sources Sci. Technol. 2008, 17, 024012. [Google Scholar] [CrossRef]
- Suslow, T. Oxidation-Reduction Potential (ORP) for Water Disinfection Monitoring. Control and Documentation, University of California, Division of Agriculture and Natural Resources. Available online: https://doi.org/10.3733/ucanr.8149 (accessed on 10 April 2021).
- Strom, M.; Crowley, T.; Shigdar, S. Novel detection of nasty bugs, prevention is better than cure. Int. J. Mol. Sci. 2021, 22, 149. [Google Scholar] [CrossRef] [PubMed]
- Spampinato, C.; Leonardi, D. Candida infections, causes, targets, and resistance mechanisms: Traditional and alternative antifungal agents. Biomed. Res. Int. 2013, 2013, 204237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Xu, Z.; Cheng, C.; Wei, J.; Lan, Y.; Ni, G.; Sun, Q.; Qian, S.; Zhang, H.; Xia, W.; et al. Bactericidal effects of plasma induced reactive species in dielectric barrier gas–liquid discharge. Plasma Chem. Plasma Process. 2017, 37, 415–431. [Google Scholar] [CrossRef]
- Pemen, A.J.M.; van Ooij, P.P.; Beckers, F.J.C.M.; Hoeben, W.F.L.M.; Koonen-Reemst, A.M.C.B.; Huiskamp, T.; Leenders, P.H.M. Power modulator for high-yield production of plasma-activated water. IEEE Trans. Plasma Sci. 2017, 45, 2725–2733. [Google Scholar] [CrossRef]
- Balan, G.G.; Roşca, I.; Ursu, E.-L.; Doroftei, F.; Bostănaru, A.-C.; Hnatiuc, E.; Năstasă, V.; Şandru, V.; Ştefănescu, G.; Mareş, M. Plasma-activated water: A new and effective alternative for duodenoscope reprocessing. Infect. Drug Resit. 2018, 11, 727–733. [Google Scholar] [CrossRef] [Green Version]
- Laurita, R.; Barbieri, D.; Gherardi, M.; Colombo, V.; Lukes, P. Chemical analysis of reactive species and antimicrobial activity of water treated by nanosecond pulsed DBD air plasma. Clin. Plasma Med. 2015, 3, 53–61. [Google Scholar] [CrossRef]
- Garcia-Rubio, R.; de Oliveira, H.C.; Rivera, J.; Trevijano-Contador, N. The fungal cell wall: Candida, Cryptococcus, and Aspergillus species. Front. Microbiol. 2020, 10, 2993. [Google Scholar] [CrossRef]
- Hoeben, W.F.L.M.; van Ooij, P.P.; Schram, D.C.; Huiskamp, T.; Pemen, A.J.M.; Lukeš, P. On the possibilities of straightforward characterization of plasma activated water. Plasma Chem. Plasma Process. 2019, 39, 597–626. [Google Scholar] [CrossRef] [Green Version]
- Hefny, M.M.; Pattyn, C.; Lukes, P.; Benedikt, J. Atmospheric plasma generates oxygen atoms as oxidizing species in aqueous solutions. J. Phys. D Appl. Phys. 2016, 49, 404002. [Google Scholar] [CrossRef]
- Naïtali, M.; Kamgang-Youbi, G.; Herry, J.-M.; Bellon-Fontaine, M.-N.; Brisset, J.-L. Combined effects of long-living chemical species during microbial inactivation using atmospheric plasma-treated water. Appl. Environ. Microbiol. 2010, 76, 7662–7664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, L.; Guun, C.; Beckman, J.S. Bactericidal activity of peroxynitrite. Arch. Biochem. Biophys. 1992, 298, 452–457. [Google Scholar] [CrossRef]
- Thirumdas, R.; Kothakota, A.; Annapure, U.; Siliveru, K.; Blundell, R.; Gatt, R.; Valdramidis, V.P. Plasma activated water (PAW): Chemistry, physico-chemical properties, applications in food and agriculture. Trends Food Sci. Technol. 2018, 77, 21–31. [Google Scholar] [CrossRef]








| Time after PAW | Sensor Position | pH (±0.05) | Temperature (±1 °C) | ORP (±7 mV) | σ (±5 μS/cm) | TDS (±5 ppm) |
|---|---|---|---|---|---|---|
| Control sample | ||||||
| - | A to E | 6.55 | 25 | 27 | 220 | 150 |
| PAW sample—Stagnant water | ||||||
| 5 min | A | 2.77 | 43 | 239 | 540 | 370 |
| B | 2.80 | 43 | 237 | 480 | 330 | |
| C | 3.40 | 43 | 217 | 400 | 280 | |
| D | 5.55 | 39 | 75 | 280 | 190 | |
| E | 6.05 | 37 | 51 | 220 | 150 | |
| 2 h | A | 3.33 | 25 | 212 | 240 | 160 |
| B | 3.98 | 25 | 167 | 230 | 152 | |
| C | 3.96 | 25 | 165 | 220 | 150 | |
| D | 3.95 | 25 | 165 | 220 | 150 | |
| E | 3.96 | 25 | 165 | 220 | 150 | |
| 24 h | A | 3.85 | 25 | 183 | 220 | 150 |
| B | 3.85 | 25 | 183 | 220 | 150 | |
| C | 3.85 | 25 | 183 | 220 | 150 | |
| D | 3.85 | 25 | 183 | 220 | 150 | |
| E | 3.85 | 25 | 183 | 220 | 150 | |
| PAW sample—Stirred water | ||||||
| 5 min | A | 3.21 | 34 | 221 | 210 | 80 |
| B | 3.33 | 33 | 212 | 210 | 90 | |
| C | 3.32 | 33 | 211 | 220 | 150 | |
| D | 3.31 | 32 | 211 | 220 | 150 | |
| E | 3.48 | 32 | 199 | 220 | 150 | |
| 2 h | A | 3.53 | 25 | 215 | 220 | 150 |
| B | 3.53 | 25 | 215 | 220 | 150 | |
| C | 3.53 | 25 | 215 | 220 | 150 | |
| D | 3.53 | 25 | 215 | 220 | 150 | |
| E | 3.53 | 25 | 215 | 220 | 150 | |
| 24 h | A | 3.53 | 25 | 215 | 220 | 150 |
| B | 3.53 | 25 | 215 | 220 | 150 | |
| C | 3.53 | 25 | 215 | 220 | 150 | |
| D | 3.53 | 25 | 215 | 220 | 150 | |
| E | 3.53 | 25 | 215 | 220 | 150 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiappim, W.; Sampaio, A.d.G.; Miranda, F.; Fraga, M.; Petraconi, G.; da Silva Sobrinho, A.; Kostov, K.; Koga-Ito, C.; Pessoa, R. Antimicrobial Effect of Plasma-Activated Tap Water on Staphylococcus aureus, Escherichia coli, and Candida albicans. Water 2021, 13, 1480. https://doi.org/10.3390/w13111480
Chiappim W, Sampaio AdG, Miranda F, Fraga M, Petraconi G, da Silva Sobrinho A, Kostov K, Koga-Ito C, Pessoa R. Antimicrobial Effect of Plasma-Activated Tap Water on Staphylococcus aureus, Escherichia coli, and Candida albicans. Water. 2021; 13(11):1480. https://doi.org/10.3390/w13111480
Chicago/Turabian StyleChiappim, William, Aline da Graça Sampaio, Felipe Miranda, Mariana Fraga, Gilberto Petraconi, Argemiro da Silva Sobrinho, Konstantin Kostov, Cristiane Koga-Ito, and Rodrigo Pessoa. 2021. "Antimicrobial Effect of Plasma-Activated Tap Water on Staphylococcus aureus, Escherichia coli, and Candida albicans" Water 13, no. 11: 1480. https://doi.org/10.3390/w13111480
APA StyleChiappim, W., Sampaio, A. d. G., Miranda, F., Fraga, M., Petraconi, G., da Silva Sobrinho, A., Kostov, K., Koga-Ito, C., & Pessoa, R. (2021). Antimicrobial Effect of Plasma-Activated Tap Water on Staphylococcus aureus, Escherichia coli, and Candida albicans. Water, 13(11), 1480. https://doi.org/10.3390/w13111480









