Size Spectra of Pelagic Fish Populations in a Deep Lake—Methodological Comparison between Hydroacoustics and Midwater Trawling
Abstract
:1. Introduction
2. Materials and Methods
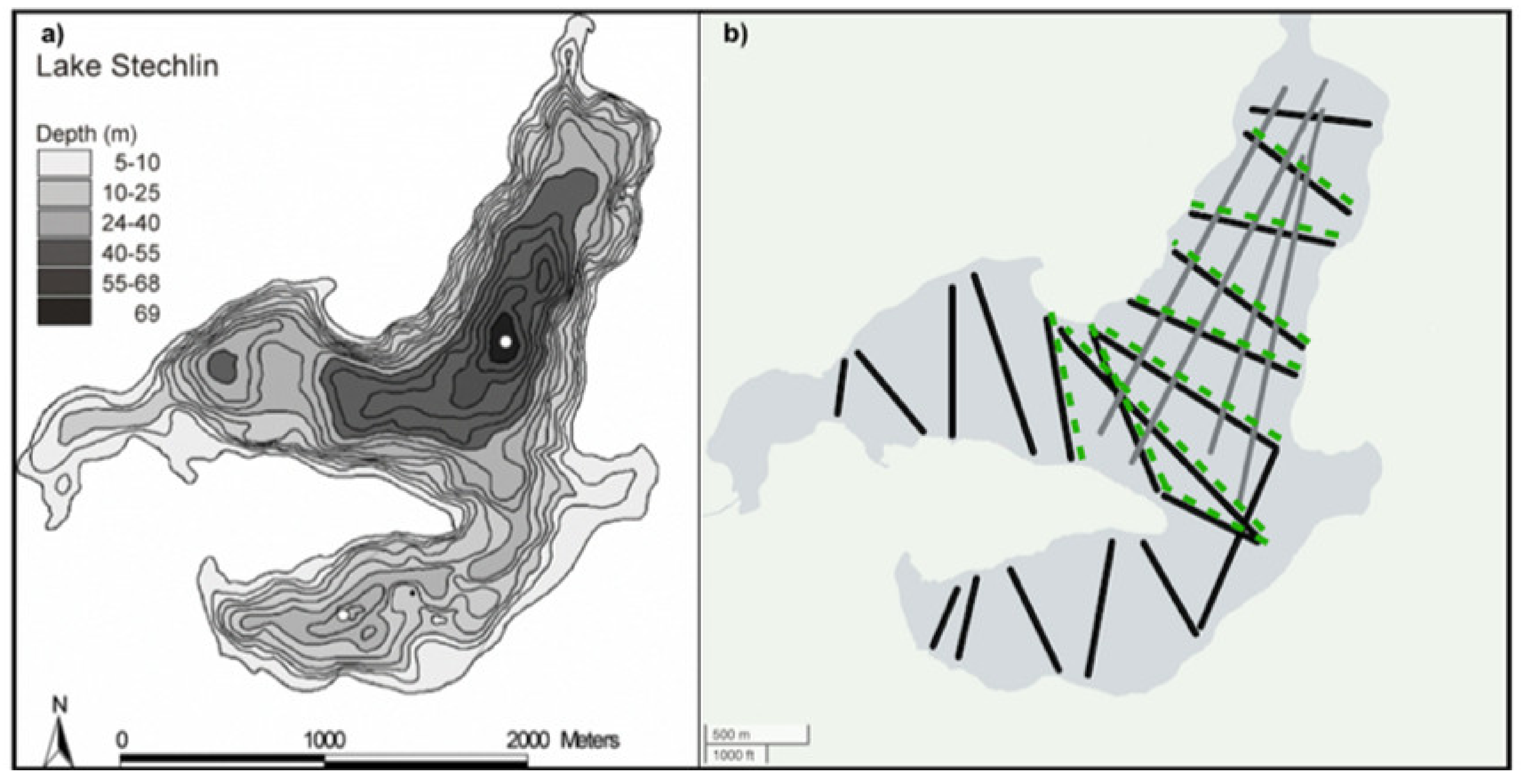
2.1. Study Site and Sampling
2.2. Fish Assessment—Trawling
2.3. Hydroacoustic Fish Assessment
2.4. Hydroacoustic Data Processing
2.5. Hydroacoustic Data Analysis Methods
2.6. Fish Size Distribution
2.7. Size Spectrum Fitting
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
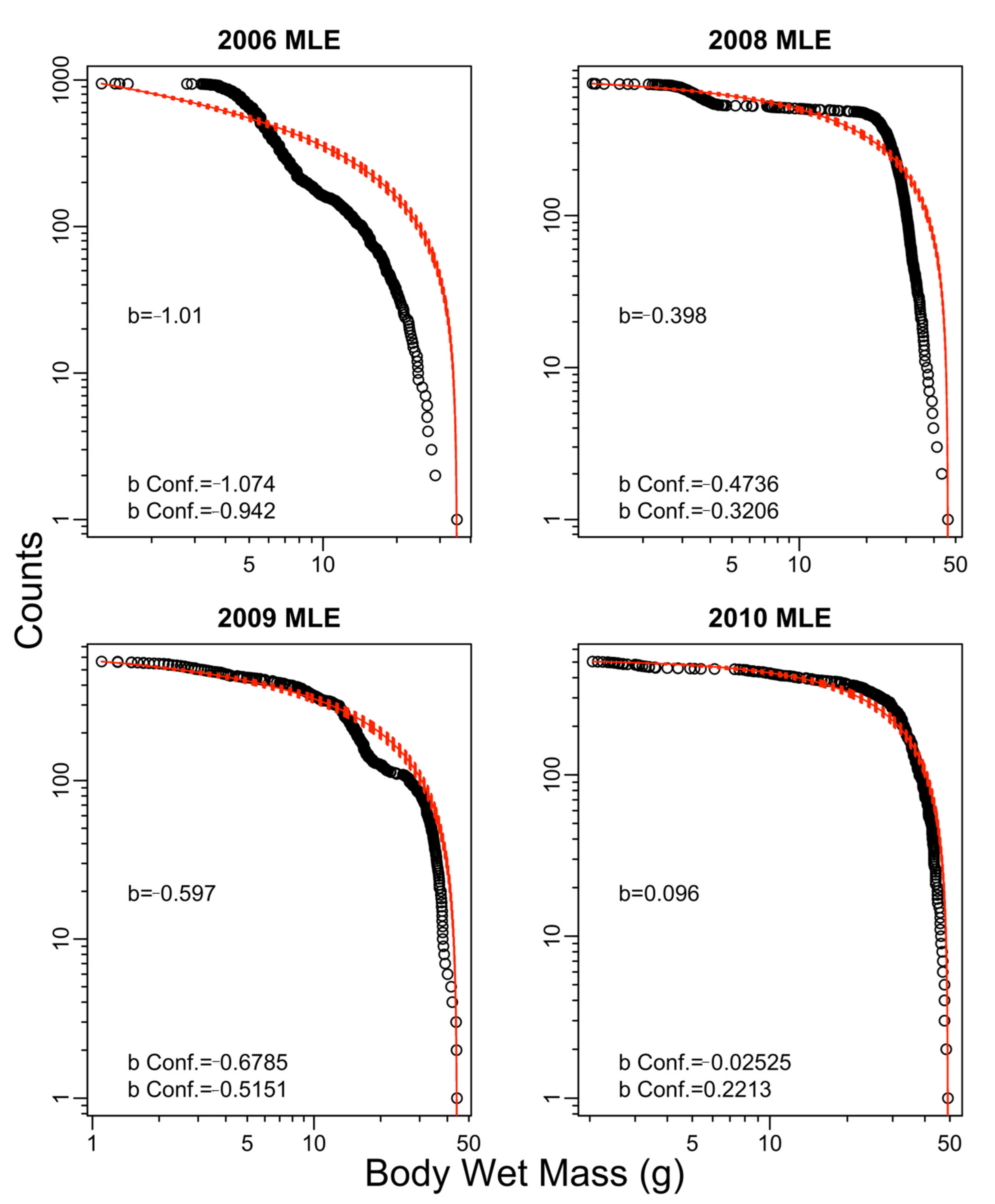


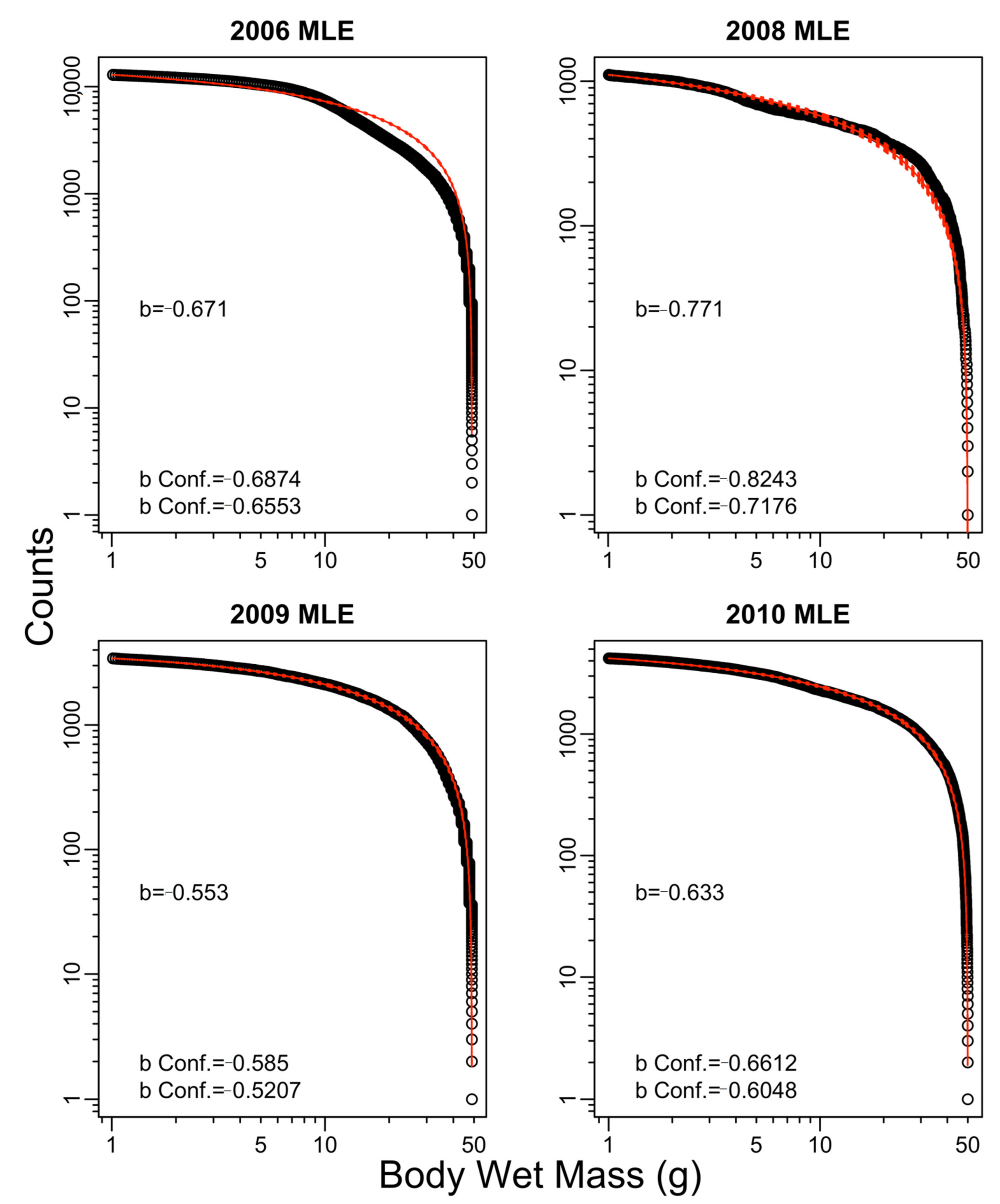
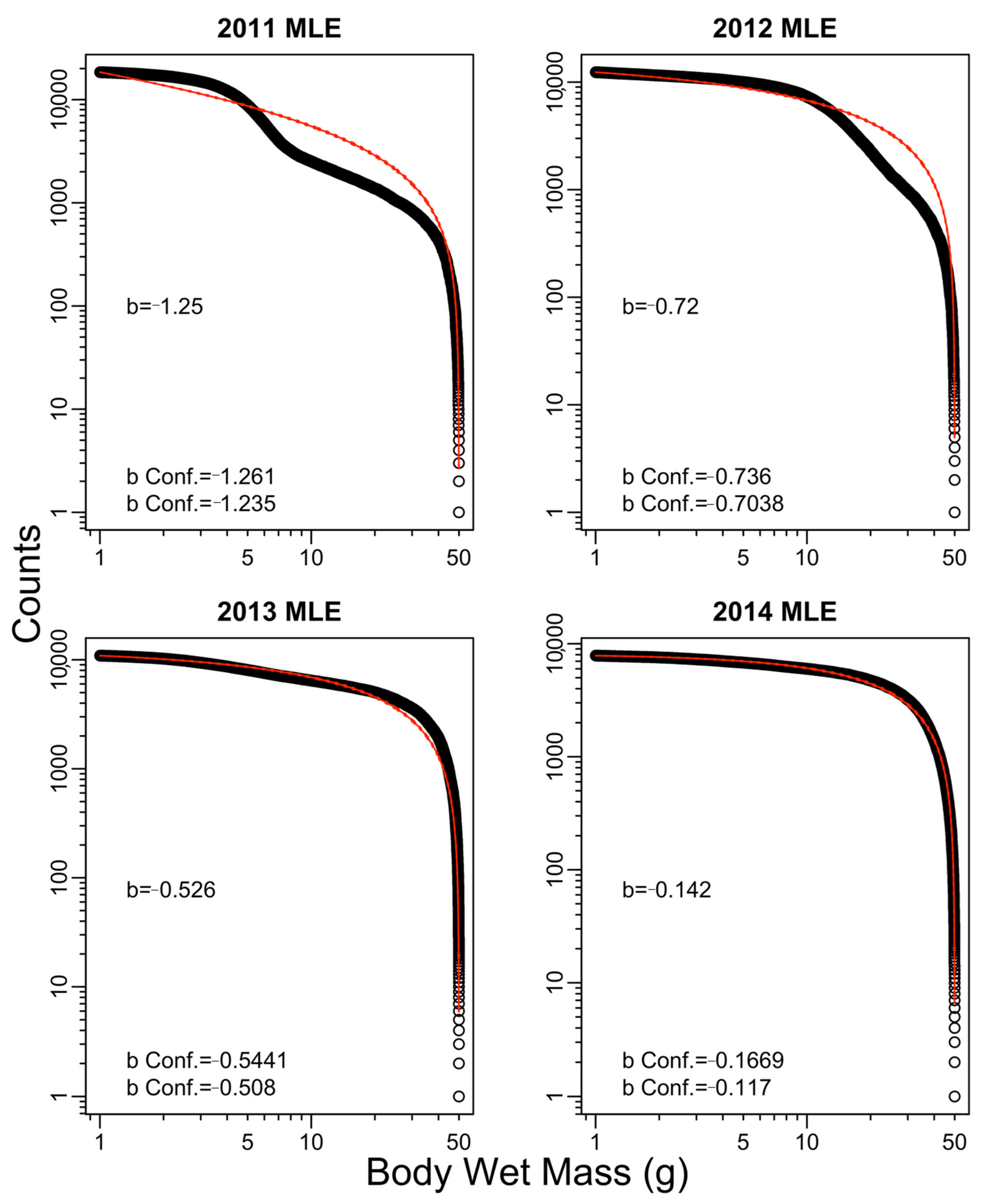


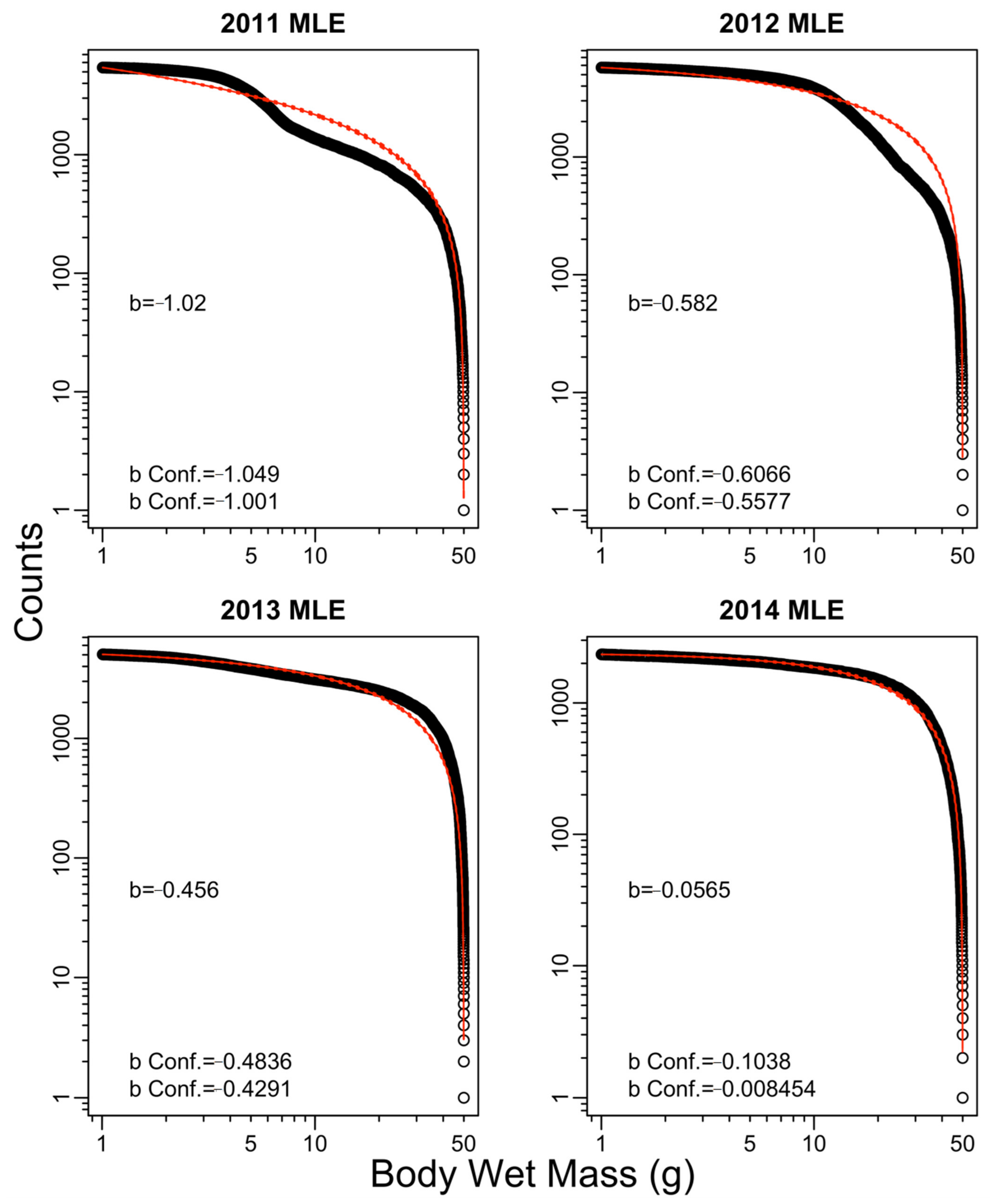

Appendix B
| Year | Exponent b | ||||
|---|---|---|---|---|---|
| TEG (WL) | SED (WL) | TEG (CB) | SED (CB) | Trawl | |
| 2006 | −0.56 | −0.67 | −0.61 | −0.71 | −1.01 |
| 2008 | −0.61 | −0.77 | −0.69 | −0.88 | −0.40 |
| 2009 | −0.41 | −0.55 | −0.32 | −0.50 | −0.60 |
| 2010 | −0.42 | −0.63 | −0.43 | −0.65 | 0.10 |
| 2011 | −1.02 | −1.25 | −1.03 | −1.25 | −0.68 |
| 2012 | −0.58 | −0.72 | −0.59 | −0.72 | −1.44 |
| 2013 | −0.46 | −0.53 | −0.45 | −0.54 | −0.76 |
| 2014 | −0.06 | −0.14 | −0.09 | −0.16 | 0.42 |
| 2015 | −0.43 | −0.67 | −0.45 | −0.67 | 0.04 |
| 2017 | −0.24 | −0.77 | −0.29 | −0.73 | −2.01 |
| 2018 | −0.15 | −0.24 | −0.15 | −0.22 | −0.79 |
| 2019 | −0.35 | −0.56 | −0.38 | −0.57 | 0.31 |
References
- Emmrich, M.; Brucet, S.; Ritterbusch, D.; Mehner, T. Size spectra of lake fish assemblages: Responses along gradients of general environmental factors and intensity of lake-use. Freshw. Biol. 2011, 56, 2316–2333. [Google Scholar] [CrossRef]
- Murry, B.A.; Farrell, J.M. Resistance of the size structure of the fish community to ecological perturbations in a large river ecosystem. Freshw. Biol. 2014, 59, 155–167. [Google Scholar] [CrossRef]
- Marquet, P.A.; Quiñones, R.A.; Abades, S.; Labra, F.; Tognelli, M.; Arim, M.; Rivadeneira, M. Scaling and power-laws in ecological systems. J. Exp. Biol. 2005, 208, 1749–1769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woodward, G.; Ebenman, B.; Emmerson, M.; Montoya, J.M.; Olesen, J.M.; Valido, A.; Warren, P.H. Body size in ecological networks. Trends Ecol. Evol. 2005, 20, 402–409. [Google Scholar] [CrossRef] [Green Version]
- Peters, R. The Ecological Implications of Body Size; Cambridge University Press: Cambridge, UK, 1983. [Google Scholar]
- Calder, W. Size, Function, and Life History; Harvard University Press: Cambridge, MA, USA, 1984. [Google Scholar]
- Thiebaux, M.L.; Dickie, L.M. Models of aquatic biomass size spectra and the common structure of their solutions. J. Theor. Biol. 1992, 159, 147–161. [Google Scholar] [CrossRef]
- Trebilco, R.; Baum, J.K.; Salomon, A.K.; Dulvy, N.K. Ecosystem ecology: Size-based constraints on the pyramids of life. Trends Ecol. Evol. 2013, 28, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.W.; Prakash, A.; Sutcliffe, W.H. The size distribution of particles in the ocean. Limnol. Oceanogr. 1972, 17, 327–340. [Google Scholar] [CrossRef] [Green Version]
- Kerr, S.; Dickie, L. The Biomass Spectrum: A Predator-Prey Theory of Aquatic Production; Columbia University Press: New York, NY, USA, 2001. [Google Scholar]
- Shin, Y.-J.; Rochet, M.-J.; Jennings, S.; Field, J.G.; Gislason, H. Using size-based indicators to evaluate the ecosystem effects of fishing. ICES J. Mar. Sci. 2005, 62, 384–396. [Google Scholar] [CrossRef] [Green Version]
- Ahrens, M.A.; Peters, R.H. Plankton community respiration: Relationships with size distribution and lake trophy. Hydrobiologia 1991, 224, 77–87. [Google Scholar] [CrossRef]
- Sprules, W.G.; Barth, L.E. Surfing the biomass size spectrum: Some remarks on history, theory, and application. Can. J. Fish. Aquat. Sci. 2016, 73, 477–495. [Google Scholar] [CrossRef]
- Blanchard, J.L.; Dulvy, N.K.; Jennings, S.; Ellis, J.R.; Pinnegar, J.K.; Tidd, A.; Kell, L.T. Do climate and fishing influence size-based indicators of Celtic Sea fish community structure? ICES J. Mar. Sci. 2005, 62, 405–411. [Google Scholar] [CrossRef]
- Bianchi, G.; Gislason, H.; Graham, K.; Hill, L.; Jin, X.; Koranteng, K.; Manickchand-Heileman, S.; Payá, I.; Sainsbury, K.; Sanchez, F.; et al. Impact of fishing on size composition and diversity of demersal fish communities. ICES J. Mar. Sci. 2000, 57, 558–571. [Google Scholar]
- Cottingham, K. Nutrients and zooplankton as multiple stressors of phytoplankton communities: Evidence from size structure. Limnol. Oceanogr. 1999, 44, 810–827. [Google Scholar] [CrossRef]
- Cózar, A.; García, C.M.; Gálvez, J.A. Analysis of plankton size spectra irregularities in two subtropical shallow lakes (Esteros del Iberá, Argentina). Can. J. Fish. Aquat. Sci. 2003, 60, 411–420. [Google Scholar] [CrossRef]
- Chopin, F.S.; Arimoto, T. The condition of fish escaping from fishing gears—A review. Fish. Res. 1995, 21, 315–327. [Google Scholar] [CrossRef]
- Olin, M.; Malinen, T.; Ruuhijärvi, J. Gillnet catch in estimating the density and structure of fish community-Comparison of gillnet and trawl samples in a eutrophic lake. Fish. Res. 2009, 88–94. [Google Scholar] [CrossRef]
- Bonvechio, T.F.; Pouder, W.F.; Hale, M.M. Variation between Electrofishing and Otter Trawling for Sampling Black Crappies in Two Florida Lakes. N. Am. J. Fish. Manag. 2008, 28, 188–192. [Google Scholar] [CrossRef]
- Prchalová, M.; Mrkvička, T.; Peterka, J.; Čech, M.; Berec, L.; Kubečka, J. A model of gillnet catch in relation to the catchable biomass, saturation, soak time and sampling period. Fish. Res. 2011, 107, 201–209. [Google Scholar] [CrossRef]
- Simmonds, J.; MacLennan, D.N. Fisheries Acoustics Theory and Practice, 2nd ed.; John Wiley & Sons: New York, NY, USA, 2008. [Google Scholar]
- Wheeland, L.J.; Rose, G.A. Acoustic measures of lake community size spectra. Can. J. Fish. Aquat. Sci. 2016, 73, 557–564. [Google Scholar] [CrossRef] [Green Version]
- De Kerckhove, D.T.; Shuter, B.J.; Milne, S. Acoustically derived fish size spectra within a lake and the statistical power to detect environmental change. Can. J. Fish. Aquat. Sci. 2016, 73, 565–574. [Google Scholar] [CrossRef] [Green Version]
- Mehner, T.; Kasprzak, P.; Hölker, F. Exploring ultimate hypotheses to predict diel vertical migrations in coregonid fish. Can. J. Fish. Aquat. Sci. 2007, 64, 874–886. [Google Scholar] [CrossRef]
- Emmrich, M.; Helland, I.P.; Busch, S.; Schiller, S.; Mehner, T. Hydroacoustic estimates of fish densities in comparison with stratified pelagic trawl sampling in two deep, coregonid-dominated lakes. Fish. Res. 2010, 105, 178–186. [Google Scholar] [CrossRef]
- Emmrich, M.; Winfield, I.J.; Guillard, J.; Rustabakken, A.; Verges, C.; Volta, P.; Jeppesen, E.; Lauridsen, T.L.; Brucet, S.; Holmgren, K.; et al. Strong correspondence between gillnet catch per unit effort and hydroacoustically derived fish biomass in stratified lakes. Freshw. Biol. 2012, 57, 2436–2448. [Google Scholar] [CrossRef] [Green Version]
- Draštík, V.; Godlewska, M.; Balk, H.; Clabburn, P.; Kubečka, J.; Morrissey, E.; Hateley, J.; Winfield, I.J.; Mrkvička, T.; Guillard, J. Fish hydroacoustic survey standardization: A step forward based on comparisons of methods and systems from vertical surveys of a large deep lake. Limnol. Oceanogr. Methods 2017, 15, 836–846. [Google Scholar] [CrossRef]
- Braun, L.-M.; Brucet, S.; Mehner, T. Top-down and bottom-up effects on zooplankton size distribution in a deep stratified lake. Aquat. Ecol. 2021, 8, 1–17. [Google Scholar] [CrossRef]
- Selmeczy, G.B.; Tapolczai, K.; Casper, P.; Krienitz, L.; Padisák, J. Spatial-and niche segregation of DCM-forming cyanobacteria in Lake Stechlin (Germany). Hydrobiologia 2016, 764, 229–240. [Google Scholar] [CrossRef]
- Bartrons, M.; Mehner, T.; Argillier, C.; Beklioglu, M.; Blabolil, P.; Hesthagen, T.; Holmgren, K.; Jeppesen, E.; Krause, T.; Podgornik, S.; et al. Energy-based top-down and bottom-up relationships between fish community energy demand or production and phytoplankton across lakes at a continental scale. Limnol. Oceanogr. 2020, 65, 892–902. [Google Scholar] [CrossRef]
- Koschel, R.; Adams, D. Lake Stechlin—An approach to understanding an oligotrophic lowland lake. Adv. Limnol. 2003, 58, 1–311. [Google Scholar]
- Anwand, K.; Valentin, M.; Mehner, T. Species composition, growth and feeding ecology of fish community in Lake Stechlin—An overview. Adv. Limnol. 2003, 58, 237–246. [Google Scholar]
- Mehner, T.; Schulz, M. Monthly variability of hydroacoustic fish stock estimates in a deep lake and its correlation to gillnet catches. J. Fish Biol. 2002, 61, 1109–1121. [Google Scholar] [CrossRef]
- Helland, I.P.; Freyhof, J.; Kasprzak, P.; Mehner, T. Temperature sensitivity of vertical distributions of zooplankton and planktivorous fish in a stratified lake. Oecologia 2007, 151, 322–330. [Google Scholar] [CrossRef]
- Wanke, T.; Brämick, U.; Mehner, T. High stock density impairs growth, female condition and fecundity, but not quality of early reproductive stages in vendace (Coregonus albula). Fish. Res. 2017, 186, 159–167. [Google Scholar] [CrossRef]
- Mehner, T. Prediction of hydroacoustic target strength of vendace (Coregonus albula) from concurrent trawl catches. Fish. Res. 2006, 79, 162–169. [Google Scholar] [CrossRef]
- Balk, H.; Lindem, T. Sonar-4 and Sonar-5 Post Processing System; Operator Manual Version 604; Lindem Data Acquisition: Oslo, Norway, 2017. [Google Scholar]
- Edwards, A.M.; Robinson, J.P.W.; Plank, M.J.; Baum, J.K.; Blanchard, J.L. Testing and recommending methods for fitting size spectra to data. Methods Ecol. Evol. 2017, 8, 57–67. [Google Scholar] [CrossRef]
- Page, R. Aftershocks and microaftershocks of the great Alaska earthquake of 1964. Bull. Seismol. Soc. Am. 1968, 58, 1131–1168. [Google Scholar]
- Edwards, A.M. Overturning conclusions of Lévy flight movement patterns by fishing boats and foraging animals. Ecology 2011, 92, 1247–1257. [Google Scholar] [CrossRef]
- Hilborn, R.; Mangel, M. The Ecological Detective. Confronting Models with Data; Princeton University Press: Princeton, NJ, USA, 1997; Volume 28. [Google Scholar]
- Shapiro, S.S.; Wilk, M.B. An analysis of variance test for normality (complete samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- Team R Core. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.r-project.org/ (accessed on 8 March 2020).
- Edwards A Accounting for the bin structure of data removes bias when fitting size spectra. Mar. Ecol. Prog. Ser. 2020, 636, 19–33. [CrossRef]
- Peck, M.A.; Buckley, L.J.; Bengtson, D.A. Effects of temperature and body size on the swimming speed of larval and juvenile Atlantic cod (Gadus morhua): Implications for individual-based modelling. Environ. Biol. Fishes 2006, 75, 419–429. [Google Scholar] [CrossRef]
- Schmidt, M.B. Reactions of vendace (Coregonus albula, Linnaeus 1758) towards an approaching pelagic pair-trawl observed by split-beam echosounding. Fish. Res. 2009, 96, 95–101. [Google Scholar] [CrossRef]
- Viljanen, M.; Väisänen, P.; Turunen, T. Fluctuations in year-class strength and growth of the vendace (Coregonus albula (L.)) in the small, mesohumic, oligotrophic Suomunjärvi, a lake in eastern Finland. Ann. Zool. Fennici 2004, 41, 241–248. [Google Scholar]
- Helminen, H.; Sarvala, J. Population regulation of vendace (Coregonus albula) in Lake Pyhäjärvi, southwest Finland. J. Fish Biol. 1994, 45, 387–400. [Google Scholar] [CrossRef]
- Auvinen, H.; Karjalainen, J.; Viljanen, M. Internationale Vereinigung für theoretische und angewandte Limnologie: Verhandlungen Fluctuation of year-class strength of vendace (Coregonus albula (L.)) in Lake Onkamo, eastern Finland. Verh. Inrernat. Verein. Limnol 2000, 27, 2057–2062. [Google Scholar] [CrossRef]
- Mehner, T.; Emmrich, M.; Kasprzak, P. Discrete thermal windows cause opposite response of sympatric cold-water fish species to annual temperature variability. Ecosphere 2011, 2, 1–16. [Google Scholar] [CrossRef]
- Arranz, I.; Mehner, T.; Benejam, L.; Argillier, C.; Holmgren, K.; Jeppesen, E.; Lauridsen, T.L.; Volta, P.; Winfield, I.J.; Brucet, S. Density-dependent effects as key drivers of intraspecific size structure of six abundant fish species in lakes across Europe. Can. J. Fish. Aquat. Sci. 2016, 73, 519–534. [Google Scholar] [CrossRef] [Green Version]
- Mehner, T.; Busch, S.; Helland, I.P.; Emmrich, M.; Freyhof, J. Temperature-related nocturnal vertical segregation of coexisting coregonids. Ecol. Freshw. Fish 2010, 19, 408–419. [Google Scholar] [CrossRef]
- Kubečka, J.; Hohausová, E.; Matěna, J.; Peterka, J.; Amarasinghe, U.S.; Bonar, S.A.; Hateley, J.; Hickley, P.; Suuronen, P.; Tereschenko, V.; et al. The true picture of a lake or reservoir fish stock: A review of needs and progress. Fish. Res. 2009, 96, 1–5. [Google Scholar] [CrossRef]




| Year | Depth (m) | Volume [m3] | WF | Rounded (WF) |
|---|---|---|---|---|
| 2009 | 14 | 15,167 | 1 | 1 |
| 2009 | 20 | 10,500 | 1.44 | 1 |
| 2009 | 32 | 5000 | 3.03 | 3 |
| 2009 | 40 | 8000 | 1.89 | 2 |
| Parameter | Value |
|---|---|
| Single-echo detection (SED) | |
| Target Strength threshold | −55 dB |
| Minimum target size | −80 dB |
| Minimum echo length | 0.5 |
| Maximum echo length | 1.6 |
| Maximum gain compensation | 3.0 dB |
| Maximum angle standard deviation | 0.80 |
| Tracked-echo group (TEG) | |
| Minimum number of pings in track | 3 pings |
| Maximum gap between single targets | 1 ping |
| Vertical gating range | 0.3 m |
| Exponent b | ||||
|---|---|---|---|---|
| Method | Mean | SD | Min | Max |
| Trawl | −0.56 | 0.72 | −2.00 | 0.41 |
| SEDWL | −0.73 | 0.27 | −1.37 | −0.36 |
| TEGWL | −0.44 | 0.24 | −1.2 | −0.05 |
| SEDCB | −0.63 | 0.28 | −1.25 | −0.16 |
| TEGCB | −0.45 | 0.25 | −1.03 | −0.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Braun, L.-M.; Mehner, T. Size Spectra of Pelagic Fish Populations in a Deep Lake—Methodological Comparison between Hydroacoustics and Midwater Trawling. Water 2021, 13, 1559. https://doi.org/10.3390/w13111559
Braun L-M, Mehner T. Size Spectra of Pelagic Fish Populations in a Deep Lake—Methodological Comparison between Hydroacoustics and Midwater Trawling. Water. 2021; 13(11):1559. https://doi.org/10.3390/w13111559
Chicago/Turabian StyleBraun, Lisa-Marie, and Thomas Mehner. 2021. "Size Spectra of Pelagic Fish Populations in a Deep Lake—Methodological Comparison between Hydroacoustics and Midwater Trawling" Water 13, no. 11: 1559. https://doi.org/10.3390/w13111559
APA StyleBraun, L. -M., & Mehner, T. (2021). Size Spectra of Pelagic Fish Populations in a Deep Lake—Methodological Comparison between Hydroacoustics and Midwater Trawling. Water, 13(11), 1559. https://doi.org/10.3390/w13111559






