Assessing the Impacts of Chloride and Sulfate Ions on Macroinvertebrate Communities in Ohio Streams
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Set
2.2. Individual Taxa Responses
2.3. Species Sensitivity Distributions
2.4. Bayesian Mixed Logistic Regression Model
3. Results
3.1. Taxa Sensitivities
3.2. Bayesian Logistic Regression Model

4. Discussion
Application of Results to Management
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Model Code for Bayesian Logistic Regression
- sink(“lrmod.txt”)cat(“model{for(i in 1:N){ac[i]~dbern(p[i])logit(p[i])<-alpha + beta1 * cl[i] + beta2 * qhei[i] + beta3 * tkn[i] + beta4 * mn[i] + beta5 * so4[i] + u[REG[i]]}alpha ~ dnorm(0, 100)sigma_a ~ dunif(0, 100)tau_a <- 1 / (sigma_a * sigma_a)for(j in 1:M){u[j]~dnorm(0,tau_a)}beta1 ~ dnorm(0.0,0.00001)beta2 ~ dnorm(0.0,0.00001)beta3 ~ dnorm(0.0,0.00001)beta4 ~ dnorm(0.0,0.00001)beta5 ~ dnorm(0.0,0.00001)}“, fill = TRUE)sink()
- line_init<-list(list(alpha = 40,beta1 = −2,beta2 = 0.05,beta3 = −2.8,beta4 = −0.5,beta5 = −1.1),list(alpha = 1,beta1 = −0.1,beta2 = 1,beta3 = −10,beta4 = −10,beta5 = −10),list(alpha = 0,beta1 = −0.5,beta2 = 0.01,beta3 = −1,beta4 = −1,beta5 = −0.5))
- modout<-autorun.jags(model = “lrmod.txt”,monitor = c(“alpha”, “beta1”,”beta2”,”beta3”,”beta4”,”beta5”,”u”),
- data = hwdata, n.chains = 3, inits = line_init, startsample = 51,000)
Appendix B. Correlations Between Water Chemistry Measures and Habitat Quality Scores Stratified by Ecoregion. Cl—Chloride; TKN—Total KJELDAHL Nitrogen; Mn—Manganese; SO4—Sulfate; Hard—Hardness
- Huron-Erie Lake PlainCl TKN Mn SO4 Hard QHEICl 1.00 0.04 −0.09 0.23 0.21 0.10TKN 0.04 1.00 0.28 −0.14 0.04 −0.06Mn −0.09 0.28 1.00 −0.05 0.21 0.10SO4 0.23 −0.14 −0.05 1.00 0.51 0.12Hard 0.21 0.04 0.21 0.51 1.00 0.09QHEI 0.10 −0.06 0.10 0.12 0.09 1.00------------------------------------------------------------
- Interior PlateauCl TKN Mn SO4 Hard QHEICl 1.00 0.17 −0.24 0.65 0.31 −0.16TKN 0.17 1.00 0.39 −0.07 −0.53 −0.24Mn −0.24 0.39 1.00 −0.42 −0.32 −0.22SO4 0.65 −0.07 −0.42 1.00 0.54 −0.07Hard 0.31 −0.53 −0.32 0.54 1.00 0.12QHEI −0.16 −0.24 −0.22 −0.07 0.12 1.00------------------------------------------------------------
- Erie-Ontario Lake PlainCl TKN Mn SO4 Hard QHEICl 1.00 0.27 −0.48 0.29 0.21 0.06TKN 0.27 1.00 0.07 0.02 −0.13 −0.11Mn −0.48 0.07 1.00 −0.10 0.02 −0.23SO4 0.29 0.02 −0.10 1.00 0.83 −0.09Hard 0.21 −0.13 0.02 0.83 1.00 −0.08QHEI 0.06 −0.11 −0.23 −0.09 −0.08 1.00------------------------------------------------------------
- Western Allegheny PlateauCl TKN Mn SO4 Hard QHEICl 1.00 0.42 0.10 0.24 0.32 −0.05TKN 0.42 1.00 0.46 0.14 0.23 −0.28Mn 0.10 0.46 1.00 0.13 0.16 −0.24SO4 0.24 0.14 0.13 1.00 0.85 0.01Hard 0.32 0.23 0.16 0.85 1.00 0.01QHEI −0.05 −0.28 −0.24 0.01 0.01 1.00------------------------------------------------------------
- Eastern Corn Belt PlainsCl TKN Mn SO4 Hard QHEICl 1.00 0.51 0.14 0.26 −0.10 −0.10TKN 0.51 1.00 0.43 0.21 −0.26 −0.31Mn 0.14 0.43 1.00 0.29 −0.04 −0.36SO4 0.26 0.21 0.29 1.00 0.30 −0.25Hard −0.10 −0.26 −0.04 0.30 1.00 0.00QHEI −0.10 −0.31 −0.36 −0.25 0.00 1.00
Appendix C. Frequencies of Taxa Groups Occurring at Less Than or Greater Than, the Hazard Concentration (HC5) for Chloride and Sulfate. Taxa Groups Are: C—Caddisfly, D—Dipterans, M—Mayfly, N—Non-Insects, O—Other, S—Stonefly, T—Midges in the Tribe Tanytarsini. Expected Frequencies are Based on the Formula for χ-Square
| Observed | Expected | |||
| Taxa Group | ≤HC5 | >HC5 | ≤HC5 | >HC% |
| Chloride | ||||
| C | 3 | 67 | 3.5121107 | 66.48789 |
| D | 7 | 176 | 9.1816609 | 173.8183 |
| M | 10 | 55 | 3.2612457 | 61.73875 |
| N | 3 | 108 | 5.5692042 | 105.4308 |
| O | 3 | 105 | 5.4186851 | 102.5813 |
| S | 2 | 16 | 0.9031142 | 17.09689 |
| T | 1 | 22 | 1.1539792 | 21.84602 |
| Sulfate | ||||
| C | 1 | 69 | 3.5251799 | 66.47482 |
| D | 11 | 163 | 8.7625899 | 165.2374 |
| M | 5 | 58 | 3.1726619 | 59.82734 |
| N | 9 | 94 | 5.1870504 | 97.81295 |
| O | 1 | 105 | 5.3381295 | 100.6619 |
| S | 1 | 16 | 0.8561151 | 16.14388 |
| T | 0 | 23 | 1.1582734 | 21.84173 |
Appendix D
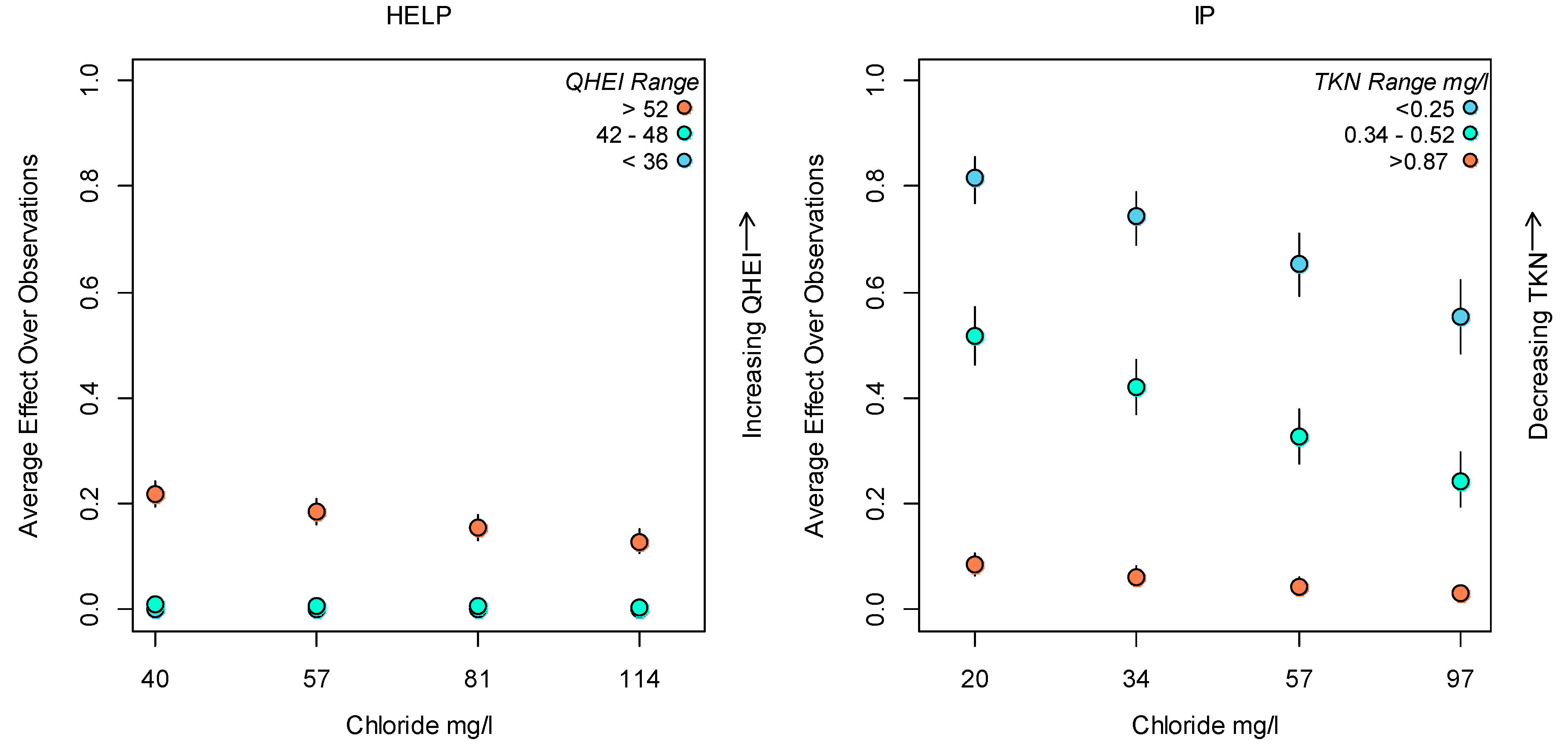
References
- Kefford, B.J.; Hickey, G.L.; Gasith, A.; Ben-David, E.; Dunlop, J.E.; Palmer, C.; Allan, K.; Choy, S.C.; Piscart, C. Global Scale Variation in the Salinity Sensitivity of Riverine Macroinvertebrates: Eastern Australia, France, Israel and South Africa. PLoS ONE 2012, 7, e35224. [Google Scholar] [CrossRef]
- Dowse, R.; Palmer, C.; Hills, K.; Torpy, F.; Kefford, B.J. The mayfly nymph Austrophlebioides pusillus Harker defies common osmoregulatory assumptions. R. Soc. Open Sci. 2017, 4, 160520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Timpano, A.J.; Schoenholtz, S.H.; Soucek, D.J.; Zipper, C.E. Benthic macroinvertebrate community response to salinization in headwater streams in Appalachia USA over multiple years. Ecol. Indic. 2018, 91, 645–656. [Google Scholar] [CrossRef]
- Kefford, B.J. Why are mayflies (Ephemeroptera) lost following small increases in salinity? Three conceptual osmophysiological hypotheses. Philos. Trans. R. Soc. B Biol. Sci. 2019, 374, 20180021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nowghani, F.; Chen, C.C.; Jonusaite, S.; Watson-Leung, T.; Kelly, S.P.; Donini, A. Impact of salt-contaminated freshwater on osmoregulation and tracheal gill function in nymphs of the mayfly Hexagenia rigida. Aquat. Toxicol. 2019, 211, 92–104. [Google Scholar] [CrossRef] [PubMed]
- Van Dam, R.A.; Hogan, A.C.; McCullough, C.D.; Houston, M.A.; Humphrey, C.L.; Harford, A. Aquatic toxicity of magnesium sulfate, and the influence of calcium, in very low ionic concentration water. Environ. Toxicol. Chem. 2010, 29, 410–421. [Google Scholar] [CrossRef]
- Mount, D.R.; Gulley, D.D.; Hockett, J.R.; Garrison, T.D.; Evans, J.M. Statistical models to predict the toxicity of major ions to Ceriodaphnia dubia, Daphnia magna and Pimephales promelas (fathead minnows). Environ. Toxicol. Chem. 1997, 16, 2009–2019. [Google Scholar] [CrossRef]
- Scheibener, S.; Conley, J.M.; Buchwalter, D. Sulfate transport kinetics and toxicity are modulated by sodium in aquatic insects. Aquat. Toxicol. 2017, 190, 62–69. [Google Scholar] [CrossRef]
- Soucek, D.J.; Kennedy, A.J. Effects of Hardness, Chloride, and Acclimation on the Acute Toxicity of Sulfate to Freshwater Invertebrates. Environ. Toxicol. Chem. 2005, 24, 1204–1210. [Google Scholar] [CrossRef]
- Buchwalter, D.; Scheibener, S.; Chou, H.; Soucek, D.; Elphick, J. Are sulfate effects in the mayfly Neocloeon triangulifer driven by the cost of ion regulation? Philos. Trans. R. Soc. B Biol. Sci. 2019, 374, 20180013. [Google Scholar] [CrossRef] [Green Version]
- Johnson, B.R.; Weaver, P.C.; Nietch, C.T.; Lazorchak, J.; Struewing, K.A.; Funk, D.H. Elevated major ion concentrations inhibit larval mayfly growth and development. Environ. Toxicol. Chem. 2014, 34, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Hassell, K.L.; Kefford, B.; Nugegoda, D. Sub-lethal and chronic salinity tolerances of three freshwater insects: Cloeon sp. and Centroptilum sp. (Ephemeroptera: Baetidae) and Chironomus sp. (Diptera: Chironomidae). J. Exp. Biol. 2006, 209, 4024–4032. [Google Scholar] [CrossRef] [Green Version]
- Clements, W.H.; Kotalik, C. Effects of major ions on natural benthic communities: An experimental assessment of the US Environmental Protection Agency aquatic life benchmark for conductivity. Freshw. Sci. 2016, 35, 126–138. [Google Scholar] [CrossRef]
- Fleetwood, M.J. Effect of Winter Road Salt Application and Episodic Pulses on Southern Appalachian Headwater Stream Macroinvertebrates. Ph.D. Thesis, Appalachian State University, Boone, NC, USA, 2017. [Google Scholar]
- Boehme, E.A.; Zipper, C.; Schoenholtz, S.H.; Soucek, D.J.; Timpano, A.J. Temporal dynamics of benthic macroinvertebrate communities and their response to elevated specific conductance in Appalachian coalfield headwater streams. Ecol. Indic. 2016, 64, 171–180. [Google Scholar] [CrossRef] [Green Version]
- Hintz, W.D.; Jones, D.K.; Relyea, R.A. Evolved tolerance to freshwater salinization in zooplankton: Life-history trade-offs, cross-tolerance and reducing cascading effects. Philos. Trans. R. Soc. B Biol. Sci. 2019, 374, 20180012. [Google Scholar] [CrossRef] [Green Version]
- Cormier, S.M.; Ii, G.W.S.; Zheng, L. Derivation of a benchmark for freshwater ionic strength. Environ. Toxicol. Chem. 2012, 32, 263–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Robertson, D.; Garrison, P.J. Linkages between Nutrients and Assemblages of Macroinvertebrates and Fish in Wadeable Streams: Implication to Nutrient Criteria Development. Environ. Manag. 2007, 39, 194–212. [Google Scholar] [CrossRef]
- Govenor, H.; Krometis, L.A.H.; Willis, L.; Angermeier, P.L.; Hession, W.C. Macroinvertebrate sensitivity thresholds for sediment in Virginia streams. Integr. Environ. Assess. Manag. 2018, 15, 77–92. [Google Scholar] [CrossRef] [PubMed]
- Van Ael, E.; De Cooman, W.; Blust, R.; Bervoets, L. Use of a macroinvertebrate based biotic index to estimate critical metal concentrations for good ecological water quality. Chemosphere 2015, 119, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Charles, E.S.; Donald, I.M.; David, J.H.; John, R.G.; Gary, A.C.; William, A.B. Guidelines for Deriving Numerical National Water Quality Criteria for the Protection of Aquatic Organisms and Their Uses; US Environmental Protection Agency: Washington, DC, USA, 1985.
- USEPA. A Practitioner’s Guide to the Biological Condition Gradient: A Framework to Describe Incremental Change in Aquatic Ecosystems; U.S. Environmental Protection Agency: Washington, DC, USA, 2016.
- Rankin, E.T. Habitat Indices in Water Resource Quality Assessments. Biological Assessment and Criteria: Tools for Water Resource Planning and Decision Making; CRC Press: Boca Raton, FL, USA, 1995; pp. 181–208. [Google Scholar]
- Rankin, E.T. Methods for Assessing Habitat in Flowing Waters: Using the Qualitative Habitat Evaluation Index (QHEI); Ohio EPA, Division of Surface Water: Groveport, OH, USA, 2006. [Google Scholar]
- Standard Methods for the Examination of Water and Wastewater, 19th ed.; American Public Health Association: Washington, DC, USA, 1998.
- Ohio, E.P.A. Manual of Ohio EPA Surveillance Methods and Quality Assurance Practices; Division of Surface Water: Columbus, OH, USA, 2012.
- Barbour, M.T.; Gerritsen, J.; Snyder, B.D.; Stribling, J.B. Rapid Bioassessment Protocols for Use in Streams and Wadeable Rivers: Periphyton, Benthic Macroinvertebrates and Fish; US Environmental Protection Agency, Office of Water: Washington, DC, USA, 1999; Volume 339.
- Ohio EPA. 2006 Updates to Biological Criteria for the Protection of Aquatic Life: Volume II and Volume II Addendum. Users Manual for Biological Field Assessment of Ohio Surface Waters; Divisionof Surface Water, Ecological Assessment Section: Columbus, OH, USA, 2006.
- Miltner, R.; McLaughlin, D. Management of headwaters based on macroinvertebrate assemblages and environ-mental attributes. Sci. Total Environ. 2019, 650, 438–451. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; Available online: http://www.R-project.org/ (accessed on 6 July 2019).
- Cormier, S.M.; Ii, G.W.S. A method for deriving water-quality benchmarks using field data. Environ. Toxicol. Chem. 2012, 32, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Hintz, W.D.; Relyea, R.A. A review of the species, community, and ecosystem impacts of road salt salinisation in fresh waters. Freshw. Biol. 2019, 64, 1081–1097. [Google Scholar] [CrossRef] [Green Version]
- Raftery, A.E.; Lewis, S.M. The number of iterations, convergence diagnostics and generic Metropolis algorithms. Pract. Markov. Chain Monte. Carlo. 1995, 7, 763–773. [Google Scholar]
- King, G.; Tomz, M.; Wittenberg, J. Making the most of statistical analyses: Improving interpretation and presentation. Am. J. Politicol. Sci. 2000, 44, 347–361. [Google Scholar] [CrossRef]
- Hosmer, D.W.; Lemesbow, S. Goodness of fit tests for the multiple logistic regression model. Commun. Stat. Theory Meth. 1980, 9, 1043–1069. [Google Scholar] [CrossRef]
- National Recommended Water Quality Criteria—Aquatic Life Criteria Table. Available online: https://www.epa.gov/wqc/national-recommended-water-quality-criteria-aquatic-life-criteria-table (accessed on 12 December 2019).
- Water Quality Standards Review: Chloride, Sulfate and Total Dissolved Solids. Available online: http://www.iowadnr.gov/portals/idnr/uploads/water/standards/ws_review.pdf (accessed on 12 December 2019).
- Soucek, D.J.; Mount, D.R.; Dickinson, A.; Hockett, J.R. Influence of dilution water ionic composition on acute major ion toxicity to the mayfly Neocloeon triangulifer. Environ. Toxicol. Chem. 2018, 37, 1330–1339. [Google Scholar] [CrossRef]
- McLaughlin, D.B.; Reckhow, K.H. A Bayesian network assessment of macroinvertebrate responses to nutrients and other factors in streams of the Eastern Corn Belt Plains, Ohio, USA. Ecol. Model. 2017, 345, 21–29. [Google Scholar] [CrossRef]
- May, M.; Drost, W.; Germer, S.; Juffernholz, T.; Hahn, S. Evaluation of acute-to-chronic ratios of fish and Daphnia to predict acceptable no-effect levels. Environ. Sci. Eur. 2016, 28, 16. [Google Scholar] [CrossRef] [PubMed]
- Wallace, A.M.; Biastoch, R.G. Detecting changes in the benthic invertebrate community in response to increasing chloride in streams in Toronto, Canada. Freshw. Sci. 2016, 35, 353–363. [Google Scholar] [CrossRef]
- Schröder, M.; Sondermann, M.; Sures, B.; Hering, D. Effects of salinity gradients on benthic invertebrate and diatom communities in a German lowland river. Ecol. Indic. 2015, 57, 236–248. [Google Scholar] [CrossRef]
- Wichard, W.; Tsui, P.; Komnick, H. Effect of different salinities on the coniform chloride cells of mayfly larvae. J. Insect Physiol. 1973, 19, 1825–1835. [Google Scholar] [CrossRef]
- Drover, D.R.; Zipper, C.E.; Soucek, D.J.; Schoenholtz, S.H. Using density, dissimilarity, and taxonomic replacement to characterize mining-influenced benthic macroinvertebrate community alterations in central Appalachia. Ecol. Indic. 2019, 106, 105535. [Google Scholar] [CrossRef]
- Clements, W.H.; Kashian, D.R.; Kiffney, P.M.; Zuellig, R.E. Perspectives on the context-dependency of stream community responses to contaminants. Freshw. Biol. 2016, 61, 2162–2170. [Google Scholar] [CrossRef]
- Mount, D.R.; Erickson, R.J.; Highland, T.L.; Hockett, J.R.; Hoff, D.J.; Jenson, C.T.; Norberg-King, T.; Peterson, K.N.; Polaske, Z.M.; Wisniewski, S. The acute toxicity of major ion salts to Ceriodaphnia dubia: I. influence of background water chemistry. Environ. Toxicol. Chem. 2016, 35, 3039–3057. [Google Scholar] [CrossRef]
- Wang, N.; Consbrock, R.A.; Ingersoll, C.G.; Hardesty, D.K.; Brumbaugh, W.G.; Hammer, E.J.; Bauer, C.R.; Mount, D.R. Acute and chronic toxicity of sodium sulfate to four freshwater organisms in water-only exposures. Environ. Toxicol. Chem. 2015, 35, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Hanmer, M.J.; Kalkan, K.O. Behind the Curve: Clarifying the Best Approach to Calculating Predicted Probabilities and Marginal Effects from Limited Dependent Variable Models. Am. J. Polit. Sci. 2012, 57, 263–277. [Google Scholar] [CrossRef]
- Gazendam, E.; Gharabaghi, B.; Jones, F.C.; Whiteley, H. Evaluation of the Qualitative Habitat Evaluation Index as a Planning and Design Tool for Restoration of Rural Ontario Waterways. Can. Water Resour. J. Rev. Can. Ressour. Hydr. 2011, 36, 149–158. [Google Scholar] [CrossRef] [Green Version]
- Moerke, A.H.; Gerard, K.J.; Latimore, J.; Hellenthal, R.A.; Lamberti, G.A. Restoration of an Indiana, USA, stream: Bridging the gap between basic and applied lotic ecology. J. N. Am. Benthol. Soc. 2004, 23, 647–660. [Google Scholar] [CrossRef]
- Fanelli, R.M.; Prestegaard, K.L.; Palmer, M.A. Urban legacies: Aquatic stressors and low aquatic biodiversity persist despite implementation of regenerative stormwater conveyance systems. Freshw. Sci. 2019, 38, 818–833. [Google Scholar] [CrossRef]
- Lee, J.H.; An, K.-G. Integrative restoration assessment of an urban stream using multiple modeling approaches with physical, chemical, and biological integrity indicators. Ecol. Eng. 2014, 62, 153–167. [Google Scholar] [CrossRef]
- Gazendam, E.; Gharabaghi, B.; Ackerman, J.D.; Whiteley, H. Integrative neural networks models for stream assessment in restoration projects. J. Hydrol. 2016, 536, 339–350. [Google Scholar] [CrossRef]
- Mueller, M.; Pander, J.; Geist, J. The ecological value of stream restoration measures: An evaluation on ecosystem and target species scales. Ecol. Eng. 2014, 62, 129–139. [Google Scholar] [CrossRef]
- Schuler, M.S.; Relyea, R.A. A Review of the Combined Threats of Road Salts and Heavy Metals to Freshwater Systems. BioScience 2018, 68, 327–335. [Google Scholar] [CrossRef]
- Neculita, C.M.; Rosa, E. A review of the implications and challenges of manganese removal from mine drainage. Chemosphere 2019, 214, 491–510. [Google Scholar] [CrossRef] [PubMed]
- Skousen, J.; Zipper, C.; Rose, A.; Ziemkiewicz, P.F.; Nairn, R.; McDonald, L.M.; Kleinmann, R.L. Review of Passive Systems for Acid Mine Drainage Treatment. Mine Water Environ. 2017, 36, 133–153. [Google Scholar] [CrossRef] [Green Version]
- Rose, A.W.; Means, B.; Shah, P.J. Methods for Passive Removal of Manganese from Acid Mine Drainage. In Proceedings of the 24th West Virginia Surface Mine Drainage Task Force Symposium, Morgantown, WV, USA, 15 April 2003; pp. 71–82. [Google Scholar]



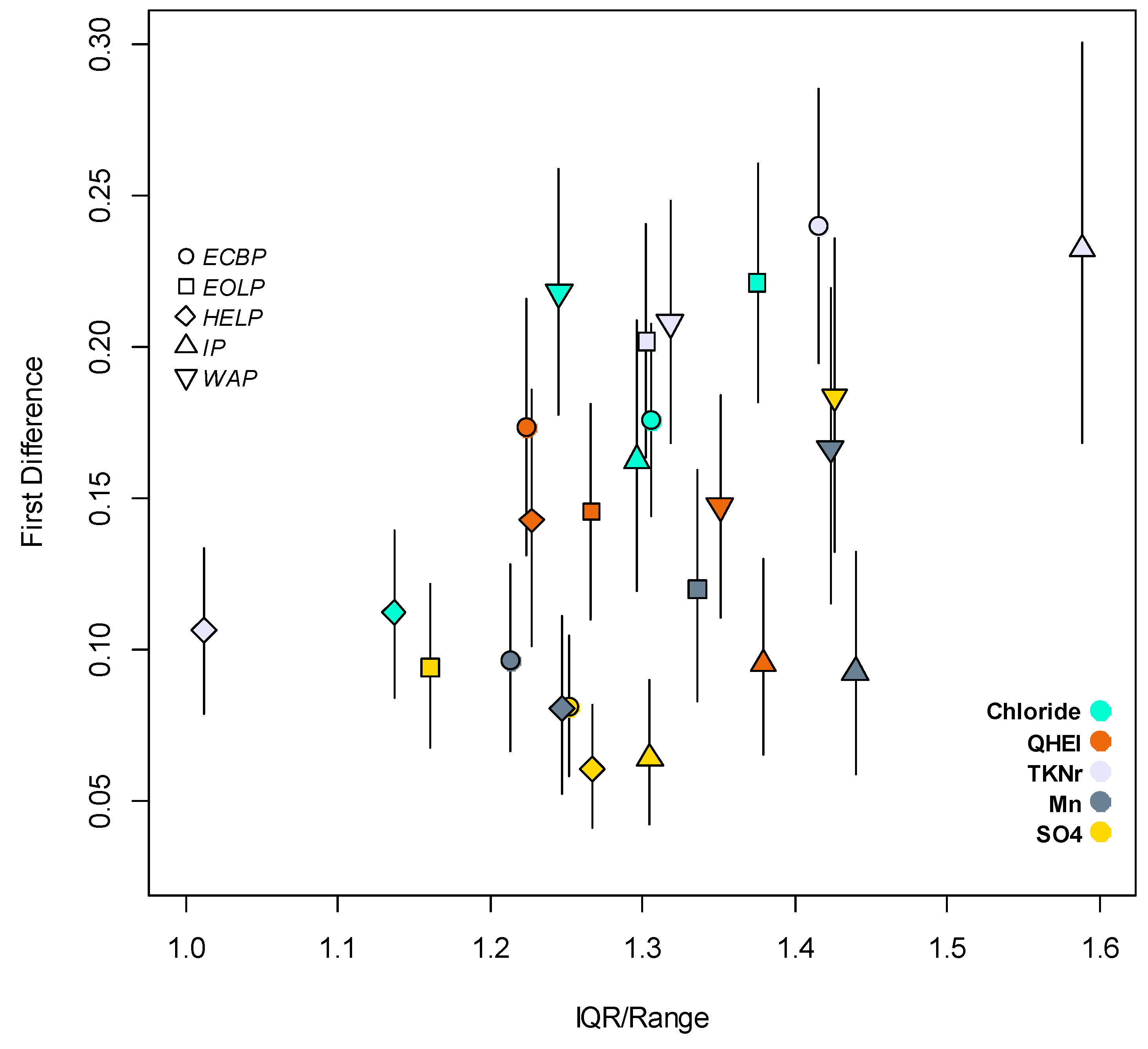





| Posterior Means and (SD) [95% Credible Interval] | Gelman-Rubin Point and Upper C.I. | Effective Size | |
|---|---|---|---|
| Intercept | 0.00 (0.010) | 1.00–1.00 | 29,242 |
| [−0.19; 0.20] | |||
| Chloride | −2.00 * (0.189) | 1.01–1.03 | 1034 |
| [−2.37; −1.63] | |||
| QHEI | 0.04 * (0.005) | 1.00–1.00 | 6816 |
| [0.03; 0.05] | |||
| rTKN | −3.15 * (0.315) | 1.00–1.00 | 10,256 |
| [−3.77; −2.53] | |||
| Mn | −0.85 * (0.141) | 1.00–1.00 | 1423 |
| [−1.13; −0.59] | |||
| SO4 | −1.09 * (0.159) | 1.00–1.01 | 1354 |
| [−1.40; −0.78] | |||
| HELP | 16.83 * (1.375) | 1.01–1.01 | 484 |
| [14.19; 19.62] | |||
| IP | 16.70 * (1.364) | 1.01–1.01 | 465 |
| [14.09; 19.47] | |||
| EOLP | 16.53 * (1.397) | 1.01–1.01 | 451 |
| [13.84; 19.37] | |||
| WAP | 15.45 * (1.342) | 1.01–1.01 | 465 |
| [12.89; 18.18] | |||
| ECBP | 15.44 * (1.322) | 1.01–1.01 | 463 |
| [12.90; 18.13] |
| HL χ2 | P | AUC | |
|---|---|---|---|
| HELP | 5.31 | 0.72 | 61.8 |
| IP | 9.49 | 0.30 | 78.3 |
| EOLP | 3.72 | 0.88 | 76.1 |
| WAP | 18.92 | 0.02 | 83.6 |
| ECBP | 6.91 | 0.55 | 70.1 |
| Over-all | 9.89 | 0.27 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miltner, R. Assessing the Impacts of Chloride and Sulfate Ions on Macroinvertebrate Communities in Ohio Streams. Water 2021, 13, 1815. https://doi.org/10.3390/w13131815
Miltner R. Assessing the Impacts of Chloride and Sulfate Ions on Macroinvertebrate Communities in Ohio Streams. Water. 2021; 13(13):1815. https://doi.org/10.3390/w13131815
Chicago/Turabian StyleMiltner, Robert. 2021. "Assessing the Impacts of Chloride and Sulfate Ions on Macroinvertebrate Communities in Ohio Streams" Water 13, no. 13: 1815. https://doi.org/10.3390/w13131815
APA StyleMiltner, R. (2021). Assessing the Impacts of Chloride and Sulfate Ions on Macroinvertebrate Communities in Ohio Streams. Water, 13(13), 1815. https://doi.org/10.3390/w13131815





