Abstract
Stormwater runoff management is challenging in a highly urbanised tropical environment due to the unique space constraints and tropical climate conditions. A modular bioretention tree (MBT) with a small footprint and a reduced on-site installation time was explored for application in a tropical environment. Tree species used in the pilot studies were Talipariti tiliaceum (TT1) and Sterculia macrophylla (TT2). Both of the MBTs could effectively remove total suspended solids (TSS), total phosphorus (TP), zinc, copper, cadmium, and lead with removal efficiencies of greater than 90%. Total nitrogen (TN) removal was noted to be significantly higher in the wet period compared to the dry period (p < 0.05). Variation in TN removal between TT1 and TT2 were attributed to the nitrogen uptake and the root formation of the trees species. A field study MBT using Talipariti tiliaceum had a very clean effluent quality, with average TSS, TP, and TN effluent EMC of 4.8 mg/L, 0.04 mg/L, and 0.27 mg/L, respectively. Key environmental factors were also investigated to study their impact on the performance of BMT. It was found that the initial pollutant concentration, the dissolved fraction of influent pollutants, and soil moisture affect the performance of the MBT. Based on the results from this study, the MBT demonstrates good capability in the improvement of stormwater runoff quality.
1. Introduction
Rapid urbanization has resulted in an increase of impervious surface area with a drastic loss of green spaces;, ensuing in a reduction in surface infiltration and evapotranspiration, causing surface runoff to increase in volume [1]. Due to these surface modifications, the urban environment often faces flash floods and problems of nutrient contamination in the surface water [2,3]. Hence, there is a pressing need for a solution to mitigate flooding events and manage nutrient pollution. Urban tropical countries like Singapore pose particular challenges due to their high amount of rainfall, land shortage, and rapid urbanisation. Singapore has a mean annual rainfall of more than 2000 mm [4], with an increase in the occurrence of reported flash floods contemporarily (post-2000), compared to preceding (1984–1999) periods [5]. Land scarcity is also a perennial challenge for highly urbanised countries [6].
A bioretention system (BRS) is a viable treatment option that is able to reduce stormwater runoff volume by reducing infiltration runoff and is aesthetically pleasing [7]. BRS makes use of an engineered environment that integrates with the natural biota to maximize infiltration and support vegetative growth [1]. BRS has shown great potential for the removal of a large variety of pollutants such as suspended solids, heavy metals, and nutrients through the trapping of particle-bound metals, filtration, and sorption methods [8,9,10,11]. Factors that affect BRS performance include environmental factors (e.g., the intensity of rain events and evapotranspiration), site selections, and design parameters such as submerged zone depth, filter material, and plant selection [1,12]. A typical filter media mixture includes materials such as sand, silt, clay, and waste materials [1]. The composition of the filter media influences both the hydraulic characteristics (e.g., infiltration rate, detention volume) and the biogeochemical processes that take place in the BRS for surface runoff treatment [12], whereas the vegetation absorbs nutrients and heavy metals from the infiltrated stormwater runoff [13].
Interactions between plants and soil were reported to influence the soil structure, hydrologic processes, and nutrient cycling of the entire ecosystem [14,15,16,17]. The plants used in the BRS had no clear distinction between woody and leafy species [17]. Tirpak et al. [18] studied the performance of a bioretention mesocosm planted with trees native to the USA (Acer rubrum, Pinus taeda and Quercus palustris) and found that nutrient uptake via the tree roots is minimal compared to the soil/microbial process. No significant differences were observed for ammonium, nitrite, nitrate, and phosphate in the effluent between all of studied the tree species. The authors explained that the removal of ammonium and phosphate were attributed to the aerobic nature of the bioretention media and the chemical sorption mechanism, respectively. Frosi et al. [19] monitored street tree-pits in Canada and concluded that the systems were able to remove a mass flux of contaminants (such as Na, Cu, and Zn) effectively. The authors also noted that tree pits with high soil organic matter (SOM) could decrease the mass flux of Na and Cu. For instance, with depth, the mass flux of Na and Cu decreased by 66% and 73% in tree pits with less SOM and by 87% and 86% in tree pits with more SOM. The authors recommended the increase of surface permeability and SOM in street tree pits for the improvement of runoff quality and quantity. Elliot et al. [20] measured forty tree pits representing the variety of physical conditions commonly seen in New York City and found that higher infiltration rates in tree pits were associated with larger pit areas, built-up surface elevations, and the combined presence of ground cover planting and mulch. The EcosolTM tree pit is a commercial MBT that can provide the tertiary treatment of stormwater flows in one compact device [21]. It can achieve suspended solids removal (95%), particulate heavy metals removal (90%), and total petroleum hydrocarbon removal (99%). Stockholm Treepits uses structural soils that can provide a solid base for surfacing while allowing large voids to remain for water movement [22]. A Tree pit (or tree wall) designed by StormTree could provide healthy and thriving trees and stormwater management. It has a unique open design that allows unrestricted tree root growth [23]. However, the reported studies were performed in the temperate regions with limited research on BRS in the tropics. Climate conditions are a key factor that affects the performance of low impact development practices [24]. Climate could affect a wide range of parameters such as plant metabolism, the adsorption capacity of media, microbial composition, and metabolism rate. Tropical climates have a higher rainfall, and higher rainfall frequency, and higher average temperature than temperate regions. In addition, Blecken et al. [25] have demonstrated that extended dry periods worsen the performance of biofilters. Rahman et al. [26] reported that a shorter antecedent dry period (ADP) led to nitrate and nitrite removals, and a longer ADP resulted in nitrate and nitrite export. Denitrification rates were also reported to be lower at low temperatures [27,28]. Clearly, these variations could impact the design and performance of bioretention tree systems in tropical areas and has a need to be further studied.
Furthermore, the design of a conventional BRS also requires longer construction time and larger land area for construction, which is unsuitable for highly urbanized environment such as Singapore. A BRS is also generally constructed on-site, and limited studies have explored integrating existing urban landscapes with BRS. For instance, Brown et al. [29] combined a BRS with pervious concrete with an internal water storage (IWS) layer and found improvement in hydrologic performance. A volume reduction approaching 100% was noted for sand cells, with reductions of 87% (1.03 m IWS depth) and 75% (0.73 m IWS depth) for the sandy clay loam in the underlying soil. The authors concluded that (a) the hydraulic conductivity of the underlying soil, (b) the IWS zone depth, and (c) the surface infiltration rate were the primary factors that controlled the outflow volume. Kazemi et al. [30] coupled BRS with permeable pavement, which showed beneficial downstream irrigation impacts. In this study, the sodium adsorption index (SAR) for high salinity runoff (1500 mg/L) was reduced from 196.45 meq/L to 6.68 meq/L when discharged from the bioretention basin. The authors concluded that retention time in both the permeable pavement and the bioretention basin storage zone are important, and they recommended that local design guidelines should include data on plants, with focus on their salinity threshold and salt stress.
Hence, this study proposes the use of a unique modular bioretention tree system (MBT) in an urban tropical context. MBT is a treatment device that consists of a tree planted in soil media in a compact module that can be applied to manage non-point source pollution from stormwater runoff. The MBT unique deep soil media helps to sustain a healthy tree and to provide extended stormwater runoff treatment. An MBT has a small footprint and amalgamates with existing urban infrastructure for the treatment of stormwater. To the best of our knowledge, this is the first study that systematically investigated bioretention trees with engineered soil in tropical conditions. In-depth and comprehensive measurements of the performance of an MBT under various ‘wet’ and ‘dry’ periods would provide valuable information for water professionals in conceptualizing stormwater runoff management strategies in the tropics. This study also monitored the MBT on a pilot- and field-scale to obtain key insights for the pollutant removal capabilities of the MBT in various scaled operations.
2. Materials and Methods
2.1. Methodology
In this work, there were 3 phases to study the performance of the Modular Bioretention Tree (MBT) system. In the first phase, the interactive effects of the engineered soil and trees were investigated for their system performance with synthetic storm events. The second phase involved the testing of the pilot-scale biofilters with real stormwater runoff. Finally, the last phase was the real application of a scaled-up MBT system with actual urban stormwater runoff. The selected tree used in Phase 3 of the study was screened during the first two phases for its performance.
2.2. Design of Modular Bioretention Tree (MBT) System
For Phases 1 and 2, the pilot MBT biofilters consisted of an impermeable concrete tank with a cross-sectional area of 0.36 m2, with and had an extended detention depth of 0.2 m (Figure 1a). The MBT biofilter consisted of a filter media layer, a transition layer, and a drainage layer. The top filter media was composed of engineered soil, and the preparation of it followed the patent “An Engineered Soil Composition and a Method of Preparing the Same” patent [31]. Engineered soil consists of a proprietary blend of coconut fibres, water treatment residue (WTR), soil, and sand. Coconut fibre acts as a source of organic matter and encourages denitrification to occur within the bioretention system. Studies by Barrett and Burke [32] showed that coconut fibre increases TN removal by increasing the immobilization of nitrogen within the fibres. WTR consists of aluminium oxides, which provide adsorption sites for pollutants removal [33]. Dissolved phosphorus can be adsorbed readily and reversibly to the surface of aluminium oxides during rain events. This slow and usually irreversible adsorption occurs as the phosphorus that is adsorbed on the surface diffuses deeper into the matrix of the aluminium oxides and becomes deposited [34,35].
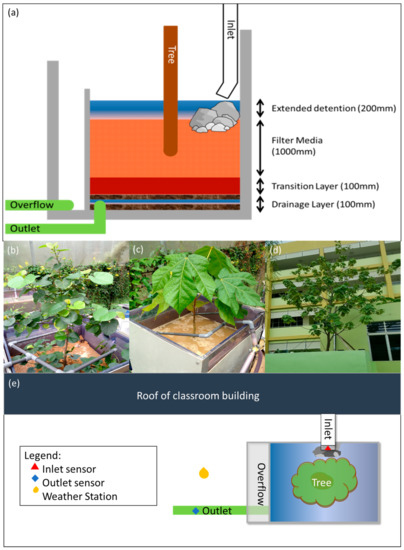
Figure 1.
(a) Cross-sectional area for MBT system, (b) Talipariti tiliaceum used in the pilot study (c) Sterculia macrophylla used in the pilot study, (d) MBT system in the field study, (e) overview of MBT on site.
The unique design of the MBT system’s unique design aims to increase the storage capacity of the BRS per surface area by having a deeper filter media layer compared to conventional systems, allowing it to treat more water in the system. Generally, the media layer was reported to be around 0.4 m [9,36]. A deeper filter media layer of 1.0 m also allows sufficient space and soil depth for the root growth of the tree species planted in the MBT system. The drainage layer consists of 0.1 m of gravel, and the transition layer consists of 0.1 m of coarse sand. The drainage layer facilitates the collection of the effluent from the system, while the transition layer minimizes the probability of the filter medium being washed out of the system during each dosing event.
For the pilot-study, water was directed using a pump through the inlet of the system and dispersed using a small layer of rocks on the top of the filter media. The tree species used in the pilot studies were Talipariti tiliaceum (TT1), a coastal species (Figure 1b)), and Sterculia macrophylla, a forest tree species (TT2) (Figure 1c). TT1 and TT2 were previously screened in a prior study for the best performance and retention rate in a tropical context [37]. A detailed breakdown of the screened tree species is shown in the Appendix A.
In this study, 3-sided transparent tanks were constructed so that root growth could be monitored, based on a proposed method by Judd et al. [38]. New root shoots and their corresponding depth were observed and recorded. The spatial distribution of root depth within the bioretention system was also recorded in terms of the percentage of new roots formed. The spatial distribution of roots has been shown to affect the removal efficiency of nitrogen species, with deeper roots favoring higher removal efficiency [39,40]. The potted systems used in BRS limit the growth of trees [41,42], hence the decision of keeping the system compact and modular, especially for space-constrained locations. Traditional BRS requires regular maintenance and favors fast-growing plants, as they are assumed to take up nutrients quickly and efficiently to support their fast growth [37,43]. Due to the space constraints in MBT, it is important to balance the growth rate to keep the system modular while having an adequate growth rate for high removal efficiency.
For the field study, the study area was located in an educational institute in the west of Singapore. The MBT system was situated between an open field, school field, and classroom blocks. Talipariti tiliaceum has been selected for the field study due to its better TN removal performance. Roof catchment from the surrounding classroom buildings (100% imperviousness, 90 m2) was channeled into the MBT system. The MBT system had a surface area of 3.7 m2 and a filter media depth of 1.0 m. The transition and drainage layers were 0.1 m and 0.3 m, respectively. The overflow manhole allowed for a maxi-mum detention depth of 0.2 m (Figure 1d). This unique engineering design allowed for a small compact system, which limited the spread of roots within the system, allowing for a deeper root system. The MBT system was also constructed offsite and was brought to the study area for installation, which reduced the time needed for on-site construction. As such, the MBT could be positioned and retrofitted easily in an urban setting dominated by impervious infrastructures.
A series of sensors were placed around the MBT system to monitor and assess the system’s performance (Figure 1e). Automatic water samplers (900 MAX, Sigma, CO, USA) were used to collect 1L of stormwater runoff samples with 6-min intervals for both the inlet and the outlet of the MBT. Area-velocity sensors (AV sensor, Sigma, CO, USA) were installed at the inlet and outlet (subsoil drainage pipe) to monitor the flow velocity and volume. Weather conditions at the site were also monitored using a rain gauge (Sigma, Colorado, USA). Rainfall information from the rain gauge was logged at 1-min intervals. The soil moisture and water potential of the soil were also measured with a soil measure sensor (EC5, Decagon, Pullman, WA, USA) and tensiometer (Tensiometer with pressure transducer, SMS, CA, USA). The monitoring study was conducted for 3 months after the commission of the system in order to take the initial stabilisation period of the system into account, wherein nutrient content was leached from the filter media. The entire monitoring spanned over 15 months and covered both the dry and wet seasons in Singapore, which are caused by the northeast and southwest monsoon.
2.3. Preparation of Synthetic Stormwater Runoff and Actual Stormwater Runoff
In Phase 1 of the study, synthetic stormwater was made based on the average range of urban stormwater pollutant concentrations found in the Singaporean environment [44,45]. This allowed for minimal fluctuation of the inflow concentration while ensuring a realistic composition for the study [45]. Whereas for Phase 2, the stormwater runoff was collected from a canal located in the north of Singapore and dosed into the pilot system. For both phases, the flow rate and volume of the dosed water were based on the average monthly Singapore rainfall data from the years 2003 to 2007. As study [46] has shown that different ADPs could affect the water quality performance of BRS, the pilot biofilter studies were conducted in 2 different dosing regimens (e.g., 2 times per week for the dry dosing event and 3 times per week for the wet dosing event). The dosing of synthetic water lasted for approximately 8 weeks and 10 weeks for Phase 1 and Phase 2, respectively. Doses of synthetic stormwater runoff were introduced a total of 20 times (10 dry dosing events and 10 wet dosing events) in Phase 1 of the study. Each pilot MBT biofilter received 24 dosing events (12 dry dosing events and 12 wet dosing events) in Phase 2 of the study. The average pollutant concentration and dosing regimen is shown in Table 1.

Table 1.
Average pollutant concentration and dosing regimens for Phase 1 and Phase 2.
The chemicals dosed in Phase 1 and the detailed pollutant concentration used in Phase 2 are summarised in Appendix B. The peak flow rate was computed based on the formula shown in Appendix C, which summarizes the frequency of dosing, flow rate, and volume of synthetic runoff, which were dosed to reflect the dry and wet periods of Singapore.
2.4. Analytical Procedures
For each dosing event in Phase 1 and Phase 2, 20 L inflow samples were taken to form a composite sample. The water quality analytical tests were done following the Standard Methods for the Examination of Water and Wastewater [47]. Both influent and effluent water samples were tested for key water quality parameters such as total phosphorus (TP) (DR 6000, Hach, CO, USA), total nitrogen (TN) (TOC-L, Shimadzu, Kyoto, Japan), and total suspended solids (TSS). For heavy metal analysis, samples were filtered through 0.45 um Millipore PTFE filter paper, and 2% ultrapure nitric acid was added to the samples. Inductively coupled plasma mass spectrometry (ICP-MS) (7700, Agilent Technologies, CA, USA) was used to analyse the concentration of copper (Cu), lead (Pb), cadmium (Cd), and zinc (Zn).
After a storm event during Phase 3, water samples were collected and transported to the laboratory inside a cooler box with ice packs. Only TSS, TP, and TN were tested for the water samples collected from the field study. Heavy metals in the field study were found to be in negligible concentrations (although not reported in this study), as the study area did not have a source of heavy metal pollutants. Heavy metals pollutants are generally sourced from industrial emissions or activities and are commonly found in road runoff, which are mainly contributed to by vehicle emissions or the wear and tear of tyres or brake linings [48]. In terms of plant growth characteristics, Fv:Fm ratio, chlorophyll meter, the height of tree growth, and leaf growth were used as indicators of the health of the tree in the MBT. The Fv:Fm ratio was measured using a chlorophyll fluorometer (PAM-210, Walz, Effeltrich, Germany) to estimate the photosynthetic performance of the leaves [9,49,50]. A Fv:Fm ratio of above 0.75 indicates healthy leaves. The chlorophyll leaf colour changes were measured using the SPAD-502 Plus chlorophyll meter (Konica-Minolta, Tokyo, Japan), which can show the relationship between leaf chlorophyll-a and nitrogen levels [51,52]. SPAD provides a rapid method to determine the chlorophyll content of the plants while the Fv:Fm ratio is able to indicate plant health via chlorophyll fluorescence [53]. The hydraulic conductivity of the soil (K) was measured based on the method adapted from the Facility for Advancing Water Biofiltration (FAWB) [54]. Event mean concentration (EMC) analysis was performed and used to benchmark performance in this study.
3. Results
3.1. Pilot Biofilter Study (Phase 1)
The detailed breakdown of the overall performance efficiency of the system for Phases 1 and 2 is shown in Appendix D. Both systems demonstrated good removal efficiencies of TSS, TP, and TN, as shown in Figure 2. On an overall note, there was not much difference between the performance of TT1 and TT2 in terms of removing the TSS, TP, and TN from synthetic stormwater. The average difference of EMC removal for TSS, TP, and TN (including both the wet and dry periods) between the two biofilters was 2.2%, 0.1%, and 1.8%, respectively. Detailed statistical difference analysis is shown in Appendix E.
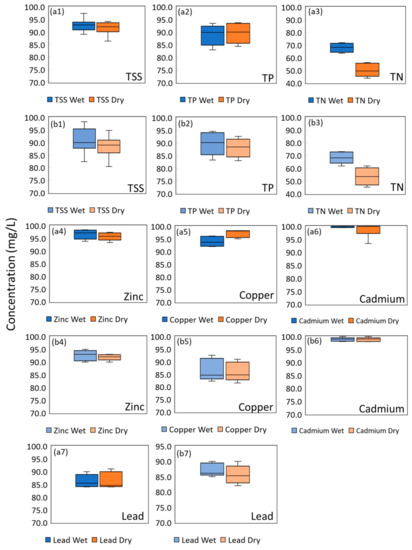
Figure 2.
Phase 1 EMC removal % for (a1) TT1 TSS, (a2) TT1 TP, (a3) TT1 TN, (a4) for TT1 Zinc, (a5) TT1 Copper, (a6) TT1 Cadmium, (a7) TT1 Lead, (b1) EMC removal % for TT2 TSS, (b2) TT2 TP, (b3) TT2 TN, (b4) TT2 Zinc, (b5) TT2 Copper, (b6) TT2 Cadmium, and (b7) TT2 Lead.
Both biofilters were highly effective in removing TSS, and their performance was consistent over time, as reported was also found in other reported studies [55,56]. The EMC removal of all of the sampling runs was mostly greater than 90%, with a coefficient of variation (CV) below 5%. No significant difference (p > 0.05) was found between the effluent of both biofilters. The removal of TSS however is attributed more to simple filtration from the media itself within the BRS itself. The plant uptake by plants was reported to not be significant [55,57]. This was similarly reported by Bratieres et al. [55], who reported that the TSS removal for bioretention systems with plants was similar to the soil only control. [41]. The EMC removal for TP was noted with a mean value of 89.4% and 89.3% for TT1 and TT2, respectively (CV less than 5%). The exceptional performance for TP removal was attributed to the application of WTR in the filter media. A study found that systems using Al-WTR showed a PO43− removal of approximately 99% [58].
For TN, EMC removal was relatively worse, with a mean value of 61.0% and 59.2% for TT1 and TT2, respectively. TN constitutes highly mobile and soluble species like nitrites and nitrates, which are reported to be less efficiently removed as compared to TSS [28]. Dissolved nitrogen species are reported to have sorption to filter media [59,60,61]. Tirpak et al. [18] concluded that TN removal in a large mesocosm was largely attributed to soil adsorption or microbial processes (nitrification and denitrification). In addition, previous studies also noted that plant uptake is an important mechanism for TN removal [62,63]. The slight difference in the TN removal between the two pilot biofilter studies could be due to the different root structures or traits of the plants that were used [64,65]. The role of plant uptake on TN removal will be further discussed in Section 3.2.
The EMC removal percentages of TSS and TP were likely to be independent of the wet/dry periods. The difference in the mean EMC removal percentages for TSS and TP were 1.0% and 0.7% (both p > 0.05) for TT1 between the different periods, while the difference in the mean EMC removal percentages for TSS and TP were 2.5% and 2.0% (both p > 0.05) for TT2 between the different periods. This suggested that the removal mechanisms for TP and TSS were not affected by the frequency of rainfall events or the volume and intensity of the rainfall. The adsorption of phosphorous was also likely to be unaffected by the weather conditions. The removals of TN have the most distinctive difference in terms of removal efficiency between the wet and dry periods. A significant difference (14.9% for TT1, 17.7% for TT2, both p < 0.05) was found between the EMC removal percentage of TN in the wet and dry periods. When wet period testing was conducted, the larger volume of influent resulted in a higher volume of retained water in the system. The retention of water in the system created pockets of anoxic submerged zones, which promoted denitrification to occur [66]. NO3− undergoes denitrification and is hence removed from the system through its conversion to nitrogen gas.
In terms of heavy metal removal, the findings are agreeable with various laboratory studies in the literature [25,67]. The heavy metals were removed effectively by both pilot biofilters. In this case, the average EMC removal of heavy metals for both biofilters was more than 85%. A relatively high variable of cadmium (Cd) removals was obtained (65.5–100.0% for TT1 and 67.2–100.0% for TT2) and may be attributed to the low effluent concentration, which was always below the detection limit (3 and 5 events are detectable, respectively for TT1 and TT2). Overall, there were no obvious differences between TT1 and TT2 heavy metals removal. Heavy metals in particulate form are mostly intercepted by the filter media surface layer, while the dissolved form of heavy metals is mostly removed via the sorption process by the filter media layer [68]. Heavy metals uptake by plants (mainly via the roots) is relatively lower [58,69], which may explain the similarities in heavy metals removal between the two pilot biofilters.
For this study, there was no significant difference (p > 0.05) in EMC removal efficiency of heavy metals between the wet and dry periods. A statistical test for Cd was not conducted due to limited datasets (most dosing events have effluent concentrations below the detection limit). Hatt et al. [70] also found no difference in heavy metals during the wetting and drying regime for their column tests. However, this phenomenon was different from the intermittent wet and dry column study by Blecken et al. [25], which reported that heavy metal removal efficiency was significantly lower (relative to wet period performance) after a prolonged drying period. Possible reasons for this include the mobilisation of fine sediments, preferential flow paths, and the reduced metal uptake by plants during the dry period. The difference between this study and the study by Blecken et al. [25] could be due to the span of the dry period for their experiments, which ranged from 1 to 7 dry weeks.
3.2. Pilot Biofilter Study (Phase 2)
Similar to the trend found in the previous section, the MBT system had a comparable EMC removal of pollutants for real stormwater runoff. Figure 3 shows the EMC removals for both TT1 and TT2 during Phase 2. Consistent and excellent removal of TSS and TP (mean removal > 90%) were noted for both pilot biofilters. The difference in the mean EMC removal percentages for TSS and TP were within 0.3% and 1.2% (both p > 0.05) for TT1 between the different periods, whereas the difference in the mean EMC removal percentages for TSS and TP were 0.8% and 0.9% (both p > 0.05) for TT2 between the different periods. Similar to TN, EMC removal showed the most distinct difference, with a mean EMC difference of 22.4% for TT1 and 10.2% for TT2 (both p < 0.05). The overall EMC removal performance of TT1 and TT2 for the two conditions was reflected in Appendix E. Similar observations were found whereby TN removal was significantly higher in the wet period compared to the dry period when actual stormwater runoff was used for the MBT systems.
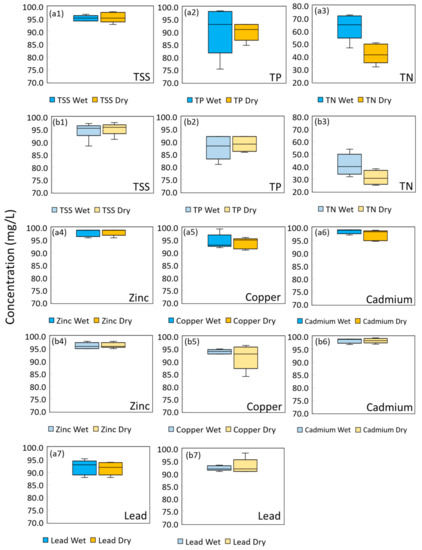
Figure 3.
Phase 2 of EMC removal % for (a1) TT1 TSS, (a2) TT1 TP, (a3) TT1 TN, (a4) for TT1 Zinc, (a5) TT1 Copper, (a6) TT1 Cadmium, (a7) TT1 Lead, (b1) EMC removal % for TT2 TSS, (b2) TT2 TP, (b3) TT2 TN, (b4) TT2 Zinc, (b5) TT2 Copper, (b6) TT2 Cadmium, and (b7) TT2 Lead.
Heavy metal removal in Phase 2 for both biofilters is excellent, with an average EMC removal of more than 90%. Similar to Phase 1, the EMC removal of heavy metals is shown to be independent of the different wet/dry periods whereby there is no significant difference (p > 0.05) between the wet and dry period, except cadmium for TT1. Despite this, the effluent concentration of TT1 during wet period dosing is similar to that of dry period dosing (average of 0.05 µg/L and 0.09 µg/L, respectively).
On an overall note, investigating the performance of BRS systems in general, on a laboratory or pilot scale, has the edge of flexible configurations and modes of operations. However, the results may not always correspond to the findings of field application. In a controlled environment and setting (e.g., consistent temperature and routine dosing of water), both Phase 1 and Phase 2 had a similar range of TSS and TP pollutant removal efficiency. The difference in TN removal efficiency could be due to the variability of nitrogen concentration and the dissolved composition of the influent water that was used for the pilot biofilter studies [10,61]. While Phase 1 of the study indicated the most ideal and controlled environment, synthetic stormwater may not be perfect for the actual representation of stormwater runoff pollutants. For instance, TN performance in TT2 using real stormwater runoff is lower compared to synthetic stormwater runoff. This could be due to the lower NO3− concentration in the influent of Phase 2. In addition, competition from other pollutants in stormwater runoff and the root growth pattern could limit the process of denitrification in TT2. Other studies also reported differences in BRS performance when synthetic stormwater and actual stormwater runoff were used as influents, with most studies performing laboratory experiments using simulated stormwater followed by a field study [59,67]. Hence, this showed that the usage of simulated stormwater as a screening tool might not necessarily be sufficient.
Figure 4 and Figure 5 show the EMC removal efficiency of the biofilters for Phase 1 and Phase 2, respectively. Comparing the different tree species used in this study, TT1 and TT2 had similar removal efficiencies of TSS (89.8–93.6%), TP (85.7–89.3%), Zn (92.3–98.2%), Cu (84.2–93.8%), Cd (95.0–98.3%), and Pb (84.7–92.2%), whereas TT1 showed a significantly higher removal of TN. This is likely due to the removal mechanism of the MBT. TSS and TP removals were consistent over time for Phase 1 and Phase 2. It was reported that TSS and TP removals were independent of the tree species used and were more influenced by the media used [36,71]. Likewise, heavy metal removals were more dependent on the media used and were less contributed to by different plant species. The bulk of phosphorus pollutants was likely removed via adsorption within the filter media, which in this study, contained WTR as amendments, whereas the differences in the removal of TN could likely be accounted for by the uptake of nitrogenous species by the plants [37] as well as how the tree grows within the soil media. The removal of TN in both phases were variable over time. Landsman and Davis [72] also noted the varied removal efficiency of TN. This could be attributed to diversity of N forms and the multiple internal treatment mechanisms within MBT. It was noted that TN removal for TT1 was better than that of TT2. Deeper plant roots were reported to increase nitrogen retention in the bioretention system [12,73] as well as strengthen microbial activity in the soil [74]. The smaller TN removal variation observed in Figure 4 and Figure 5 compared to previous studies [72,75] could be attributed to the different settings. Phase 1 and Phase 2 of this study were conducted in a controlled environment. Environmental variables and different N species in the field studies could confound the results and could cause larger variation in TN removal.
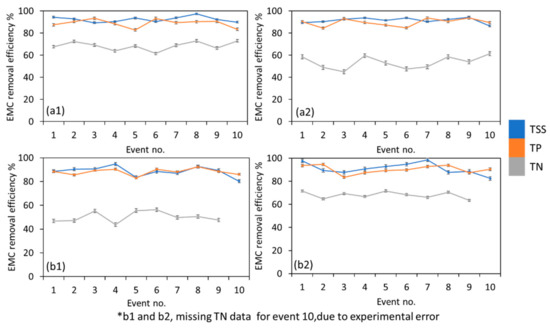
Figure 4.
Phase 1 EMC removal efficiency of TSS, TP and TN for (a1) TT1 in dry condition, (a2) TT2 in dry condition, (b1) TT1 in wet condition, (b2) TT2 in wet condition.
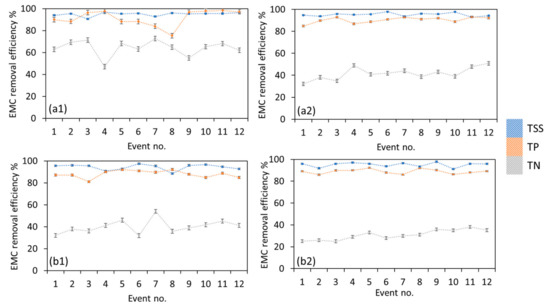
Figure 5.
Phase 2 EMC removal efficiency of TSS, TP and TN for (a1) TT1 in dry condition, (a2) TT2 in dry condition, (b1) TT1 in wet condition, (b2) TT2 in wet condition.
New root growth in Phase 2 was recorded and summarised in Figure 6. The new root growth for the 7th and 10th month was not recorded due to an obstruction blocking the access to the system caused by other ongoing experiments. This root study provides insights into tree selection based on root growth for BRS applications. The root growth rate of the tree was observed to stabilise from the 3rd month onwards, with no new root growth observed for the first 2 months in TT2. Root growth for TT2 was significantly slower, with the vast majority of root growth occurring in the shallower regions of the system. Throughout the study period, >50% of new root growth was found to be below the 600 mm depth for TT1, with a high percentage (>30%) of the new root growth being observed in the deeper regions (800–1000 mm). The total number of new root tips for TT2 was also lesser than that of TT1, with TT1 having about twice that of TT2. This correlated well with the higher degree of removal of TN for TT1 as compared to TT2. Root depth was found to correlated well to the removal efficiency of nitrogen species, with deeper roots favoring higher removal efficiency [39,40]. Kristian-Thorup-Kristensen [40] reported that subsoil nitrate was well correlated to root intensity and rooting depth. Additionally, McMurtrie et. al. [39] reported that root depth and overall root mass had a high correlation to the removal efficiency of nitrogen species. Due to the variation in the environment that the tree species proliferate in, the root structure of the two species shows a significant difference. Coastal plants such as the tree species used in TT1 have deeper roots compared to rainforest plants such as the tree species used in TT2. Hence, in the selection of tree species for a BRS, there needs to be a balance between the depth of the filter media to support plant growth as well as to improve pollutant removal. It may be advantageous to plant a forest species in a BRS with shallower filter depths. On the other hand, when the infiltration rate of the filter media is of concern, coastal tree species may be more valuable, as the continuous high root growth could provide many passageways and pockets for runoff to flow through. In this study, TT1 has better pollutant removal performance with a deeper filter media design, hence the plant species Talipariti tiliaceum would be used in the subsequent field study (Phase 3).
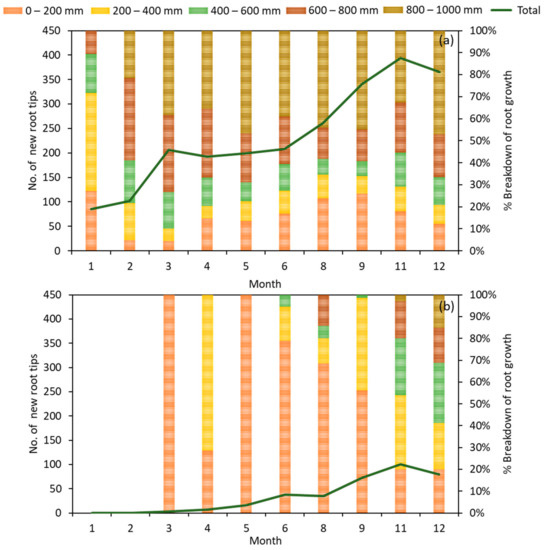
Figure 6.
(a) The number of new root tips observed at different depths of the biofilters planted with Talipalitri tiliaceum, TT1 and (b) Sterculia macrophylla, TT2 in the bioretention system for the pilot study over a period of 12 months.
3.3. Field Study (Phase 3)
The performance of the field-scale MBT and the factors impacting its performance will be discussed in this section.
3.3.1. Tree-Soil Relationship
The T. tiliaceum tree in the MBT was monitored for the first 12 months, and the indices for plant growth are reflected in Figure 7a. The height of tree, Fv:Fm ratio, and soil plant analysis development (SPAD) factor remained fairly constant throughout the study. The average height of the tree was noted to be 3.84 m while the average Fv:Fm ratio was 0.823 over 12 months. Tree height remained consistent, with a minute difference in growth height throughout the period of study. The tree in this study depicted a Fv:Fm ratio range from 0.73 to 0.88. This result is similar to other tree phytoremediation studies in the tropics [37,76], which shows that the plants are healthy. Furthermore, SPAD sampled at the top of the tree also had a fairly constant value, with an average of 39.32, indicating that the tree maintained its chlorophyll levels. A demarcated branch near the top of the tree was selected to monitor new leaf growth. Results showed a steady increase in the number of new leaves throughout the study. Based on the definition by Chen et al. [37], the T. tiliaceum used in this study was found to have moderate growth with a controlled increase in the number of new leaves. Overall, this suggests that the T. tiliaceum tree is suitable for the MBT, due to its controlled growth and good indication of health.
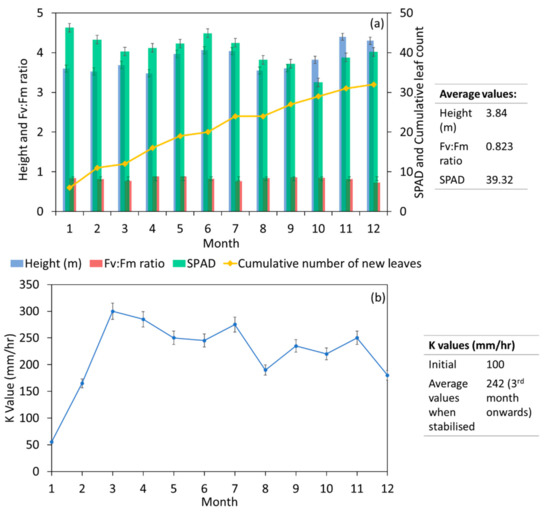
Figure 7.
(a) Plant growth indices in the MBT system and (b) Soil conductivity indices.
The hydraulic conductivity of soil is affected by other factors such as the antecedent dry period [77] and plant characteristics, especially root properties [78,79]. Archer et al. [78] found that plants with thick roots can form macropores and can facilitate water to percolate deeper, which leads to higher hydraulic conductivity. The hydraulic conductivity of the soil in the field study was monitored for 12 months to observe key changes to the infiltration rate of the soil due to the growth of the tree in the system (Figure 7b). The first measurement (0 months) of 100 mm/h was taken before the planting of the tree. It can be later seen that the K values at the initial stage decreased slightly and subsequently increased to a stabilised range. The initial decrease in saturated hydraulic conductivity is a typical trait of BRS and was similar to other field studies [46,70], which could be caused by the natural compaction of the filter media layer due to hydraulic loading from stormwater runoff. Furthermore, washed-off sediments that accumulated and were deposited due to the installation of the MBT system could also decrease the K value, which is the primary reason for the surface clogging of such soil-based systems [80,81]. When the hydraulic conductivity is too low, the low infiltration rate might promote high levels of ponding and even overflow, causing runoff to bypass the system and go untreated. Thus, the surface soil layer was removed was conducted after 1 month of operation, resulting in an improvement in the subsequent measurement of hydraulic conductivity. As the tree continued to grow, root growthpenetrated the filter media, creating pores and reducing the clogging potential of the BRS [82,83]. The K values stabilised between the range of 180 and 296 mm/h from the 3rd month onwards, which corresponds well with the recommended range (100 mm/h to 300 mm/h) of the technical guidelines in Singapore [84].
3.3.2. Pollutants Removal Performance
A detailed breakdown of the field performance efficiency of the system is shown in Appendix F. The field system generally performed well in TSS removal, with the effluent TSS EMCs (ranged from 0.3 mg/L to 28.4 mg/L) having an average removal efficiency of 43.6%, as shown in Figure 8. This efficiency result was lower compared to other conducted field studies conducted [70,85,86]. Poor removal efficiency was obtained due to low influent EMCs (further discussed in Section 4.2.1). For several events during the monitoring period, the TSS effluent concentration was much higher than the influent TSS concentration. This phenomenon was also observed in another field study [87]. During a prolonged dry period, soil aggregates can break down into fine particles that can migrate to deeper levels or can be washed out in the next storm event. Besides that, antecedent dry weather can also lead to low soil moisture, forming cracks within the filter media due to the shrinkage of soil aggregates. This modifies the preferential flow path of the stormwater runoff, reducing the maximum treatment area of the bioretention system [46]. Effluent TP concentration from the bioretention tree system varied from 0.01 mg/L to 0.13 mg/L. Overall, satisfactory removal of TP was observed with an average of 32.1%. Occasional negative removal efficiency was observed during the monitoring of the bioretention tree system, suggesting that phosphorus in the filter media might be washed out with the effluent. However, the low effluent TP EMC might be due to low phosphorus loading into the bioretention tree system. Roof runoff generally had a lower TP concentration than other catchment types such as lawns and roads. Similar to what was being observed for TSS pollutants, low inflow TP EMC in this study led to a negative removal efficiency for some of the rainfall events [88]. Furthermore, the WTR within the soil mix also aided in the effective removal of TP, as seen by other field studies [89,90]. For nitrogen, effluent TN ranged from 0.08 mg/L to 3.22 mg/L with an average TN removal efficiency of 45.6%. The ability of the bioretention tree system to act as an effective sink for nitrogen could be due to the choice of plant species used. The tree planted in the bioretention tree system is a coastal plant species that required a large amount of nitrogen for growth. As such, a large amount of nitrogen in the stormwater runoff was used by the tree. Additionally, tree species typically have deeper roots and a vast root network that can aid in the uptake of dissolved nitrogen in water [17].
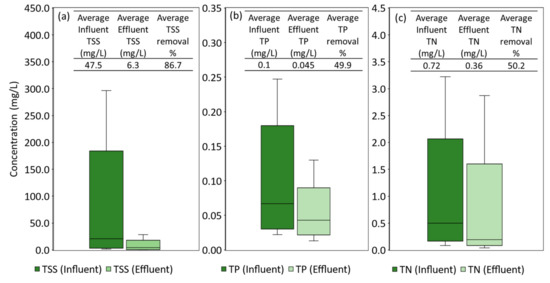
Figure 8.
(a) TSS removal performance of field MBT, (b) TP removal performance of field MBT, and (c) TN removal performance of field MBT.
4. Discussion
4.1. Comparison to Other Bioretention Systems in the Tropics
Generally, the effluent of the field MBT system was very clean with average TSS, TP, and TN effluent EMC of 4.8 mg/L, 0.04 mg/L and 0.27 mg/L, respectively. Compared to other conventional BRS systems in the tropics, this MBT showed comparable removal performance, as summarised in Table 1.
The studies by Ong et al. [91] and Wang et al. [92] were conducted in a region with similar rainfall and tropical conditions. It is important to note that both studies monitored a BRS with a submerged zone, which was reported to typically have a much more significant removal of TN [90,92]. This highlights that despite not having a submerged zone, the pollutant removal performance of our MBT was comparable to that of other reported studies, which may be due to the thicker depth of the filter media being used for the MBT. A deeper filter media increases the retention time of the system and contains more sorption sites for nitrogen removal [10]. Compared to other pilot studies of conventional BRS in the tropics, the average effluent of the MBT is also shown to be significantly cleaner. Since there were no reported studies on tree systems in a tropical climate, the results were benchmarked against bioretention tree systems in temperate conditions (Table 2). The performance of the MBT in this study was comparable to other studies.

Table 2.
Effluent pollutants EMC of the field MBT system and comparison with literature studies.
However, in comparison to Phase 1 and 2, the pollutant removal efficiency of the field study was not remarkable, and this is likely due to various factors that will be discussed in the following sections.
4.2. Environmental Influences on Performance of MBT
4.2.1. Initial Pollutant Concentration
Various environmental factors were explored to have a better understanding of MBT performance in field conditions, and these are summarized in Figure 9. Understanding design and environmental factors is essential to better assess the performance of MBT. This is particularly important for the prediction of the MBT performance and further improvements can be made to optimize this design for other locations with different environmental conditions. Influent pollutant loading is an important factor that affects the removal performance of the BRS [93]. As seen in Figure 9a, as influent pollutant concentration increased, higher removal efficiency was achieved. This is likely due to the strong influence of ‘background’ pollutants. McNett et al. [94] found that effluent nutrient pollutant concentration was strongly influenced by the ‘background’ pollutant concentration and less affected by the influent pollutant concentration. The effluent concentration is controlled by the characteristics of the media filter and not by pollutant loading, though this phenomenon is more prominent for phosphorus than nitrogen.
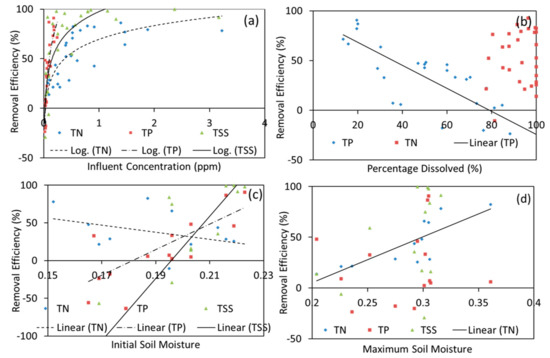
Figure 9.
Environmental factors that affect the performance of MBT: (a) influent of pollutant, (b) % of dissolved pollutant, (c) initial soil moisture, and (d) maximum soil moisture.
The influent runoff pollutant concentration in this study is significantly lower compared to other studies reported for urban areas [95,96] (Appendix G). Roof runoff generally has a smooth surface, resulting in a cleaner runoff compared to other runoff sources such as pedestrian footpath and road [97]. In the context of Singapore, the high degree of variability in the runoff quality may also be due to the frequency and seasonal severity of storm events [44]. Furthermore, the contributing catchment area in this study is quite small (90 m2) compared to other field studies e.g., 2200 m2 [29]; 2400 m2 [87], leading to a lower pollutant accumulation. This indicates that the field MBT system could potentially manage and treat runoff with higher pollutant concentration, such as industrial and road runoffs. Earlier pilot biofilter studies also noted a much higher pollutant removal efficiency compared to the field study, which is likely due to the higher influent pollutant concentration. Further study to evaluate the performance of MBT systems in a larger catchment area would be recommended. Simulated storm events with higher pollutant inflow levels can be conducted to further validate the performance of the MBT.
4.2.2. Dissolved Percentage of Influent Pollutants
The dissolved percentage of influent pollutants and their respective removal efficiency are visualized in Figure 7b with more detailed results shown in Appendix H. The removal efficiency of TP was observed to be affected by the dissolved fraction in the influent sample. In this study, the influent dissolved percentage of phosphorus (DP/TP) ranged between 14% to 85%. A higher dissolved fraction of influent phosphorus resulted in the lower phosphorus removal performance of the MBT. As adsorption is the major removal mechanism for phosphorus in BRS [10,36,98], the phosphorus adsorption capacity of the filter media is an important parameter in managing and removing dissolved phosphorus.
On the other hand, little relationship was observed between TN removal efficiency and the dissolved fraction of the pollutants in this study. The difference in observation could be due to the narrow range of the observed results. Most of the observed results had a dissolved nitrogen content ranging from 80% to 100%, with little data below 80%. This led to a skewed result due to the poor spread of the collected data. Similar to most reported studies, the particulate fraction of nitrogen accounted for the lowest fraction of TN in urban stormwater [99,100]. Nitrogen loading is mainly from atmospheric loading, giving rise to a predominantly dissolved percentage of nitrogen in stormwater runoff [60,101]. While dissolved nitrogen forms a large fraction of TN in this study, the removal of TN is shown to have a great degree of variability, due to the relatively complicated TN removal mechanism. The removal of dissolved nitrogen species such as nitrate and nitrite often posed great difficulty, as these nitrogen species are highly soluble and do not readily adsorb into the BRS soil media readily [102].
It is also noted that environmental influences such as weather conditions and the number of antecedent dry days can affect the health of both plants and soil microbial communities, while soil moisture can affect the mobility of the dissolved pollutants. Tropical countries like Singapore have high rainfall, but the time interval between consecutive rainfall events is short [103]. Thus, with varying environmental conditions, the removal of dissolved pollutants may be highly inconsistent and difficult.
4.2.3. Soil Moisture
In this study, soil moisture content is used as an indicator to represent the wet and dry conditions that are experienced by the MBT. The removal efficiency of TP and TSS displayed a positive relationship with the initial volumetric water content in the soil before the rainfall event, while that of TN was more influenced by the maximum soil moisture content during the storm event. This difference in influencing parameters could be due to the different removal mechanisms involved.
TSS and the particulate fraction of phosphorus were removed via a filtration process through the soil column. Higher initial soil moisture prevented the shrinking and cracking of the soil particles to ensure an effective and efficient removal process. TSS and TP have seemingly no relationship with the maximum soil moisture experienced by the MBT during a storm event. Higher maximum soil moisture suggests that the MBT receives a larger volume of stormwater runoff. The field study results coincide with the results of the pilot biofilter studies in which wet and dry conditions did not necessarily affect TSS and TP performance.
On the other hand, nitrogen fate and transport are highly regulated by the moisture content [61,104]. Higher soil moisture would increase the mobility and bioavailability of dissolved nitrogen species. Nitrogen (nitrate species) can be removed through the denitrification process, which is encouraged by an anoxic environment. With higher maximum moisture content in the soil, there is less air in the spaces between soil particles, promoting a temporary anoxic environment for denitrification to occur. Higher initial soil moisture also increases the bioavailability and mobility of dissolved nutrients as well as maintains a healthy microbial consortium to promote nutrient uptake and improves nutrient removal [105].
5. Conclusions and Impact of Studies
Overall, this study has provided insights into the performance of a MBT system in terms of stormwater runoff quality improvements at both the pilot and field scale in the tropical region. MBT was shown to be successful in highly urbanized areas and was able to do so with low construction time while also maintaining high removal performance without the addition of submerged zone layer. Wet or dry dosing conditions had a significant influence on the TN removal performance of both TT1 and TT2 in both phases. While the effective removal of TSS, TP, and heavy metals was found to be independent of wet and dry dosing conditions, variations in TN removal between Phase 1 and 2 was found to be due to the differences in organic speciation found in the stormwater runoff compared to synthetic water. Hence, this study showed that the usage of simulated stormwater as a screening tool might not necessarily be sufficiently representative of field conditions compared to real stormwater runoff. Variation in TN removal was also attributed to the plant species used. Coastal plants such as Talipalitri tiliaceum are more suitable for MBT in the tropics due to their moderate growth rate, high root infiltration within the soil media, and high uptake of TN.
The field study showed that the tree retained a healthy growth rate while maintaining the optimal infiltration rate of the system to treat incoming stormwater runoff effectively. Pollutant removal performance of MBT during the field monitoring of 15 months was comparable with other reported studies in the tropics, with the average EMC removal of TSS, TP and TN being 44%, 32% and 46%, respectively. Environmental factors such as low influent pollutant concentrations, soil moisture, and dissolved species were found to affect the performance of MBT in the field. While the present study only explores the simulation of a single rewetting–drying alternative condition based on real rainfall, it might not be fully representative of the removal efficiency for the natural cycle of drying and rewetting caused by climate changes. Future challenge tests with controlled dosing conditions can be further explored in field MBT to monitor the performance of multiple periods of wetting and drying.
Author Contributions
Conceptualization, F.Y.L., H.G., and S.Z.G.; methodology development, F.Y.L. and S.Z.G.; investigation, F.Y.L., S.Z.G.; writing—data analysis and draft preparation, T.H.N. and B.C.Y.L.; supervision and project administration, J.H. and S.L.O., G.S.O., and C.X.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by PUB, Singapore’s National Water Agency under the ‘Novel Bioretention Systems Development for Sustainable Stormwater Management’ research project, grant number WBS: R-706-000-020-490.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study is available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A
Tree Species Screened

Table A1.
Summary of tree species screened for suitability for bioretention tree system.
Table A1.
Summary of tree species screened for suitability for bioretention tree system.
| S/N | Trees | Growth Rate | Flow Rate | Nitrate Removal | Phosphate Removal |
|---|---|---|---|---|---|
| 1 | Ardisia elliptica | Slow | No change | Effective | Ineffective |
| 2 | Baccaurea minor | Slow | Improved | Effective | Ineffective |
| 3 | Barringtonia asiaticum | Moderate | Improved | Ineffective | Ineffective |
| 4 | Bhesa paniculata | Slow | No change | Effective | Ineffective |
| 5 | Bhesa robusta | Fast | No change | Ineffective | Ineffective |
| 6 | Diospyros discolor | Slow | Improved | Ineffective | Ineffective |
| 7 | Dipterocarpus kerrii | Slow | No change | Ineffective | Ineffective |
| 8 | Elateriospermum tapos | Moderate | Improved | Effective | Ineffective |
| 9 | Garcinia cowa | Slow | No change | Ineffective | Ineffective |
| 10 | Garcinia subelliptica | Slow | Worsened | Ineffective | Ineffective |
| 11 | Gardenia tubifera | Slow | No change | Effective | Ineffective |
| 12 | Hopea ferrea | Moderate | Worsened | Effective | Ineffective |
| 13 | Kopsia arborea | Slow | Improved | Ineffective | Ineffective |
| 14 | Lithocarpus sundaicus | Fast | Improved | Effective | Ineffective |
| 15 | Magnolia coco | Slow | Improved | Ineffective | Ineffective |
| 16 | Memecylon edule | Slow | No change | Ineffective | Ineffective |
| 17 | Magnolia coco | Slow | Improved | Ineffective | Ineffective |
| 18 | Memecylon edule | Slow | No change | Ineffective | Ineffective |
| 19 | Sterculia macrophylla | Moderate | No change | Ineffective | Ineffective |
| 20 | Suregada multiflora | Moderate | Improved | Ineffective | Ineffective |
| 21 | Syzygium acuminatissimum | Moderate | Improved | Effective | Ineffective |
| 22 | Syzygium gratum | Fast | Improved | Ineffective | Ineffective |
| 23 | Syzygium leucoxylon | Fast | No change | Effective | Ineffective |
| 24 | Syzygium myrtifolium | Moderate | Worsened | Effective | Ineffective |
| 25 | Talipalitri tiliaceum (red-leaf variety) | Moderate | Improved | Effective | Ineffective |
| 26 | Tristaniopsis whiteana | Moderate | No change | Ineffective | Ineffective |
The growth rate and flow rate of the plant systems were determined using various non-destructive techniques such as SPAD-502 readings, new leaf growth and leaf length as well as destructive techniques such as root and shoot analysis. Destructive analysis in this context refers to irreversible damage to the plant after the observation. SPAD-502 chlorophyll meter (Konica Minolta, Japan) can be used to determine leaf colour changes, which have been reported to be related to leaf chlorophyll-a and nitrogen levels.
Appendix B
Synthetic Stormwater Composition and Actual Stormwater Runoff Concentration
Tap water was left overnight in order to dechlorinate the water samples and subsequently dosed with the chemicals shown in the table below.

Table A2.
Characteristics of simulated water.
Table A2.
Characteristics of simulated water.
| Pollutant | Chemical Used | Target Concentration of the Pollutant (mg/L) |
|---|---|---|
| Total Suspended Soils (TSS) | Sediment and sand | 100 |
| Phosphate (PO43−) | H2KO4P | 1.80 |
| Nitrogen (N) | MgN2O6∙6H2O | 0.80 |
| C6H5NO2 | 1.30 | |
| NH4Cl | 0.40 | |
| Copper (Cu) | Cl2Cu | 0.241 |
| Lead (Pb) | Cl2Pb | 0.09025 |
| Zinc (Zn) | Cl2Zn | 1.127 |
| Cadmium (Cd) | CdN2O6∙4H2O | 0.00457 |
The range of the pollutants for the stormwater runoff is shown below.

Table A3.
Characteristics of stormwater runoff used for Phase 2.
Table A3.
Characteristics of stormwater runoff used for Phase 2.
| Pollutant | Min–Max (Mean) Concentration |
| TSS (mg/L) | 78.0–164.0 (121.8) |
| TP (mg/L) | 1.63–3.06 (2.20) |
| TN (mg/L) | 2.24–4.56 (3.45) |
| NO3− (mg/L) | 0.25–0.78 (0.50) |
| NH3 (mg/L) | 0.12–0.60 (0.33) |
| Zinc, Zn (µg/L) | 666.36–1957.32 (1034.35) |
| Cadmium, Cd (µg/L) | 1.21–6.67 (3.74) |
| Copper, Cu (µg/L) | 87.26–514.98 (232.28) |
| Lead, Pb (µg/L) | 35.21–263.98 (100.26) |
Appendix C
Peak Runoff Flow Rate Computation
The peak runoff flow rate resulting from the precipitation event is calculated using the rational formula shown in equation below, where C is the runoff coefficient, I is the average rainfall intensity, and A is the catchment area served.
The runoff coefficient is obtained by assuming the catchment area to be comprised of 50% urban areas both fully and closely built-up and 50% residential or industrial areas densely built up. The coefficient was then obtained as 0.85 from the weighted average of the runoff coefficient of these two areas [94].
Appendix D
EMC Removal Efficiency for Pilot Biofilter Study—TT1 and TT2 (Phase 1 and 2)

Table A4.
EMC removal efficiency of pilot bioretention tree biofilter study (TT1: Talipariti tiliaceum; TT2: Sterculia macrophylla) for Phase 1.
Table A4.
EMC removal efficiency of pilot bioretention tree biofilter study (TT1: Talipariti tiliaceum; TT2: Sterculia macrophylla) for Phase 1.
| Phase 1 | ||||||
|---|---|---|---|---|---|---|
| TT1 | ||||||
| Pollutant | EMC Removal Efficiency (%) | |||||
| Mean | Minimum | Maximum | ||||
| Wet | Dry | Wet | Dry | Wet | Dry | |
| TSS | 92.5 | 91.5 | 89.1 | 86.4 | 97.4 | 94.2 |
| TP | 89.0 | 89.7 | 83.0 | 84.3 | 93.4 | 93.7 |
| TN | 68.4 | 53.6 | 61.8 | 45.1 | 73.0 | 61.7 |
| Zn | 97.2 | 96.3 | 93.8 | 93.4 | 98.4 | 97.4 |
| Cu | 89.4 | 88.6 | 76.1 | 76.6 | 92.1 | 91.9 |
| Cd a | 95.3 | 94.6 | 65.5 | 67.2 | 99.6 | 99.6 |
| Pb | 85.1 | 84.3 | 72.1 | 71.6 | 90.8 | 91.0 |
| TT2 | ||||||
| Pollutant | EMC Removal Efficiency (%) | |||||
| Mean | Minimum | Maximum | ||||
| Wet | Dry | Wet | Dry | Wet | Dry | |
| TSS | 91.0 | 88.6 | 82.4 | 80.4 | 98.2 | 94.8 |
| TP | 90.3 | 88.2 | 83.3 | 83.0 | 94.5 | 92.6 |
| TN | 68.0 | 50.3 | 63.5 | 44.0 | 71.8 | 56.2 |
| Zn | 92.5 | 92.1 | 83.2 | 85.5 | 98.8 | 96.9 |
| Cu | 83.9 | 84.5 | 74.3 | 75.3 | 92.5 | 91.0 |
| Cd b | 94.3 | 96.1 | 84.1 | 84.0 | 99.5 | 99.6 |
| Pb | 87.0 | 85.9 | 80.3 | 78.4 | 98.5 | 97.2 |
a only 3 events are detectable, others are below detection limit; b only 5 events are detectable, others are below detection limit.

Table A5.
EMC removal efficiency of pilot bioretention tree biofilter study (TT1: Talipariti tiliaceum; TT2: Sterculia macrophylla) for Phase 2.
Table A5.
EMC removal efficiency of pilot bioretention tree biofilter study (TT1: Talipariti tiliaceum; TT2: Sterculia macrophylla) for Phase 2.
| Phase 2 | ||||||
|---|---|---|---|---|---|---|
| TT1 | ||||||
| Pollutant | EMC Removal Efficiency (%) | |||||
| Mean | Minimum | Maximum | ||||
| Wet | Dry | Wet | Dry | Wet | Dry | |
| TSS | 95.0 | 95.3 | 90.6 | 92.9 | 96.9 | 97.9 |
| TP | 91.5 | 90.3 | 75.3 | 84.8 | 98.4 | 93.1 |
| TN | 94.1 | 41.7 | 47.0 | 32.1 | 72.8 | 50.9 |
| Zn | 98.2 | 98.2 | 96.0 | 97.0 | 99.0 | 99.0 |
| Cu | 93.8 | 93.7 | 92.0 | 91.0 | 99.5 | 96.0 |
| Cd | 98.6 | 96.9 | 98.1 | 94.7 | 99.1 | 99.0 |
| Pb | 92.0 | 91.8 | 88.0 | 88.0 | 95.4 | 94.0 |
| TT2 | ||||||
| Pollutant | EMC Removal Efficiency (%) | |||||
| Mean | Minimum | Maximum | ||||
| Wet | Dry | Wet | Dry | Wet | Dry | |
| TSS | 94.3 | 95.1 | 88.5 | 91.2 | 97.5 | 97.8 |
| TP | 88.0 | 88.9 | 81.0 | 85.9 | 92.1 | 92.2 |
| TN | 40.1 | 30.9 | 31.9 | 24.9 | 54.0 | 38.1 |
| Zn | 96.2 | 96.3 | 95.0 | 95.0 | 98.0 | 98.0 |
| Cu | 93.8 | 92.3 | 93.0 | 84.1 | 95.0 | 96.5 |
| Cd | 98.3 | 98.3 | 96.9 | 97.0 | 99.0 | 99.5 |
| Pb | 91.9 | 92.4 | 91.0 | 91.0 | 93.5 | 98.3 |
Appendix E
Statistical Analysis for Phase 1 and Phase 2

Table A6.
Paired sample test for Phase 1 of study.
Table A6.
Paired sample test for Phase 1 of study.
| TT1 | |||||
|---|---|---|---|---|---|
| Paired Samples Test | |||||
| Mean | Std Dev | Std Error Mean | df | Sig. (2-tailed) | |
| TT1 (TSS Wet)–TT1 (TSS Dry) | 0.953 | 3.539 | 1.119 | 9 | 0.417 |
| TT1 (TP Wet)–TT1 (TP Dry) | −0.716 | 4.689 | 1.483 | 9 | 0.641 |
| TT1 (TN Wet)–TT1 (TN Dry) | 14.850 | 6.178 | 1.954 | 9 | 0.000 |
| TT1 (Zinc Wet)–TT1 (Zinc Dry) | −0.018 | 1.723 | 0.581 | 9 | 0.468 |
| TT1 (Copper Wet)–TT1 (Copper Dry) | 0.147 | 2.264 | 0.532 | 9 | 0.154 |
| TT1 (Cadmium Wet)–TT1 (Cadmium Dry) | - | - | - | - | - |
| TT1 (Lead Wet)–TT1 (Lead Dry) | 0.142 | 2.701 | 0.628 | 9 | 0.573 |
| TT2 | |||||
| Paired Samples Test | |||||
| Mean | Std Dev | Std Error Mean | df | Sig. (2-tailed) | |
| TT2 (TSS Wet)–TT2 (TSS Dry) | 2.450 | 6.077 | 1.922 | 9 | 0.234 |
| TT2 (TP Wet)–TT2 (TP Dry) | 2.021 | 4.633 | 1.465 | 9 | 0.201 |
| TT2 (TN Wet)–TT2 (TN Dry) | 17.706 | 4.044 | 1.348 | 9 | 0.000 |
| TT2 (Zinc Wet)–TT2 (Zinc Dry) | −0.084 | 1.203 | 0.416 | 9 | 0.457 |
| TT2 (Copper Wet)–TT2 (Copper Dry) | 1.552 | 2.413 | 0.315 | 9 | 0.224 |
| TT2 (Cadmium Wet)–TT2 (Cadmium Dry) | - | - | - | 9 | - |
| TT2 (Lead Wet)–TT2 (Lead Dry) | −0.544 | 1.892 | 0.621 | 9 | 0.654 |

Table A7.
Paired sample test for Phase 2 of study.
Table A7.
Paired sample test for Phase 2 of study.
| TT1 | |||||
|---|---|---|---|---|---|
| Paired Samples Test | |||||
| Mean | Std Dev | Std Error Mean | df | Sig. (2-tailed) | |
| TT1 (TSS Wet)–TT1 (TSS Dry) | −0.333 | 2.245 | 0.648 | 11 | 0.617 |
| TT1 (TP Wet)–TT1 (TP Dry) | 1.150 | 7.595 | 2.192 | 11 | 0.610 |
| TT1 (TN Wet)–TT1 (TN Dry) | 22.408 | 10.749 | 3.103 | 11 | 0.000 |
| TT1 (Zinc Wet)–TT1 (Zinc Dry) | −0.025 | 1.781 | 0.514 | 11 | 0.962 |
| TT1 (Copper Wet)–TT1 (Copper Dry) | 0.133 | 2.570 | 0.742 | 11 | 0.861 |
| TT1 (Cadmium Wet)–TT1 (Cadmium Dry) | 1.642 | 1.794 | 0.518 | 11 | 0.009 |
| TT1 (Lead Wet)–TT1 (Lead Dry) | 0.158 | 2.578 | 0.744 | 11 | 0.835 |
| TT2 | |||||
| Paired Samples Test | |||||
| Mean | Std Dev | Std Error Mean | df | Sig. (2-tailed) | |
| TT2 (TSS Wet)–TT2 (TSS Dry) | −0.742 | 3.631 | 1.048 | 11 | 0.494 |
| TT2 (TP Wet)–TT2 (TP Dry) | −0.809 | 3.351 | 0.967 | 11 | 0.421 |
| TT2 (TN Wet)–TT2 (TN Dry) | 9.229 | 5.749 | 1.660 | 11 | 0.000 |
| TT2 (Zinc Wet)–TT2 (Zinc Dry) | −0.084 | 1.505 | 0.435 | 11 | 0.851 |
| TT2 (Copper Wet)–TT2 (Copper Dry) | 1.552 | 3.306 | 0.954 | 11 | 0.132 |
| TT2 (Cadmium Wet)–TT2 (Cadmium Dry) | 0.024 | 1.109 | 0.320 | 11 | 0.942 |
| TT2 (Lead Wet)–TT2 (Lead Dry) | −0.544 | 2.423 | 0.699 | 11 | 0.453 |
Appendix F

Table A8.
Performance of MBT for Phase 3 (Field Study).
Table A8.
Performance of MBT for Phase 3 (Field Study).
| Event | TSS | TP | TN | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Influent(mg/L) | Effluent(mg/L) | Removal Efficiency (%) | Influent(mg/L) | Effluent(mg/L) | Removal Efficiency (%) | Influent(mg/L) | Effluent(mg/L) | Removal Efficiency (%) | |
| 1 | 99.8 | 2.6 | 97.4 | 0.19 | 0.03 | 82.4 | 1.47 | 0.34 | 76.9 |
| 2 | 99.8 | 2.1 | 97.9 | 0.19 | 0.02 | 90.9 | 1.47 | 0.52 | 64.6 |
| 3 | 296.3 | 0.4 | 99.9 | 0.07 | 0.04 | 46.3 | 0.08 | 0.06 | 25.0 |
| 4 | 106.6 | 0.8 | 99.2 | 0.10 | 0.01 | 87.0 | 0.46 | 0.33 | 28.3 |
| 5 | 4.8 | 4.1 | 14.6 | 0.03 | 0.02 | 46.9 | 0.10 | 0.08 | 20.0 |
| 6 | 6.1 | 18.7 | −206.6 | 0.04 | 0.05 | −19.0 | 0.10 | 0.07 | 30.0 |
| 7 | 1.4 | 2.1 | −50.0 | 0.02 | 0.03 | −22.7 | 0.29 | 0.23 | 20.7 |
| 8 | 4.0 | 10.0 | −150.0 | 0.06 | 0.05 | 7.0 | 0.53 | 0.09 | 83.0 |
| 9 | 1.3 | 0.3 | 76.9 | 0.05 | 0.03 | 34.0 | 0.22 | 0.55 | −150.0 |
| 10 | 9.0 | 7.5 | 16.7 | 0.05 | 0.08 | −57.1 | 0.61 | 0.32 | 47.5 |
| 11 | 3.1 | 4.0 | −29.0 | 0.04 | 0.04 | 2.6 | 0.41 | 0.14 | 65.9 |
| 12 | 42.0 | 6.8 | 83.8 | 0.14 | 0.13 | 7.1 | 0.27 | 0.30 | −11.1 |
| 13 | 4.6 | 3.0 | 34.8 | 0.04 | 0.04 | −19.4 | 0.21 | 0.12 | 42.9 |
| 14 | 11.3 | 12.0 | −6.2 | 0.03 | 0.03 | 9.1 | 3.22 | 0.70 | 78.3 |
| 15 | 28.4 | 0.7 | 97.5 | 0.09 | 0.05 | 46.0 | 0.79 | 0.14 | 82.3 |
| 16 | 8.4 | 0.7 | 91.7 | 0.03 | 0.02 | 50.0 | 1.88 | 0.39 | 79.3 |
| 17 | 84.3 | 5.7 | 93.2 | 0.11 | 0.04 | 63.6 | 2.39 | 1.42 | 40.6 |
| 18 | 7.2 | 6.0 | 16.7 | 0.03 | 0.03 | 6.3 | 0.16 | 0.13 | 18.8 |
| 19 | 3.1 | 0.8 | 74.2 | 0.19 | 0.04 | 78.6 | 0.25 | 0.19 | 24.0 |
| 20 | 11.9 | 5.3 | 55.5 | 0.17 | 0.05 | 72.8 | 0.40 | 0.14 | 65.0 |
| 21 | 20.5 | 3.0 | 85.4 | 0.05 | 0.05 | 6.0 | 0.52 | 0.12 | 76.9 |
| 22 | 52.3 | 10.0 | 80.9 | 0.11 | 0.06 | 46.9 | 0.52 | 0.15 | 71.2 |
| 23 | 10.9 | 7.3 | 33.0 | 0.06 | 0.04 | 39.3 | 0.54 | 0.04 | 92.6 |
| 24 | 71.3 | 11.3 | 84.2 | 0.19 | 0.06 | 66.8 | 0.91 | 0.16 | 82.4 |
| 25 | 47.5 | 2.0 | 95.8 | 0.05 | 0.04 | 30.2 | 0.50 | 0.23 | 54.0 |
| 26 | 144.0 | 2.7 | 98.1 | 0.09 | 0.05 | 43.0 | 1.39 | 0.19 | 86.3 |
| 27 | 3.3 | 0.3 | 90.9 | 0.06 | 0.05 | 21.7 | 0.26 | 0.17 | 34.6 |
Appendix G
Stormwater Runoff Water Quality and Comparison with Literature Studies

Table A9.
Runoff water quality of the study area and comparison with literature studies.
Table A9.
Runoff water quality of the study area and comparison with literature studies.
| Min-Max (Mean) | ||||
|---|---|---|---|---|
| TSS EMC (mg/L) | TP EMC (mg/L) | TN EMC (mg/L) | References | |
| This Study | 1.3–296.3 (47.5) | 0.02–0.25 (0.09) | 0.08–3.22 (0.72) | - |
| High Urban Areas | 155 | 0.32 | 2.63 | [95] |
| Ang Mo Ki—commercial, residential, carparks, road | 112.07 | 0.17 | 1.85 | [96] |
| Pemimpin—residential | 31.92 | 0.07 | 1.16 | |
| Lower Seletar—parkland | 147.34 | 0.31 | 3.02 | |
Appendix H
Dissolved Nutrients Composition

Table A10.
Dissolved nutrient composition and respective removal efficiency.
Table A10.
Dissolved nutrient composition and respective removal efficiency.
| Dissolved Phosphorus (DP)/Total Phosphorus (TP) | TP Removal Efficiency (%) | Dissolved Nitrogen (DN)/Total Nitrogen (TN) | TN Removal Efficiency (%) |
|---|---|---|---|
| 13.5 | 71.4 | 77.6 | 21.4 |
| 15.8 | 66.7 | 78.6 | 52.2 |
| 19.6 | 90.7 | 80.4 | 76.2 |
| 19.6 | 82.1 | 80.4 | 76.2 |
| 20.0 | 86.7 | 81.7 | −10.8 |
| 29.0 | 42.1 | 84.8 | 63.6 |
| 30.3 | 63.6 | 85.0 | 42.6 |
| 31.8 | 32.7 | 87.8 | 64.4 |
| 35.7 | 7.1 | 87.8 | 76.9 |
| 39.2 | 5.9 | 87.8 | 76.9 |
| 47.1 | 47.1 | 87.9 | 40.6 |
| 50.0 | 46.2 | 91.5 | 86.1 |
| 50.0 | 42.9 | 91.7 | 70.7 |
| 50.6 | 48.1 | 92.6 | 28.6 |
| 57.8 | −17.9 | 93.1 | 79.3 |
| 60.0 | 46.0 | 93.1 | 79.3 |
| 60.0 | 40.2 | 93.6 | 47.9 |
| 63.7 | 33.3 | 94.9 | 21.3 |
| 68.8 | 31.3 | 96.4 | 92.5 |
| 70.4 | 33.3 | 96.6 | 82.0 |
| 73.3 | 6.7 | 98.0 | 24.3 |
| 77.8 | 22.2 | 99.3 | 78.3 |
| 81.2 | 2.5 | 99.5 | 83.0 |
| 84.8 | 5.0 | 99.9 | 52.7 |
| 100.0 | 13.9 | ||
| 100.0 | 25.5 | ||
| 100.0 | 28.2 | ||
| 100.0 | 34.8 | ||
| 100.0 | 43.8 | ||
| 100.0 | 66.0 | ||
| 100.0 | 78.7 | ||
| 100.0 | 82.5 |
References
- Roy-Poirier, A.; Champagne, P.; Filion, Y. Review of Bioretention System Research and Design: Past, Present, and Future. J. Environ. Eng. 2010, 136, 878–889. [Google Scholar] [CrossRef]
- Luell, S.K.; Hunt, W.F.; Winston, R.J. Evaluation of undersized bioretention stormwater control measures for treatment of highway bridge deck runoff. Water Sci. Technol. 2011, 64, 974–979. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.D.; Hutchins, M. The impacts of urbanisation and climate change on urban flooding and urban water quality: A review of the evidence concerning the United Kingdom. J. Hydrol. Reg. Stud. 2017, 12, 345–362. [Google Scholar] [CrossRef] [Green Version]
- Goh, H.W.; Zakaria, N.A.; Lau, T.L.; Foo, K.Y.; Chang, C.K.; Leow, C.S. Mesocosm Study of Enhanced Bioreten-tion Media in Treating Nutrient Rich Stormwater for Mixed Development Area. Urban Water J. 2017, 14, 134–142. [Google Scholar] [CrossRef]
- Chow, W.T.L.; Cheong, B.D.; Ho, B.H. A Multimethod Approach towards Assessing Urban Flood Patterns and Its Associated Vulnerabilities in Singapore. Adv. Meteorol. 2016, 2016, 1–11. [Google Scholar] [CrossRef]
- Gerber, J.D.; Hartmann, T.; Hengstermann, A. Instruments of Land Policy: Dealing with Scarcity of Land; Routledge: London, UK, 2018. [Google Scholar]
- Lopez-Ponnada, E.V.; Lynn, T.J.; Ergas, S.J.; Mihelcic, J.R. Long-term field performance of a conventional and modified bioretention system for removing dissolved nitrogen species in stormwater runoff. Water Res. 2020, 170, 115336. [Google Scholar] [CrossRef]
- Davis, A.P.; Hunt, W.F.; Traver, R.G.; Clar, M. Bioretention Technology: Overview of Current Practice and Future Needs. J. Environ. Eng. 2009, 135, 109–117. [Google Scholar] [CrossRef]
- Goh, H.W.; Lem, K.S.; Azizan, N.A.; Chang, C.K.; Talei, A.; Leow, C.S.; Zakaria, N.A. A review of bioretention components and nutrient removal under different climates—Future directions for tropics. Environ. Sci. Pollut. Res. 2019, 26, 14904–14919. [Google Scholar] [CrossRef]
- LeFevre, G.; Paus, K.H.; Natarajan, P.; Gulliver, J.; Novak, P.J.; Hozalski, R. Review of Dissolved Pollutants in Urban Storm Water and Their Removal and Fate in Bioretention Cells. J. Environ. Eng. 2015, 141, 04014050. [Google Scholar] [CrossRef]
- Lim, H.S.; Lim, W.; Hu, J.Y.; Ziegler, A.; Ong, S.L. Comparison of Filter Media Materials for Heavy Metal Remov-al from Urban Stormwater Runoff Using Biofiltration Systems. J. Environ. Manag. 2015, 147, 24–33. [Google Scholar] [CrossRef]
- Barrett, M.E.; Limouzin, M.; Lawler, D.F. Effects of Media and Plant Selection on Biofiltration Performance. J. Environ. Eng. 2013, 139, 462–470. [Google Scholar] [CrossRef]
- Angers, D.A.; Caron, J. Plant-induced Changes in Soil Structure: Processes and Feedbacks. Biogeochemistry 1998, 42, 55–72. [Google Scholar] [CrossRef]
- Binkley, D.; Giardina, C. Why Do Tree Species Affect Soils? The Warp and Woof of Tree-Soil Interactions. In Plant-Induced Soil Changes: Processes and Feedbacks; Springer: Dordrecht, The Netherlands, 1998; Volume 42, pp. 89–106. [Google Scholar]
- Van Breemen, N.; Finzi, A.C. Plant-Soil Interactions: Ecological Aspects and Evolutionary Implications. Biogeochemistry 1998, 42, 1–19. [Google Scholar] [CrossRef]
- Skorobogatov, A.; He, J.; Chu, A.; Valeo, C.; van Duin, B. The impact of media, plants and their interactions on bioretention performance: A review. Sci. Total Environ. 2020, 715, 136918. [Google Scholar] [CrossRef]
- Dagenais, D.; Brisson, J.; Fletcher, T.D. The role of plants in bioretention systems; does the science underpin current guidance? Ecol. Eng. 2018, 120, 532–545. [Google Scholar] [CrossRef]
- Tirpak, R.A.; Hathaway, J.M.; Franklin, J.A. Investigating the Hydrologic and Water Quality Performance of Trees in Bioretention Mesocosms. J. Hydrol. 2019, 576, 65–71. [Google Scholar] [CrossRef]
- Frosi, M.H.; Kargar, M.; Jutras, P.; Prasher, S.O.; Clark, O.G. Street tree pits as bioretention units: Effects of soil organic matter and area permeability on the volume and quality of urban runoff. Water Air Soil Pollut. 2019, 230, 152. [Google Scholar] [CrossRef]
- Elliott, R.M.; Adkins, E.R.; Culligan, P.J.; Palmer, M.I. Stormwater infiltration capacity of street tree pits: Quantifying the influence of different design and management strategies in New York City. Ecol. Eng. 2017, 111, 157–166. [Google Scholar] [CrossRef]
- EcosolTM Tree Pit Technical Specification. Available online: https://urbanassetsolutions.com.au/wp-content/uploads/UAS-Tree-Pit-Technical-Specifications-2018.pdf (accessed on 20 June 2021).
- Stockholm Tree Pits. Available online: https://stockholmtreepits.co.uk/ (accessed on 20 June 2021).
- StormTree. Available online: https://www.storm-tree.com/ (accessed on 20 June 2021).
- Macedo, M.B.D.; Lago, C.A.F.D.; Mendiondo, E.M.; Giacomoni, M.H. Bioretention performance under different rainfall regimes in substropical conditions: A case study in Sao Carlos, Brazil. J. Environ. Manag. 2019, 248, 109266. [Google Scholar] [CrossRef]
- Blecken, G.-T.; Zinger, Y.; Deletetić, A.; Fletcher, T.D.; Viklander, M. Influence of Intermittent Wetting and Drying Conditions on Heavy Metal Removal by Stormwater Biofilters. Water Res. 2009, 43, 4590–4598. [Google Scholar] [CrossRef]
- Rahman, Y.A.; Nachabe, M.H.; Ergas, S.J. Biochar amendment of stormwater bioretention systems for nitrogen and Escherichia coli removal: Effect of hydraulic loading rates and antecedent dry periods. Bioresour. Technol. 2020, 310, 123428. [Google Scholar] [CrossRef]
- Manka, B.; Hathaway, J.; Tirpak, R.; He, Q.; Hunt, W. Driving forces of effluent nutrient variability in field scale bioretention. Ecol. Eng. 2016, 94, 622–628. [Google Scholar] [CrossRef]
- Hunt, W.F.; Jarrett, A.R.; Smith, J.T.; Sharkey, L.J. Evaluating Bioretention Hydrology and Nutrient Removal at Three Field Sites in North Carolina. J. Irrig. Drain. Eng. 2006, 132, 600–608. [Google Scholar] [CrossRef]
- Brown, R.A.; Hunt, W.F. Underdrain Configuration to Enhance Bioretention Exfiltration to Reduce Pollutant Loads. J. Environ. Eng. 2011, 137, 1082–1091. [Google Scholar] [CrossRef]
- Kazemi, F.; Golzarian, M.R.; Myers, B. Potential of combined water sensitive urban design systems for salinity treat-ment in urban environments. J. Environ. Manag. 2018, 209, 169–175. [Google Scholar] [CrossRef]
- An Engineered Soil Composition and a Method of Preparing the Same. Singapore Patent Application No. SG2013082722A, 27 August 2013.
- Barrett, J.E.; Burke, I.C. Potential Nitrogen Immobilization in Grassland Soils across a Soil Organic Matter Gradient. Soil Biol. Biochem. 2000, 32, 1707–1716. [Google Scholar] [CrossRef]
- O’Neill, S.W.; Davis, A.P. Water Treatment Residual as a Bioretention Amendment for Phosphorus. I: Evaluation Studies. J. Environ. Eng. 2012, 138, 318–327. [Google Scholar] [CrossRef]
- McGechan, M.B.; Lewis, D.R. Sorption of Phosphorus by Soil, Part 1: Principles, Equations and Models. Biosyst. Eng. 2002, 82, 1–24. [Google Scholar] [CrossRef]
- Xu, D.; Lee, L.Y.; Lim, F.Y.; Lyu, Z.; Zhu, H.; Ong, S.L.; Hu, J. Water treatment residual: A critical review of its applications on pollutant removal from stormwater runoff and future perspectives. J. Environ. Manag. 2020, 259, 109649. [Google Scholar] [CrossRef]
- Hermawan, A.A.; Talei, A.; Leong, J.Y.C.; Jayatharan, M.; Goh, H.W.; Alaghmand, S. Performance Assessment of a Laboratory Scale Prototype Biofiltration System in Tropical Region. Sustainability 2019, 11, 1947. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.C.; Huang, L.; Chang, T.H.A.; Ong, B.L.; Ong, S.L.; Hu, J. Plant Traits for Phytoremediation in the Tropics. Engineering 2019, 5, 841–848. [Google Scholar] [CrossRef]
- Judd, L.A.; Jackson, B.E.; Fonteno, W.C. Advancements in Root Growth Measurement Technologies and Observation Capabilities for Container-Grown Plants. Plants 2015, 4, 369–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McMurtrie, R.E.; Iversen, C.M.; Dewar, R.C.; Medlyn, B.E.; Näsholm, T.; Pepper, D.A.; Norby, R.J. Plant root distributions and nitrogen uptake predicted by a hypothesis of optimal root foraging. Ecol. Evol. 2012, 2, 1235–1250. [Google Scholar] [CrossRef]
- Thorup-Kristensen, K. Are differences in root growth of nitrogen catch crops important for their ability to reduce soil nitrate-N content, and how can this be measured? Plant Soil 2001, 230, 185–195. [Google Scholar] [CrossRef]
- Barwick, M.; Van der Schans, A.; Claudy, J.B. Tropical & Subtropical Trees: A Worldwide Encyclopaedic Guide; Thames & Hudson: London, UK, 2004. [Google Scholar]
- Poorter, H.; Bühler, J.; Van Dusschoten, D.; Climent, J.; Postma, J.A. Pot Size Matters: A Meta-Analysis of the Effects of Rooting Volume on Plant Growth. Funct. Plant Biol. 2012, 39, 839–850. [Google Scholar] [CrossRef] [Green Version]
- Qiu, Z.C.; Wang, M.; Lai, W.L.; He, F.H.; Chen, Z.H. Plant Growth and Nutrient Removal in Constructed Monoculture and Mixed Wetlands. Hydrobiologia 2011, 661, 251–260. [Google Scholar] [CrossRef]
- Lim, H.S. Variations in the Water Quality of a Small Urban Tropical Catchment: Implications for Load Estimation and Water Quality Monitoring. In The Interactions between Sediments and Water; Springer: Dordrecht, The Netherlands, 2003; pp. 57–63. [Google Scholar]
- Chui, P. Characteristics of Stormwater Quality from Two Urban Watersheds in Singapore. Environ. Monit. Assess. 1997, 44, 173–181. [Google Scholar] [CrossRef]
- Zinger, Y.; Prodanovic, V.; Zhang, K.; Fletcher, T.D.; Deletic, A. The effect of intermittent drying and wetting stormwater cycles on the nutrient removal performances of two vegetated biofiltration designs. Chemosphere 2021, 267, 129294. [Google Scholar] [CrossRef]
- American Public Health Association (APHA). Standard Methods for Examination of Water and Wastewater, 22nd ed.; APHA: Washington, DC, USA, 2012. [Google Scholar]
- Joshi, U.M.; Balasubramanian, R. Characteristics and Environmental Mobility of Trace Elements in Urban Runoff. Chemosphere 2010, 80, 310–318. [Google Scholar] [CrossRef]
- Gorbe, E.; Calatayud, A. Applications of chlorophyll fluorescence imaging technique in horticultural research: A review. Sci. Hortic. 2012, 138, 24–35. [Google Scholar] [CrossRef]
- Makarova, V.; Kazimirko, Y.; Krendeleva, T.; Kukarskikh, G.; Lavrukhina, O.; Pogosyan, S.; Yakovleva, O. Fv/Fm as a Stress Indicator for Woody Plants from Urban-Ecosystem. In Photosynthesis: Mechanisms and Effects; Springer: Dordrecht, The Netherlands, 1998; pp. 4065–4068. [Google Scholar]
- Netto, A.T.; Campostrini, E.; de Oliveira, J.G.; Bressan-Smith, R.E. Photosynthetic pigments, nitrogen, chlorophyll a fluorescence and SPAD-502 readings in coffee leaves. Sci. Hortic. 2005, 104, 199–209. [Google Scholar] [CrossRef]
- Richardson, A.D.; Duigan, S.P.; Berlyn, G.P. An evaluation of noninvasive methods to estimate foliar chlorophyll content. New Phytol. 2002, 153, 185–194. [Google Scholar] [CrossRef] [Green Version]
- Sepúlveda, P.; Johnstone, D.M. A Novel Way of Assessing Plant Vitality in Urban Trees. Forests 2018, 10, 2. [Google Scholar] [CrossRef] [Green Version]
- FAWB. Guidelines for Filter Media in Biofiltration Systems (Version 3.01); FAWB: Clayton, Australia, 2009; pp. 1–8. [Google Scholar]
- Bratieres, K.; Fletcher, T.; Deletic, A.; Zinger, Y. Nutrient and sediment removal by stormwater biofilters: A large-scale design optimisation study. Water Res. 2008, 42, 3930–3940. [Google Scholar] [CrossRef]
- Hatt, B.; Fletcher, T.; Deletic, A. Hydraulic and pollutant removal performance of stormwater filters under variable wetting and drying regimes. Water Sci. Technol. 2007, 56, 11–19. [Google Scholar] [CrossRef]
- Read, J.; Wevill, P.; Fletcher, T.; Deletic, A. Variation among plant species in pollutant removal from stormwater in biofiltration systems. Water Res. 2008, 42, 893–902. [Google Scholar] [CrossRef]
- Lucas, W.C.; Greenway, M. Phosphorus Retention by Bioretention Mesocosms Using Media Formulated for Phosphorus Sorption: Response to Accelerated Loads. J. Irrig. Drain. Eng. 2011, 137, 144–153. [Google Scholar] [CrossRef]
- Ergas, S.J.; Sengupta, S.; Siegel, R.; Pandit, A.; Yao, Y.; Yuan, X.; Davis, A.P.; Shokouhian, M.; Sharma, H.; Minami, C. Water Quality Improvement through Bioretention Media: Nitrogen and Phosphorus Removal. Water Environ. Res. 2006, 78, 284–293. [Google Scholar]
- Taylor, G.D.; Fletcher, T.D.; Wong, T.H.; Breen, P.F.; Duncan, H.P. Nitrogen composition in urban runoff—implications for stormwater management. Water Res. 2005, 39, 1982–1989. [Google Scholar] [CrossRef]
- Zhang, H.; Ahmad, Z.; Shao, Y.; Yang, Z.; Jia, Y.; Zhong, H. Bioretention for removal of nitrogen: Processes, operational conditions, and strategies for improvement. Environ. Sci. Pollut. Res. 2021, 28, 10519–10535. [Google Scholar] [CrossRef]
- Rycewicz-Borecki, M.; McLean, J.E.; Dupont, R.R. Nitrogen and phosphorus mass balance, retention and uptake in six plant species grown in stormwater bioretention microcosms. Ecol. Eng. 2017, 99, 409–416. [Google Scholar] [CrossRef]
- Barron, N.J.; Hatt, B.; Jung, J.; Chen, Y.; Deletic, A. Seasonal operation of dual-mode biofilters: The influence of plant species on stormwater and greywater treatment. Sci. Total Environ. 2020, 715, 136680. [Google Scholar] [CrossRef]
- Eissenstat, D. Root structure and function in an ecological context. New Phytol. 2000, 148, 353–354. [Google Scholar] [CrossRef]
- Read, J.; Fletcher, T.D.; Wevill, P.; Deletic, A. Plant Traits that Enhance Pollutant Removal from Stormwater in Biofiltration Systems. Int. J. Phytoremed. 2010, 12, 34–53. [Google Scholar] [CrossRef]
- Collins, K.A.; Lawrence, T.J.; Stander, E.K.; Jontos, R.J.; Kaushal, S.S.; Newcomer, T.A.; Grimm, N.B.; Ekberg, M.L.C. Opportunities and Challenges for Managing Nitrogen in Urban Stormwater: A Review and Synthesis. Ecol. Eng. 2010, 36, 1507–1519. [Google Scholar] [CrossRef]
- Hsieh, C.-H.; Davis, A.P. Evaluation and Optimization of Bioretention Media for Treatment of Urban Storm Water Runoff. J. Environ. Eng. 2005, 131, 1521–1531. [Google Scholar] [CrossRef] [Green Version]
- Chu, Y.; Yang, L.; Wang, X.; Wang, X.; Zhou, Y. Research on Distribution Characteristics, Influencing Factors, and Maintenance Effects of Heavy Metal Accumulation in Bioretention Systems: Critical Review. J. Sustain. Water Built Environ. 2021, 7, 03120001. [Google Scholar] [CrossRef]
- Muthanna, T.M.; Viklander, M.; Blecken, G.-T.; Thorolfsson, S.T. Snowmelt pollutant removal in bioretention areas. Water Res. 2007, 41, 4061–4072. [Google Scholar] [CrossRef] [PubMed]
- Hatt, B.E.; Fletcher, T.; Deletic, A. Hydrologic and pollutant removal performance of stormwater biofiltration systems at the field scale. J. Hydrol. 2009, 365, 310–321. [Google Scholar] [CrossRef]
- Brix, H. Functions of Macrophytes in Constructed Wetlands. Water Sci. Technol. 1994, 29, 71–78. [Google Scholar] [CrossRef]
- Landsman, M.R.; Davis, A.P. Evaluation of Nutrients and Suspended Solids Removal by Stormwater Control Measures Using High-Flow Media. J. Environ. Eng. 2018, 144, 04018106. [Google Scholar] [CrossRef]
- Gold, A.C.; Thompson, S.P.; Piehler, M.F. Nitrogen Cycling Processes within Stormwater Control Measures: A Review and Call for Research. Water Res. 2019, 149, 578–587. [Google Scholar] [CrossRef]
- Xu, B.; Wang, X.; Liu, J.; Wu, J.; Zhao, Y.; Cao, W. Improving Urban Stormwater Runoff Quality by Nutrient Removal through Floating Treatment Wetlands and Vegetation Harvest. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Davis, A.P. Water Quality Improvement through Reductions of Pollutant Loads Using Bioretention. J. Environ. Eng. 2009, 135, 567–576. [Google Scholar] [CrossRef]
- Chen, X.C.; Huang, L.; Ong, B.L. The Phytoremediation Potential of a Singapore Forest Tree for Bioretention Systems. J. Mater. Sci. Eng. A 2014, 4, 220–227. [Google Scholar]
- Gadi, V.K.; Tang, Y.R.; Das, A.; Monga, C.; Garg, A.; Berretta, C.; Sahoo, L. Spatial and Temporal Variation of Hydraulic Conductivity and Vegetation Growth in Green Infrastructures Using Infiltrometer and Visual Technique. Catena 2017, 155, 20–29. [Google Scholar] [CrossRef]
- Archer, N.A.L.; Quinton, J.N.; Hess, T. Below-ground relationships of soil texture, roots and hydraulic conductivity in two-phase mosaic vegetation in South-East Spain. J. Arid Environ. 2002, 52, 535–553. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.; Zhang, Q.; Werner, A.D.; Li, Y.; Jiang, S.; Tan, Z. Root-induced changes of soil hydraulic properties—A review. J. Hydrol. 2020, 589, 125203. [Google Scholar] [CrossRef]
- Le Coustumer, S.; Fletcher, T.; Deletic, A.; Barraud, S.; Lewis, J.F. Hydraulic performance of biofilter systems for stormwater management: Influences of design and operation. J. Hydrol. 2009, 376, 16–23. [Google Scholar] [CrossRef]
- Le Coustumer, S.; Fletcher, T.; Deletic, A.; Barraud, S.; Poelsma, P. The influence of design parameters on clogging of stormwater biofilters: A large-scale column study. Water Res. 2012, 46, 6743–6752. [Google Scholar] [CrossRef]
- Langergraber, G.; Haberl, R.; Laber, J.; Pressl, A. Evaluation of Substrate Clogging Processes in Vertical Flow Constructed Wetlands. Water Sci. Technol. 2003, 48, 25–34. [Google Scholar] [CrossRef]
- Siriwardene, N.R.; Deletic, A.; Fletcher, T.D. Clogging of Stormwater Gravel Infiltration Systems and Filters: Insights from a Laboratory Study. Water Res. 2007, 41, 1433–1440. [Google Scholar] [CrossRef]
- PUB. ABC Waters Design Guidelines; PUB: Singapore, 2018. [Google Scholar]
- Hsieh, C.-H.; Davis, A.P.; Needelman, B.A. Bioretention Column Studies of Phosphorus Removal from Urban Stormwater Runoff. Water Environ. Res. 2007, 79, 177–184. [Google Scholar] [CrossRef]
- Yan, Q.; James, B.R.; Davis, A.P. Lab-Scale Column Studies for Enhanced Phosphorus Sorption from Synthetic Ur-ban Stormwater Using Modified Bioretention Media. J. Environ. Eng. 2017, 143, 04016073. [Google Scholar] [CrossRef]
- Davis, A.P. Field Performance of Bioretention: Water Quality. Environ. Eng. Sci. 2007, 24, 1048–1064. [Google Scholar] [CrossRef]
- Zhou, N.; Bi, C.-J.; Chen, Z.-L.; Yu, Z.-J.; Wang, J.; Han, J.-C. Phosphorus loads from different urban storm runoff sources in southern China: A case study in Wenzhou City. Environ. Sci. Pollut. Res. 2013, 20, 8227–8236. [Google Scholar] [CrossRef]
- Liu, J.; Davis, A.P. Phosphorus Speciation and Treatment Using Enhanced Phosphorus Removal Bioretention. Environ. Sci. Technol. 2014, 48, 607–614. [Google Scholar] [CrossRef]
- Qiu, F.; Zhao, S.; Zhao, D.; Wang, J.; Fu, K. Enhanced nutrient removal in bioretention systems modified with water treatment residuals and internal water storage zone. Environ. Sci. Water Res. Technol. 2019, 5, 993–1003. [Google Scholar] [CrossRef]
- Ong, G.S.; Kalyanaraman, G.; Wong, K.L.; Wong, T.H.F. Monitoring Singapore’s First Bioretention System: Rain Garden at Balam Estate. In WSUD 2012: Water Sensitve Urban Design, Proceedings of the Building the Water Sensitve Community, Seventh International Conference on Water Sensitive Urban Design, Melbourne, Australia, 21–23 February 2012; ACT: Barton, Australia, 2012; pp. 601–608. [Google Scholar]
- Wang, M.; Zhang, D.; Li, Y.; Hou, Q.; Yu, Y.; Qi, J.; Fu, W.; Dong, J.; Cheng, Y. Effect of a Submerged Zone and Carbon Source on Nutrient and Metal Removal for Stormwater by Bioretention Cells. Water 2018, 10, 1629. [Google Scholar] [CrossRef] [Green Version]
- Barrett, M.E. Comparison of BMP Performance Using the International BMP Database. J. Irrig. Drain. Eng. 2008, 134, 556–561. [Google Scholar] [CrossRef]
- McNett, J.K.; Hunt, W.F.; Davis, A.P. Influent Pollutant Concentrations as Predictors of Effluent Pollutant Concentrations for Mid-Atlantic Bioretention. J. Environ. Eng. 2011, 137, 790–799. [Google Scholar] [CrossRef]
- Duncan, H.P. Urban Stormwater Quality: A Statistical Overview; CRC for Catchment Hydrology: Victoria, Australia, 1999; 134p. [Google Scholar]
- Song, H.; Qin, T.; Wang, J.; Wong, T.H.F. Characteristics of Stormwater Quality in Singapore Catchments in 9 Different Types of Land Use. Water 2019, 11, 1089. [Google Scholar] [CrossRef] [Green Version]
- Wei, Z.; Simin, L.; Fengbing, T. Characterization of Urban Runoff Pollution between Dissolved and Particulate Phases. Sci. World J. 2013, 2013, 964737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hermawan, A.A.; Jung, D.Y.; Talei, A. Removal Process of Nutrients and Heavy Metals in Tropical Biofilters. E3S Web Conf. 2018, 65, 05026. [Google Scholar] [CrossRef] [Green Version]
- Jani, J.; Yang, Y.Y.; Lusk, M.G.; Toor, G.S. Composition of Nitrogen in Urban Residential Stormwater Runoff: Concentrations, Loads, and Source Characterization of Nitrate and Organic Nitrogen. PLoS ONE 2020, 15, e0229715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miguntanna, N.P.; Liu, A.; Egodawatta, P.; Goonetilleke, A. Characterising nutrients wash-off for effective urban stormwater treatment design. J. Environ. Manag. 2013, 120, 61–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müller, A.; Österlund, H.; Marsalek, J.; Viklander, M. The pollution conveyed by urban runoff: A review of sources. Sci. Total Environ. 2020, 709, 136125. [Google Scholar] [CrossRef]
- Clark, S.E.; Pitt, R. Targeting treatment technologies to address specific stormwater pollutants and numeric discharge limits. Water Res. 2012, 46, 6715–6730. [Google Scholar] [CrossRef]
- Chui, T.F.M.; Trinh, D.H. Modelling infiltration enhancement in a tropical urban catchment for improved stormwater management. Hydrol. Process. 2016, 30, 4405–4419. [Google Scholar] [CrossRef]
- Mangangka, I.; Liu, A.; Egodawatta, P.; Goonetilleke, A. Performance characterisation of a stormwater treatment bioretention basin. J. Environ. Manag. 2015, 150, 173–178. [Google Scholar] [CrossRef] [Green Version]
- Hsieh, C.-H.; Davis, A.P.; Needelman, B.A. Nitrogen Removal from Urban Stormwater Runoff Through Layered Bioretention Columns. Water Environ. Res. 2007, 79, 2404–2411. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).