Effects of the Filter-Feeding Benthic Bivalve Corbicula fluminea on Plankton Community and Water Quality in Aquatic Ecosystems: A Mesocosm Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Mesocosm Set-Up
2.2. Sampling and Analysis
2.3. Statistical Analyses
3. Results
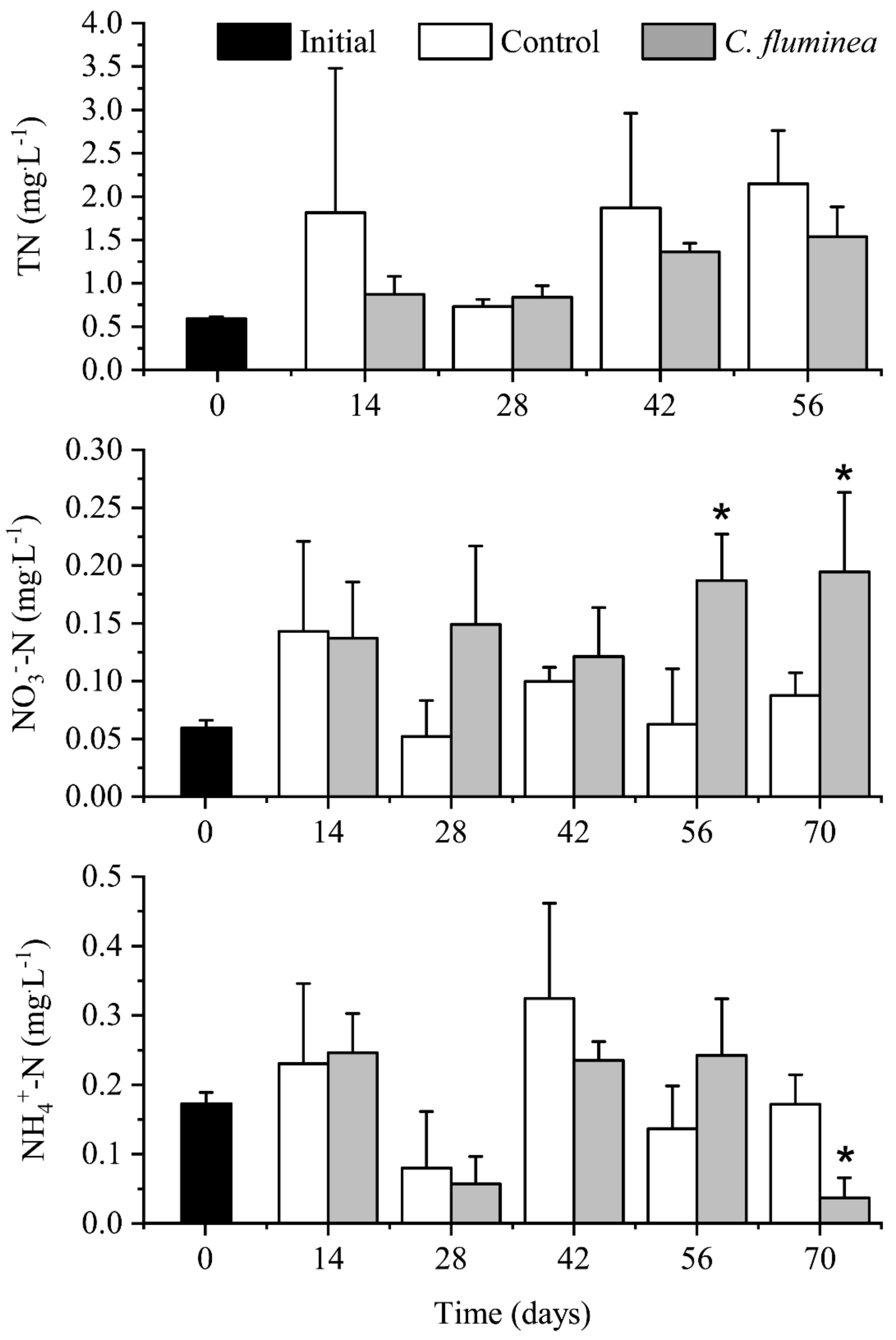
3.1. Nutrients
3.2. OSS and Light Intensity
3.3. Phytoplankton Biomass and Community Structure
3.4. Zooplankton Community
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lauritsen, D.D.; Mozley, S.C. Nutrient excretion by the Asiatic clam Corbicula fluminea. J. N. Am. Benthol. Soc. 1989, 8, 134–139. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, H.S.; Zhang, T.; Liu, S.L.; Zhang, S.M.; Liu, Q.; Xiang, J.H.; Zhang, F.S. Influence of filtering and biodeposition by the cultured scallops Chlamys farreri on benthic-pelagic coupling in a eutrophic bay in China. Mar. Ecol. Prog. Ser. 2006, 317, 127–141. [Google Scholar] [CrossRef] [Green Version]
- Sousa, R.; Antunes, C.; Guilhermino, L. Ecology of the invasive Asian clam Corbicula fluminea (Müller, 1774) in aquatic ecosystems: An overview. Ann. Limnol. Int. J. Lim. 2008, 44, 85–94. [Google Scholar] [CrossRef] [Green Version]
- Jørgensen, C. Bivalve filter feeding revisited. Mar. Ecol. Prog. Ser. 1996, 142, 287–302. [Google Scholar] [CrossRef]
- Reid, R.; McMahon, R.F.; Foighil, D.O.; Finnigan, R. Anterior inhalant currents and pedal feeding in bivalves. Veliger 1992, 35, 93–104. [Google Scholar]
- Gulati, R.D.; Pires, M.D.; Donk, E.V. Lake restoration studies: Failures, bottlenecks and prospects of new ecotechnological measures. Limnologica 2008, 38, 233–247. [Google Scholar] [CrossRef] [Green Version]
- Klocker, C.A.; Strayer, D.L. Interactions among an invasive Crayfish (Orconectes rusticus), a native crayfish (Orconectes limosus), and native bivalves (Sphaeriidae and Unionidae). Northeast. Nat. 2004, 11, 167–178. [Google Scholar] [CrossRef]
- Burlakova, L.E.; Karatayev, A.Y.; Karatayev, V.A. Invasive mussels induce community changes by increasing habitat complexity. Hydrobiologia 2012, 685, 121–134. [Google Scholar] [CrossRef]
- Yamamuro, M.; Koike, I. Nitrogen metabolism of the filter-feeding bivalve Corbicula japonica and its significance in primary production of a brackish lake in Japan. Limnol. Oceanogr. 1993, 38, 997–1007. [Google Scholar] [CrossRef]
- Nakamura, Y.; Kerciku, F. Effects of filter-feeding bivalves on the distribution of water quality and nutrient cycling in a eutrophic coastal lagoon. J. Mar. Syst. 2000, 26, 209–221. [Google Scholar] [CrossRef]
- Ruesink, J.L.; Lenihan, H.S.; Trimble, A.C.; Heiman, K.W.; Micheli, F.; Byers, J.E.; Kay, M.C. Introduction of non-native Oysters: Ecosystem effects and restoration implications. Annu. Rev. Ecol. Evol. Syst. 2005, 36, 643–689. [Google Scholar] [CrossRef] [Green Version]
- Lopez, C.B.; Cloern, J.E.; Schraga, T.S.; Little, A.J.; Thompson, J.K.; Burau, J.R. Ecological values of shallow-water habitats: Implications for the restoration of disturbed ecosystems. Ecosystems 2006, 9, 422–440. [Google Scholar] [CrossRef]
- Beaver, J.R.; Crisman, T.L.; Brock, R.J. Grazing effects of an exotic bivalve (Corbicula fluminea) on hypereutrophic lake water. Lake Reserv. Manage. 1991, 7, 45–51. [Google Scholar] [CrossRef]
- Caraco, N.F.; Cole, J.J.; Raymond, P.A.; Strayer, D.L.; Pace, M.L.; Findlay, S.E.G.; Fischer, D.T. Zebra mussel invasion in a large, turbid river: Phytoplankton response to increased grazing. Ecology 1997, 78, 588–602. [Google Scholar] [CrossRef]
- Fahnenstiel, G.L.; Lang, G.A.; Nalepa, T.F.; Johengen, T.H. Effects of zebra mussel (Dreissena polymorpha) colonization on water quality parameters in Saginaw Bay, Lake Huron. J. Great Lakes Res. 1995, 21, 435–448. [Google Scholar] [CrossRef] [Green Version]
- MacIsaac, H.J. Potential abiotic and biotic impacts of zebra mussels on the inland waters of North America. Amer. Zool. 1996, 36, 287–299. [Google Scholar] [CrossRef] [Green Version]
- Rosa, M.; Ward, J.E.; Shumway, S.E. Selective capture and ingestion of particles by suspension-feeding bivalve molluscs: A review. J. Shellfish Res. 2018, 37, 727–746. [Google Scholar] [CrossRef]
- Baker, S.M.; Levinton, J.S. Selective feeding by three native North American freshwater mussels implies food competition with zebra mussels. Hydrobiologia 2003, 505, 97–105. [Google Scholar] [CrossRef]
- Iglesias, J.I.P.; Navarro, E.; Alvarez Jorna, P.; Armentia, I. Feeding, particle selection and absorption in cockles Cerastoderma edule (L.) exposed to variable conditions of food concentration and quality. J. Exp. Mar. Biol. Ecol. 1992, 162, 177–198. [Google Scholar] [CrossRef]
- Stenton-Dozey, J.M.E.; Brown, A.C. Clearance and retention efficiency of natural suspended particles by the rock-pool bivalve Venerupis corrugatus in relation to tidal availability. Mar. Ecol. Prog. Ser. 1992, 82, 175–186. [Google Scholar] [CrossRef]
- Fei, Z.L.; Wu, J.; Zhao, Q.; Tang, J.Q.; Huang, C. Effects of filtration and digestion of Hyriopsis cumingii to algae. Freshw. Fish. 2006, 5, 24–27. [Google Scholar]
- Vaughn, C.C.; Nichols, S.J.; Spooner, D.E. Community and foodweb ecology of freshwater mussels. J. N. Am. Benthol. Soc. 2008, 27, 409–423. [Google Scholar] [CrossRef]
- Horsted, S.J.; Nielsen, T.G.; Riemann, B.; Pock-Steen, J.; Bjørnsen, P.K. Regulation of zooplankton by suspension-feeding bivalves and fish in estuarine enclosures. Mar. Ecol. Prog. Ser. 1988, 48, 217–224. [Google Scholar] [CrossRef]
- Pace, M.L.; Findlay, S.E.G.; Fischer, D. Effects of an invasive bivalve on the zooplankton community of the Hudson River. Freshw. Biol. 1998, 39, 103–116. [Google Scholar] [CrossRef]
- Green, S.; Visser, A.W.; Titelman, J.; Kiørboe, T. Escape responses of copepod napulii in the flow field of the blue mussel. Mytilus edulis. Mar. Biol. 2003, 142, 727–733. [Google Scholar] [CrossRef]
- Rojas Molina, F.; de Paggi, S.J.; Boltovskoy, D. Vulnerability of microcrustaceans to predation by the invasive filter-feeding mussel Limnoperna fortune (Dunker). Mar. Freshw. Behav. Phy. 2011, 44, 329–338. [Google Scholar] [CrossRef]
- Covich, A.P.; Palmer, M.A.; Crowl, T.A. The role of benthic invertebrate species in freshwater ecosystems: Zoobenthic species influence energy flows and nutrient cycling. BioScience 1999, 49, 119–127. [Google Scholar] [CrossRef] [Green Version]
- Karatayev, A.Y.; Burlakova, L.E.; Padilla, D.K. Zebra versus quagga mussels: A review of their spread, population dynamics, and ecosystem impacts. Hydrobiologia 2014, 746, 97–112. [Google Scholar] [CrossRef]
- Phelps, H.L. The Asiatic clam (Corbicula fluminea) invasion and system-level ecological change in the Potomac River Estuary near Washington, D.C. Estuaries 1994, 17, 614–621. [Google Scholar] [CrossRef]
- Strayer, D.L. Effects of alien species on freshwater mollusks in North America. J. N. Am. Benthol. Soc. 1999, 18, 74–98. [Google Scholar] [CrossRef]
- Vaughn, C.C.; Hakenkamp, C.C. The functional role of burrowing bivalves in freshwater ecosystems. Freshw. Biol. 2001, 46, 1431–1446. [Google Scholar] [CrossRef] [Green Version]
- Benelli, S.; Bartoli, M.; Zilius, M.; Vybernaite-Lubiene, I.; Ruginis, T.; Vaiciute, D.; Petkuviene, J.; Fano, E.A. Stoichiometry of regenerated nutrients differs between native and invasive freshwater mussels with implications for algal growth. Freshw. Biol. 2019, 64, 619–631. [Google Scholar] [CrossRef]
- Zhang, L.; Gu, X.Z.; Shao, S.G.; Hu, H.Y.; Zhong, J.C.; Fan, C.X. Impacts of Asian clams (Corbicula fluminea) on lake sediment properties and phosphorus movement. J. Environ. Sci. 2011, 32, 88–95. [Google Scholar] [CrossRef]
- Hakenkamp, C.C.; Palmer, M.A. Introduced bivalves in freshwater ecosystem: The impact of Corbicula on organic matter dynamics in a sandy stream. Oecologia 1999, 119, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Zieritz, A.; Chan, W.N.; McGowan, S.; Gibbins, C. High rates of biodeposition and N-excretion indicate strong functional effects of mussels (Bivalvia: Unionida) in certain anthropogenic tropical freshwater habitats. Hydrobiologia 2020, 1–14. [Google Scholar] [CrossRef]
- Vaughn, C.C.; Hoellein, T.J. Bivalve impacts in freshwater and marine ecosystems. Annu. Rev. Ecol. Evol. Syst. 2018, 49, 183–208. [Google Scholar] [CrossRef] [Green Version]
- Strayer, D.L. Understanding how nutrient cycles and freshwater mussels (Unionoida) affect one another. Hydrobiologia 2014, 735, 277–292. [Google Scholar] [CrossRef]
- Pigneur, L.M.; Falisse, E.; Roland, K.; Everbecq, E.; Deliege, J.F.; Smitz, J.S.; Van Doninck, K.; Descy, J.P. Impact of invasive Asian clams, Corbicula spp., on a large river ecosystem. Freshw. Bio. 2014, 59, 573–583. [Google Scholar] [CrossRef]
- Vaughn, C.C. Ecosystem services provided by freshwater mussels. Hydrobiologia 2018, 810, 15–27. [Google Scholar] [CrossRef]
- Richardson, T.D. The ecological consequences of nonindigenous Corbicula fluminea establishment on a benthic macroinvertebrate community. Aquat. Invasions 2020, 15, 382–407. [Google Scholar] [CrossRef]
- Cataldo, D.; Boltovskoy, D.; Stripeikis, J.; Pose, M. Condition index and growth rates of field caged Corbicula fluminea (Bivalvia) as biomarkers of pollution gradients in the Paraná River delta (Argentina). Aquat. Ecosyst. Health Manag. 2001, 4, 187–201. [Google Scholar] [CrossRef]
- Zhang, X.F.; Liu, Z.W.; Jeppesen, E.; Taylor, W.D.; Rudstam, L.G. Effects of benthic-feeding common carp and filter-feeding silver carp on benthic-pelagic coupling: Implications for shallow lake management. Ecol. Eng. 2016, 88, 256–264. [Google Scholar] [CrossRef]
- Zhang, X.F.; Liu, Z.W.; Jeppesen, E.; Taylor, W.D. Effects of deposit-feeding tubificid worms and filter-feeding bivalves on benthic-pelagic coupling: Implications for the restoration of eutrophic shallow lakes. Water Res. 2014, 50, 135–146. [Google Scholar] [CrossRef]
- Cai, Y.J.; Gong, Z.J.; Qin, B.Q. Community structure and diversity of macrozoobenthos in Lake Taihu, a large shallow eutrophic lake in China. Biodivers. Sci. 2010, 18, 50–59. [Google Scholar] [CrossRef]
- China State Environmental Protection Administration. HJ 897-2017, Water Quality-Determination of Chlorophylla-Spectrophotometric Method; China Environment Science Press: Beijing, China, 2017. [Google Scholar]
- American Public Health Association (APHA). Standard Methods for the Examination of Water and Wastewater, 20th ed.; American Public Health Association: Washington, DC, USA, 1998; ISBN 0875530788. [Google Scholar]
- Jin, X.C.; Tu, Q.Y. Specification for Lake Eutrophication Survey, 2nd ed.; China Environment Science Press: Beijing, China, 1990; ISBN 7-80010-675-6. [Google Scholar]
- Huang, X.F.; Chen, W.M.; Cai, Q.M. Survey, Observation and Analysis of Lake Ecology; Standards Press of China: Beijing, China, 1999; p. 247. [Google Scholar]
- Wang, J.J. Fauna of Freshwater Rotifera of China; Science Press: Beijing, China, 1961. (In Chinese) [Google Scholar]
- Chiang, S.C.; Du, N.S. Fauna Sinica: Crustacea: Freshwater Cladocera; Science Press: Beijing, China, 1979. (In Chinese) [Google Scholar]
- Crooks, J.A. Characterizing ecosystem-level consequences of biological invasions: The role of ecosystem engineers. Oikos 2002, 97, 153–166. [Google Scholar] [CrossRef] [Green Version]
- Karatayev, A.Y.; Padilla, D.K.; Minchin, D.; Boltovskoy, D.; Burlakova, L.E. Changes in global economies and trade: The potential spread of exotic freshwater bivalves. Biol. Invasions 2007, 9, 161–180. [Google Scholar] [CrossRef]
- Shen, R.J.; Gu, X.H.; Chen, H.H.; Mao, Z.G.; Zeng, Q.F.; Jeppesen, E. Combining bivalve (Corbicula fluminea) and filter-feeding fish (Aristichthys nobilis) enhances the bioremediation effect of algae: An outdoor mesocosm study. Sci. Total Environ. 2020, 727, 138692. [Google Scholar] [CrossRef] [PubMed]
- Modesto, V.; Dias, E.; Ilarri, M.; Lopes-Lima, M.; Teixeira, A.; Varandas, S.; Castro, P.; Antunes, C.; Sousa, R. Trophic niche overlap between native freshwater mussels (Order: Unionida) and the invasive Corbicula fluminea. Aquat. Conserv. Mar. Freshw. Ecosyst. 2021, 1, 1–14. [Google Scholar] [CrossRef]
- Rollwagen-Bollens, G.; Bolam, B.A.; Bollens, S.M.; Henricksen, S.; Sandison, C.; Zimmerman, J. Temperature-dependent functional response of the invasive Asian clam, Corbicula fluminea, feeding on natural phytoplankton. Inland Waters 2021. [Google Scholar] [CrossRef]
- Ulanowicz, R.E.; Tuttle, J.H. The trophic consequences of oyster stock rehabilitation in Chesapeake Bay. Estuaries 1992, 15, 298–306. [Google Scholar] [CrossRef]
- Cohen, R.R.H.; Dresler, P.V.; Phillips, E.J.P.; Cory, R.L. The effect of the Asiatic clam, Corbicula fluminea, on phytoplankton of the Potomac River, Maryland. Limnol. Oceanogr. 1984, 29, 170–180. [Google Scholar] [CrossRef]
- Lauritsen, D.D. Filter-feeding in Corbicula fluminea and its effect on seston removal. J. N. Am. Benthol. Soc. 1986, 5, 165–172. [Google Scholar] [CrossRef]
- Bolam, B.A.; Rollwagen-Bollens, G.; Bollens, S.M. Feeding rates and prey selection of the invasive Asian clam, Corbicula fluminea, on microplankton in the Columbia River, USA. Hydrobiologia 2019, 833, 107–123. [Google Scholar] [CrossRef]
- Minaudo, C.; Abonyi, A.; Leitão, M.; Lançon, A.M.; Floury, M.; Descy, J.P.; Moatar, F. Long-term impacts of nutrient control, climate change, and invasive clams on phytoplankton and cyanobacteria biomass in a large temperate river. Sci. Total Environ. 2021, 756, 144074. [Google Scholar] [CrossRef] [PubMed]
- Boltovskoy, D.; Izaguirre, I.; Correa, N. Feeding selectivity of Corbicula fluminea (Bivalvia) on natural phytoplankton. Hydrobiologia 1995, 312, 171–182. [Google Scholar] [CrossRef]
- Belanger, S.E.; Farris, J.L.; Cherry, D.S.; Cairns, J. Sediment preference of the freshwater Asiatic clam Corbicula fluminea. Nautilus 1985, 99, 66–73. [Google Scholar] [CrossRef]
- Callieri, C.; Stockner, J.G. Freshwater autotrophic picoplankton: A review. J. Limnol. 2002, 61, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Kamburska, L.; Lauceri, R.; Beltrami, M.; Boggero, A.; Cardeccia, A.; Guarneri, I.; Manca, M.; Riccardi, N. Establishment of Corbicula fluminea (O.F. Müller, 1774) in Lake Maggiore: A spatial approach to trace the invasion dynamics. Bioinvasions Rec. 2013, 2, 105–117. [Google Scholar] [CrossRef]
- Wong, W.H.; Levinton, J.S. The trophic linkage between zooplankton and benthic suspension feeders: Direct evidence from analyses of bivalve faecal pellets. Mar. Biol. 2006, 148, 799–805. [Google Scholar] [CrossRef]
- Marroni, S.; Mazzeo, N.; Pacheco, J.P.; Clemente, J.; Iglesias, C. Interactions between bivalves and zooplankton: Competition or intraguild predation? Implications for biomanipulation in subtropical shallow lakes. Mar. Freshw. Res. 2016, 68, 1036–1043. [Google Scholar] [CrossRef]
- Rojas Molina, F.; de Paggi, S.J.; Frau, D. Impacts of the invading golden mussel Limnoperna fortunei on zooplankton: A mesocosm experiment. Zool. Stud. 2012, 51, 733–744. [Google Scholar] [CrossRef] [Green Version]
- Rothhaupt, K.O. Differences in particle size-dependent feeding efficiencies of closely related rotifer species. Limnol. Oceanogr. 1990, 35, 16–23. [Google Scholar] [CrossRef]
- Sousa, R.; Gutiérrez, J.L.; Aldridge, D.C. Non-indigenous invasive bivalves as ecosystem engineers. Biol. Invasions 2009, 11, 2367–2385. [Google Scholar] [CrossRef]
- Novais, A.; Souza, A.T.; Ilarri, M.; Pascoal, C.; Sousa, R. Effects of the invasive clam Corbicula fluminea (Müller, 1774) on an estuarine microbial community. Sci. Total Environ. 2016. [Google Scholar] [CrossRef] [PubMed]
- Patrick, C.H.; Waters, M.N.; Golladay, S.W. The distribution and ecological role of Corbicula fluminea (Müller, 1774) in a large and shallow reservoir. Bioinvasions Rec. 2017, 6, 39–48. [Google Scholar] [CrossRef]
- Zhang, L.; Shen, Q.S.; Hu, H.Y.; Shao, S.G.; Fan, C.X. Impacts of Corbicula fluminea on oxygen uptake and nutrient fluxes across the sediment-water interface. Water Air Soil Poll. 2011, 220, 399–411. [Google Scholar] [CrossRef]
- Holland, R.E.; Johengen, T.H.; Beeton, A.M. Trends in nutrient concentrations in Hatchery Bay, western Lake Erie, before and after Dreissena polymorpha. Can. J. Fish. Aquat. Sci. 1995, 52, 1202–1209. [Google Scholar] [CrossRef] [Green Version]
- Bruesewitz, D.A.; Tank, J.L.; Bernot, M.J. Delineating the effects of zebra mussels (Dreissena polymorpha) on N transformation rates using laboratory mesocosms. J. N. Am. Benthol. Soc. 2008, 27, 236–251. [Google Scholar] [CrossRef]
- Yang, D.M.; Chen, Y.W.; Liu, Z.W.; Wu, Q.L. Top-down effects of Anodonta woodiana on nutrient concentration & phytoplankton community composition in a microcosm ecosystem. J. Lake Sci. 2008, 20, 228–234. [Google Scholar] [CrossRef]
- Li, P.; Zhang, X.F.; Mo, S.Q. Effects of mussel (Anodonta woodiana), submerged macrophyte (Vallisneria natans) and their coexistence on water quality. Chin. J. Ecol. 2016, 35, 1589–1594. [Google Scholar] [CrossRef]
- Kolar, C.S.; Lodge, D.M. Progress in invasion biology: Predicting invaders. Trends Ecol. Evol. 2001, 16, 199–204. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rong, Y.; Tang, Y.; Ren, L.; Taylor, W.D.; Razlutskij, V.; Naselli-Flores, L.; Liu, Z.; Zhang, X. Effects of the Filter-Feeding Benthic Bivalve Corbicula fluminea on Plankton Community and Water Quality in Aquatic Ecosystems: A Mesocosm Study. Water 2021, 13, 1827. https://doi.org/10.3390/w13131827
Rong Y, Tang Y, Ren L, Taylor WD, Razlutskij V, Naselli-Flores L, Liu Z, Zhang X. Effects of the Filter-Feeding Benthic Bivalve Corbicula fluminea on Plankton Community and Water Quality in Aquatic Ecosystems: A Mesocosm Study. Water. 2021; 13(13):1827. https://doi.org/10.3390/w13131827
Chicago/Turabian StyleRong, Yuqin, Yali Tang, Lijuan Ren, William D Taylor, Vladimir Razlutskij, Luigi Naselli-Flores, Zhengwen Liu, and Xiufeng Zhang. 2021. "Effects of the Filter-Feeding Benthic Bivalve Corbicula fluminea on Plankton Community and Water Quality in Aquatic Ecosystems: A Mesocosm Study" Water 13, no. 13: 1827. https://doi.org/10.3390/w13131827
APA StyleRong, Y., Tang, Y., Ren, L., Taylor, W. D., Razlutskij, V., Naselli-Flores, L., Liu, Z., & Zhang, X. (2021). Effects of the Filter-Feeding Benthic Bivalve Corbicula fluminea on Plankton Community and Water Quality in Aquatic Ecosystems: A Mesocosm Study. Water, 13(13), 1827. https://doi.org/10.3390/w13131827








