Treatment of Purified Terephthalic Acid Wastewater by Ozone Catalytic Oxidation Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Catalyst Preparation and Characterization
2.3. Ozone Catalytic Oxidation Experiment
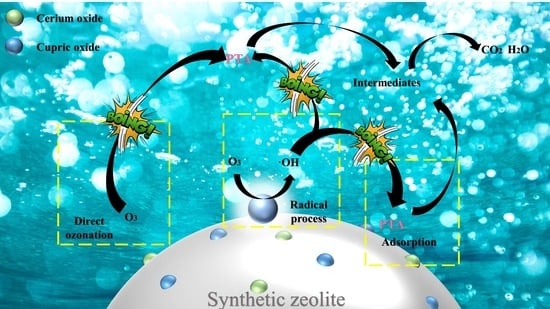
3. Results and Discussion
3.1. Preparation of Cu–Ce@Az Catalyst
3.2. Physical and Chemical Properties of Cu–Ce@Az Ozone Catalyst
3.3. Degradation of PTA Wastewater by Cu–Ce@Az Ozone Catalyst
3.3.1. Effect of Reaction Time on Catalytic Performance
3.3.2. Effect of pH on Catalytic Performance
3.3.3. Effect of Ozone Dosage on Catalytic Performance
3.3.4. Effect of Catalyst Dosage on Catalytic Performance
3.4. Stability Analysis of Cu–Ce@Az Ozone Catalyst
3.5. Effect of Tert-Butanol Dosage on Catalytic Oxidation Performance
3.6. 3D Fluorescence Spectrum Analysis
3.7. Ultraviolet Absorption Peak of PTA Wastewater
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, W.; Zhou, S.; Sun, Y.; Xu, Y.; Zheng, H. W-Ag-Ti@γ-Al2O3 particle electrodes for enhanced electrocatalytic pretreatment of coal chemical wastewater. J. Environ. Chem. Eng. 2021, 9, 104681. [Google Scholar] [CrossRef]
- Sun, W.; Sun, Y.; Zhu, H.; Zheng, H. Catalytic activity and evaluation of Fe-Mn@Bt for ozonizing coal chemical biochemical tail water. Sep. Purif. Technol. 2020, 239, 116524. [Google Scholar] [CrossRef]
- Krishan, K.G.; Basheshwar, P. Treatment of toxic pollutants of purified terephthalic acid waste water: A review. Environ. Technol. Innov. 2017, 8, 191–217. [Google Scholar]
- Wang, D.; Ma, W.; Han, H.; Li, K.; Xu, H.; Fang, F.; Hou, B.; Jia, S. Enhanced anaerobic degradation of Fischer–Tropsch wastewater by integrated UASB system with Fe-C micro-electrolysis assisted. Chemosphere 2016, 164, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Qiu, W.; Xu, H.; Lu, X.; Ma, J.; Lu, D. Highly-efficient and stable MgCo2O4 spinel for bisphenol a removal by activating peroxymonosulfate via radical and non-radical pathways. Chem. Eng. J. 2021, 421, 129498. [Google Scholar] [CrossRef]
- Sheikhi, S.; Dehghanzadeh, R.; Maryamabadi, A.; Aslani, H. Chlorpyrifos removal from aqueous solution through sequential use of coagulation and advanced oxidation processes: By-products, degradation pathways, and toxicity assessment. Environ. Technol. Innov. 2021, 23, 101564. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhao, H.; Liang, J.; Yue, L.; Li, T.; Luo, Y.; Liu, Q.; Lu, S.; Asiri, A.M.; Gong, Z.; et al. Anodic oxidation for the degradation of organic pollutants: Anode materials, operating conditions and mechanisms. A mini review. Electrochem. Commun. 2021, 123, 106912. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, Y.; Yao, B.; Yang, J.; Zhi, D. Current progress in electrochemical anodic-oxidation of pharmaceuticals: Mechanisms, influencing factors, and new technique. J. Hazard. Mater. 2021, 418, 126313. [Google Scholar] [CrossRef]
- Saad, M.E.K.; Rabaaoui, N.; Elaloui, E.; Moussaoui, Y. Mineralization of p-methylphenol in aqueous medium by anodic oxidation with a boron-doped diamond electrode. Sep. Purif. Technol. 2016, 171, 157–163. [Google Scholar] [CrossRef]
- Ridruejo, C.; Salazar, C.; Cabot, P.L.; Centellas, F.; Brillas, E.; Sirés, I. Electrochemical oxidation of anesthetic tetracaine in aqueous medium. Influence of the anode and matrix composition. Chem. Eng. J. 2017, 326, 811–819. [Google Scholar] [CrossRef] [Green Version]
- Wu, M.; Kwok, Y.H.; Zhang, Y.; Szeto, W.; Huang, H.; Leung, D.Y. Synergetic effect of vacuum ultraviolet photolysis and ozone catalytic oxidation for toluene degradation over MnO2-rGO composite catalyst. Chem. Eng. Sci. 2021, 231, 116288. [Google Scholar] [CrossRef]
- Gopi, T.; Swetha, G.; Shekar, S.C.; Krishna, R.; Ramakrishna, C.; Saini, B.; Rao, P. Ozone catalytic oxidation of toluene over 13X zeolite supported metal oxides and the effect of moisture on the catalytic process. Arab. J. Chem. 2019, 12, 4502–4513. [Google Scholar] [CrossRef] [Green Version]
- Pang, L.; Fan, C.; Shao, L.; Song, K.; Yi, J.; Cai, X.; Wang, J.; Kang, M.; Li, T. The Ce doping Cu/ZSM-5 as a new superior catalyst to remove NO from diesel engine exhaust. Chem. Eng. J. 2014, 253, 394–401. [Google Scholar] [CrossRef]
- Shao, Q.; Dong, H.; Zhang, J.; Xu, B.; Wu, Y.; Long, C. Manganese supported on controlled dealumination Y-zeolite for ozone catalytic oxidation of low concentration toluene at low temperature. Chemosphere 2021, 271, 129604. [Google Scholar] [CrossRef]
- Sun, W.; Zhou, S.; Sun, Y.; Tang, J.; Zheng, H. Ozone catalytic oxidation capacity of Ti-Co@Al 2 O 3 for the treatment of biochemical tail water from the coal chemical industry. Water Environ. Res. 2020, 92, 1283–1292. [Google Scholar] [CrossRef]
- Kim, J.; Kwon, E.E.; Lee, J.E.; Jang, S.-H.; Jeon, J.-K.; Song, J.; Park, Y.-K. Effect of zeolite acidity and structure on ozone oxidation of toluene using Ru-Mn loaded zeolites at ambient temperature. J. Hazard. Mater. 2021, 403, 123934. [Google Scholar] [CrossRef]
- Sun, W.; Sun, Y.; Shah, K.; Zheng, H.; Ma, B. Electrochemical degradation of oxytetracycline by Ti-Sn-Sb/γ-Al2O3 three-dimensional electrodes. J. Environ. Manag. 2019, 241, 22–31. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Luan, P.; Mo, L.; Xu, J.; Li, J.; Zhu, L.; Zeng, J. Mineralization of Recalcitrant Organic Pollutants in Pulp and Paper Mill Wastewaters through Ozonation Catalyzed by Cu-Ce Supported on Al2O3. Bioresources 2018, 13, 3686–3703. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Chen, A.; Zhu, S.; Sun, W.; Shah, K.; Zheng, H. Degradation of chloramphenicol using Ti-Sb/attapulgite ceramsite particle electrodes. Water Environ. Res. 2019, 91, 756–769. [Google Scholar] [CrossRef]
- Dou, B.; Liu, D.; Zhang, Q.; Zhao, R.; Hao, Q.; Bin, F.; Cao, J. Enhanced removal of toluene by dielectric barrier discharge coupling with Cu-Ce-Zr supported ZSM-5/TiO2/Al2O3. Catal. Commun. 2017, 92, 15–18. [Google Scholar] [CrossRef] [Green Version]
- Águila, G.; Gracia, F.; Araya, P. CuO and CeO2 catalysts supported on Al2O3, ZrO2, and SiO2 in the oxidation of CO at low temperature. Appl. Catal. A Gen. 2008, 343, 16–24. [Google Scholar] [CrossRef]
- Park, J.W.; Jeong, J.H.; Yoon, W.L.; Rhee, Y.W. Selective oxidation of carbon monoxide in hydrogen-rich stream over Cu-Ce/γ-Al2O3 catalysts promoted with cobalt in a fuel processor for proton exchange membrane fuel cells. J. Power Sources 2004, 132, 18–28. [Google Scholar] [CrossRef]
- Francisco, M.S.P.; Mastelaro, V.; Nascente, P.A.P.; Florentino, A.O. Activity and Characterization by XPS, HR-TEM, Raman Spectroscopy, and BET Surface Area of CuO/CeO2-TiO2 Catalysts. J. Phys. Chem. B 2001, 105, 10515–10522. [Google Scholar] [CrossRef]
- Chen, L.; Zhu, Y.; Zheng, H.; Zhang, C.; Zhang, B.; Li, Y. Catalytic degradation of oxygenates in Fischer-Tropsch aqueous phase effluents to fuel gas via hydrodeoxygenation over Ru/AC catalyst. J. Chem. Technol. Biotechnol. 2011, 87, 112–122. [Google Scholar] [CrossRef]
- Bae, S.; Jung, J.; Lee, W. The effect of pH and zwitterionic buffers on catalytic nitrate reduction by TiO2-supported bimetallic catalyst. Chem. Eng. J. 2013, 232, 327–337. [Google Scholar] [CrossRef]
- Qi, F.; Chen, Z.; Xu, B.; Shen, J.; Ma, J.; Joll, C.; Heitz, A. Influence of surface texture and acid–base properties on ozone decomposition catalyzed by aluminum (hydroxyl) oxides. Appl. Catal. B Environ. 2008, 84, 684–690. [Google Scholar] [CrossRef]
- Lan, Q.; Cao, M.; Ye, Z.; Zhu, J.; Chen, M.; Chen, X.; Liu, C. Effect of oxalate and pH on photodegradation of pentachlorophenol in heterogeneous irradiated maghemite System. J. Photochem. Photobiol. A Chem. 2016, 328, 198–206. [Google Scholar] [CrossRef]
- Zhang, L.-C.; Jia, Z.; Lyu, F.; Liang, S.-X.; Lu, J. A review of catalytic performance of metallic glasses in wastewater treatment: Recent progress and prospects. Prog. Mater. Sci. 2019, 105, 100576. [Google Scholar] [CrossRef]
- Xia, F.; Xu, X.; Li, X.; Zhang, L.; Qiu, H.; Wang, W.; Liu, Y.; Gao, J. Preparation of Bismuth Nanoparticles in Aqueous Solution and Its Catalytic Performance for the Reduction of 4-Nitrophenol. Ind. Eng. Chem. Res. 2014, 53, 10576–10582. [Google Scholar] [CrossRef]
- Chen, H.-S.; Zhang, Q.-M.; Yang, Z.-J.; Liu, Y.-S. Research on Treatment of Oily Sludge from the Tank Bottom by Ball Milling Combined with Ozone-Catalyzed Oxidation. ACS Omega 2020, 5, 12259–12269. [Google Scholar] [CrossRef]
- Sun, Y.; Zhu, S.; Sun, W.; Zheng, H. Degradation of high-chemical oxygen demand concentration pesticide wastewater by 3D electrocatalytic oxidation. J. Environ. Chem. Eng. 2019, 7, 103276. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, A.; Sun, W.; Zhou, J.; Shah, K.J.; Zheng, H.; Shen, H. Degradation of chloramphenicol by Ti-Ag/gamma-Al2O3 particle electrode using three-dimensional reactor. Desalin. Water. Treat. 2019, 163, 96–108. [Google Scholar] [CrossRef]
- Zhu, G.; Wang, C.; Dong, X. Fluorescence excitation–emission matrix spectroscopy analysis of landfill leachate DOM in coagulation–flocculation process. Environ. Technol. 2016, 38, 1489–1497. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Huang, T.; Zhang, S.; Zheng, J.; Zheng, H. Synthesis of an amphoteric chitosan-based flocculant and its flocculation performance in the treatment of dissolved organic matter from drinking water. Desalination Water Treat. 2020, 174, 171–177. [Google Scholar] [CrossRef]










| Test Index | Unit | Detection Value |
|---|---|---|
| COD | mg/L | 178.6 |
| NH4+-N | mg/L | 0.24 |
| pH | / | 8.50 |
| Turbidity | NTU | 7.63 |
| Conductivity | μS/cm | 11.76 |
| Total phosphorus | mg/L | 0.93 |
| Total nitrogen | mg/L | 0.65 |
| TOC | mg/L | 59.94 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, X.; Xie, S.; Li, S.; Zhou, J.; Sun, W.; Xu, Y.; Sun, Y. Treatment of Purified Terephthalic Acid Wastewater by Ozone Catalytic Oxidation Method. Water 2021, 13, 1906. https://doi.org/10.3390/w13141906
Lu X, Xie S, Li S, Zhou J, Sun W, Xu Y, Sun Y. Treatment of Purified Terephthalic Acid Wastewater by Ozone Catalytic Oxidation Method. Water. 2021; 13(14):1906. https://doi.org/10.3390/w13141906
Chicago/Turabian StyleLu, Xi, Shuqian Xie, Shuai Li, Jun Zhou, Wenquan Sun, Yanhua Xu, and Yongjun Sun. 2021. "Treatment of Purified Terephthalic Acid Wastewater by Ozone Catalytic Oxidation Method" Water 13, no. 14: 1906. https://doi.org/10.3390/w13141906
APA StyleLu, X., Xie, S., Li, S., Zhou, J., Sun, W., Xu, Y., & Sun, Y. (2021). Treatment of Purified Terephthalic Acid Wastewater by Ozone Catalytic Oxidation Method. Water, 13(14), 1906. https://doi.org/10.3390/w13141906