Nopalea cochenillifera Biomass as Bioadsorbent in Water Purification
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experiment Design
2.2. Preparation of Cloudy Aqueous Solution
2.3. Origin and Biomass Preparation
2.4. Experimental Procedure and Evaluations
2.4.1. Alcoholic Extracts
2.4.2. Total Carbohydrates
2.4.3. Non-Structural Carbohydrates
Reducing and Non-Reducing Sugars
Alcohol Insoluble Solids
2.4.4. Structural Carbohydrate
Pectin
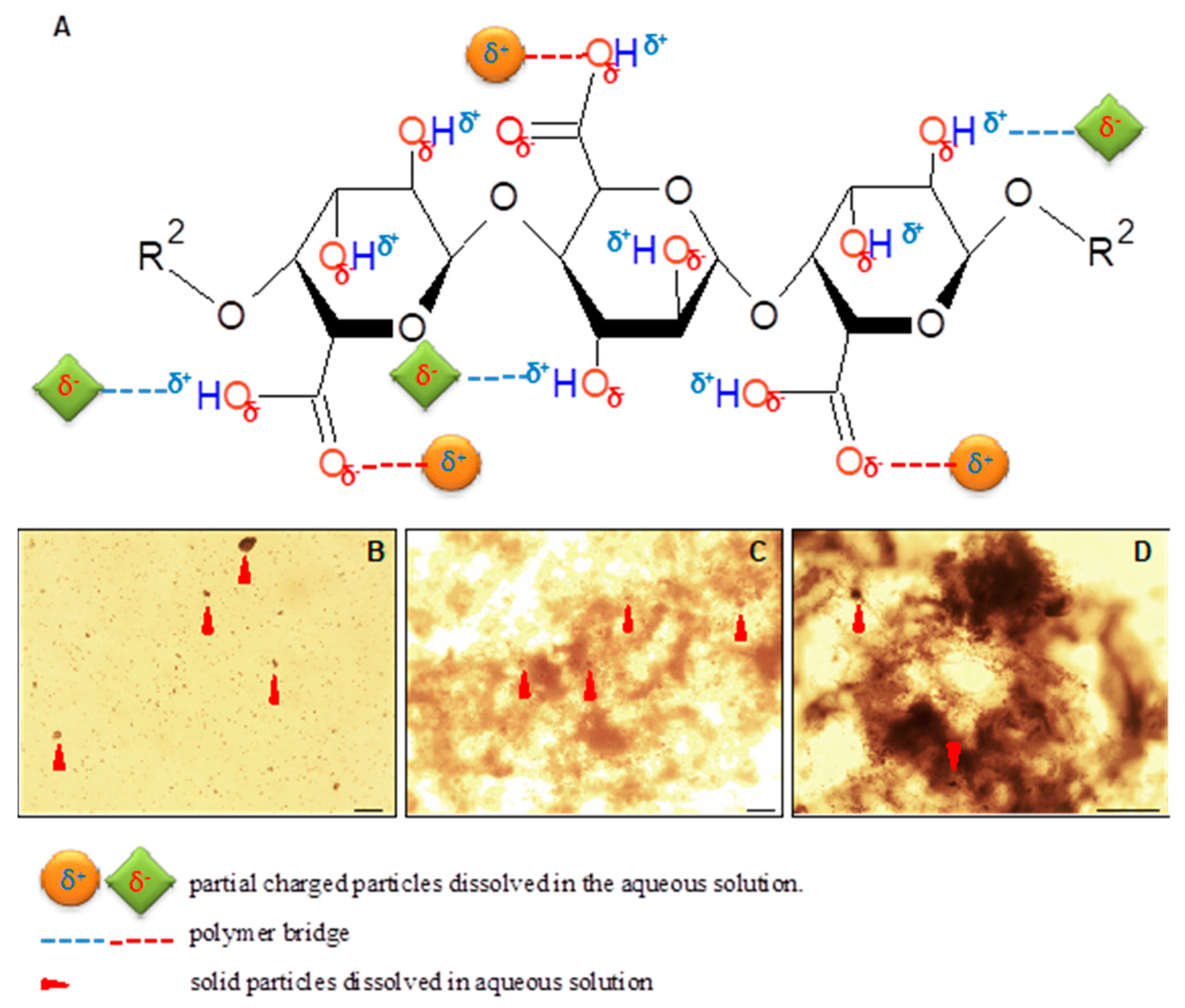
2.4.5. Adsorption kinetics
2.4.6. Turbidity
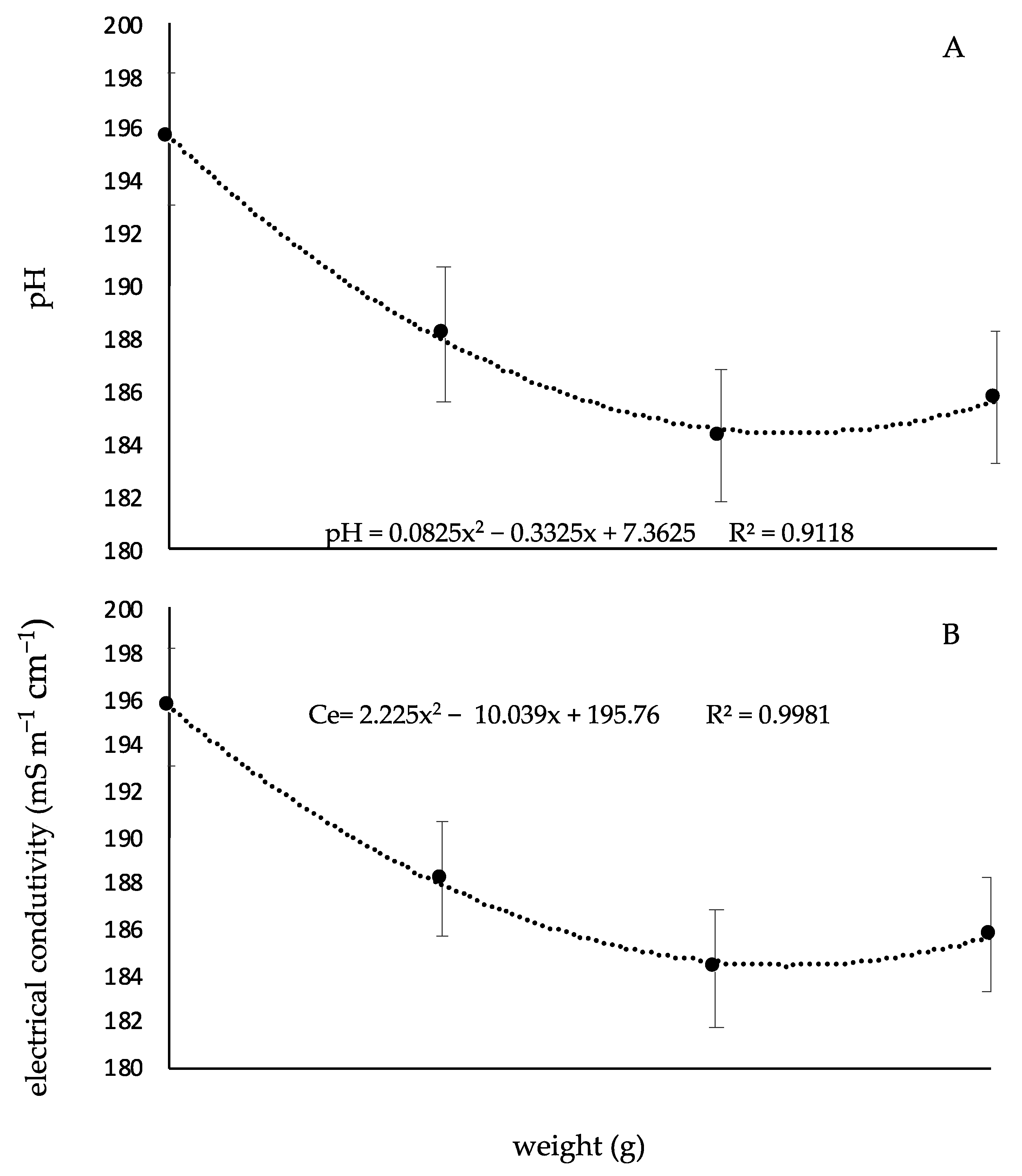
2.4.7. pH and Electric Conductivity
2.4.8. Zeta Potential
2.4.9. Total Coliforms Presence
2.5. Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Drinking-Water. Available online: https://www.who.int/news-room/fact-sheets/detail/drinking-water (accessed on 10 July 2020).
- United Nations. Office of the High Commissioner for Human Rights (OHCHR). United Nations Human Settlements Programme (UN-HABITAT). Available online: http://www.ohchr.org/Documents/Publications/FactSheet35en.pdf (accessed on 10 July 2020).
- United Nations. The Human Right to Water and Sanitation. Available online: http://www.un.org/waterforlifedecade/pdf/human_right_to_water_and_sanitation_media_brief.pdf (accessed on 10 July 2020).
- Di Bernardo, L.; Di Bernardo, A.; Centurione Filho, P.L. Ensaios de Tratabilidade de Água e dos Resíduos Gerados em Estações de Tratamento de Água; RiMa: São Carlos, Brazil, 2002. [Google Scholar]
- Gleick, P.H. Dirty Water: Estimated Deaths from Water-Related Diseases 2000–2020. Pac. Inst. Res. Rep. Pub. Health 2014, 128, 404–409. [Google Scholar] [CrossRef] [Green Version]
- Mara, D.; Lane, J.; Scott, B.; Trouba, D. Sanitation and Health. PLoS Med. 2010, 7, e1000363. [Google Scholar] [CrossRef] [Green Version]
- Ministério da Saúde. Saúde Brasil. 2013. Available online: http://portalarquivos2.saude.gov.br/images/pdf/2015/janeiro/28/saude-brasil-2013-analise-situacao-saude.pdf (accessed on 10 July 2020).
- Ministério da Integração Nacional. Available online: http://www.mi.gov.br/download/download.asp?endereco=/pdf/desenvolvimentoregional/cartilha_delimitacao_semi_arido.pdf&nome_arquivo=cartilha_delimitacao_semi_arido.pdf (accessed on 10 July 2020).
- Ministério da Saúde. Biblioteca Virtual em Saúde. Available online: http://bvsms.saude.gov.br/bvs/publicacoes/vigilancia_controle_qualidade_agua.pdf (accessed on 10 July 2020).
- Nicola, E.; Aburizaiza, O.S.; Siddique, A.; Khwaja, H.; Carpenter, D.O. Climate change and water scarcity: The case of Saudi Arabia. Ann. Glob. Health 2015, 81, 42–353. [Google Scholar] [CrossRef]
- Scanlon, B.R.; Jolly, I.; Sophocleous, M.; Zhang, L. Global impacts of conversions from natural to agricultural ecosystems on water resources: Quantity versus quality. Water Resour. Res. 2007, 43, e3437. [Google Scholar] [CrossRef] [Green Version]
- Todd, A.S.; Manning, A.H.; Verplanck, P.L.; Crouch, C.; Mcknight, D.M.; Dunham, R. Climate-change-driven deterioration of water quality in a mineralized watershed. Environ. Sci. Technol. 2012, 46, 9324–9332. [Google Scholar] [CrossRef] [PubMed]
- John, V.; Jain, P.; Rahate, M.; Labhasetwar, P. Assessment of deterioration in water quality from source to household storage in semi-urban settings of developing countries. Environ. Monit. Assess. 2014, 186, 725–734. [Google Scholar] [CrossRef] [PubMed]
- Fettweis, M.; Lee, B.J. Spatial and seasonal variation of biomineral suspended particulate matter properties in high-turbid nearshore and low-turbid offshore zones. Water 2017, 9, 694. [Google Scholar] [CrossRef] [Green Version]
- Ribas, A.; Jollivet, C.; Morand, S.; Thongmalayvong, B.; Somphavong, S.; Siew, C.C.; Ting, P.J.; Suputtamongkol, S.; Saensombath, V.; Sanguankiat, S.; et al. Intestinal parasitic infections and environmental water contamination in a rural village of northern lao pdr. Korean J. Parasitol. 2017, 55, 523–532. [Google Scholar] [CrossRef] [Green Version]
- Kinyua, E.M.; Mwangi, I.W.; Wanjau, R.N.; Ngila, J.C. Clarification of colloidal and suspended material in water using triethanolamine modified maize tassels. Environ. Sci. Pollut. Res. 2016, 23, 5214–5221. [Google Scholar] [CrossRef] [Green Version]
- Richter, C.A. Água: Métodos e Tecnologia de Tratamento, 5th ed.; Edgard Blucher: São Paulo, Brazil, 2009; p. 339. [Google Scholar]
- Rachdi, R.; Srarfi, F.; Shimi, N.S. Cactus Opuntia as natural flocculant for urban wastewater treatment. Water Sci. Technol. 2017, 76, 1875–1883. [Google Scholar] [CrossRef]
- Yin, C.H. Emerging usage of plant based coagulants for water and wastewater treatment. Process Biochem. 2010, 45, 1437–1444. [Google Scholar] [CrossRef] [Green Version]
- Khedimallah, N.; Zazoua, A.; Sbartai, A.; Jaffrezic-Renault, N. A high sensitivity impedimetric biosensor using the tannin from Quercus macrolepis as Biorecognition element for heavy metals detection. IEEE Trans. NanoBiosci. 2015, 14, e694. [Google Scholar] [CrossRef] [PubMed]
- Zazoua, A.; Bouraoui, S.; Jaffrezic-Renault, N.J. Cu(II) adsorption onto a biopolymer extracted from a vegetable waste: Application to a miniaturized electrochemical sensor. J. Inorg. Organomet. Polym. Mater. 2018, 28, 1–10. [Google Scholar] [CrossRef]
- Santos, F.A.; IulianellI, G.C.; Tavares, M.I. Effect of microcrystalline and nanocrystals cellulose fillers in materials based on PLA matrix. Polym. Test. 2017, 61, 280–288. [Google Scholar] [CrossRef]
- Theodoro, J.D.P.; Lenz, G.F.; Zara, R.F.; Bergamasco, R. Coagulants and natural polymers: Perspectives for the treatment of water. Plast. Polym. Technol. 2013, 2, 55–62. [Google Scholar]
- Tripathi, P.N.; Chaudhari, M.; Bokil, S.D. Nirmali seed—a naturally occurring coagulant. Indian J. Environ. Health 1976, 18, 272–281. [Google Scholar]
- WHO. Preventing Travellers’ Diarrhoea: How to Make Drinking Water Safe. Available online: http://www.who.int/water_sanitation_health/publications/traveldiarrh/en/ (accessed on 10 July 2020).
- Gonçalves, C.; Rodriguez-Jasso, R.M.; Gomes, N.; Gomes, N.; Teixeira, J.A.; Belo, I. Adaptation of dinitrosalicylic acid method to microtiter plates. Anal. Methods 2010, 2, 2046–2048. [Google Scholar] [CrossRef] [Green Version]
- La Bonte, D.R.; Picha, D.H.; Johnson, H.A. Carbohydrate-related changes in sweetpotato storage roots during development. J. Am. Soc. Hortic. Sci. 2000, 125, 200–204. [Google Scholar] [CrossRef]
- Farid, M.A.A.; Hassan, M.A.; Taufiq-Yap, Y.H.; Shirai, Y.; Hasan, M.Y.; Zakaria, M.R. Waterless purification using oil palm biomass-derived bioadsorbent improved the quality of biodiesel from waste cooking oil. J. Clean. Prod. 2017, 165, 262–272. [Google Scholar] [CrossRef]
- Dos Santos, H.G.; Jacomine, P.K.T.; dos Anjos, L.H.; de Oliveira, V.A.; de Oliveira, J.B.; Coelho, M.R.; Lumbreras, J.F.; Cunha, T.J.F. Sistema Brasileiro de Classificação de Solos, 2rd ed.; Empraba: Rio de Janeiro, Brasil, 2006; p. 353. [Google Scholar]
- Dąbrowski, A. Adsorption—From theory to practice. Adv. Coll. Interf. Sci. 2001, 93, 135–224. [Google Scholar] [CrossRef]
- Crittenden, J.C.; TrusselL, R.R.; Hand, D.W.; Howe, K.J.; Tchobanoglous, G. Water Treatment Principles and Design, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2005; p. 380. [Google Scholar]
- Crini, G. Recent developments in polysaccharide-based materials used as adsorbents in wastewater treatment. Prog. Polym. Sci. 2005, 30, 38–70. [Google Scholar] [CrossRef]
- Ramirez-Orduna, R.; Ramirez, R.G.; Gonzalez-Rodriguez, H.; Haenlein, G.F.W. Mineral content of browse species from Baja California Sur, Mexico. Small Rumin. Res. 2005, 57, 1–10. [Google Scholar] [CrossRef]
- Lefsih, K.; Giacomazza, D.; Passantino, R.; Costa, M.A.; Bulone, D.; Mangione, M.R.; Madani, K. Biochemical and biophysical characterization of water-soluble pectin from Opuntia ficus-indica and its potential cytotoxic activity. Phytochemistry 2018, 154, 47–55. [Google Scholar] [CrossRef]
- Sepulveda, E.; Saenz, C.; Aliaga, E.; Aceituno, C. Extraction and characterization of mucilage in Opuntia spp. J. Arid Environ. 2007, 68, 534–545. [Google Scholar] [CrossRef]
- Khotimchenko, M.; Kovalev, V.; Khotimchenko, Y. Equilibrium studies of sorption of lead (II) ions by different pectin compounds. J. Hazard. Mater. 2007, 149, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Nougbodé, Y.A.E.I.; Sessou, P.; Alassane, A.; Youssao, A.K.A.; Agbangnan, C.P.; Mama, D.; Sohounhloue, K.C.D. Evaluation of aloe vera leaf gel as a natural flocculant: Phytochemical screening and turbidity removal trials of water by coagulation flocculation. Res. J. Recent Sci. 2016, 5, 9–15. [Google Scholar]
- Tan, W.; Li, Q.; Dong, F.; Chen, Q.; Guo, Z. Preparation and characterization of novel cationic chitosan derivatives bearing quaternary ammonium and phosphonium salts and assessment of their antifungal properties. Molecules 2017, 22, 1438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sankar, M.U.; Aigal, S.; Maliyekkal, S.M.; Chaudhary, A.; Anshup, A.A.; Chaudhari, K.; Pradeep, T. Biopolymer-reinforced synthetic granular nanocomposites for affordable point-of-use water purification. Proc. Natl. Acad. Sci. USA 2013, 110, 8459–8464. [Google Scholar] [CrossRef] [Green Version]
- Weber, W.J. Physiochemical Processes for Water Quality Control, 1st ed.; John Wiley & Sons: New York, NY, USA, 1972; p. 640. [Google Scholar]
- Jakóbik-Kolon, A.; Bok-Badura, J.; Karoń, K.; Mitko, K.; Milewski, A. Hybrid pectin-based biosorbents for zinc ions removal. Carbohydr. Polym. 2017, 169, 213–219. [Google Scholar] [CrossRef]
- Mallampati, R.; Valiyaveettil, S. Apple peels—A versatile biomass for water purification. ACS Appl. Mater. Interfaces 2013, 5, 4443–4449. [Google Scholar] [CrossRef]
- Jayalakshmi, G.; Saritha, V.; DwarapureddI, B.K. A review on native plant based coagulants for water purification. Int. J. Appl. Environ. Sci. 2017, 12, 469–487. [Google Scholar]
- Houghton, J.I.; Quarmby, J. Biopolymers in wastewater treatment. Cur. Opin. Biotechnol. 1999, 10, 259–262. [Google Scholar] [CrossRef]
- Sarode, S.; Upadhyay, P.; Khosa, M.A.; Mak, T.; Shakir, A.; Song, S.; Ullah, A. Overview of wastewater treatment methods with special focus on biopolymer chitin-chitosan. Int. J. Biol. Macromol. 2019, 121, 1086–1100. [Google Scholar] [CrossRef]
- Choudhary, M.; Ray, M.B.; Neogi, S. Evaluation of the potential application of cactus (Opuntia ficus-indica) as a bio-coagulant for pre-treatment of oil sands process-affected water. Sep. Purif. Technol. 2019, 1, 714–724. [Google Scholar] [CrossRef]
- Prasertsan, P.; Dermlim, W.; Doelle, H.; Kennedy, J.F. Screening, characterization and flocculating property of carbohydrate polymer from newly isolated Enterobacter cloacae WD7. Carbohyd. Polym. 2006, 66, 289–297. [Google Scholar] [CrossRef]
- Mittal, H.; Jindal, R.; Kaith, B.S.; Maity, A.; Ray, S.S. Synthesis and flocculation properties of gum ghatti and poly(acrylamide-co-acrylonitrile) based biodegradable hydrogels. Carbohydr. Polym. 2014, 114, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Dubeux, J.C.B., Jr.; dos Santos, M.V.F.; da Cunha, M.V.; dos Santos, D.C.; Souza, R.T.A.; de Mello, A.C.L.; de Souza, T.C. Cactus (Opuntia and Nopalea) nutritive value: A review. Anim. Feed Sci. Technol. 2021, 275, e114890. [Google Scholar] [CrossRef]
- Huang, X.; Li, X.; Li, Y.; Wang, X. Biopolymer as stabilizer and adhesive to in situ precipitate cus nanocrystals on cellulose nanofibers for preparing multifunctional composite papers. ACS Omega 2018, 19, 8083–8090. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Das, S.; Singh, S.; Pradhan, N.; Gajamer, V.R.; Kumar, S.; Lepcha, Y.D.; Tiwari, H.K. Physicochemical parameters and alarming coliform count of the potable water of eastern himalayan state sikkim: An indication of severe fecal contamination and immediate health risk. Front. Public Health 2019, 7, e174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taa, N.; Benyahya, M.; Chaouch, M. Using a bio-flocculent in the process of coagulation flocculation for optimizing the chromium removal from the polluted water. J. Mater. Environ. Sci. 2016, 7, 1581–1588. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.T.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]


| Compounds | Content |
|---|---|
| Total carbohydrates | 66.80 ± 8.60% |
| Non-structural carbohydrates Reducing sugars Non-reducing sugars Alcohol insoluble solids | 1.95 ± 0.33% 47.28 ± 2.22% 12.13 ± 0.98% |
| Structural carbohydrates Pectin | 5.44 ± 0.03% |
| pH | 4.70 ± 0.03% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lima, V.R.d.N.; Silva, Á.G.F.d.; Cruz, R.R.P.; Barbosa, L.d.S.; Junior, N.R.d.S.; Sales, G.N.B.; Limão, M.A.R.; Costa, F.B.d.; Souza, P.A.d.; Lopes, K.P.; et al. Nopalea cochenillifera Biomass as Bioadsorbent in Water Purification. Water 2021, 13, 2012. https://doi.org/10.3390/w13152012
Lima VRdN, Silva ÁGFd, Cruz RRP, Barbosa LdS, Junior NRdS, Sales GNB, Limão MAR, Costa FBd, Souza PAd, Lopes KP, et al. Nopalea cochenillifera Biomass as Bioadsorbent in Water Purification. Water. 2021; 13(15):2012. https://doi.org/10.3390/w13152012
Chicago/Turabian StyleLima, Vitória Régia do Nascimento, Álvaro Gustavo Ferreira da Silva, Renata Ranielly Pedroza Cruz, Luana da Silva Barbosa, Neilier Rodrigues da Silva Junior, Giuliana Naiara Barros Sales, Marcelo Augusto Rocha Limão, Franciscleudo Bezerra da Costa, Pahlevi Augusto de Souza, Kilson Pinheiro Lopes, and et al. 2021. "Nopalea cochenillifera Biomass as Bioadsorbent in Water Purification" Water 13, no. 15: 2012. https://doi.org/10.3390/w13152012
APA StyleLima, V. R. d. N., Silva, Á. G. F. d., Cruz, R. R. P., Barbosa, L. d. S., Junior, N. R. d. S., Sales, G. N. B., Limão, M. A. R., Costa, F. B. d., Souza, P. A. d., Lopes, K. P., Bruno, R. d. L. A., Andrade, A. P. d., & Ribeiro, W. S. (2021). Nopalea cochenillifera Biomass as Bioadsorbent in Water Purification. Water, 13(15), 2012. https://doi.org/10.3390/w13152012







