Multiple-Facet Diversity Patterns of Aquatic Vegetation in Lakes along a Trophic Gradient
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area and Vegetation Data
2.2. Lake Trophic Status Parameters
2.3. Diversity Analysis
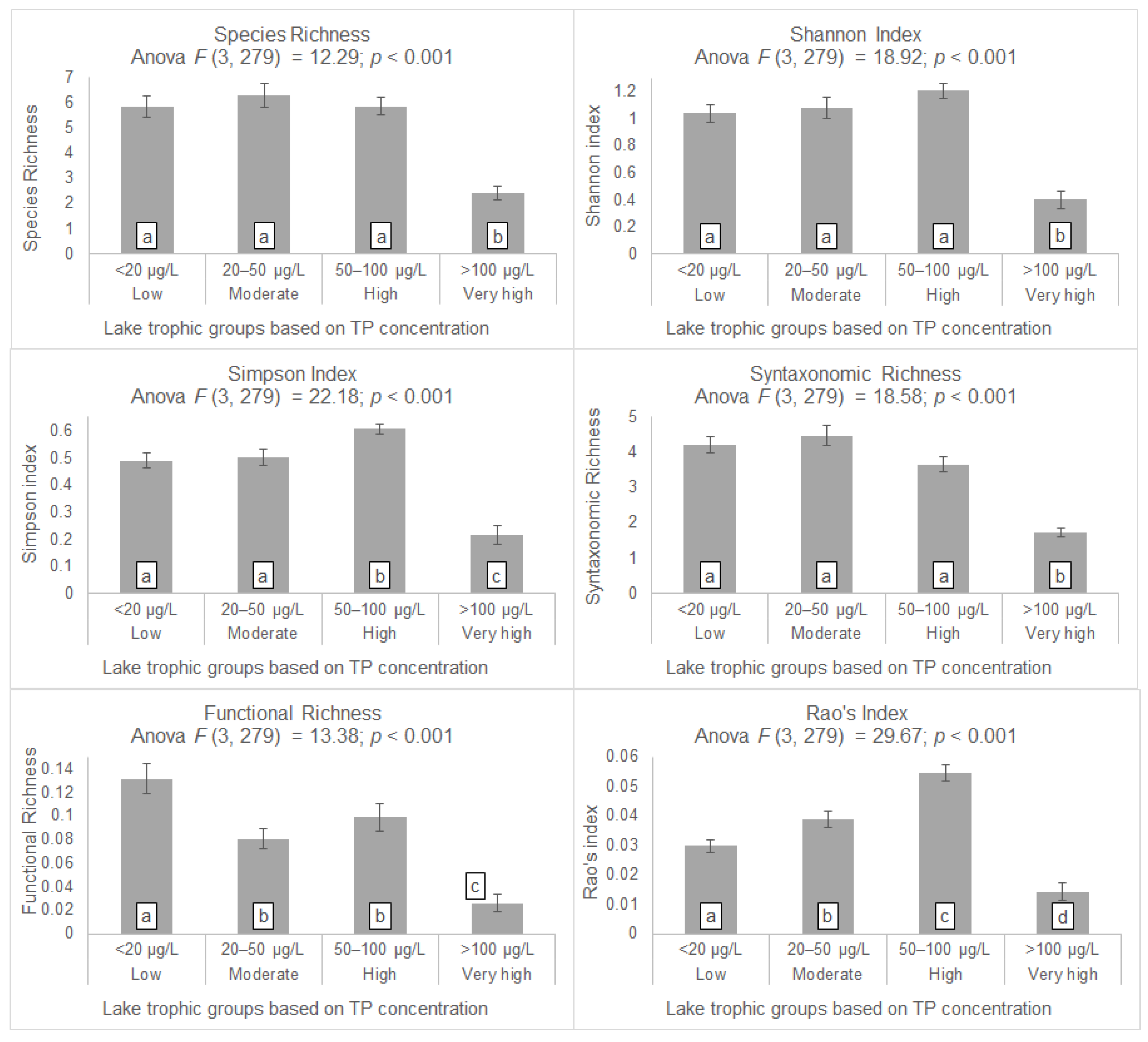
3. Results
4. Discussion
4.1. Taxonomic Alpha Diversity Patterns
4.2. Taxonomic Beta Diversity Patterns
4.3. Syntaxonomic Diversity Patterns
4.4. Functional Alpha Diversity Patterns
4.5. Functional Beta Diversity Patterns
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dudgeon, D.; Arthington, A.H.; Gessner, M.O.; Kawabata, Z.-I.; Knowler, D.J.; Lévêque, C.; Naiman, R.J.; Prieur-Richard, A.-H.; Soto, D.; Stiassny, M.L. Freshwater biodiversity: Importance, threats, status and conservation challenges. Biol. Rev. 2006, 81, 163–182. [Google Scholar] [CrossRef]
- Reid, A.J.; Carlson, A.K.; Creed, I.F.; Eliason, E.J.; Gell, P.A.; Johnson, P.T.; Kidd, K.A.; MacCormack, T.J.; Olden, J.D.; Ormerod, S.J. Emerging threats and persistent conservation challenges for freshwater biodiversity. Biol. Rev. 2019, 94, 849–873. [Google Scholar] [CrossRef] [Green Version]
- Sala, O.E.; Chapin, F.S.; Armesto, J.J.; Berlow, E.; Bloomfield, J.; Dirzo, R.; Huber-Sanwald, E.; Huenneke, L.F.; Jackson, R.B.; Kinzig, A. Global biodiversity scenarios for the year 2100. Science 2000, 287, 1770–1774. [Google Scholar] [CrossRef]
- Vörösmarty, C.J.; McIntyre, P.B.; Gessner, M.O.; Dudgeon, D.; Prusevich, A.; Green, P.; Glidden, S.; Bunn, S.E.; Sullivan, C.A.; Liermann, C.R. Global threats to human water security and river biodiversity. Nature 2010, 467, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Geist, J. Integrative freshwater ecology and biodiversity conservation. Ecol. Indic. 2011, 11, 1507–1516. [Google Scholar] [CrossRef]
- Strayer, D.L.; Dudgeon, D. Freshwater biodiversity conservation: Recent progress and future challenges. J. N. Am. Benthol. Soc. 2010, 29, 344–358. [Google Scholar] [CrossRef] [Green Version]
- European Community. Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 establishing a framework for Community action in the field of water policy. Off. J. Eur. Communities 2000, 327, 1–72. [Google Scholar]
- Moss, B. The Water Framework Directive: Total environment or political compromise? Sci. Total Environ. 2008, 400, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Voulvoulis, N.; Arpon, K.D.; Giakoumis, T. The EU Water Framework Directive: From great expectations to problems with implementation. Sci. Total Environ. 2017, 575, 358–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reyjol, Y.; Argillier, C.; Bonne, W.; Borja, A.; Buijse, A.D.; Cardoso, A.C.; Daufresne, M.; Kernan, M.; Ferreira, M.T.; Poikane, S. Assessing the ecological status in the context of the European Water Framework Directive: Where do we go now? Sci. Total Environ. 2014, 497, 332–344. [Google Scholar] [CrossRef]
- Birk, S.; Bonne, W.; Borja, A.; Brucet, S.; Courrat, A.; Poikane, S.; Solimini, A.; Van De Bund, W.; Zampoukas, N.; Hering, D. Three hundred ways to assess Europe’s surface waters: An almost complete overview of biological methods to implement the Water Framework Directive. Ecol. Indic. 2012, 18, 31–41. [Google Scholar] [CrossRef]
- Poikane, S.; Birk, S.; Böhmer, J.; Carvalho, L.; de Hoyos, C.; Gassner, H.; Hellsten, S.; Kelly, M.; Solheim, A.L.; Olin, M. A hitchhiker’s guide to European lake ecological assessment and intercalibration. Ecol. Indic. 2015, 52, 533–544. [Google Scholar] [CrossRef]
- Poikane, S.; van den Berg, M.; Hellsten, S.; de Hoyos, C.; Ortiz-Casas, J.; Pall, K.; Portielje, R.; Phillips, G.; Solheim, A.L.; Tierney, D. Lake ecological assessment systems and intercalibration for the European Water Framework Directive: Aims, achievements and further challenges. Procedia Environ. Sci. 2011, 9, 153–168. [Google Scholar] [CrossRef] [Green Version]
- Noss, R.F. Indicators for monitoring biodiversity: A hierarchical approach. Conserv. Biol. 1990, 4, 355–364. [Google Scholar] [CrossRef]
- Jarzyna, M.A.; Jetz, W. Detecting the multiple facets of biodiversity. Trends Ecol. Evol. 2016, 31, 527–538. [Google Scholar] [CrossRef]
- Petchey, O.L.; Gaston, K.J. Functional diversity (FD), species richness and community composition. Ecol. Lett. 2002, 5, 402–411. [Google Scholar] [CrossRef]
- Cardoso, P.; Rigal, F.; Borges, P.A.; Carvalho, J.C. A new frontier in biodiversity inventory: A proposal for estimators of phylogenetic and functional diversity. Methods Ecol. Evol. 2014, 5, 452–461. [Google Scholar] [CrossRef] [Green Version]
- Baselga, A. Partitioning the turnover and nestedness components of beta diversity. Glob. Ecol. Biogeogr. 2010, 19, 134–143. [Google Scholar] [CrossRef]
- Epele, L.B.; Brand, C.; Miserendino, M.L. Ecological drivers of alpha and beta diversity of freshwater invertebrates in arid and semiarid Patagonia (Argentina). Sci. Total Environ. 2019, 678, 62–73. [Google Scholar] [CrossRef]
- Cardoso, P.; Rigal, F.; Carvalho, J.C.; Fortelius, M.; Borges, P.A.; Podani, J.; Schmera, D. Partitioning taxon, phylogenetic and functional beta diversity into replacement and richness difference components. J. Biogeogr. 2014, 41, 749–761. [Google Scholar] [CrossRef] [Green Version]
- Legendre, P. Interpreting the replacement and richness difference components of beta diversity. Glob. Ecol. Biogeogr. 2014, 23, 1324–1334. [Google Scholar] [CrossRef]
- Dίaz, S.; Cabido, M. Vive la différence: Plant functional diversity matters to ecosystem processes. Trends Ecol. Evol. 2001, 16, 646–655. [Google Scholar] [CrossRef]
- Villéger, S.; Grenouillet, G.; Brosse, S. Decomposing functional β-diversity reveals that low functional β-diversity is driven by low functional turnover in E uropean fish assemblages. Glob. Ecol. Biogeogr. 2013, 22, 671–681. [Google Scholar] [CrossRef]
- Villéger, S.; Novack-Gottshall, P.M.; Mouillot, D. The multidimensionality of the niche reveals functional diversity changes in benthic marine biotas across geological time. Ecol. Lett. 2011, 14, 561–568. [Google Scholar] [CrossRef]
- Izco, J.; Amigo, J. Syntaxonomical diversity: Relationships between syntaxa richness and area. Plant Biosyst. Int. J. Deal. Asp. Plant. Biol. 2011, 145, 38–45. [Google Scholar] [CrossRef]
- Redžić, S. Syntaxonomic diversity as an indicator of ecological diversity—case study Vranica Mts in the Central Bosnia. Biologia 2007, 62, 173–184. [Google Scholar] [CrossRef]
- Lachavanne, J.-B. The influence of accelerated eutrophication on the macrophytes of Swiss lakes: Abundance and distribution: With 2 figures in the text. Int. Ver. Theor. Angew. Limnol. Verh. 1985, 22, 2950–2955. [Google Scholar] [CrossRef]
- Murphy, K. Plant communities and plant diversity in softwater lakes of northern Europe. Aquat. Bot. 2002, 73, 287–324. [Google Scholar] [CrossRef]
- Rørslett, B. Principal determinants of aquatic macrophyte richness in northern European lakes. Aquat. Bot. 1991, 39, 173–193. [Google Scholar] [CrossRef]
- Penning, W.E.; Dudley, B.; Mjelde, M.; Hellsten, S.; Hanganu, J.; Kolada, A.; van den Berg, M.; Poikane, S.; Phillips, G.; Willby, N. Using aquatic macrophyte community indices to define the ecological status of European lakes. Aquat. Ecol. 2008, 42, 253–264. [Google Scholar] [CrossRef]
- Penning, W.E.; Mjelde, M.; Dudley, B.; Hellsten, S.; Hanganu, J.; Kolada, A.; Van Den Berg, M.; Poikane, S.; Phillips, G.; Willby, N. Classifying aquatic macrophytes as indicators of eutrophication in European lakes. Aquat. Ecol. 2008, 42, 237–251. [Google Scholar] [CrossRef]
- Søndergaard, M.; Johansson, L.S.; Lauridsen, T.L.; Jørgensen, T.B.; Liboriussen, L.; Jeppesen, E. Submerged macrophytes as indicators of the ecological quality of lakes. Freshw. Biol. 2010, 55, 893–908. [Google Scholar] [CrossRef]
- Chappuis, E.; Ballesteros, E.; Gacia, E. Distribution and richness of aquatic plants across E urope and M editerranean countries: Patterns, environmental driving factors and comparison with total plant richness. J. Veg. Sci. 2012, 23, 985–997. [Google Scholar] [CrossRef]
- Bolpagni, R.; Laini, A.; Stanzani, C.; Chiarucci, A. Aquatic plant diversity in Italy: Distribution, drivers and strategic conservation actions. Front. Plant Sci. 2018, 9, 116. [Google Scholar] [CrossRef] [Green Version]
- Chappuis, E.; Gacia, E.; Ballesteros, E. Environmental factors explaining the distribution and diversity of vascular aquatic macrophytes in a highly heterogeneous Mediterranean region. Aquat. Bot. 2014, 113, 72–82. [Google Scholar] [CrossRef]
- García-Girón, J.; Fernández-Aláez, M.; Fernández-Aláez, C. Redundant or complementary? Evaluation of different metrics as surrogates of macrophyte biodiversity patterns in Mediterranean ponds. Ecol. Indic. 2019, 101, 614–622. [Google Scholar] [CrossRef]
- Zervas, D.; Tsiaoussi, V.; Tsiripidis, I. HeLM: A macrophyte-based method for monitoring and assessment of Greek lakes. Environ. Monit. Assess. 2018, 190, 326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klein Tank, A.; Wijngaard, J.; Können, G.; Böhm, R.; Demarée, G.; Gocheva, A.; Mileta, M.; Pashiardis, S.; Hejkrlik, L.; Kern-Hansen, C. Daily dataset of 20th-century surface air temperature and precipitation series for the European Climate Assessment. Int. J. Climatol. J. R. Meteorol. Soc. 2002, 22, 1441–1453. [Google Scholar] [CrossRef]
- Lyche Solheim, A.; Persson, J.; Austnes, K.; Moe, J.; Kampa, E.; Stein, U.; Feher, J.; Kristensen, P. European Freshwater Ecosystem Assessment: Cross-Walk between the Water Framework Directive and Habitats Directive Types, Status and Pressures. ETC 2015, 176. Available online: http://icm.eionet.europa.eu/ETC_Reports/FreshwaterEcosystemAssessmentReport_201509 (accessed on 21 October 2019).
- Mavromati, E.; Kagalou, I.; Kemitzoglou, D.; Apostolakis, A.; Seferlis, M.; Tsiaoussi, V. Relationships among land use patterns, hydromorphological features and physicochemical parameters of surface waters: WFD lake monitoring in Greece. Environ. Process. 2018, 5, 139–151. [Google Scholar] [CrossRef]
- Zervas, D.; Tsiaoussi, V.; Kallimanis, A.S.; Dimopoulos, P.; Tsiripidis, I. Exploring the relationships between aquatic macrophyte functional traits and anthropogenic pressures in freshwater lakes. Acta Oecologica 2019, 99, 103443. [Google Scholar] [CrossRef]
- Palmer, M.; Bell, S.; Butterfield, I. A botanical classification of standing waters in Britain: Applications for conservation and monitoring. Aquat. Conserv. Mar. Freshw. Ecosyst. 1992, 2, 125–143. [Google Scholar] [CrossRef]
- Cardoso, A.; Solimini, A.; Premazzi, G.; Carvalho, L.; Lyche, A.; Rekolainen, S. Phosphorus reference concentrations in European lakes. In Shallow Lakes in a Changing World; Springer: Berlin/Heidelberg, Germany, 2007; pp. 3–12. [Google Scholar]
- Kagalou, I.; Ntislidou, C.; Latinopoulos, D.; Kemitzoglou, D.; Tsiaoussi, V.; Bobori, D.C. Setting the Phosphorus Boundaries for Greek Natural Shallow and Deep Lakes for Water Framework Directive Compliance. Water 2021, 13, 739. [Google Scholar] [CrossRef]
- Wetzel, R.G. Limnology: Lake and River Ecosystems; Elsevier Academic Press: Amsterdam, The Netherlands, 2001. [Google Scholar]
- Ntislidou, C.; Lazaridou, M.; Tsiaoussi, V.; Bobori, D.C. A new multimetric macroinvertebrate index for the ecological assessment of Mediterranean lakes. Ecol. Indic. 2018, 93, 1020–1033. [Google Scholar] [CrossRef]
- De Hoyos, C.; Catalan, J.; Dörflinger, G.; Ferreira, J.; Kemitzoglou, D.; Laplace-Treyture, C.; López, P.; Marchetto, A.; Mihail, O.; Morabito, G. Mediterranean Lake Phytoplankton Ecological Assessment Methods; Water Framework Directive Intercalibration Technical Report; Joint Research Centre Technical Reports; Joint Research Centre: Ispra, Italy, 2014. [Google Scholar]
- Pahissa, J.; Catalan, J.; Morabito, G.; Dörflinger, G.; Ferreira, J.; Laplace-Treyture, C.; Gîrbea, R.; Marchetto, A.; Polykarpou, P.; de Hoyos, C. Benefits and limitations of an intercalibration of phytoplankton assessment methods based on the Mediterranean GIG reservoir experience. Sci. Total Environ. 2015, 538, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Vollenweider, R.; Kerekes, J. Eutrophication of Waters. In Monitoring, Assessment and Control; Organization for Economic Co-Operation and Development (OECD): Paris, France, 1982; Volume 156. [Google Scholar]
- Schleuter, D.; Daufresne, M.; Massol, F.; Argillier, C. A user’s guide to functional diversity indices. Ecol. Monogr. 2010, 80, 469–484. [Google Scholar] [CrossRef] [Green Version]
- Villéger, S.; Mason, N.W.; Mouillot, D. New multidimensional functional diversity indices for a multifaceted framework in functional ecology. Ecology 2008, 89, 2290–2301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsieh, T.; Ma, K.; Chao, A. iNEXT: An R package for rarefaction and extrapolation of species diversity (Hill numbers). Methods Ecol. Evol. 2016, 7, 1451–1456. [Google Scholar] [CrossRef]
- Chao, A.; Chiu, C.-H.; Jost, L. Unifying species diversity, phylogenetic diversity, functional diversity, and related similarity and differentiation measures through Hill numbers. Annu. Rev. Ecol. Evol. Syst. 2014, 45, 297–324. [Google Scholar] [CrossRef] [Green Version]
- Oksanen, J.; Blanchet, F.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.; O’Hara, R.; Simpson, G.; Solymos, P.; et al. Vegan: Community Ecology Package. R Package Version 2.4–6. 2018. Available online: http://cran.nexr.com/web/packages/vegan/vegan.pdf (accessed on 9 October 2019).
- Zervas, D.; Tsiripidis, I.; Bergmeier, E.; Tsiaoussi, V. A phytosociological survey of aquatic vegetation in the main freshwater lakes of Greece. Veg. Classif. Surv. 2020, 1, 53. [Google Scholar] [CrossRef]
- Botta-Dukát, Z. Rao’s quadratic entropy as a measure of functional diversity based on multiple traits. J. Veg. Sci. 2005, 16, 533–540. [Google Scholar] [CrossRef]
- Laliberté, E.; Legendre, P. A distance-based framework for measuring functional diversity from multiple traits. Ecology 2010, 91, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Podani, J. Extending Gower’s general coefficient of similarity to ordinal characters. Taxon 1999, 48, 331–340. [Google Scholar] [CrossRef]
- Laliberté, E.; Legendre, P.; Shipley, B.; Laliberté, M.E. Package ‘FD’. Measuring Functional Diversity from Multiple Traits, and Other Tools for Functional Ecology. 2014. Available online: https://cran.r-project.org/web/packages/FD/FD.pdf (accessed on 9 October 2019).
- Ensing, D.J.; Pither, J. A novel multiple-site extension to pairwise partitioned taxonomic beta diversity. Ecol. Complex. 2015, 21, 62–69. [Google Scholar] [CrossRef]
- Baselga, A.; Orme, C.D.L. betapart: An R package for the study of beta diversity. Methods Ecol. Evol. 2012, 3, 808–812. [Google Scholar] [CrossRef]
- Chambers, J.M.; Freeny, A.E.; Heiberger, R.M. Analysis of variance; designed experiments. In Statistical Models in S; Routledge: London, UK, 2017; pp. 145–193. [Google Scholar]
- Tukey, J.W. Comparing individual means in the analysis of variance. Biometrics 1949, 5, 99–114. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Ting, W.; Shiqiang, Z. Study on linear correlation coefficient and nonlinear correlation coefficient in mathematical statistics. Stud. Math. Sci. 2011, 3, 58–63. [Google Scholar]
- Grime, J.P. Plant Strategies, Vegetation Processes, and Ecosystem Properties; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Toivonen, H.; Huttunen, P. Aquatic macrophytes and ecological gradients in 57 small lakes in southern Finland. Aquat. Bot. 1995, 51, 197–221. [Google Scholar] [CrossRef]
- Alahuhta, J.; Kanninen, A.; Vuori, K.-M. Response of macrophyte communities and status metrics to natural gradients and land use in boreal lakes. Aquat. Bot. 2012, 103, 106–114. [Google Scholar] [CrossRef]
- Kolada, A.; Hellsten, S.; Søndergaard, M.; Mjelde, M.; Dudley, B.; Van Geest, G.; Goldsmith, B.; Davidson, T.; Bennion, H.; Noges, P. Report on the Most Suitable Lake Macrophyte Based Assessment Methods for Impacts of Eutrophication and Water Level Fluctuations; Wiser Deliverable D3.2-3; Institute of Environmental Protection: Warsaw, Poland, 2011. [Google Scholar]
- Jeppesen, E.; Søndergaard, M.; Jensen, J.P.; Lauridsen, T.L.; Howard-Williams, C.; Kelly, D. Recovery from eutrophication. In Freshwater Management; Springer: Berlin/Heidelberg, Germany, 2003; pp. 135–175. [Google Scholar]
- Romo, S.; Miracle, M.R.; Villena, M.J.; Rueda, J.; Ferriol, C.; Vicente, E. Mesocosm experiments on nutrient and fish effects on shallow lake food webs in a Mediterranean climate. Freshw. Biol. 2004, 49, 1593–1607. [Google Scholar] [CrossRef] [Green Version]
- Badosa, A.; Boix, D.; Brucet, S.; López-Flores, R.; Gascón, S.; Quintana, X.D. Zooplankton taxonomic and size diversity in Mediterranean coastal lagoons (NE Iberian Peninsula): Influence of hydrology, nutrient composition, food resource availability and predation. Estuar. Coast. Shelf Sci. 2007, 71, 335–346. [Google Scholar] [CrossRef]
- Romo, S.; Villena, M.-J. Phytoplankton strategies and diversity under different nutrient levels and planktivorous fish densities in a shallow Mediterranean lake. J. Plankton Res. 2005, 27, 1273–1286. [Google Scholar] [CrossRef] [Green Version]
- Townsend, C.R.; Scarsbrook, M.R.; Dolédec, S. The intermediate disturbance hypothesis, refugia, and biodiversity in streams. Limnol. Oceanogr. 1997, 42, 938–949. [Google Scholar] [CrossRef] [Green Version]
- Alahuhta, J.; Heino, J. Spatial extent, regional specificity and metacommunity structuring in lake macrophytes. J. Biogeogr. 2013, 40, 1572–1582. [Google Scholar] [CrossRef]
- Mikulyuk, A.; Sharma, S.; Van Egeren, S.; Erdmann, E.; Nault, M.E.; Hauxwell, J. The relative role of environmental, spatial, and land-use patterns in explaining aquatic macrophyte community composition. Can. J. Fish. Aquat. Sci. 2011, 68, 1778–1789. [Google Scholar] [CrossRef]
- Depew, D.C.; Houben, A.J.; Guildford, S.J.; Hecky, R.E. Distribution of nuisance Cladophora in the lower Great Lakes: Patterns with land use, near shore water quality and dreissenid abundance. J. Great Lakes Res. 2011, 37, 656–671. [Google Scholar] [CrossRef]
- Kane, D.D.; Conroy, J.D.; Richards, R.P.; Baker, D.B.; Culver, D.A. Re-eutrophication of Lake Erie: Correlations between tributary nutrient loads and phytoplankton biomass. J. Great Lakes Res. 2014, 40, 496–501. [Google Scholar] [CrossRef]
- Alahuhta, J.; Kosten, S.; Akasaka, M.; Auderset, D.; Azzella, M.M.; Bolpagni, R.; Bove, C.P.; Chambers, P.A.; Chappuis, E.; Clayton, J. Global variation in the beta diversity of lake macrophytes is driven by environmental heterogeneity rather than latitude. J. Biogeogr. 2017, 44, 1758–1769. [Google Scholar] [CrossRef]
- Qian, H.; Ricklefs, R.E. A latitudinal gradient in large-scale beta diversity for vascular plants in North America. Ecol. Lett. 2007, 10, 737–744. [Google Scholar] [CrossRef]
- Soininen, J.; Lennon, J.J.; Hillebrand, H. A multivariate analysis of beta diversity across organisms and environments. Ecology 2007, 88, 2830–2838. [Google Scholar] [CrossRef]
- Pereira, S.A.; Trindade, C.R.T.; Albertoni, E.F.; Palma-Silva, C. Aquatic macrophytes as indicators of water quality in subtropical shallow lakes, Southern Brazil. Acta Limnol. Bras. 2012, 24, 52–63. [Google Scholar] [CrossRef] [Green Version]
- Pereto, S.S.; Cervi, A.C.; Bona, C.; Padial, A.A. Aquatic macrophyte community varies in urban reservoirs with different degrees of eutrophication. Acta Limnol. Bras. 2014, 26, 129–142. [Google Scholar]
- Kraft, N.J.; Comita, L.S.; Chase, J.M.; Sanders, N.J.; Swenson, N.G.; Crist, T.O.; Stegen, J.C.; Vellend, M.; Boyle, B.; Anderson, M.J. Disentangling the drivers of β diversity along latitudinal and elevational gradients. Science 2011, 333, 1755–1758. [Google Scholar] [CrossRef] [Green Version]
- Cardinale, B.J.; Palmer, M.A.; Collins, S.L. Species diversity enhances ecosystem functioning through interspecific facilitation. Nature 2002, 415, 426–429. [Google Scholar] [CrossRef]
- Hooper, D.U.; Chapin, F., III; Ewel, J.J.; Hector, A.; Inchausti, P.; Lavorel, S.; Lawton, J.H.; Lodge, D.; Loreau, M.; Naeem, S. Effects of biodiversity on ecosystem functioning: A consensus of current knowledge. Ecol. Monogr. 2005, 75, 3–35. [Google Scholar] [CrossRef]
- Elmqvist, T.; Folke, C.; Nyström, M.; Peterson, G.; Bengtsson, J.; Walker, B.; Norberg, J. Response diversity, ecosystem change, and resilience. Front. Ecol. Environ. 2003, 1, 488–494. [Google Scholar] [CrossRef]
- Mori, A.S.; Furukawa, T.; Sasaki, T. Response diversity determines the resilience of ecosystems to environmental change. Biol. Rev. 2013, 88, 349–364. [Google Scholar] [CrossRef] [PubMed]
- Lavorel, S. Ecological diversity and resilience of Mediterranean vegetation to disturbance. Divers. Distrib. 1999, 5, 3–13. [Google Scholar] [CrossRef]
- Mason, N.W.; de Bello, F.; Mouillot, D.; Pavoine, S.; Dray, S. A guide for using functional diversity indices to reveal changes in assembly processes along ecological gradients. J. Veg. Sci. 2013, 24, 794–806. [Google Scholar] [CrossRef]
- Mouchet, M.A.; Villéger, S.; Mason, N.W.; Mouillot, D. Functional diversity measures: An overview of their redundancy and their ability to discriminate community assembly rules. Funct. Ecol. 2010, 24, 867–876. [Google Scholar] [CrossRef]
- Arthaud, F.; Vallod, D.; Robin, J.; Bornette, G. Eutrophication and drought disturbance shape functional diversity and life-history traits of aquatic plants in shallow lakes. Aquat. Sci. 2012, 74, 471–481. [Google Scholar] [CrossRef]
- Fu, H.; Zhong, J.; Fang, S.; Hu, J.; Guo, C.; Lou, Q.; Yuan, G.; Dai, T.; Li, Z.; Zhang, M. Scale-dependent changes in the functional diversity of macrophytes in subtropical freshwater lakes in south China. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef]
- Zhang, M.; García Molinos, J.; Zhang, X.; Xu, J. Functional and taxonomic differentiation of macrophyte assemblages across the Yangtze River floodplain under human impacts. Front. Plant Sci. 2018, 9, 387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walker, B.; Kinzig, A.; Langridge, J. Plant attribute diversity, resilience, and ecosystem function: The nature and significance of dominant and minor species. Ecosystems 1999, 2, 95–113. [Google Scholar] [CrossRef]
- Siefert, A.; Ravenscroft, C.; Weiser, M.D.; Swenson, N.G. Functional beta-diversity patterns reveal deterministic community assembly processes in eastern N orth A merican trees. Glob. Ecol. Biogeogr. 2013, 22, 682–691. [Google Scholar] [CrossRef]
- Fu, H.; Yuan, G.; Jeppesen, E.; Ge, D.; Li, W.; Zou, D.; Huang, Z.; Wu, A.; Liu, Q. Local and regional drivers of turnover and nestedness components of species and functional beta diversity in lake macrophyte communities in China. Sci. Total Environ. 2019, 687, 206–217. [Google Scholar] [CrossRef]
- Elo, M.; Alahuhta, J.; Kanninen, A.; Meissner, K.K.; Seppälä, K.; Mönkkönen, M. Environmental characteristics and anthropogenic impact jointly modify aquatic macrophyte species diversity. Front. Plant Sci. 2018, 9, 1001. [Google Scholar] [CrossRef]
- Salgado, J.; Sayer, C.D.; Brooks, S.J.; Davidson, T.A.; Goldsmith, B.; Patmore, I.R.; Baker, A.G.; Okamura, B. Eutrophication homogenizes shallow lake macrophyte assemblages over space and time. Ecosphere 2018, 9, e02406. [Google Scholar] [CrossRef]
- Salgado, J.; Sayer, C.D.; Brooks, S.J.; Davidson, T.A.; Okamura, B. Eutrophication erodes inter-basin variation in macrophytes and co-occurring invertebrates in a shallow lake: Combining ecology and palaeoecology. J. Paleolimnol. 2018, 60, 311–328. [Google Scholar] [CrossRef] [Green Version]


| No | Lake | Code | Centroid Longitude | Centroid Latitude | Mean Altitude (masl) | Area (km2) | Mean-Max Depth (m) | Aver. Annual Temp. (°C) | Annual Precipitation (mm) | Climate Zone | Survey Period | No of Transects/Relevés Recorded |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Volvi | VOL | 23.47368 | 40.67740 | 37 | 75.5 | 13–28 | 15.6 | 458 | Csa | August 2016 | 20/317 |
| 2 | Doirani * | DOI | 22.76487 | 21.23853 | 146 | 30.7 | 4–8 | 14.3 | 453 | Cfa | August 2016 | 10/173 |
| 3 | Vegoritida | VEG | 21.78442 | 40.74464 | 517 | 46.5 | 25–52 | 11.5 | 530 | Cfb | June 2016 | 20/509 |
| 4 | Petres | PET | 21.69612 | 40.72604 | 573 | 12 | 3–6 | 11.5 | 562 | Cfb | June 2016 | 16/227 |
| 5 | Zazari | ZAZ | 21.54690 | 4062507 | 600 | 3 | 5–8 | 11.5 | 595 | Cfb | July 2016 | 12/124 |
| 6 | Chimaditida | CHI | 21.56585 | 40.59258 | 592 | 9.1 | 1–5 | 11.5 | 595 | Cfb | July 2016 | 16/239 |
| 7 | Kastoria | KAS | 21.30080 | 40.52269 | 627 | 31.2 | 4–9 | 11.4 | 697 | Cfb | August 2014 | 20/312 |
| 8 | Megali Prespa * | MEP | 20.98875 | 40.85057 | 845 | 39.4 | ~16–26 | 10.2 | 750 | Cfb | August 2015 | 12/206 |
| 9 | Mikri Prespa * | MIP | 21.10128 | 40.77031 | 850 | 46.7 | 4–10 | 10.2 | 728 | Cfb | August 2015 | 15/294 |
| 10 | Pamvotida | PAM | 20.88518 | 39.66270 | 469 | 22.6 | 5–12 | 13.2 | 1081 | Csa | September 2013 | 20/74 |
| 11 | Amvrakia | AMV | 21.17941 | 38.75113 | 20 | 13.5 | 22–54 | 17.3 | 930 | Csa | June 2014 | 20/331 |
| 12 | Ozeros | OZE | 21.22294 | 38.65358 | 24 | 10.5 | 4–7 | 17.2 | 931 | Csa | June 2014 | 20/178 |
| 13 | Lysimachia | LYS | 21.37665 | 38.56234 | 15 | 13 | 4–8 | 17.1 | 909 | Csa | June 2014 | 20/215 |
| 14 | Trichonida | TRI | 21.54813 | 38.57309 | 16 | 93.4 | 30–56 | 17.1 | 902 | Csa | July 2015 | 20/792 |
| 15 | Paralimni | PAR | 23.35285 | 38.45862 | 37 | 10.6 | 5–8 | 17.5 | 527 | Csa | July 2014 | 20/503 |
| 16 | Yliki | YLI | 23.27973 | 38.39764 | 75 | 22.5 | 22–34 | 17.5 | 527 | Csa | July 2014 | 20/29 |
| 17 | Feneos | FEN | 22.28513 | 37.92861 | 872 | 0.5 | 10–29 | 11.5 | 862 | Csb | August 2014 | 10/373 |
| 18 | Kourna | KOU | 24.27776 | 35.33180 | 16 | 0.6 | ~15–22 | 18.2 | 831 | Csa | May 2014 | 14/794 |
| TP | SpeRich | Shan | Simp | Syntax | FunRich | Rao | BetOver | BetTurn | BetNest | FunBeta | FunTurn | FunNest | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TP | 1 | ||||||||||||
| SpeRich | 0.33 | 1 | |||||||||||
| Shan | 0.38 | 0.86 | 1 | ||||||||||
| Simp | 0.41 | 0.73 | 0.96 | 1 | |||||||||
| Syntax | 0.34 | 0.80 | 0.81 | 0.74 | 1 | ||||||||
| FunRich | 0.39 | 0.73 | 0.63 | 0.5 | 0.54 | 1 | |||||||
| Rao | 0.58 | 0.49 | 0.70 | 0.78 | 0.43 | 0.33 | 1 | ||||||
| BetOver | 0.52 | 0.12 | 0.26 | 0.29 | 0.24 | 0.21 | 0.31 | 1 | |||||
| BetTurn | 0.71 | 0.29 | 0.44 | 0.44 | 0.37 | 0.33 | 0.39 | 0.87 | 1 | ||||
| BetNest | 0.79 | 0.34 | 0.44 | 0.45 | 0.36 | 0.36 | 0.46 | 0.73 | 0.96 | 1 | |||
| FunBeta | 0.71 | 0.23 | 0.34 | 0.40 | 0.29 | 0.17 | 0.31 | 0.72 | 0.75 | 0.63 | 1 | ||
| FunTurn | 0.79 | 0.27 | 0.32 | 0.28 | 0.41 | 0.15 | 0.38 | 0.82 | 0.68 | 0.58 | 0.72 | 1 | |
| FunNest | 0.75 | 0.13 | 0.31 | 0.33 | 0.30 | 0.18 | 0.39 | 0.65 | 0.76 | 0.68 | 0.71 | 0.77 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zervas, D.; Tsiaoussi, V.; Kallimanis, A.S.; Dimopoulos, P.; Bergmeier, E.; Tsiripidis, I. Multiple-Facet Diversity Patterns of Aquatic Vegetation in Lakes along a Trophic Gradient. Water 2021, 13, 2281. https://doi.org/10.3390/w13162281
Zervas D, Tsiaoussi V, Kallimanis AS, Dimopoulos P, Bergmeier E, Tsiripidis I. Multiple-Facet Diversity Patterns of Aquatic Vegetation in Lakes along a Trophic Gradient. Water. 2021; 13(16):2281. https://doi.org/10.3390/w13162281
Chicago/Turabian StyleZervas, Dimitrios, Vasiliki Tsiaoussi, Athanasios S. Kallimanis, Panayotis Dimopoulos, Erwin Bergmeier, and Ioannis Tsiripidis. 2021. "Multiple-Facet Diversity Patterns of Aquatic Vegetation in Lakes along a Trophic Gradient" Water 13, no. 16: 2281. https://doi.org/10.3390/w13162281
APA StyleZervas, D., Tsiaoussi, V., Kallimanis, A. S., Dimopoulos, P., Bergmeier, E., & Tsiripidis, I. (2021). Multiple-Facet Diversity Patterns of Aquatic Vegetation in Lakes along a Trophic Gradient. Water, 13(16), 2281. https://doi.org/10.3390/w13162281








