Emerging Contaminants: An Overview of Recent Trends for Their Treatment and Management Using Light-Driven Processes
Abstract
1. Introduction
1.1. Global Occurrence of Contaminants of Emerging Concern (CECs) and Their Categorization
1.2. Conventional Detection of CECs in Water Bodies
2. Overview of Light-Driven Processes
2.1. Mechanism of Light-Driven Processes
2.2. Critical Factors That Affect Light-Driven Processes
2.3. UV/Oxidant
2.4. UV/Ozone
2.5. Photo-Fenton
2.6. Photocatalysis
2.7. Light-Driven Detection of CEC in Treatment Systems
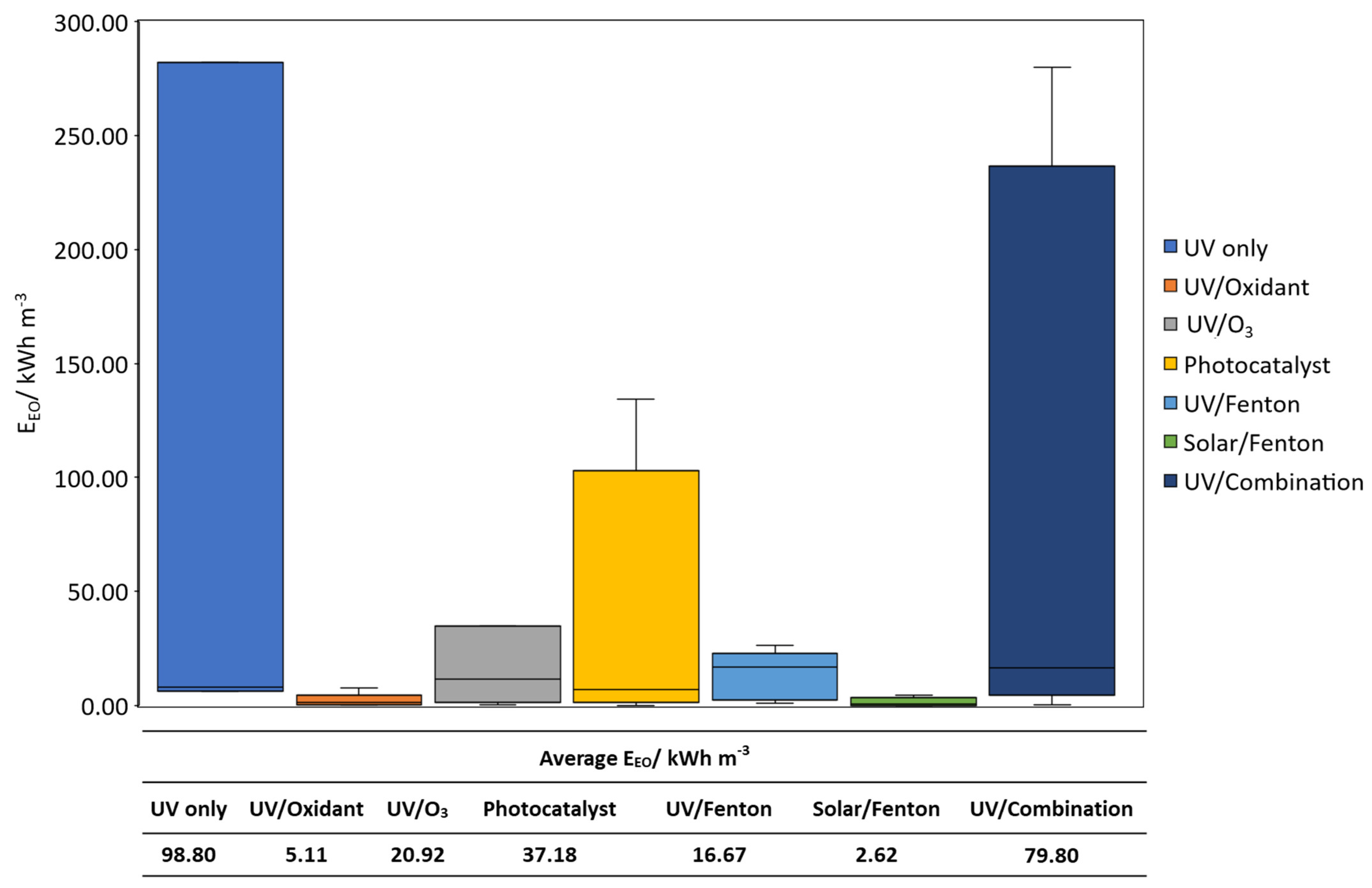
3. Cost–Benefit Analysis of Light-Driven AOPs on the Treatment of CECs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gavrilescu, M.; Demnerová, K.; Aamand, J.; Agathos, S.; Fava, F. Emerging pollutants in the environment: Present and future challenges in biomonitoring, ecological risks and bioremediation. New Biotechnol. 2015, 32, 147–156. [Google Scholar] [CrossRef]
- Richardson, S.D.; Ternes, T.A. Water Analysis: Emerging Contaminants and Current Issues. Anal. Chem. 2017, 90, 398–428. [Google Scholar] [CrossRef]
- Parida, V.K.; Saidulu, D.; Majumder, A.; Srivastava, A.; Gupta, B.; Gupta, A.K. Emerging contaminants in wastewater: A critical review on occurrence, existing legislations, risk assessment, and sustainable treatment alternatives. J. Environ. Chem. Eng. 2021, 9, 105966. [Google Scholar] [CrossRef]
- Choi, Y.Y.; Baek, S.R.; Kim, J.L.; Choi, J.W.; Hur, J.; Lee, T.U.; Park, C.J.; Lee, B.J. Characteristics and Biodegradability of Wastewater Organic Matter in Municipal Wastewater Treatment Plants Collecting Domestic Wastewater and Industrial Discharge. Water (Switz.) 2017, 9, 409. [Google Scholar] [CrossRef]
- Ahearn, A. A Regrettable Substitute: The Story of GenX. Pod. Res. Perspect. 2019, 2019. [Google Scholar] [CrossRef]
- Sharma, S.; Tolley, H.D.; Farnsworth, P.B.; Lee, M.L. LED-based UV absorption detector with low detection limits for capillary liquid chromatography. Anal. Chem. 2015, 87, 1381–1386. [Google Scholar] [CrossRef]
- Safari, G.H.; Yetilmezsoy, K.; Mahvi, A.H.; Zarrabi, M. Post-treatment of secondary wastewater treatment plant effluent using a two-stage fluidized bed bioreactor system. J. Environ. Health Sci. Eng. 2013, 11, 10. [Google Scholar] [CrossRef] [PubMed]
- Watkinson, A.J.; Murby, E.J.; Kolpin, D.W.; Costanzo, S.D. The occurrence of antibiotics in an urban watershed: From wastewater to drinking water. Sci. Total Environ. 2009, 407, 2711–2723. [Google Scholar] [CrossRef] [PubMed]
- Mutiyar, P.K.; Mittal, A.K. Occurrences and fate of an antibiotic amoxicillin in extended aeration-based sewage treatment plant in Delhi, India: A case study of emerging pollutant. Desalination Water Treat. 2013, 51, 6158–6164. [Google Scholar] [CrossRef]
- Azanu, D.; Styrishave, B.; Darko, G.; Weisser, J.J.; Abaidoo, R.C. Occurrence and risk assessment of antibiotics in water and lettuce in Ghana. Sci. Total Environ. 2018, 622, 293–305. [Google Scholar] [CrossRef] [PubMed]
- Ashfaq, M.; Li, Y.; Rehman, M.S.U.; Zubir, M.; Mustafa, G.; Nazar, M.F.; Yu, C.P.; Sun, Q. Occurrence, spatial variation and risk assessment of pharmaceuticals and personal care products in urban wastewater, canal surface water, and their sediments: A case study of Lahore, Pakistan. Sci. Total Environ. 2019, 688, 653–663. [Google Scholar] [CrossRef]
- Ali, A.M.; Rønning, H.T.; al Arif, W.M.; Kallenborn, R.; Kallenborn, R. Occurrence of pharmaceuticals and personal care products in effluent-dominated Saudi Arabian coastal waters of the Red Sea. Chemosphere 2017, 175, 505–513. [Google Scholar] [CrossRef]
- Kermia, A.E.B.; Fouial-Djebbar, D.; Trari, M. Occurrence, fate and removal efficiencies of pharmaceuticals in wastewater treatment plants (WWTPs) discharging in the coastal environment of Algiers. Comptes Rendus Chim. 2016, 19, 963–970. [Google Scholar] [CrossRef]
- Rosal, R.; Rodríguez, A.; Perdigón-Melón, J.A.; Petre, A.; García-Calvo, E.; Gómez, M.J.; Agüera, A.; Fernández-Alba, A.R. Occurrence of emerging pollutants in urban wastewater and their removal through biological treatment followed by ozonation. Water Res. 2009, 44, 578–588. [Google Scholar] [CrossRef]
- Ngubane, N.P.; Naicker, D.; Ncube, S.; Chimuka, L.; Madikizela, L.M. Determination of naproxen, diclofenac and ibuprofen in Umgeni estuary and seawater: A case of northern Durban in KwaZulu–Natal Province of South Africa. Reg. Stud. Mar. Sci. 2019, 29, 100675. [Google Scholar] [CrossRef]
- Giang, C.N.D.; Sebesvari, Z.; Renaud, F.; Rosendahl, I.; Minh, Q.H.; Amelung, W. Occurrence and dissipation of the antibiotics sulfamethoxazole, sulfadiazine, trimethoprim, and enrofloxacin in the Mekong Delta, Vietnam. PLoS ONE 2015, 10, e0131855. [Google Scholar] [CrossRef]
- Wei, R.; Ge, F.; Huang, S.; Chen, M.; Wang, R. Occurrence of veterinary antibiotics in animal wastewater and surface water around farms in Jiangsu Province, China. Chemosphere 2011, 82, 1408–1414. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.C.; Krasner, S.W. Occurrence of primidone, carbamazepine, caffeine, and precursors for N-nitrosodimethylamine in drinking water sources impacted by wastewater. J. Am. Water Resour. Assoc. 2009, 45, 58–67. [Google Scholar] [CrossRef]
- Huerta-Fontela, M.; Galceran, M.T.; Ventura, F. Occurrence and removal of pharmaceuticals and hormones through drinking water treatment. Water Res. 2011, 45, 1432–1442. [Google Scholar] [CrossRef] [PubMed]
- Rimayi, C.; Odusanya, D.; Weiss, J.M.; de Boer, J.; Chimuka, L. Contaminants of emerging concern in the Hartbeespoort Dam catchment and the uMngeni River estuary 2016 pollution incident, South Africa. Sci. Total Environ. 2018, 627, 1008–1017. [Google Scholar] [CrossRef]
- Gan, Z.; Sun, H.; Feng, B.; Wang, R.; Zhang, Y. Occurrence of seven artificial sweeteners in the aquatic environment and precipitation of Tianjin, China. Water Res. 2013, 47, 4928–4937. [Google Scholar] [CrossRef]
- Kahl, S.; Kleinsteuber, S.; Nivala, J.; van Afferden, M.; Reemtsma, T. Emerging Biodegradation of the Previously Persistent Artificial Sweetener Acesulfame in Biological Wastewater Treatment. Environ. Sci. Technol. 2018, 52, 2717–2725. [Google Scholar] [CrossRef]
- Ouyang, W.; Zhang, Y.; Gu, X.; Tysklind, M.; Lin, G.; Wang, B.; Xin, M. Occurrence, transportation, and distribution difference of typical herbicides from estuary to bay. Environ. Int. 2019, 130, 104858. [Google Scholar] [CrossRef]
- Bachetti, R.A.; Ursela, N.; Morgante, V.; Damilano, G.; Porporatta, C.; Agostini, E.; Morgante, C. Monitoring of Atrazine Pollution and its Spatial-Seasonal Variation on Surface Water Sources of an Agricultural River Basin. Bull. Environ. Contam. Toxicol. 2021, 106, 929–935. [Google Scholar] [CrossRef] [PubMed]
- Rimayi, C.; Odusanya, D.; Weiss, J.M.; de Boer, J.; Chimuka, L. Seasonal variation of chloro-s-triazines in the Hartbeespoort Dam catchment, South Africa. Sci. Total Environ. 2018, 613-614, 472–482. [Google Scholar] [CrossRef] [PubMed]
- Carrera, G.; Vegué, L.; Ventura, F.; Hernández-Valencia, A.; Devesa, R.; Boleda, M.R. Dioxanes and dioxolanes in source waters: Occurrence, odor thresholds and behavior through upgraded conventional and advanced processes in a drinking water treatment plant. Water Res. 2019, 156, 404–413. [Google Scholar] [CrossRef] [PubMed]
- Stepien, D.K.; Diehl, P.; Helm, J.; Thoms, A.; Püttmann, W. Fate of 1,4-dioxane in the aquatic environment: From sewage to drinking water. Water Res. 2014, 48, 406–419. [Google Scholar] [CrossRef]
- Kunacheva, C.; Fujii, S.; Tanaka, S.; Seneviratne, S.T.M.L.D.; Lien, N.P.H.; Nozoe, M.; Kimura, K.; Shivakoti, B.R.; Harada, H. Worldwide surveys of perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA) in water environment in recent years. Water Sci. Technol. 2012, 66, 2764–2771. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Yu, J.; Tanaka, S.; Fujii, S. Perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA) in water environment of Singapore. Water Air Soil Pollut. 2010, 216, 179–191. [Google Scholar] [CrossRef]
- Dalahmeh, S.; Tirgani, S.; Komakech, A.J.; Niwagaba, C.B.; Ahrens, L. Per- and polyfluoroalkyl substances (PFASs) in water, soil and plants in wetlands and agricultural areas in Kampala, Uganda. Sci. Total Environ. 2018, 631, 660–667. [Google Scholar] [CrossRef]
- Huang, Z.; Zhao, J.L.; Yang, Y.Y.; Jia, Y.W.; Zhang, Q.Q.; Chen, C.E.; Liu, Y.S.; Yang, B.; Xie, L.; Ying, G.G. Occurrence, mass loads and risks of bisphenol analogues in the Pearl River Delta region, South China: Urban rainfall runoff as a potential source for receiving rivers. Environ. Pollut. 2020, 263, 114361. [Google Scholar] [CrossRef]
- Lalwani, D.; Ruan, Y.; Taniyasu, S.; Yamazaki, E.; Kumar, N.J.I.; Lam, P.K.S.; Wang, X.; Yamashita, N. Nationwide distribution and potential risk of bisphenol analogues in Indian waters. Ecotoxicol. Environ. Saf. 2020, 200, 110718. [Google Scholar] [CrossRef] [PubMed]
- AlAmmari, A.M.; Khan, M.R.; Aqel, A. Trace identification of endocrine-disrupting bisphenol A in drinking water by solid-phase extraction and ultra-performance liquid chromatography-tandem mass spectrometry. J. King Saud Univ. Sci. 2019, 32, 1634–1640. [Google Scholar] [CrossRef]
- Santos, M.M.d.; Hoppe-Jones, C.; Snyder, S.A. DEET occurrence in wastewaters: Seasonal, spatial and diurnal variability - mismatches between consumption data and environmental detection. Environ. Int. 2019, 132, 105038. [Google Scholar] [CrossRef]
- Chase, D.A.; Karnjanapiboonwong, A.; Fang, Y.; Cobb, G.P.; Morse, A.N.; Anderson, T.A. Occurrence of synthetic musk fragrances in effluent and non-effluent impacted environments. Sci. Total Environ. 2011, 416, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Qin, F.; Boyd, J.M.; Anichina, J.; Li, X.F. Characterization and determination of chloro- and bromo-benzoquinones as new chlorination disinfection byproducts in drinking water. Anal. Chem. 2010, 82, 4599–4605. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Long, K.; Sha, Y.; Lu, D.; Xia, Y.; Mo, Y.; Yang, Q.; Zheng, W.; Yang, M.; Wei, X. Occurrence and toxicity of halobenzoquinones as drinking water disinfection byproducts. Sci. Total Environ. 2021, 770, 145277. [Google Scholar] [CrossRef] [PubMed]
- Tugulea, A.M.; Aranda-Rodriguez, R.; Bérubé, D.; Giddings, D.; Lemieux, F.; Hnatiw, J.; Dabeka, L.; Breton, F. The influence of precursors and treatment process on the formation of Iodo-THMs in Canadian drinking water. Water Res. 2018, 130, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Allard, S.; Charrois, J.W.A.; Joll, C.A.; Heitz, A. Simultaneous analysis of 10 trihalomethanes at nanogram per liter levels in water using solid-phase microextraction and gas chromatography mass-spectrometry. J. Chromatogr. A 2012, 1238, 15–21. [Google Scholar] [CrossRef]
- National Research Council. A Framework to Guide Selection of Chemical Alternatives; The National Academies Press: Washington, DC, USA, 2014. [Google Scholar] [CrossRef]
- DiGuiseppi, W.; Walecka-Hutchison, C.; Hatton, J. 1,4-Dioxane Treatment Technologies. Remediat. J. 2016, 27, 71–92. [Google Scholar] [CrossRef]
- Behnajady, M.A.; Eskandarloo, H.; Modirshahla, N.; Shokri, M. Influence of the chemical structure of organic pollutants on photocatalytic activity of TiO2 nanoparticles: Kinetic analysis and evaluation of electrical energy per order (EEO). Dig. J. Nanomater. Biostruct. 2011, 6, 1887–1895. [Google Scholar]
- Sivaranjanee, R.; Kumar, P.S. A review on remedial measures for effective separation of emerging contaminants from wastewater. Environ. Technol. Innov. Environ. Technol. Innov. 2021, 23, 101741. [Google Scholar] [CrossRef]
- Moschet, C.; Lew, B.M.; Hasenbein, S.; Anumol, T.; Young, T.M. LC- and GC-QTOF-MS as Complementary Tools for a Comprehensive Micropollutant Analysis in Aquatic Systems. Environ. Sci. Technol. 2017, 51, 1553–1561. [Google Scholar] [CrossRef] [PubMed]
- Perez, E.R.; Knapp, J.A.; Horn, C.K.; Stillman, S.L.; Evans, J.E.; Arfsten, D.P. Comparison of LC-MS-MS and GC-MS analysis of benzodiazepine compounds included in the drug demand reduction urinalysis program. J. Anal. Toxicol. 2016, 40, 201–207. [Google Scholar] [CrossRef] [PubMed]
- García-Córcoles, M.T.; Rodríguez-Gómez, R.; Alarcón-Gómez, B.D.; Çipa, M.; Martín-Pozo, L.; Kauffmann, J.M.; Zafra-Gómez, A. Chromatographic Methods for the Determination of Emerging Contaminants in Natural Water and Wastewater Samples: A Review. Crit. Rev. Anal. Chem. 2018, 49, 160–186. [Google Scholar] [CrossRef] [PubMed]
- Andersson, A.; Ashiq, M.J.; Shoeb, M.; Karlsson, S.; Bastviken, D.; Kylin, H. Evaluating gas chromatography with a halogen-specific detector for the determination of disinfection by-products in drinking water. Environ. Sci. Pollut. Res. 2018, 26, 7305–7314. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, X. Current trends in the analysis and identification of emerging disinfection byproducts. Trends Environ. Anal. Chem. 2016, 10, 24–34. [Google Scholar] [CrossRef]
- Ryu, H.; Li, B.; de Guise, S.; McCutcheon, J.; Lei, Y. Recent progress in the detection of emerging contaminants PFASs. J. Hazard. Mater. 2020, 408, 124437. [Google Scholar] [CrossRef]
- Calvert, J.G.; Pms, J.N., Jr. Photochemistry; John Wiley and Sons Inc.: Hoboken, NJ, USA, 1967; Volume 6, p. 601. [Google Scholar]
- Zhang, W.; Xiao, Z.; An, T.; Song, Z.; Fu, J.; Sheng, G.; Cui, M. Kinetics, degradation pathway and reaction mechanism of advanced oxidation of 4-nitrophenol in water by a UV/H2O2 process. J. Chem. Technol. Biotechnol. 2003, 78, 788–794. [Google Scholar] [CrossRef]
- Liu, Y.; He, X.; Fu, Y.; Dionysiou, D.D. Kinetics and mechanism investigation on the destruction of oxytetracycline by UV-254 nm activation of persulfate. J. Hazard. Mater. 2016, 305, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Watts, M.J.; Linden, K.G. Chlorine photolysis and subsequent OH radical production during UV treatment of chlorinated water. Water Res. 2007, 41, 2871–2878. [Google Scholar] [CrossRef] [PubMed]
- Serpone, N.; Artemev, Y.M.; Ryabchuk, V.K.; Emeline, A.V.; Horikoshi, S. Light-driven advanced oxidation processes in the disposal of emerging pharmaceutical contaminants in aqueous media: A brief review. Curr. Opin. Green Sustain. Chem. 2017, 6, 18–33. [Google Scholar] [CrossRef]
- Cai, Q.Q.; Lee, B.C.Y.; Ong, S.L.; Hu, J.Y. Fluidized-bed Fenton technologies for recalcitrant industrial wastewater treatment–Recent advances, challenges and perspective. Water Res. 2020, 190, 116692. [Google Scholar] [CrossRef]
- Clarizia, L.; Russo, D.; di Somma, I.; Marotta, R.; Andreozzi, R. Homogeneous photo-Fenton processes at near neutral pH: A review. Appl. Catal. B Environ. 2017, 209, 358–371. [Google Scholar] [CrossRef]
- Dominguez, S.; Huebra, M.; Han, C.; Campo, P.; Nagagouda, M.N.; Rivero, M.J.; Ortiz, I.; Dionysiou, D.D. Magnetically recoverable TiO2-WO3 photocatalyst to oxidize bisphenol A from model wastewater under simulated solar light. Environ. Sci. Pollut. Res. 2016, 24, 12589–12598. [Google Scholar] [CrossRef]
- Cai, Q.; Hu, J. Decomposition of sulfamethoxazole and trimethoprim by continuous UVA/LED/TiO2 photocatalysis: Decomposition pathways, residual antibacterial activity and toxicity. J. Hazard. Mater. 2017, 323, 527–536. [Google Scholar] [CrossRef]
- Vela, N.; Calín, M.; Yáñez-Gascón, M.J.; Garrido, I.; Pérez-Lucas, G.; Fenoll, J.; Navarro, S. Photocatalytic oxidation of six endocrine disruptor chemicals in wastewater using ZnO at pilot plant scale under natural sunlight. Environ. Sci. Pollut. Res. 2018, 25, 34995–35007. [Google Scholar] [CrossRef]
- Ding, H.; Hu, J. Degradation of ibuprofen by UVA-LED/TiO2/persulfate process: Kinetics, mechanism, water matrix effects, intermediates and energy consumption. Chem. Eng. J. 2020, 397, 125462. [Google Scholar] [CrossRef]
- Mirzaei, A.; Chen, Z.; Haghighat, F.; Yerushalmi, L. Magnetic fluorinated mesoporous g-C3N4 for photocatalytic degradation of amoxicillin: Transformation mechanism and toxicity assessment. Appl. Catal. B Environ. 2018, 242, 337–348. [Google Scholar] [CrossRef]
- Garcia-Segura, S.; Bellotindos, L.M.; Huang, Y.H.; Brillas, E.; Lu, M.C. Fluidized-bed Fenton process as alternative wastewater treatment technology—A review. J. Taiwan Inst. Chem. Eng. 2016, 67, 211–225. [Google Scholar] [CrossRef]
- Szpyrkowicz, L.; Juzzolino, C.; Kaul, S.N. A comparative study on oxidation of disperse dyes by electrochemical process, ozone, hypochlorite and fenton reagent. Water Res. 2001, 35, 2129–2136. [Google Scholar] [CrossRef]
- Tang, W.Z.; Huang, C.P. 2,4-Dichlorophenol Oxidation Kinetics by Fenton’s Reagent. Environ. Technol. (UK) 1996, 17, 1371–1378. [Google Scholar] [CrossRef]
- Velichkova, F.; Delmas, H.; Julcour, C.; Koumanova, B. Heterogeneous fenton and photo-fenton oxidation for paracetamol removal using iron containing ZSM-5 zeolite as catalyst. AIChE J. 2016, 63, 669–679. [Google Scholar] [CrossRef]
- Zammouri, L.; Aboulaich, A.; Capoen, B.; Bouazaoui, M.; Sarakha, M.; Stitou, M.; Mahiou, R. Enhancement under UV–visible and visible light of the ZnO photocatalytic activity for the antibiotic removal from aqueous media using Ce-doped Lu3Al5O12 nanoparticles. Mater. Res. Bull. 2018, 106, 162–169. [Google Scholar] [CrossRef]
- Funai, D.H.; Didier, F.; Giménez, J.; Esplugas, S.; Marco, P.; Machulek, A. Photo-Fenton treatment of valproate under UVC, UVA and simulated solar radiation. J. Hazard. Mater. 2017, 323, 537–549. [Google Scholar] [CrossRef]
- Eskandarian, M.R.; Choi, H.; Fazli, M.; Rasoulifard, M.H. Effect of UV-LED wavelengths on direct photolytic and TiO2 photocatalytic degradation of emerging contaminants in water. Chem. Eng. J. 2016, 300, 414–422. [Google Scholar] [CrossRef]
- Summerfelt, S.T. Ozonation and UV irradiation—An introduction and examples of current applications. Aquac. Eng. 2003, 28, 21–36. [Google Scholar] [CrossRef]
- Guerra, M.M.H.; Alberola, I.O.; Rodriguez, S.M.; López, A.A.; Merino, A.A.; Alonso, J.M.Q. Oxidation mechanisms of amoxicillin and paracetamol in the photo-Fenton solar process. Water Res. 2019, 156, 232–240. [Google Scholar] [CrossRef]
- Long, M.; Brame, J.; Qin, F.; Bao, J.; Li, Q.; Alvarez, P.J.J. Phosphate Changes Effect of Humic Acids on TiO2 Photocatalysis: From Inhibition to Mitigation of Electron-Hole Recombination. Environ. Sci. Technol. 2017, 51, 514–521. [Google Scholar] [CrossRef]
- Lu, G.; Hu, J. Effect of alpha-hydroxy acids on transformation products formation and degradation mechanisms of carbamazepine by UV/H2O2 process. Sci. Total Environ. 2019, 689, 70–78. [Google Scholar] [CrossRef]
- Ren, M.; Drosos, M.; Frimmel, F.H. Inhibitory effect of NOM in photocatalysis process: Explanation and resolution. Chem. Eng. J. 2018, 334, 968–975. [Google Scholar] [CrossRef]
- Uyguner-Demirel, C.S.; Birben, N.C.; Bekbolet, M. Elucidation of background organic matter matrix effect on photocatalytic treatment of contaminants using TiO2: A review. Catal. Today 2017, 284, 202–214. [Google Scholar] [CrossRef]
- Du, Y.; Zhou, M.; Lei, L. The role of oxygen in the degradation of p-chlorophenol by Fenton system. J. Hazard. Mater. 2007, 139, 108–115. [Google Scholar] [CrossRef]
- Duesterberg, C.K.; Cooper, W.J.; Waite, T.D. Fenton-mediated oxidation in the presence and absence of oxygen. Environ. Sci. Technol. 2005, 39, 5052–5058. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Hu, J. Transformation products formation of ciprofloxacin in UVA/LED and UVA/LED/TiO2 systems: Impact of natural organic matter characteristics. Water Res. 2018, 132, 320–330. [Google Scholar] [CrossRef]
- Nasseh, N.; Taghavi, L.; Barikbin, B.; Nasseri, M.A. Synthesis and characterizations of a novel FeNi3/SiO2/CuS magnetic nanocomposite for photocatalytic degradation of tetracycline in simulated wastewater. J. Clean. Prod. 2018, 179, 42–54. [Google Scholar] [CrossRef]
- Yamal-Turbay, E.; Graells, M.; Pérez-Moya, M. Systematic assessment of the influence of hydrogen peroxide dosage on caffeine degradation by the photo-fenton process. Ind. Eng. Chem. Res. 2012, 51, 4770–4778. [Google Scholar] [CrossRef]
- Song, C.; Wang, L.; Ren, J.; Lv, B.; Sun, Z.; Yan, J.; Li, X.; Liu, J. Comparative study of diethyl phthalate degradation by UV/H2O2 and UV/TiO2: Kinetics, mechanism, and effects of operational parameters. Environ. Sci. Pollut. Res. 2015, 23, 2640–2650. [Google Scholar] [CrossRef]
- Qi, W.; Hu, J. NDMA formation potential removal in treated effluent by UV/ H2O2 process. J. Water Reuse Desalin. 2015, 6, 156–166. [Google Scholar] [CrossRef][Green Version]
- Chu, X.; Xiao, C.; Hu, J.; Quek, E.; Xie, R.; Pang, T.; Xing, Y. Pilot-scale UV/H2O2 study for emerging organic contaminants decomposition. Rev. Environ. Health 2016, 31, 71–74. [Google Scholar] [CrossRef]
- Carotenuto, M.; Libralato, G.; Gürses, H.; Siciliano, A.; Rizzo, L.; Guida, M.; Lofrano, G. Nonylphenol deca-ethoxylate removal from wastewater by UV/H2O2: Degradation kinetics and toxicity effects. Process Saf. Environ. Prot. 2019, 124, 1–7. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, Y.; Kong, M.; Xu, E.G.; Coffin, S.; Schlenk, D.; Dionysiou, D.D. Efficient degradation of cytotoxic contaminants of emerging concern by UV/H2O2. Environ. Sci. Water Res. Technol. 2018, 4, 1272–1281. [Google Scholar] [CrossRef]
- Huang, Y.; Kong, M.; Coffin, S.; Cochran, K.H.; Westerman, D.C.; Schlenk, D.; Richardson, S.D.; Lei, L.; Dionysiou, D.D. Degradation of contaminants of emerging concern by UV/H2O2 for water reuse: Kinetics, mechanisms, and cytotoxicity analysis. Water Res. 2020, 174, 115587. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Iranmanesh, S.; Keir, I.; Achari, G. A field pilot study on treating groundwater contaminated with sulfolane using UV/H2O2. Water (Switz.) 2020, 12, 1200. [Google Scholar] [CrossRef]
- Rott, E.; Kuch, B.; Lange, C.; Richter, P.; Kugele, A.; Minke, R. Removal of emerging contaminants and estrogenic activity from wastewater treatment plant effluent with UV/chlorine and UV/H2O2 advanced oxidation treatment at pilot scale. Int. J. Environ. Res. Public Health 2018, 15, 935. [Google Scholar] [CrossRef]
- Cerreta, G.; Roccamante, M.A.; Oller, I.; Malato, S.; Rizzo, L. Contaminants of emerging concern removal from real wastewater by UV/free chlorine process: A comparison with solar/free chlorine and UV/H2O2 at pilot scale. Chemosphere 2019, 236, 124354. [Google Scholar] [CrossRef]
- Starling, M.C.V.M.; Souza, P.P.; le Person, A.; Amorim, C.C.; Criquet, J. Intensification of UV-C treatment to remove emerging contaminants by UV-C/H2O2 and UV-C/S2O82−: Susceptibility to photolysis and investigation of acute toxicity. Chem. Eng. J. 2019, 376, 120856. [Google Scholar] [CrossRef]
- Zhou, K.; Wang, Z.; Wang, X.; Jiao, G.; Li, Y.; Sun, S.P.; Chen, X.D. Degradation of emerging pharmaceutical micropollutants in municipal secondary effluents by low-pressure UVC-activated HSO5− and S2O82− AOPs. Chem. Eng. J. 2020, 393, 124712. [Google Scholar] [CrossRef]
- Dhaka, S.; Kumar, R.; Khan, M.A.; Paeng, K.J.; Kurade, M.B.; Kim, S.J.; Jeon, B.H. Aqueous phase degradation of methyl paraben using UV-activated persulfate method. Chem. Eng. J. 2017, 321, 11–19. [Google Scholar] [CrossRef]
- Ghauch, A.; Baalbaki, A.; Amasha, M.; el Asmar, R.; Tantawi, O. Contribution of persulfate in UV-254 nm activated systems for complete degradation of chloramphenicol antibiotic in water. Chem. Eng. J. 2017, 317, 1012–1025. [Google Scholar] [CrossRef]
- Hou, S.; Ling, L.; Shang, C.; Guan, Y.; Fang, J. Degradation kinetics and pathways of haloacetonitriles by the UV/persulfate process. Chem. Eng. J. 2017, 320, 478–484. [Google Scholar] [CrossRef]
- Ao, X.; Liu, W. Degradation of sulfamethoxazole by medium pressure UV and oxidants: Peroxymonosulfate, persulfate, and hydrogen peroxide. Chem. Eng. J. 2017, 313, 629–637. [Google Scholar] [CrossRef]
- Khan, S.; He, X.; Khan, J.A.; Khan, H.M.; Boccelli, D.L.; Dionysiou, D.D. Kinetics and mechanism of sulfate radical- and hydroxyl radical-induced degradation of highly chlorinated pesticide lindane in UV/peroxymonosulfate system. Chem. Eng. J. 2017, 318, 135–142. [Google Scholar] [CrossRef]
- Acero, J.L.; Benítez, F.J.; Real, F.J.; Rodríguez, E. Degradation of selected emerging contaminants by UV-activated persulfate: Kinetics and influence of matrix constituents. Sep. Purif. Technol. 2018, 201, 41–50. [Google Scholar] [CrossRef]
- Bao, Y.; Deng, S.; Jiang, X.; Qu, Y.; He, Y.; Liu, L.; Chai, Q.; Mumtaz, M.; Huang, J.; Cagnetta, G.; et al. Degradation of PFOA Substitute: GenX (HFPO-DA Ammonium Salt): Oxidation with UV/Persulfate or Reduction with UV/Sulfite? Environ. Sci. Technol. 2018, 52, 11728–11734. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, L.; Agovino, T.; Nahim-Granados, S.; Castro-Alférez, M.; Fernández-Ibáñez, P.; Polo-López, M.I. Tertiary treatment of urban wastewater by solar and UV-C driven advanced oxidation with peracetic acid: Effect on contaminants of emerging concern and antibiotic resistance. Water Res. 2018, 149, 272–281. [Google Scholar] [CrossRef]
- Zhou, S.; Li, L.; Wu, Y.; Zhu, S.; Zhu, N.; Bu, L.; Dionysiou, D.D. UV365 induced elimination of contaminants of emerging concern in the presence of residual nitrite: Roles of reactive nitrogen species. Water Res. 2020, 178, 115829. [Google Scholar] [CrossRef]
- Li, M.; Li, W.; Bolton, J.R.; Blatchley, E.R.; Qiang, Z. Organic Pollutant Degradation in Water by the Vacuum-Ultraviolet/Ultraviolet/H2O2 Process: Inhibition and Enhancement Roles of H2O2. Environ. Sci. Technol. 2018, 53, 912–918. [Google Scholar] [CrossRef]
- Li, C.; Zhang, D.; Peng, J.; Li, X. The effect of pH, nitrate, iron (III) and bicarbonate on photodegradation of oxytetracycline in aqueous solution. J. Photochem. Photobiol. A Chem. 2018, 356, 239–247. [Google Scholar] [CrossRef]
- Jing, L.; Chen, B.; Wen, D.; Zheng, J.; Zhang, B. The removal of COD and NH3-N from atrazine production wastewater treatment using UV/O3: Experimental investigation and kinetic modeling. Environ. Sci. Pollut. Res. 2018, 25, 2691–2701. [Google Scholar] [CrossRef]
- Xu, Y.; Wu, Y.; Zhang, W.; Fan, X.; Wang, Y.; Zhang, H. Performance of artificial sweetener sucralose mineralization via UV/O3 process: Kinetics, toxicity and intermediates. Chem. Eng. J. 2018, 353, 626–634. [Google Scholar] [CrossRef]
- Derco, J.; Šimovičová, K.; Dudáš, J.; Valičková, M. Removal of BTX Contaminants with O3 and O3/UV Processes. In Physico-Chemical Wastewater Treatment and Resource Recovery; IntechOpen: London, UK, 2017; Available online: https://www.intechopen.com/chapters/52713 (accessed on 3 May 2021).
- Takashina, T.A.; Leifeld, V.; Zelinski, D.W.; Mafra, M.R.; Igarashi-Mafra, L. Application of Response Surface Methodology for Coffee Effluent Treatment by Ozone and Combined Ozone/UV. Ozone Sci. Eng. 2018, 40, 293–304. [Google Scholar] [CrossRef]
- Yao, W.; Rehman, S.W.U.; Wang, H.; Yang, H.; Yu, G.; Wang, Y. Pilot-scale evaluation of micropollutant abatements by conventional ozonation, UV/O3, and an electro-peroxone process. Water Res. 2018, 138, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Xie, Z.; Dorian, B.; Gray, S.; Zhang, J. Comparative study of PFAS treatment by UV, UV/ozone, and fractionations with air and ozonated air. Environ. Sci. Water Res. Technol. 2019, 5, 1897–1907. [Google Scholar] [CrossRef]
- Rajabizadeh, K.; Yazdanpanah, G.; Dowlatshahi, S.; Malakootian, M. Photooxidation Process Efficiency (UV/O3) for P-nitroaniline Removal from Aqueous Solutions. Ozone Sci. Eng. 2019, 42, 420–427. [Google Scholar] [CrossRef]
- Fu, P.; Ma, Y.; Yang, H.; Li, G.; Lin, X. Ozone and ozone/vacuum-UV degradation of diethyl dithiocarbamate collector: Kinetics, mineralization, byproducts and pathways. RSC Adv. 2019, 9, 23579–23588. [Google Scholar] [CrossRef]
- Von Sonntag, C.; von Gunten, U. Chemistry of Ozone in Water and Wastewater Treatment: From Basic Principles to Applications; IWA Publishing: London, UK, 2015. [Google Scholar] [CrossRef]
- Molina, C.B.; Sanz-Santos, E.; Boukhemkhem, A.; Bedia, J.; Belver, C.; Rodriguez, J.J. Removal of emerging pollutants in aqueous phase by heterogeneous Fenton and photo-Fenton with Fe2O3-TiO2-clay heterostructures. Environ. Sci. Pollut. Res. 2020, 27, 38434–38445. [Google Scholar] [CrossRef]
- Du, Z.; Li, K.; Zhou, S.; Liu, X.; Yu, Y.; Zhang, Y.; He, Y.; Zhang, Y. Degradation of ofloxacin with heterogeneous photo-Fenton catalyzed by biogenic Fe-Mn oxides. Chem. Eng. J. 2020, 380, 122427. [Google Scholar] [CrossRef]
- Raizada, P.; Kumari, J.; Shandilya, P.; Dhiman, R.; Singh, V.P.; Singh, P. Magnetically retrievable Bi2WO6/Fe3O4 immobilized on graphene sand composite for investigation of photocatalytic mineralization of oxytetracycline and ampicillin. Process Saf. Environ. Prot. 2017, 106, 104–116. [Google Scholar] [CrossRef]
- Wang, X.; Wang, A.; Lu, M.; Ma, J. Synthesis of magnetically recoverable Fe0/graphene-TiO2 nanowires composite for both reduction and photocatalytic oxidation of metronidazole. Chem. Eng. J. 2018, 337, 372–384. [Google Scholar] [CrossRef]
- Alani, O.A.; Ari, H.A.; Alani, S.O.; Offiong, N.A.O.; Feng, W. Visible-light-driven bio-templated magnetic copper oxide composite for heterogeneous photo-fenton degradation of tetracycline. Water (Switz.) 2021, 13, 1918. [Google Scholar] [CrossRef]
- Santos, M.M.d.; da Silva, T.D.; de Lucena, A.L.A.; Napoleão, D.C.; Duarte, M.M.M.B. Degradation of Ketoprofen, Tenoxicam, and Meloxicam Drugs by Photo-Assisted Peroxidation and Photo-Fenton Processes: Identification of Intermediates and Toxicity Study. Water. Air. Soil Pollut. 2020, 231, 35. [Google Scholar] [CrossRef]
- Soriano-Molina, P.; Plaza-Bolaños, P.; Lorenzo, A.; Agüera, A.; García Sánchez, J.L.; Malato, S.; Sánchez Pérez, J.A. Assessment of solar raceway pond reactors for removal of contaminants of emerging concern by photo-Fenton at circumneutral pH from very different municipal wastewater effluents. Chem. Eng. J. 2019, 366, 141–149. [Google Scholar] [CrossRef]
- Soriano-Molina, P.; Sánchez, J.L.G.; Malato, S.; Plaza-Bolaños, P.; Agüera, A.; Pérez, J.A.S. On the design and operation of solar photo-Fenton open reactors for the removal of contaminants of emerging concern from WWTP effluents at neutral pH. Appl. Catal. B Environ. 2019, 256, 117801. [Google Scholar] [CrossRef]
- Sun, S.; Yao, H.; Fu, W.; Xue, S.; Zhang, W. Enhanced degradation of antibiotics by photo-fenton reactive membrane filtration. J. Hazard. Mater. 2019, 386, 121955. [Google Scholar] [CrossRef] [PubMed]
- Arzate, S.; Campos-Mañas, M.C.; Miralles-Cuevas, S.; Agüera, A.; Sánchez, J.L.G.; Pérez, J.A.S. Removal of contaminants of emerging concern by continuous flow solar photo-Fenton process at neutral pH in open reactors. J. Environ. Manag. 2020, 261, 110265. [Google Scholar] [CrossRef] [PubMed]
- Costa, E.P.; Roccamante, M.; Amorim, C.C.; Oller, I.; Pérez, J.A.S.; Malato, S. New trend on open solar photoreactors to treat micropollutants by photo-Fenton at circumneutral pH: Increasing optical pathway. Chem. Eng. J. 2020, 385, 123982. [Google Scholar] [CrossRef]
- Soriano-Molina, P.; Miralles-Cuevas, S.; García, B.E.; Plaza-Bolaños, P.; Pérez, J.A.S. Two strategies of solar photo-Fenton at neutral pH for the simultaneous disinfection and removal of contaminants of emerging concern. Comparative assessment in raceway pond reactors. Catal. Today 2019, 361, 17–23. [Google Scholar] [CrossRef]
- Mejri, A.; Soriano-Molina, P.; Miralles-Cuevas, S.; Pérez, J.A.S. Fe3+-NTA as iron source for solar photo-Fenton at neutral pH in raceway pond reactors. Sci. Total Environ. 2020, 736, 139617. [Google Scholar] [CrossRef]
- Dong, W.; Jin, Y.; Zhou, K.; Sun, S.P.; Li, Y.; Chen, X.D. Efficient degradation of pharmaceutical micropollutants in water and wastewater by FeIII-NTA-catalyzed neutral photo-Fenton process. Sci. Total Environ. 2019, 688, 513–520. [Google Scholar] [CrossRef]
- Gonçalves, N.P.F.; Minella, M.; Fabbri, D.; Calza, P.; Malitesta, C.; Mazzotta, E.; Prevot, A.B. Humic acid coated magnetic particles as highly efficient heterogeneous photo-Fenton materials for wastewater treatments. Chem. Eng. J. 2020, 390, 124619. [Google Scholar] [CrossRef]
- Fiorentino, A.; Esteban, B.; Garrido-Cardenas, J.A.; Kowalska, K.; Rizzo, L.; Aguera, A.; Pérez, J.A.S. Effect of solar photo-Fenton process in raceway pond reactors at neutral pH on antibiotic resistance determinants in secondary treated urban wastewater. J. Hazard. Mater. 2019, 378, 120737. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Zhou, C.; Li, Y.; Zhang, M.; Tao, P.; Liu, Q.; Cui, W. Flower-like FeOOH hybridized with carbon quantum dots for efficient photo-Fenton degradation of organic pollutants. Appl. Surf. Sci. 2020, 540, 148362. [Google Scholar] [CrossRef]
- Yu, X.; Yin, H.; Ye, J.S.; Peng, H.; Lu, G.; Dang, Z. Degradation of tris-(2-chloroisopropyl) phosphate via UV/TiO2 photocatalysis: Kinetic, pathway, and security risk assessment of degradation intermediates using proteomic analyses. Chem. Eng. J. 2019, 374, 263–273. [Google Scholar] [CrossRef]
- Zelinski, D.W.; Santos, T.P.M.d.; Takashina, T.A.; Leifeld, V.; Igarashi-Mafra, L. Photocatalytic Degradation of Emerging Contaminants: Artificial Sweeteners. Water. Air. Soil Pollut. 2018, 229, 207. [Google Scholar] [CrossRef]
- Ikhlef-Taguelmimt, T.; Hamiche, A.; Yahiaoui, I.; Bendellali, T.; Lebik-Elhadi, H.; Ait-Amar, H.; Aissani-Benissad, F. Tetracycline hydrochloride degradation by heterogeneous photocatalysis using TiO2(P25) immobilized in biopolymer (Chitosan) under UV irradiation. Water Sci. Technol. 2020, 82, 1570–1578. [Google Scholar] [CrossRef]
- Aissani, T.; Yahiaoui, I.; Boudrahem, F.; Chikh, S.A.; Aissani-Benissad, F.; Amrane, A. The combination of photocatalysis process (UV/TiO2(P25) and UV/ZnO) with activated sludge culture for the degradation of sulfamethazine. Sep. Sci. Technol. 2018, 53, 1423–1433. [Google Scholar] [CrossRef]
- Moreira, N.F.F.; Sampaio, M.J.; Ribeiro, A.R.; Silva, C.G.; Faria, J.L.; Silva, A.M.T. Metal-free g-C3N4 photocatalysis of organic micropollutants in urban wastewater under visible light. Appl. Catal. B Environ. 2019, 248, 184–192. [Google Scholar] [CrossRef]
- Wei, X.N.; Wang, H.L.; Wang, X.K.; Jiang, W.F. Facile fabrication of mesoporous g-C 3 N 4/TiO 2 photocatalyst for efficient degradation of DNBP under visible light irradiation. Appl. Surf. Sci. 2017, 426, 1271–1280. [Google Scholar] [CrossRef]
- Lin, L.; Wang, H.; Xu, P. Immobilized TiO2-reduced graphene oxide nanocomposites on optical fibers as high performance photocatalysts for degradation of pharmaceuticals. Chem. Eng. J. 2017, 310, 389–398. [Google Scholar] [CrossRef]
- Davididou, K.; McRitchie, C.; Antonopoulou, M.; Konstantinou, I.; Chatzisymeon, E. Photocatalytic degradation of saccharin under UV-LED and blacklight irradiation. J. Chem. Technol. Biotechnol. 2018, 93, 269–276. [Google Scholar] [CrossRef]
- Chauhan, P.S.; Kant, R.; Rai, A.; Gupta, A.; Bhattacharya, S. Facile synthesis of ZnO/GO nanoflowers over Si substrate for improved photocatalytic decolorization of MB dye and industrial wastewater under solar irradiation. Mater. Sci. Semicond. Process. 2019, 89, 6–17. [Google Scholar] [CrossRef]
- Shandilya, P.; Mittal, D.; Soni, M.; Raizada, P.; Hosseini-Bandegharaei, A.; Saini, A.S.; Singh, P. Fabrication of fluorine doped graphene and SmVO4 based dispersed and adsorptive photocatalyst for abatement of phenolic compounds from water and bacterial disinfection. J. Clean. Prod. 2018, 203, 386–399. [Google Scholar] [CrossRef]
- Shao, H.; Zhao, X.; Wang, Y.; Mao, R.; Wang, Y.; Qiao, M.; Zhao, Y.; Zhu, Y. Synergetic activation of peroxymonosulfate by Co3O4 modified g-C3N4 for enhanced degradation of diclofenac sodium under visible light irradiation. Appl. Catal. B Environ. 2017, 218, 810–818. [Google Scholar] [CrossRef]
- Anwer, H.; Park, J.W. Synthesis and characterization of a heterojunction rGO/ZrO2/Ag3PO4 nanocomposite for degradation of organic contaminants. J. Hazard. Mater. 2018, 358, 416–426. [Google Scholar] [CrossRef]
- Kumar, A.; Khan, M.; Zeng, X.; Lo, I.M.C. Development of g-C3N4/TiO2/Fe3O4@SiO2 heterojunction via sol-gel route: A magnetically recyclable direct contact Z-scheme nanophotocatalyst for enhanced photocatalytic removal of ibuprofen from real sewage effluent under visible light. Chem. Eng. J. 2018, 353, 645–656. [Google Scholar] [CrossRef]
- Xu, X.; Liu, S.; Cui, Y.; Wang, X.; Smith, K.; Wang, Y. Solar-Driven Removal of 1,4-Dioxane Using WO3/nγ-Al2O3 Nano-catalyst in Water. Catalysts 2019, 9, 389. [Google Scholar] [CrossRef]
- Cristino, V.; Longobucco, G.; Marchetti, N.; Caramori, S.; Bignozzi, C.A.; Martucci, A.; Molinari, A.; Boaretto, R.; Stevanin, C.; Argazzi, R.; et al. Photoelectrochemical degradation of pharmaceuticals at β25 modified WO3 interfaces. Catal. Today 2018, 340, 302–310. [Google Scholar] [CrossRef]
- Ghenaatgar, A.; Tehrani, R.M.A.; Khadir, A. Photocatalytic degradation and mineralization of dexamethasone using WO3 and ZrO2 nanoparticles: Optimization of operational parameters and kinetic studies. J. Water Process Eng. 2019, 32, 100969. [Google Scholar] [CrossRef]
- Alvarez-Corena, J.R.; Bergendahl, J.A.; Hart, F.L. Advanced oxidation of five contaminants in water by UV/TiO2: Reaction kinetics and byproducts identification. J. Environ. Manag. 2016, 181, 544–551. [Google Scholar] [CrossRef]
- Juang, R.S.; Yei, Y.C.; Liao, C.S.; Lin, K.S.; Lu, H.S.; Wang, S.F.; Sun, A.C. Synthesis of magnetic Fe3O4/activated carbon nanocomposites with high surface area as recoverable adsorbents. J. Taiwan Inst. Chem. Eng. 2018, 90, 51–60. [Google Scholar] [CrossRef]
- Rasheed, T.; Bilal, M.; Nabeel, F.; Adeel, M.; Iqbal, M.N. Environmentally-related contaminants of high concern: Potential sources and analytical modalities for detection, quantification, and treatment. Environ. Int. 2018, 122, 52–66. [Google Scholar] [CrossRef] [PubMed]
- Chon, K.; Salhi, E.; von Gunten, U. Combination of UV absorbance and electron donating capacity to assess degradation of micropollutants and formation of bromate during ozonation of wastewater effluents. Water Res. 2015, 81, 388–397. [Google Scholar] [CrossRef]
- Pisarenko, A.N.; Stanford, B.D.; Yan, D.; Gerrity, D.; Snyder, S.A. Effects of ozone and ozone/peroxide on trace organic contaminants and NDMA in drinking water and water reuse applications. Water Res. 2012, 46, 316–326. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; von Gunten, U. Advances in predicting organic contaminant abatement during ozonation of municipal wastewater effluent: Reaction kinetics, transformation products, and changes of biological effects. Environ. Sci. Water Res. Technol. 2016, 2, 421–442. [Google Scholar] [CrossRef]
- Zhang, Z.; Zeng, K.; Liu, J. Immunochemical detection of emerging organic contaminants in environmental waters. TrAC Trends Anal. Chem. 2017, 87, 49–57. [Google Scholar] [CrossRef]
- Sgroi, M.; Anumol, T.; Roccaro, P.; Vagliasindi, F.G.A.; Snyder, S.A. Modeling emerging contaminants breakthrough in packed bed adsorption columns by UV absorbance and fluorescing components of dissolved organic matter. Water Res. 2018, 145, 667–677. [Google Scholar] [CrossRef]
- Sierra, M.M.D.; Giovanela, M.; Parlanti, E.; Soriano-Sierra, E.J. Fluorescence fingerprint of fulvic and humic acids from varied origins as viewed by single-scan and excitation/emission matrix techniques. Chemosphere 2005, 58, 715–733. [Google Scholar] [CrossRef]
- Yu, H.W.; Park, M.; Wu, S.; Lopez, I.J.; Ji, W.; Scheideler, J.; Synder, S.A. Strategies for selecting indicator compounds to assess attenuation of emerging contaminants during UV advanced oxidation processes. Water Res. 2019, 166, 115030. [Google Scholar] [CrossRef]
- Quintelas, C.; Mesquita, D.P.; Ferreira, E.C.; Amaral, A.L. Quantification of pharmaceutical compounds in wastewater samples by near infrared spectroscopy (NIR). Talanta 2018, 194, 507–513. [Google Scholar] [CrossRef]
- Wang, T.; Wang, S.; Cheng, Z.; Wei, J.; Yang, L.; Zhong, Z.; Hu, H.; Wang, Y.; Zhou, B.; Li, P. Emerging core–shell nanostructures for surface-enhanced Raman scattering (SERS) detection of pesticide residues. Chem. Eng. J. 2021, 424, 130323. [Google Scholar] [CrossRef]
- Fang, Q.; Li, Y.; Miao, X.; Zhang, Y.; Yan, J.; Yu, T.; Liu, J. Sensitive detection of antibiotics using aptamer conformation cooperated enzyme-assisted SERS technology. Analyst 2019, 144, 3649–3658. [Google Scholar] [CrossRef]
- Cennamo, N.; D’Agostino, G.; Sequira, F.; Mattiello, F.; Porto, G.; Biasiolo, A.; Nogueira, R.; Bilro, L.; Zeni, L. A simple and low-cost optical fiber intensity-based configuration for perfluorinated compounds in water solution. Sensors (Switz.) 2018, 18, 3009. [Google Scholar] [CrossRef]
- Cennamo, N.; D’Agostino, G.; Porto, G.; Biasiolo, A.; Perri, C.; Arcadio, F.; Zeni, L. A molecularly imprinted polymer on a plasmonic plastic optical fiber to detect perfluorinated compounds in water. Sensors (Switz.) 2018, 18, 1836. [Google Scholar] [CrossRef]
- Tagg, A.S.; Sapp, M.; Harrison, J.P.; Ojeda, J.J. Identification and Quantification of Microplastics in Wastewater Using Focal Plane Array-Based Reflectance Micro-FT-IR Imaging. Anal. Chem. 2015, 87, 6032–6040. [Google Scholar] [CrossRef] [PubMed]
- Li, W.T.; Jin, J.; Li, Q.; Wu, C.F.; Lu, H.; Zhou, Q.; Li, A.M. Developing LED UV fluorescence sensors for online monitoring DOM and predicting DBPs formation potential during water treatment. Water Res. 2016, 93, 1–9. [Google Scholar] [CrossRef]
- Jaria, G.; Calisto, V.; Otero, M.; Esteves, V.I. Monitoring pharmaceuticals in the aquatic environment using enzyme-linked immunosorbent assay (ELISA)—A practical overview. Anal. Bioanal. Chem. 2020, 412, 3983–4008. [Google Scholar] [CrossRef]
- Vacher, M.; Fdez. Galván, I.; Ding, B.W.; Schramm, S.; Berraud-Pache, R.; Naumov, P.; Ferre, N.; Liu, Y.J.; Navizet, I.; Roca-Sanjuan, D.; et al. Chemi- and Bioluminescence of Cyclic Peroxides. Chem. Rev. 2018, 118, 6927–6974. [Google Scholar] [CrossRef]
- Lodeiro, P.; Achterberg, E.P.; El-Shahawi, M.S. Detection of silver nanoparticles in seawater at ppb levels using UV–visible spectrophotometry with long path cells. Talanta 2017, 164, 257–260. [Google Scholar] [CrossRef]
- Cai, Q.; Lee, B.C.Y.; Ong, S.L.; Hu, J. Application of a Multiobjective Artificial Neural Network (ANN) in Industrial Reverse Osmosis Concentrate Treatment with a Fluidized Bed Fenton Process: Performance Prediction and Process Optimization. ACS ES&T Water 2021, 1, 847–858. [Google Scholar] [CrossRef]
- Shen, W.; Huang, F.; Zhang, X.; Zhu, Y.; Chen, X.; Akbarjon, N. On-line chemical oxygen demand estimation models for. Water Sci. Technol. 2018, 78, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Bolton, J.R.; Bircher, K.G.; Tumas, W.; Tolman, C.A. Figures-of-merit for the technical development and application of advanced oxidation technologies for both electric- and solar-driven systems. Pure Appl. Chem. 2001, 73, 627–637. [Google Scholar] [CrossRef]
- Jankunaite, D.; Tichonovas, M. Removal of Diclofenac, Ketoprofen, and Carbamazepine from Simulated Drinking Water by Advanced Oxidation in a Model Reactor. Water Air Soil Pollut. 2017, 228, 353. [Google Scholar] [CrossRef]
- Guo, K.; Wu, Z.; Yan, S.; Yao, B.; Song, W.; Hua, Z.; Zhang, X.; Kong, X.; Li, X.; Fang, J. Comparison of the UV/chlorine and UV/H2O2 processes in the degradation of PPCPs in simulated drinking water and wastewater: Kinetics, radical mechanism and energy requirements. Water Res. 2018, 147, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Wardenier, N.; Liu, Z.; Nikiforov, A.; van Hulle, S.W.H.; Leys, C. Micropollutant elimination by O3, UV and plasma-based AOPs: An evaluation of treatment and energy costs. Chemosphere 2019, 234, 715–724. [Google Scholar] [CrossRef]
- Sgroi, M.; Snyder, S.A.; Roccaro, P. Comparison of AOPs at pilot scale: Energy costs for micro-pollutants oxidation, disinfection by-products formation and pathogens inactivation. Chemosphere 2020, 273, 128527. [Google Scholar] [CrossRef]
- Expósito, A.J.; Patterson, D.A.; Monteagudo, J.M.; Durán, A. Sono-photo-degradation of carbamazepine in a thin falling film reactor: Operation costs in pilot plant. Ultrason. Sonochem. 2017, 34, 496–503. [Google Scholar] [CrossRef]
- Miklos, D.B.; Remy, C.; Jekel, M.; Linden, K.G.; Drewes, J.E.; Hübner, U. Evaluation of advanced oxidation processes for water and wastewater treatment—A critical review. Water Res. 2018, 139, 118–131. [Google Scholar] [CrossRef] [PubMed]
- Ahn, Y.; Lee, D.; Kwon, M.; Choi, I.h.; Nam, S.N.; Kang, J.W. Characteristics and fate of natural organic matter during UV oxidation processes. Chemosphere 2017, 184, 960–968. [Google Scholar] [CrossRef]
- Tang, W.Z.; An, H. Photocatalytic degradation kinetics and mechanism of acid blue 40 by TiO2/UV in aqueous solution. Chemosphere 1995, 31, 4171–4183. [Google Scholar] [CrossRef]
- Scaria, J.; Gopinath, A.; Nidheesh, P.V. A versatile strategy to eliminate emerging contaminants from the aqueous environment: Heterogeneous Fenton process. J. Clean. Prod. 2020, 278, 124014. [Google Scholar] [CrossRef]
- Ortega-Liébana, M.C.; Sánchez-López, E.; Hidalgo-Carrillo, J.; Marinas, A.; Marinas, J.M.; Urbano, F.J. A comparative study of photocatalytic degradation of 3-chloropyridine under UV and solar light by homogeneous (photo-Fenton) and heterogeneous (TiO 2) photocatalysis. Appl. Catal. B Environ. 2012, 127, 316–322. [Google Scholar] [CrossRef]


| Category of Contaminant | Chemical Structure within the Contaminant | Name of Contaminant | Uses in Industry | Concentration Detected | References |
|---|---|---|---|---|---|
| Pharmaceuticals | Azetidine, Benzene | Amoxicillin | Antibiotics | Queensland: 6.9 µg/L Delhi: Up to 172.6 ng/L Ghana: Up to 0.0027 ng/L | [8,9,10] |
| Halogenic-Benzene | Diclofenac | Anti-inflammatory drug | Algiers: 85.2 ± 9.3 ng/L Saudi Arabian coastal waters: 10,221 ng/L Lahore: 260–470 ng/L WWTP effleunt from South Africa: 5.56–243.6 ng/L | [11,12,13] | |
| Benzene | Ibuprofen | Painkiller | Madrid: 4.1 ng/L Algiers: 372.8 ± 19.8 ng/L Saudi Arabian coastal waters: 127–660 ng/L Lahore: 1728–2300 ng/L Sea water Durban, South Africa: <0.17 ng/L | [11,12,13,14,15] | |
| Benzene, Piperidine | Acetaminophen (ACE) | Painkiller | Saudi Arabian coastal waters: 1234 and 2346 ng/L Lahore: 12,120–13,880 ng/L | [11,12] | |
| Benzene, Pyrazine | Sulfamethoxazole (SMX) | Antibiotics | Mekong Delta: 21 ng/L Jiangsu Province: 63.6 ng/L Madrid: 162–530 ng/L Ghana: 0.013–2.861 ng/L | [10,14,16,17] | |
| Benzene, 7-member ring | Carbamazepine | Anticonvulsant | Various plants in the USA: 2–207 ng/L Spain treatment plants: <54 ng/L Hartbeespoort Dam catchment and the uMngeni River estuary: up to 94 ng/L | [18,19,20] | |
| Food additive | Oxadiazine | Acesulfame potassium (ACE-K): | Artificial sweetener in food and beverages | Jiangsu: 2.9 μg/L to 0.20 mg/L German Elbe river: 100 to 900 mg/s (mass load) | [21,22] |
| Benzothiazole | Sucralose | Jiangsu: up to 3.6 μg/L. | [21] | ||
| Pesticides | Triazine | Atrazine | Herbicide | Jiaozhou Bay: 76 ng/L Ctalamochita river basin: 0.23 to 0.26 ng/L (urban), 0.28 to 3 ng/L (rural) Hartbeespoort Dam catchment (South Africa): up to 1570 ng/L | [23,24,25] |
| Industrial chemicals | Dioxane | 1,4-Dioxane | Organic solvents | Sant Joan Despí: 4360 (average), 32,370 (max) Oder River:143–2245 ng/L Rhine/Main River:110–850 ng/L | [26,27] |
| Pyrazole, 6 membered heterocyclic ring | Caffeine | Food and beverage industry | Saudi Arabian coastal waters: 7708 ng/L Various plants in the USA: 7–687 ng/L Madrid: 5010–65 625 ng/L WWTP effluent from South Africa: 85.76–4878 ng/L | [12,14,18] | |
| - | Perfluorooctane sulfonate (PFOS) and Perfluorooctanoic acid (PFOA) | Industrial manufacturing and use and disposal of PFAS-containing products, | Worldwide: 0.2–1630.2 ng/L Singapore: 532–1060 ng/L (WWTP treated effluent) WWTP effluent from Kampala, Uganda: PFOS (1.3–1.5 ng/L) and PFOA (1.5–2.4 ng/L) | [28,29,30] | |
| - | N-Nitroso-dimethylamine (NDMA) | A by-product of industrial processes that use nitrates and/or nitrites. | Various plants in the USA: 12–321 ng/L | [18] | |
| Phenols | Bisphenol A (BPA) | Plastic formation | Yamuna/Cooum River: 1420–14,800 ng/L Zhujiang/Dongjiang River: 101–2310 ng/L Zhujiang/Dongjiang WWTP: 29,400 ng/L Riyadhm Saudi Arabia/Drinking water: 291–41,190 ng/L | [31,32,33] | |
| Personal care products (PCPs) | Benzene | Diethyltoluamide (DEET) | Insect repellent | Arizona: 1570–15,200 ng/L | [34] |
| Benzene, 5- member cycloalkane | Galaxolide | Synthetic musk | Madrid: <24 971 ng/L Lubbock: 3789–10,525 ng/L | [14,35] | |
| Disinfection by-products (DBPs) | Dihalobenzoquinones | 2,6-dichloro-1,4-benzoquinone | Disinfection by-product from water treatment | Canada WWTP: 165.1 ng/L China Drinking WTP: 2.6–19.70 ng/L | [36,37] |
| Iodotrihalomethanes | Dibromoiodomethane (DBIM), Chlorodiiodomethane (CDIM), Bromodiiodomethane (BDIM), Iodoform (TIM) | Disinfection by-product from water treatment | China Drinking WTPs: 0.007–0.23 ng/L Australia Advanced water recycling plant: <1–7 ng/L | [38,39] |
| Name of AOP | Type | Mechanism | References |
|---|---|---|---|
| UV/Oxidant | UV-H2O2 | [51] | |
| UV-persulfate | [52] | ||
| UV-chlorine | (pKa = 7.5 at 25 °C) | [53] | |
| UV/Ozone | Microbubble | + 3 | [54] |
| Photo-Fenton | Homogenous | [55] | |
| Heterogenous | [55,56] | ||
| Photocatalysis | TiO2 | [57,58] | |
| ZnO | . | [59] |
| Factors that Affect the Process | Effects on Process | References |
|---|---|---|
| Low UV-absorbance | UV/Ozone:
| [65,69] |
| Low pH | UV/Ozone:
| [63,64,65] |
| Water matrix: Presence of natural organic matter (NOM) | UV/Ozone:
| [60,62,70,71,72,73] |
| Water matrix: Presence of O2 | Photocatalysis:
| [59,74,75,76,77] |
| Excess oxidant/catalyst dosage | UV/Ozone:
| [59,78,79,80] |
| Type of irradiationof light used | Photo-Fenton:
| [66,67,68] |
| Category | Wastewater/ Compounds | Operation Condition | Removal Efficiency | References |
|---|---|---|---|---|
| UV/peroxide | Wastewater treatment plant effluent |
| The initial concentration of contaminant: ~400 ng/L DOC: 70% DON: 20–30% NDMA: ~80% | [81] |
| Wastewater treatment plant effluent |
| Initial concentration of contaminant: 150–300 ng/L and 10–20 μg/L of bisphenol A (BPA). perfluorooctanoic acid (PFOA): <30% perfluorooctane sulfonate (PFOS): <30% N-nitrosodimethylamine (NDMA): ~60% Diclofenac: ~90% (BPA): ~60% 2-Methylisoborneol (MIB): ~80% Geosmin: ~90% 17 beta-estradiol (E2): ~70% | [82] | |
| Wastewater treatment plant effluent doped CECS |
| Initial concentration of contaminant: 1 μM Nonylphenol ethoxylated: 99% removal | [83] | |
| Synthetic wastewater, simulating pharmaceuticals discharges |
| Initial concentration of contaminant: 1 μM Triclosan: ~80% (both systems) Diclofenac: ~80% (both systems) | [84] | |
| RO treatment plant effluent |
| Initial concentration of contaminant: 1 μM Ibuprofen (IBP): ~80% Triclosan (TCS): ~80% Estrone (E1): ~80% Diclofenac: ~80% (mostly contributed by UV) Bisphenol A (BPA): ~60% | [85] | |
| Sulfolane containing wastewater |
| Initial concentration of contaminant: 200 µg/L Sulfolane removal: 36–89% | [86] | |
| UV/peroxide and UV/chlorine | Wastewater treatment plant effluent |
| 4t-octylphenol: 65% (UV/Cl), 0% (UV/H2O2) bisphenol A (BPA), 4-nonylphenols, TCPP: ~90% (UV/C, UV/H2O2) Diclofenac: ~90% (UV/C, UV/H2O2) DEET, HHCB: ~40% (UV/C, UV/H2O2) | [87] |
| Wastewater treatment plant effluent |
| Initial concentration of contaminant: 1:1 dilution with real wastewater 200 μg/L Sulfamethoxazole: ~90% ((UV/Cl):3 min, (UV/H2O2): 6 min) Imidacloprid: ~90% (UV/Cl):8.5 min, (UV/H2O2): 12 min Carbamazepine: ~90% (UV/Cl): 6 min, (UV/H2O2): 25 min Diclofenac: ~90% ((UV/Cl): 3 min, (UV/H2O2): 6 min Blended CEC matrix: 82% (UV/Cl) and 87% (UV/H2O2) (60 min) | [88] | |
| UV/persulfate and UV/peroxide | Synthetic wastewater, simulating pharmaceuticals discharges |
| Initial concentration of contaminant: 2.2, 3.0, 5.2, and 5.5 µM (LP, FRSM, CAF, and CBZ) losartan potassium (LP): 85% ((UV/S2O82−): 290 mJ cm−2 (UV/H2O2): 620 mJ cm−2) furosemide (FRSM): 85% ((UV/S2O82−): 290 mJ cm−2 (UV/H2O2): 620 mJ cm−2) caffeine (CAF): 85% ((UV/S2O82−): 290 mJ cm−2 (UV/H2O2): 620 mJ cm−2) carbendazim (CBZ): 85% ((UV/S2O82−): 290 mJ cm−2 (UV/H2O2): 620 mJ cm−2) | [89] |
| Wastewater treatment plant effluent spiked with synthetic compounds |
| Initial concentration of contaminant: 500 µg/L Carbamazepine (CBZ): >90% Crotamiton (CRMT): >90% N,N-diethyl-meta-toluamide (DEET): >90% (80% UV/S2O82−) Gemfibrozil (GEM): >90% Ibuprofen (IBP): >90% Trimethoprim (TMP): >90% TOC removal: 31.8% (UV/HSO5−) and 33.7% (UV/S2O82−) | [90] | |
| UV/persulfate | Synthetic wastewater, simulating pharmaceuticals discharges |
| Initial concentration of contaminant: 32.8 μM Methyl paraben: 98.9% removal | [91] |
| Synthetic wastewater, simulating pharmaceuticals discharges |
| Initial concentration of contaminant: 31 μM Chloramphenicol: 100% removal | [92] | |
| Synthetic wastewater, simulating pharmaceuticals discharges |
| Initial concentration of contaminant: 2 μM Haloacetonitriles: 99.8% removal | [93] | |
| Synthetic wastewater, simulating pharmaceuticals discharges |
| Initial concentration of contaminant: 23.69 μM Sulfamethoxazole: 97% removal | [94] | |
| Synthetic wastewater, simulating pharmaceuticals discharges |
| Initial concentration of contaminant: 3.43 μM Lindane: ~90% removal | [95] | |
| Wastewater treatment plant effluent and surface water spiked with synthetic compounds |
| Initial concentration of contaminant: 1 μM each 1H-benzotriazole (BZ): ~0% (secondary effluent), ~80% (surface water) N,N-diethyl-m-toluamide (DEET): ~20% (secondary effluent), ~70% (surface water) Chlorophene (CP): ~90% (secondary effluent), ~90% (surface water) 3-methylindole (ML): ~90% (secondary effluent), ~90% (surface water) Nortriptyline hydrochloride (NH): ~90% (secondary effluent), ~90% (surface water) | [96] | |
| GENx |
| Initial concentration of contaminant: 1 mg/L Perfluorooctanoic acid (PFOA): <90% (UV/sulfite), ~10% (UV/S2O82−) Hexafluoropropylene oxide dimer acid (GenX): <95% (UV/sulfite), ~35% (UV/S2O82−) | [97] | |
| UV/nitrate | Wastewater treatment plant effluent doped with CECS |
| Initial concentration of contaminant: 100 μg/L Carbamazepine (CBZ): 30% (sunlight), ~70% (UVC) Diclofenac (DCF): >90% (sunlight), >90% (UVC: 2 kJ L−1) Sulfamethoxazole (SMX): >90% (sunlight), ~100% (with UVC alone) | [98] |
| Generic CECs |
| Initial concentration of contaminant: 2 μM Bisphenol A (BPA): ~80% Carbamazepine (CBZ): ~60% | [99] |
| Wastewater/ Compounds | Operation Condition | Removal Efficiency | References |
|---|---|---|---|
| Synthetic wastewater |
| Initial concentration of caffeine: 300 mg/L Color: 99.1% removal Caffeine degradation: 96.5% removal | [105] |
| Synthetic wastewater with sucralose |
| Initial concentration of sucralose: 50 mg/L Degradation: 100% (after 30 min) Mineralization: 89.8% (after 2 h) | [103] |
| Real wastewater containing atrazine |
| Initial concentration of atrazine: 0.0232 mM Initial COD: 0.447 M Initial NH3 – N: 1.44 mM Initial Cl-: 5.56 M Atrazine degradation: 95% (after 180 min) COD: 55% removal NH3-N: 65% removal | [102] |
| Groundwater/surface water/secondary effluent spiked with CECs |
| Initial concentration of micropollutants: ∼150 μg/L each Groundwater: 76–~100% removal Surface water: 84–100% removal Secondary effluent: 89–97% removal | [106] |
| Synthetic wastewater, mixing waste firefighting foam |
| Initial concentration of PFAS: 3–10 μg/L (benchtop) 3.15 μg/L (pilot plant) Benchtop: 73% removal Pilot plant: 73% removal | [107] |
| Synthetic wastewater with P-nitroaniline and coal washing plant wastewater |
| Initial concentration of nitroaniline: 10–25 mg/L PNA: 81% removal TOC: 81% removal | [108] |
| Synthetic wastewater |
| Initial concentration: Diethyl dithiocarbonate collector = 100 mg/L Degradation: 99.55% removal TOC: 34% removal | [109] |
| Category | Wastewater/ Compounds | Operation Condition | Removal Efficiency | References |
|---|---|---|---|---|
| Heterogenous | Pharmaceutical wastewater |
| Initial concentration of contaminant: 5 mg/L ACT removal: ~90% ACE removal: ~90% | [111] |
| Synthetic water containing ofloxacin (OFL) |
| Initial concentration of OFL: 30 mg/L Ofloxacin (OFL) removal: ~90% (UV) | [112] | |
| Synthetic wastewater containing tetracycline (TC) |
| Initial concentration of TC: 10 mg/L TC: 96.71% removal | [78] | |
| Synthetic wastewater containing amoxicillin (AMX) |
| Initial concentration of AMX: 0.25 mMAMX: 89% removal TOC: 60% removal | [61] | |
| Synthetic wastewater containing oxytetracycline (OTC) and ampicillin (AMP) |
| Initial concentration of OTC and AMP: 0.1 mM each OTC: 73% removal (after 1 h) COD: 62% removal (after 10 h) AMP: 73% removal (after 1 h) COD: 60% removal (after 10 h) | [113] | |
| Synthetic wastewater containing metronidazole (MNZ) |
| Initial concentration of metronidazole: 35 mg/L MNZ: 71.98% removal | [114] | |
| Synthetic wastewater containing tetracycline (TC) |
| Initial concentration of TC: 50 mg/L TC: ~90% removal | [115] | |
| Homogenous | Synthetic wastewater containing an anti-inflammatory substance |
| Initial concentration of contaminant: 10 mg/L (Ketoprofen, tenoxicam, and meloxicam drugs) COD removal: 98% removal | [116] |
| Real wastewater |
| Initial concentration of contaminant: 5000–50,000 ng/L (total CECs) COD removal: <80% removal | [117] | |
| Real wastewater |
| Initial concentration of contaminant: na Total EC removal: >80% | [118] | |
| Pharmaceuticals wastewater |
| Initial concentration of contaminant: 12 mg/L (SDZ), 5.8 mg/L (TOC) sulfadiazine (SDZ) removal: ~100%(5 min), ~80%(60 min) | [119] | |
| Real wastewater |
| Initial concentration of CECs: 59.1–77.7 ng/L Total removal: ~61% (20 min) | [120] | |
| Real wastewater |
| Initial concentration of contaminant: 1 ppm Total removal (DI): 89–94% (AMX), 92–95% (PC) Total removal (WW): ~50% (AMX), ~30% (PC) | [70] | |
| Real wastewater |
| Initial concentration of contaminant: 100 µg/L Caffeine removal: ~90% Carbamazepine removal: ~90% Diclofenac removal: ~90% Sulfamethoxazole removal: ~90% Trimethoprim removal: ~90% | [121] | |
| Real wastewater doped with SMX |
| Initial concentration of contaminant: 50 µg/L Sulfamethoxazole removal: ~80% (UV), ~40% (solar) Wild E. coli inactivation: below the detection limit | [122] | |
| Real wastewater doped with SMX and IMD |
| Initial concentration of contaminant: 50 µg/L Sulfamethoxazole removal: ~90% (UV) Imidacloprid removal: ~80% (UV) | [123] | |
| PPCPs removal in wastewater effluent |
| Initial concentration of contaminant: 452.6 (CBZ), 394.6 (CRMT), and 101.1 (IBP) μg/L CBZ removal: ~80% Crotamiton (CRMT): ~80% Ibuprofen (IBP): ~80% | [124] | |
| PPCPs removal in wastewater effluent |
| Initial concentration of contaminant: 0.2, 0.1 mM carbamazepine, 20 μmol (blended) Ibuprofen removal: ~30% Bisphenol A removal: ~80% tolylbenzotriazole removal: ~90% Carbamazepine removal: ~80% Blended mix: ~90% (in DI water) | [125] | |
| PPCPs removal in wastewater effluent |
| Initial concentration of contaminant: as per wastewater stream Blended: 73–82% Azithromycin (AZT) removal: 24% Ciprofloxacin (CIP) removal: 100% Clarithromycin (CLR) removal: 8–24% Clindamycin: 57–86% Enrofloxacin (ERF) removal: 100% Erythromycin (ENR) removal: 22–36% levofloxacin (LEV) removal: 61–75% Lincomycin (LIN) removal: 84% Metronidazole (MET) removal: 70% | [126] |
| Categories | Wastewater/ Compounds | Operation Condition | Removal Efficiency | References |
|---|---|---|---|---|
| Titanium oxide-based catalyst | Synthetic wastewater containing tris-(2-chloroisopropyl) phosphate (TCPP) |
| Initial concentration of contaminant: 1 mg/L TCPP: ~100% removal TOC: ~80% removal | [128] |
| Synthetic wastewater with acesulfame potassium (ACE-K) and sodium saccharin (SAC) |
| Initial concentration of contaminant: 20 mg/L each Degradation: Both: 100% removal Mineralization: ACE-K: 57% removal SAC: 49% removal | [129] | |
| Synthetic wastewater containing tetracycline hydrochloride (TC) |
| Initial concentration of contaminant: 40 mg/L TC: 96% removal | [130] | |
| Synthetic wastewater containing sulfamethazine (SMT) |
| Initial concentration of contaminant: 50 mg/L SMT: 42% removal COD: 34% removal | [131] | |
| Secondary treatment effluent |
| Initial concentration of contaminants: varied Contaminants: 25–90% removal | [132] | |
| Wastewater effluent or synthetic wastewater containing Bisphenol A |
| Initial concentration of BPA: 10 mg/L Synthetic wastewater: With UV/TiO2 (P25): 100% removal With UV/TiO2-WO3: 30% removal With UV/TiO2: 38% removal Real wastewater: With UV/TiO2 (P25): 62% removal With UV/TiO2-WO3: 2% removal | [57] | |
| Synthetic wastewater with dinitro butyl-phenol (DNBP) (herbicide) |
| Initial concentration of DNBP: 20 mg/L DNBP: 28% removal | [133] | |
| Synthetic wastewater containing carbamazepine (CBZ), ibuprofen (IBU,) and sulfamethoxazole (SMX) |
| Initial concentration of pharmaceuticals: 5 mg/L each IBU: 38% removal CBZ: 38% removal SMX: 64% removal | [134] | |
| Synthetic wastewater containing metronidazole (MNZ) |
| Initial concentration of MNZ: 35 mg/L MNZ: 43.02% removal | [114] | |
| Synthetic wastewater containing saccharin (SAC) |
| Initial concentration of saccharin: 5 mg/L SAC: 100% removal (after 30 min) | [135] | |
| Zinc based catalyst | Synthetic wastewater containing sulfamethazine (SMT) |
| Initial concentration of contaminant: 50 mg/L SMT: 64% removal COD: 45% removal | [131] |
| Synthetic wastewater |
| Initial concentration of contaminants: 0.3 mg/L Synthetic wastewater: Contaminants: 97.5–99.7% removal (after 90 min) Real wastewater: Contaminants: 76.0–100.0% removal (after 240 min) | [59] | |
| Synthetic wastewater with sulfathiazole (STZ) |
| Initial concentration of STZ: 0.1 M STZ: 69% removal | [66] | |
| Synthetic wastewater containing methylene blue (MB) and real industrial dye |
| Initial concentration of MB: 20 mg/L MB: ~99% removal (after 120 min) | [136] | |
| Carbon-based catalyst | Synthetic wastewater containing phenol and dinitrophenol |
| Initial concentration of phenol and DNP: 0.1 mM each Phenol: 95% removal DNP: 88% removal | [137] |
| Synthetic wastewater containing amoxicillin (AMX) |
| Initial concentration of AMX: 0.25 mM AMX: 67% removal TOC: 42% removal | [61] | |
| Synthetic wastewater with diclofenac sodium (DCF) |
| Initial concentration of DCF: 10 mg/L DCF: 100% removal (30 min in presence of 0.1 mM PMS) | [138] | |
| Synthetic wastewater containing carbamazepine, ibuprofen, and sulfamethoxazole |
| Initial concentration of pharmaceuticals: 5 mg/L each IBU: 81% removal IBU-TOC: 52% removal CBZ: 54% removal CBZ-TOC = 54% removal SMX: 92% removal SMX-TOC = 59% removal | [134] | |
| Synthetic wastewater containing phenol and dinitrophenol (DNP) |
| Initial concentration of phenol and DNP: 0.1 mM each Phenol: 36% removal DNP: 25% removal | [137] | |
| Synthetic wastewater containing 4-nitrophenol (PNP) |
| Initial concentration of PNP: 15 mg/L PNP: 97% removal | [139] | |
| Other types of catalyst | Synthetic wastewater and treated sewage effluent spiked with ibuprofen (IBU) |
| Initial concentration of IBU: 2 mg/L Synthetic wastewater: IBU: 97% removal (15 min) Real wastewater: IBU: 92% removal (180 min) | [140] |
| Synthetic wastewater with sulfathiazole (STZ) |
| Initial concentration of STZ: 0.1 M STZ: ~100% removal | [66] | |
| Synthetic wastewater with 1,4-dioxane (1,4-D) |
| Initial concentration of 1,4-D: 50 mg/L 1,4-D: >75% mineralization | [141] | |
| Synthetic wastewater with levofloxacin (LFX) and ketoprofen (KPT) |
| Initial concentration of LFX: 10 mg/L LFX: ~90% removal Initial concentration of KPT: 10 mg/L KPT: >65% removal | [142] | |
| Synthetic wastewater with dexamethasone (DXM) |
| Initial concentration of DXM: 5 mg/L DXM: ~100% removal | [143] |
| Type of Process | Name of Process | Theory | Mechanism | Advantages | Disadvantages | References |
|---|---|---|---|---|---|---|
| Adsorption of light | FT-NIR spectroscopy | Polychromatic light beam at a sample, measure the intensity of the light as a function of time. It allows simultaneous measurement over the whole wavelength range | Electromagnetic radiation (EMR) interacts with atoms and molecules in discrete ways to produce characteristic absorption profiles Planck’s constant, h = 6.63 × 10−34 Js Speed of light in a vacuum, c = 2.998 × 108 ms−1 | Able to do online analysis | Might not be the most accurate due to similar adsorption spectra of various chemical bonds | [153] |
| Infrared (IR) spectrum | Beam containing a different combination of frequencies shone on the sample and measured for light absorbed. This process is rapidly repeated with different combinations of light wavelengths. Correlations between the data points would be used to infer contaminants in the sample | Slightly more accurate due to the use of multiple combined wavelengths | - | [159] | ||
| Excitation and emission | Excitation emission matrix (EEM) | An EEM is a 3D scan, of the excitation wavelength vs. emission wavelength vs. fluorescence intensity of a sample when a given monochromatic beam of light is shone on the sample | Similar to the mechanism above, EEM also uses a similar mechanism, however, it correlates the excitation, reemission and fluoresces as well | Fast and quick analysis | At present might not be as accurate. It requires a large scale of intensive data processing | [151] |
| Size exclusion coupled UV based detection | Size exclusion coupled detection methods | This method is similar to the EEM analysis except it has an element of size exclusion | More accurate measurements, due to an additional size exclusion mechanism | Might not be as accurate presently | [160] | |
| Immunoassay | Measure changes in color or emission of light | Antibodies competitively capture dissolved targets and immobilized antigens. After the washing step, the labeled secondary antibodies bond to the corresponding antibodies. Signals can be obtained after incubation and another washing procedure | Low cost, simple procedure | Organic solvent and environmental factors may interfere with the immunoreaction | [161] | |
| Physio-chemical signal detection | Chemiluminescence | Chemical reactions occur, exhibiting light signatures | Explores the chemical signature of selected types of compounds which gives off fluorescence | Able to identify the luminescence compounds with a fairly high degree of accuracy | Highly selective to types of compounds that emit fluorescence | [162] |
| Surface-enhanced Raman scattering (SERS) | Novel spectrum analysis technology based on the combination of Raman spectroscopy and nanotechnology | The molecular fingerprint specificity of Raman spectroscopy is used for the detection of CECs. The enhancement Raman signal is achieved through electromagnetic enhancement mechanism and chemical enhancement mechanism | High sensitivity, non-destructive, capability for molecular fingerprint | Not stable, not cost-effective, Complex synthetic procedure, complicated synthetic procedure | [155] | |
| Surface plasmon resonance band | Collective electron charge oscillations of contaminants excited by light are measured | - | Only available for detection for nano-metal detection and costly | Only available for detection for nano-metal detection | [163] |
| Name of AOP | Advantages | Drawbacks | References | |
|---|---|---|---|---|
| UV/Oxidant | UV/H2O2 | Cheap and easy to implement | Low degradation performance Control of peroxide dosage needed to meet discharge limits | [51] |
| UV-persulfate | Fast reaction time Large pH range | Selective degradation at pH below 7, since SO4•−; is the dominant free radical pH adjustment needed | [52] | |
| UV-chlorine | Easy to implement Higher kinetics constants with CECs than HO• | Formation of disinfection by-products Selective degradation of electron-rich moieties compared to unsaturated C-C bonds | [53] | |
| UV/Ozone | Microbubble | Fast reaction time Low EEO | High operational and capital cost Works better at elevated pH The limited usable wavelength of light | [54] |
| Photo-Fenton | Heterogeneous | Does not require sludge treatment The large bandwidth of light usage | Slower reaction kinetics | [174] |
| Homogeneous | Fast reaction time pH range can be extended via the use of chelators like EDDS Able to use solar energy | Large surface area needed for solar processes | [70,175] | |
| Photocatalysis | TiO2 | Able to use solar energy with morphology modifications | High energy consumption is needed for the activation Requires high energy UV wavelength Conventional photocatalyst has issues with the reuse of the catalyst | [57,61,176] |
| ZnO | Able to utilize sunlight without any modifications Cheaper catalyst compared to TiO2 | High energy consumption is needed for the activation Conventional photocatalyst has issues with the reuse of the catalyst Degradation performance was not as good as the titanium-based catalyst | [59] | |
| Alternative catalyst | Able to use solar energy | Relatively new and requires further study of efficiency Suffers potential issues with conventional TiO2 and ZnO issues like the reuse and separation of catalyst | [137,138,141] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, B.C.Y.; Lim, F.Y.; Loh, W.H.; Ong, S.L.; Hu, J. Emerging Contaminants: An Overview of Recent Trends for Their Treatment and Management Using Light-Driven Processes. Water 2021, 13, 2340. https://doi.org/10.3390/w13172340
Lee BCY, Lim FY, Loh WH, Ong SL, Hu J. Emerging Contaminants: An Overview of Recent Trends for Their Treatment and Management Using Light-Driven Processes. Water. 2021; 13(17):2340. https://doi.org/10.3390/w13172340
Chicago/Turabian StyleLee, Brandon Chuan Yee, Fang Yee Lim, Wei Hao Loh, Say Leong Ong, and Jiangyong Hu. 2021. "Emerging Contaminants: An Overview of Recent Trends for Their Treatment and Management Using Light-Driven Processes" Water 13, no. 17: 2340. https://doi.org/10.3390/w13172340
APA StyleLee, B. C. Y., Lim, F. Y., Loh, W. H., Ong, S. L., & Hu, J. (2021). Emerging Contaminants: An Overview of Recent Trends for Their Treatment and Management Using Light-Driven Processes. Water, 13(17), 2340. https://doi.org/10.3390/w13172340








