Abstract
The biosorption behaviour of arsenic(V) and cadmium(II) ions by unmodified and five types of chemically modified Chlorella vulgaris and Spirulina platensis was investigated. The biosorption rates of As(V) and Cd(II) in binary metal solutions were lower than those in sole metal systems, which exhibited a competition between As(V) and Cd(II) ions to occupy the active sites of the adsorbent. Among the five chemical reagents, NaCl and ZnCl2 were the most suitable modifiers for improving the biosorption performance of C. vulgaris and S. platensis, respectively. The maximum biosorption capacities of As(V) and Cd(II) were: (a) 20.9 and 1.2 mg/g, respectively, for C. vulgaris modified with NaCl; (b) 24.8 and 29.4 mg/g, respectively, for S. platensis modified with ZnCl2, which were much higher than those using other chemically modifying methods. The pseudo-second-order kinetic model fitted well with all the biosorption processes. The SEM analysis revealed that the modification changed the surface morphologies and enhanced the porosity of the algae biomass. The FTIR analysis established the presence of diverse groups of compounds that were largely hydroxyl, carboxylate, amino, and amide groups on the adsorbents that contributed significantly to the upregulated biosorption. This work showed the potential application of chemically modified C. vulgaris and S. platensis biomasses to effectively remove both from water.
1. Introduction
Arsenic(V) and cadmium(II) are trace elements with bio-accumulative properties that pose a wide range of severe risks to human health and environmental sustainability, due to their non-degradability and persistence [1]. As(V) and Cd(II) are mostly constituents of wastewater generated by metal smelting, pesticide insecticide production, and mining activities [2,3,4]. Due to the indiscriminate discharge of wastewater in the environment, As(V) and Cd(II) can easily percolate into the soil, water bodies, and plants, and enter the human body via drinking and eating [5]. Once these toxicants get into the human system, they induce a series of serious diseases such as “black foot disease” [6] and “Itai-Itai disease” [7]. As(V) is present as HAsO42− when the solution pH is 3–6; AsO43− dominates at pH 7–10 [8], while Cd(II) is often present in the solution as Cd(II) [9]. Due to the extremely toxic effects on humans even at low concentration levels, the Chinese Standard “Sanitary Standards for Drinking Water” stipulates that the maximum allowable concentrations of As and Cd should not exceed 0.01 and 0.005 mg/L, respectively [10]. Therefore, the water treatment technologies of As(V) and Cd(II) ions have drawn great concerns.
Different treatment technologies including chemical precipitation, ion exchange, and membrane filtration have been developed to eliminate contaminants from wastewater. However, these conventional technologies have numerous disadvantages ranging from the possible generation of secondary wastes and sludge, the high capital cost, and the huge power requirements for the operation [11,12]. In contrast with conventional technologies, biosorption is suitable as a simple, efficient, and low-cost process for treating low concentrations of wastewater from several to several hundred mg/L [13]. Biosorption refers to an adsorption process using biological materials or biopolymers (including live or dead biomass) as adsorbents [8]. Biosorption materials, especially algae, are abundant in nature either in the terrestrial, marine or freshwater ecosystems and could also be cultivated under optimized conditions [14]. In addition, dead algae do not require nutrition or oxygen [15], which simplifies the experiment procedure. In addition, diverse functional groups such as hydroxyls, carboxylates, amino, and phosphate on the interface of the adsorbents enhance the biosorption capacities of algae to successfully remove various pollutants [16]. Furthermore, there is a possibility of regenerating the used biomass adsorbents after the completion of the biosorption process via desorption using various surfactants [17]. However, the biosorption capacity and efficiency of the adsorbents were not as excellent as the conventional adsorbents. Given the quantities of the adsorbents required to detoxify the wastewater under industrial application [18], there is an urgent need to explore various methods to improve the removal efficiencies of low-cost adsorbents such as algae.
Adsorbent modification is a common method that can rapidly increase the biosorption capacity of adsorbent, including physical modifications and chemical modifications. Physical modifications, such as grinding and boiling, are meant to increase the specific surface area of the adsorbents material [19]. Chemical modifications use chemical reagents such as acids, bases, salts, and organic solvents to change the functional group composition of the surface of the adsorbent [20]. The modification by NaCl could remove the impurity ions on the surface of the adsorbents, which exposed more binding sites [21]. Moreover, sodium ions can be easily replaced by heavy metal ions without affecting the subsequent biosorption process. The ZnCl2 modification could separate hemicellulose, which contains a variety of functional groups that are beneficial to the biosorption process, from biomass and cause biomass swelling [22]. It has been reported that iron-based adsorbents exhibited a strong affinity for arsenate [23], thus FeCl3 was used to enhance the efficacy of the adsorbent for the effective removal of the As. In addition, NaOH [24] and CaCl2 [25] have been reported as the modifiers that increase the biosorption capacity of adsorbents for heavy metals to different degrees. Most existing biosorption studies concentrate on the removal of toxic contaminations in mono-component systems [26,27], and the related biosorption mechanism has been thoroughly studied [9,13,28]. However, realistic wastewater is much more complex than the mono-component wastewater, which contains various metal ions and other components [29]. The interference and influence of the bimetallic system on the biosorption capacity of algae-based adsorbents are still unclear.
The aims of the study were: (1) to explore the interaction between As(V) and Cd(II) during the simultaneous adsorption process; and (2) to develop effective modification methods to improve the adsorption rate of As(V) and Cd(II) by the C. vulgaris and S. platensis. Therefore, the chemical modifications were carried out using ZnCl2, NaOH, NaCl, CaCl2, and FeCl3. The effects of pH, adsorbents dosage, contact time, and initial solution concentration in a simultaneous biosorption system were investigated by batch experiments. The adsorbents were characterized using scanning electron microscopy (SEM) and Fourier transform infrared spectroscopy (FTIR) techniques to further elucidate the morphological and physicochemical properties of the adsorbents.
2. Materials and Methods
2.1. Adsorbent Preparation and Modification
The Chlorella vulgaris (green algae) and Spirulina platensis (cyanobacterium) that were purchased from Zhengzhou Wanbo Chemical Products Co., are dominant algae species in the south of China. Disodium hydrogen arsenate (Na2HAsO4•7H2O) and cadmium nitrate (Cd(NO3)2•4H2O) were purchased from Sigma-Aldrich and Aladdin, respectively, and all the reagents used in the investigation were of analytical grades.
Chemical modification experiments were carried out using conical flasks (150 mL) with a 50 mL solution of each chemical reagent (one of ZnCl2, NaOH, NaCl, CaCl2, and FeCl3). To each 50 mL solution of the 0.1 mol/L modification reagent, 2 g of C. vulgaris and S. platensis powder were added. The suspensions were thoroughly mixed at a speed of 150 rotations per minute (rpm) for 24 h at a temperature of 25 °C. Afterwards, the modified algae slurry was washed several times with distilled water until all the impurities were successfully eliminated, and was followed by centrifugation. The modified algae biomasses were dried in an oven at a temperature of 80 °C until a constant weight was obtained and later used in biosorption studies. As a control measure, some C. vulgaris and S. platensis powders were pretreated with only distilled water and labelled as unmodified adsorbents. Before the batch experiments, the modified biomasses were ground using pestle and mortar followed by sieving (<500 μm) until the particle sizes became uniform.
2.2. Characterizations and Analysis
The samples were characterized with the SEM and FTIR techniques to elucidate the varied morphological and functional groups of characteristics on the adsorbents. The structure and shape of the modified and unmodified C. vulgaris and S. platensis were examined under a scanning electron microscope (Zeiss Ultra Plus, Zeiss, Jena, Germany). The functional groups present on cell walls of the C. vulgaris and S. platensis before and after the modification were determined with the Nicolet 6700 spectrometer (Thermo electron scientific instruments, USA) at wavenumbers from 400–4000 cm−1.
In addition, the concentration of Cd(II) was measured via flame-atomic absorption spectrometry (ZEEnit700, Analyjena, Jena, Germany) in this study.
The concentration of As(V) was measured using the colourimetric technique at a wavelength of 880 nm [30].
2.3. Biosorption Experiments
2.3.1. Effects of Initial pH and Adsorbents Dosage
To avoid the co-precipitation on biosorption, the effects of the initial solution pH on the coexistence of As(V) and Cd(II) were investigated in the pH range from 3.0 to 8.0. The initial solution pH on the biosorption capacity of As(V) and Cd(II) was conducted in a pH range of 3.0 to 6 based on a previous pH experiment, where As(V) and Cd(II) were present. After studying the adsorbents dosage in the range of 1–4 g/L with the 30 mg/L initial As(V) and Cd(II) solution, it was found that the optimum adsorbents dosage was 4 g/L and the optimum contact time was 4 h, which was used in the following experiments. The above experiments were performed using the unmodified C. vulgaris. In the batch experiments, the sorption amount of As(V) and Cd(II) was analyzed by measuring the residuals in the supernatant.
2.3.2. Effects of Different Modifications
Different modified adsorbents were added to the 30 mg/L As(V) and Cd(II) solution to evaluate the efficacy of the modification on the biosorption of the two metal species. The mixture swirled at a speed of 150 rpm, temperature of 25 °C, and 150 rpm for 4 h in a vertical full temperature oscillation incubator (ZQLY-180V, Zhichu Instrument Co., Ltd., Shanghai, China). After settling, 5 mL of the supernatant was separated using a 0.45 μm membrane filter, and the remaining As(V) and Cd(II) concentrations were determined.
2.3.3. Effects of Chemical Reagents Concentration
The chemical reagents (ZnCl2 and NaCl) which showed upregulated effects on the As(V) and Cd(II) biosorption rate, were selected for studying the different concentrations of these reagents (0, 0.1, 0.5, 1.0, and 2.0 mol/L). The modified C. vulgaris and S. platensis were used to investigate the As(V) and Cd(II) biosorption rates. Batch experiments were then carried out with the 50 mL solution of the As(V) and Cd(II) (30 mg/L) with 4 g/L biosorbent dosages.
The biosorption rate (r) of the As(V) and Cd(II) after the batch experiments was evaluated according to Equation (1):
where Ct (mg/L) represents the equilibrium concentration of either As(V) or Cd(II), and C0 (mg/L) represents the initial concentration of either As(V) or Cd(II).
2.4. Biosorption Isotherm
The optimum concentration of chemical reagents (1 M NaCl for C. vulgaris and 0.5 M ZnCl2 for S. platensis) was used for the biosorption isotherm experiments. The experiments were carried out with the adsorbents dosage of 4 g/L with varied initial As(V) and Cd(II) concentrations (10–300 mg/L) in a single system. The mixtures were agitated at a speed of 150 rpm and a temperature of 25 °C for 8 h to ensure that the biosorption system attained an equilibrium with the initial solution pH adjusted to 6.0.
The amount of equilibrium biosorption (qe, mg/g) of the As(V) and Cd(II) is calculated following Equation (2):
where V (L) denotes the volume of the solution, W (g) represents the weight of dry adsorbents, and C0 (mg/L) represents the initial As(V) and Cd(II) concentration.
The Langmuir and Freundlich models in Equations (3) and (4), respectively were employed to evaluate the biosorption isotherms to assess the performance of the adsorbents.
where qmax (mg/g) represents the maximum adsorption capacity, qe (mg/g) is the equilibrium adsorbed amount of the As(V) and Cd(II), and Ce (mg/L) is the equilibrium concentration of the solute. In the Langmuir isotherm model (Equation (3)), KL represents the Langmuir constant regarding the energy of biosorption, whereas the KF and 1/n are the Freundlich constants (Equation (4)), respectively.
2.5. Biosorption Kinetics
Biosorption kinetics studies were conducted with 50 mL of the solution containing 30 mg/L of As(V) and Cd(II) with 4 g/L of the modified adsorbents using initial pH 6. The mixtures were agitated at a speed of 150 rpm at a temperature of 25 °C with varied time intervals from 5 min to 8 h, depending on the appropriate equilibrium time.
The biosorption kinetic data on the removal of As(V) and Cd(II) were assessed via pseudo-first-order and pseudo-second-order models.
The pseudo-first-order kinetic model is expressed in Equation (5) as follows:
While the pseudo-second-order kinetic model is given as shown in Equation (6):
where qe and qt represent the equilibrium biosorption capacity (mg/g) and the biosorption capacity at t (min), k1, and k2 are the rate constants for the pseudo-first-order and pseudo-second-order models, correspondingly. All the experiments were conducted in duplicate and the averages were used for the analysis.
3. Results and Discussion
3.1. Effects of Initial pH and Adsorbents Dosage
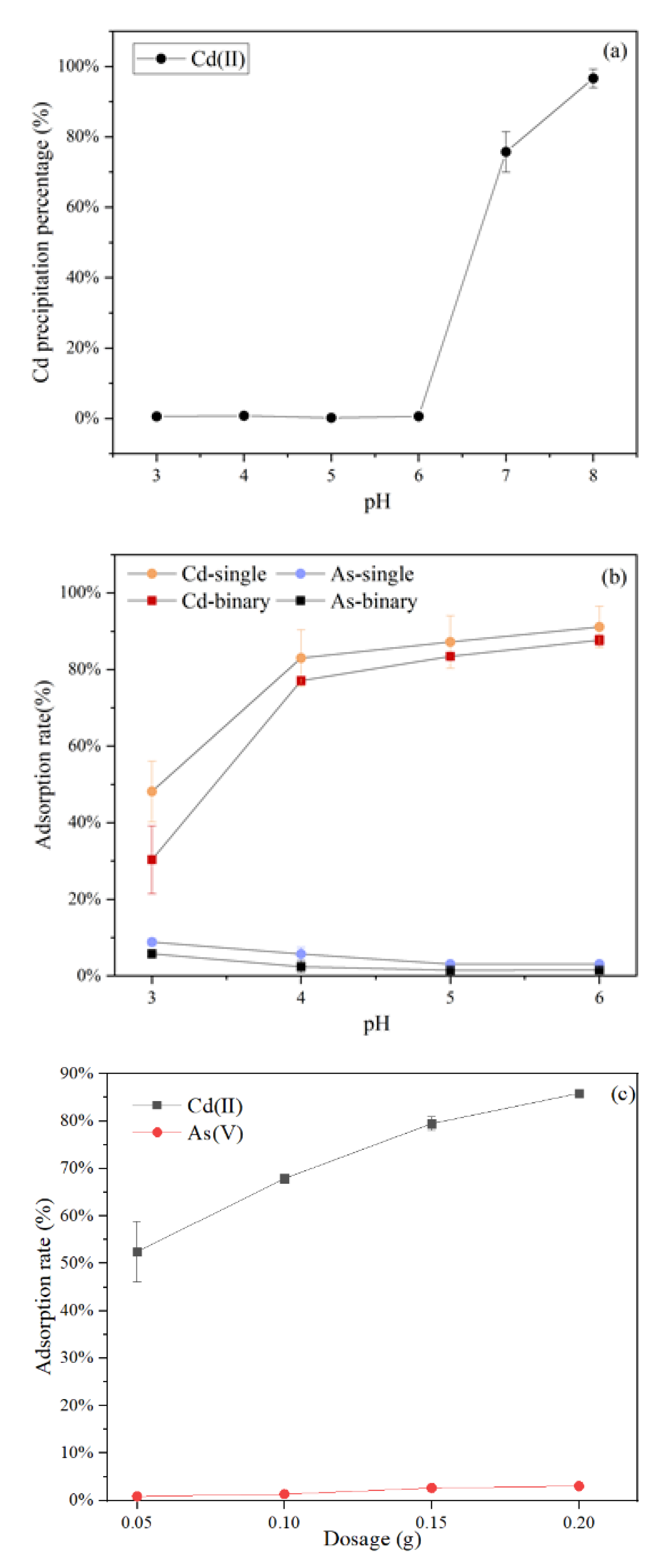
The initial pH of the metal solution is a key influencing factor during the biosorption process. The influence of the initial solution pH was investigated on the simultaneous removal of As(V) and Cd(II) using C. vulgaris, which was investigated under pH conditions of 3.0–6.0. The results in Figure 1a showed that with an increase in the initial pH from 3.0 to 6.0 involving the Cd-As solution, there was no change in the concentration of Cd(II). However, it sharply decreased when the pH increased above 6.0. Additionally, it was reported that Cd(II) started to form Cd(OH)2 precipitation at pH values above 8.0 [31]. Moreover, it suggested that the precipitation threshold of Cd(II) shifts towards a low pH in the existence of As(V). This was probably due to the fact that Cd(II) and AsO43− formed Cd3(AsO4)2 complexed with a solubility product constant value of Ksp = 5.13 × 10−34 [32]. Similar results have been reported by Jiang, Lv, Luo, Yang, Lin, Hu, Zhang, and Zhang [5], which showed that 0.1 mM Cd2+ barely co-precipitated with 0.5 mM As(V) below pH 7. Consequently, the coexistence of As(V) and Cd(II) was influenced by the initial solution pH together with the concentration of As(V) and Cd(II). To eliminate the effect of co-precipitation during the biosorption processes, the pH values were adjusted between 3 to 6. The results in Figure 1b showed opposite trends regarding the biosorption of As(V) and Cd(II) in the single system. The biosorption rate of Cd(II) was relatively low at pH 3, whereas it rapidly increased with the pH rise from 3 to 4. As the pH continued to increase, the biosorption rate gradually increased and eventually exceeded 90%. This phenomenon could be ascribed to the electrostatic attraction between Cd(II) and the various functional groups present on adsorbents surfaces. In low-pH solutions, the H+ of high concentration could compete for biosorption sites with Cd(II). An increase in pH gave rise to the deprotonation of functional groups of the adsorbents. Hence, a high affinity for positively charged Cd(II) ions [33]. The biosorption rate of As(V) and Cd(II) decreased in the binary system (Figure 1b), possibly due to the competition of biosorption sites, but the basic trend remained unchanged in the single system. When the initial pH was 6, the Cd(II) biosorption rate reached the maximum(above 90%), and the As(V) was maintained at 2%. Therefore, pH 6 was selected to be the test condition of the following experiments. The findings are indicative of the fact that the removal of Cd(II) and As(V) is exceedingly dependent on the pH.
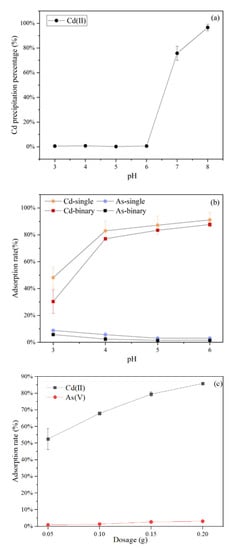
Figure 1.
The image of the As(V) and Cd(II) precipitation curves: (a) Effects of pH on co-precipitation, (b) biosorption by C. vulgaris for the single and binary solution systems, and (c) adsorbents dosage on adsorption by C. vulgaris for the binary solution systems.
The results of C. vulgaris dosage on the simultaneous biosorption are presented in Figure 1c, which suggested that the biosorption efficiency for both As(V) and Cd(II) is dependent on the C. vulgaris adsorbents dosage. A possible reason could be that more biosorption sites for heavy metals were available at higher C. vulgaris dosage [34]. However, the biosorption capacities for As(V) and Cd(II) decreased intensely with the increasing adsorbents dosage, which might be caused by the fixed total amount of ions. Therefore, for practical applications, it is essential to consider the optimum adsorbents dosage for an effective reduction in the concentration of the pollutants. When the dosage of C. vulgaris was 2.0 g/L, the removal of Cd(II) was above 80%, and As(V) reached the maximum. Therefore, the adsorbents dosage of 2.0 g/L was chosen as the optimum dosage for the subsequent experiments, including unmodified and modified algae adsorbents.
3.2. Biosorption Efficiency of Different Modified Adsorbents
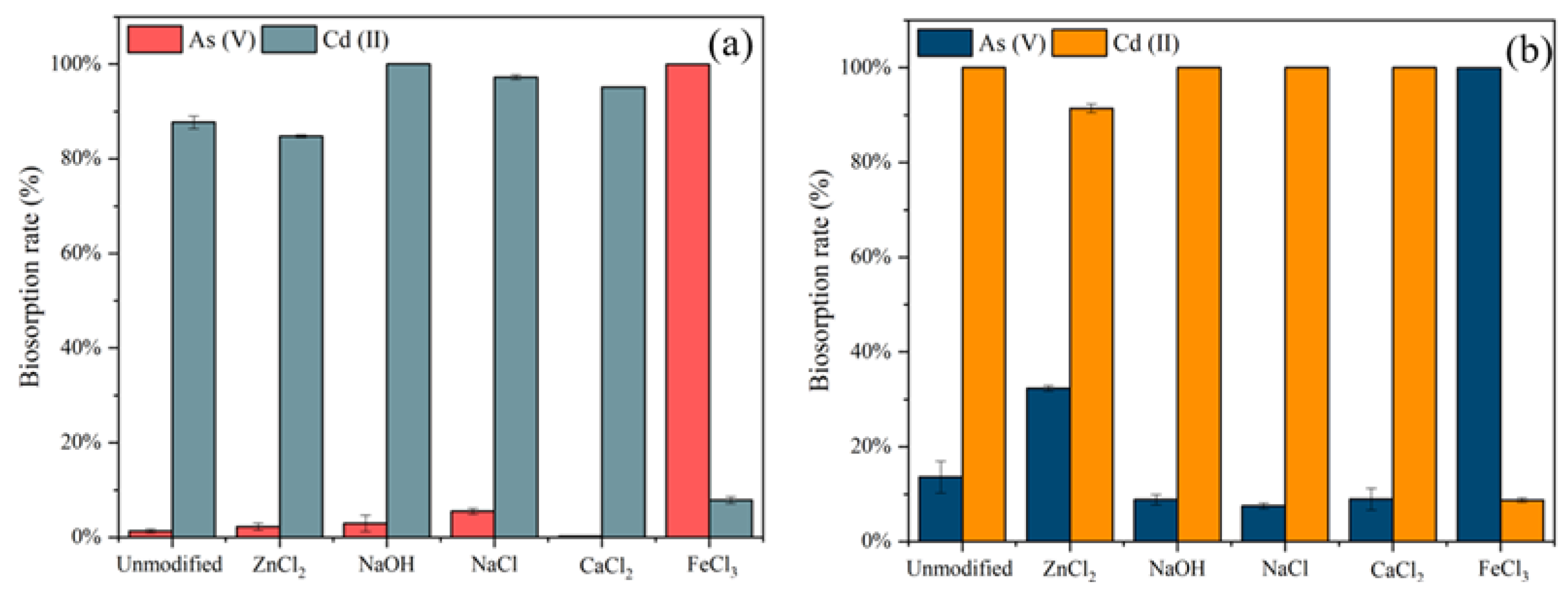
The different chemical modifications on the removal of C. vulgaris (Figure 2a) and S. platensis (Figure 2b) were investigated with five 0.1 mol/L modifiers including ZnCl2, NaOH, NaCl, CaCl2, and FeCl3.
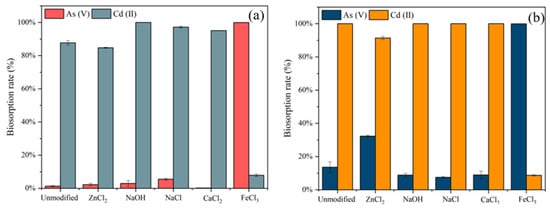
Figure 2.
Biosorption rates (%) for As(V) and Cd(II) by (a) unmodified and chemically modified C. vulgaris, and (b) unmodified and chemically modified S. platensis.
The NaOH modified adsorbents showed the enhanced removal of Cd(II) by both algae but had little effect on the removal of As(V). The hemicellulose in the biomass will be softened due to the hydrolysis reaction by alkaline compounds such as NaOH. This could be attributed to the formation of hydroxyl (-OH) and carboxylic (-COOH) groups present in the modified algae. Hence, facilitating the Cd(II) removal [35]. Similar results were observed from CaCl2 in this study, which increased the removal of Cd(II) by C. vulgaris and S. platensis but reduced the removal of As(V). In contrast to the modification by NaOH and CaCl2, the algae modified with FeCl3 could achieve almost 100% removal of As(V). However, it could only reach 8% and 9% removal of Cd(II), respectively, which was much lower than that of unmodified algae. The mechanism of As(V) biosorption could be the potential ligand exchange reactions between the As(V) and the surface hydroxyl groups in harmony with the Fe atoms [36].
Moreover, NaCl and ZnCl2 had a significant increase in the biosorption of C. vulgaris and S. platensis, respectively. The NaCl modification of C. vulgaris increased the Cd(II) removal from 93% to 97% and As(V) from 1% to 5%. The increase in biosorption efficiency after modification with NaCl could be attributed to an ion exchange process. After the modification with NaCl, Na+ may have remained on the surfaces of the algae cell walls, and then the cationic ions exchanged with the metal ions during the biosorption process [19]. Additionally, a notable difference was observed in the biosorption rate of ZnCl2 on S. platensis, which increased the As removal from 13.6% to 32.3% but decreased the Cd(II) from 100% to 91%. Two reasons may account for the enhanced biosorption efficiency: (a) These could be the swelling of the hemicelluloses and cellulose during the activation of the biomass [37]; (b) the modification of S. platensis with ZnCl2 increased the number of biosorption sites, but due to the competition effect between As(V) and Cd(II), As(V) might occupy some of the Cd(II) biosorption sites, resulting in a decrease in the biosorption rate of Cd(II).
3.3. Effects of the Concentration of Modification Reagents
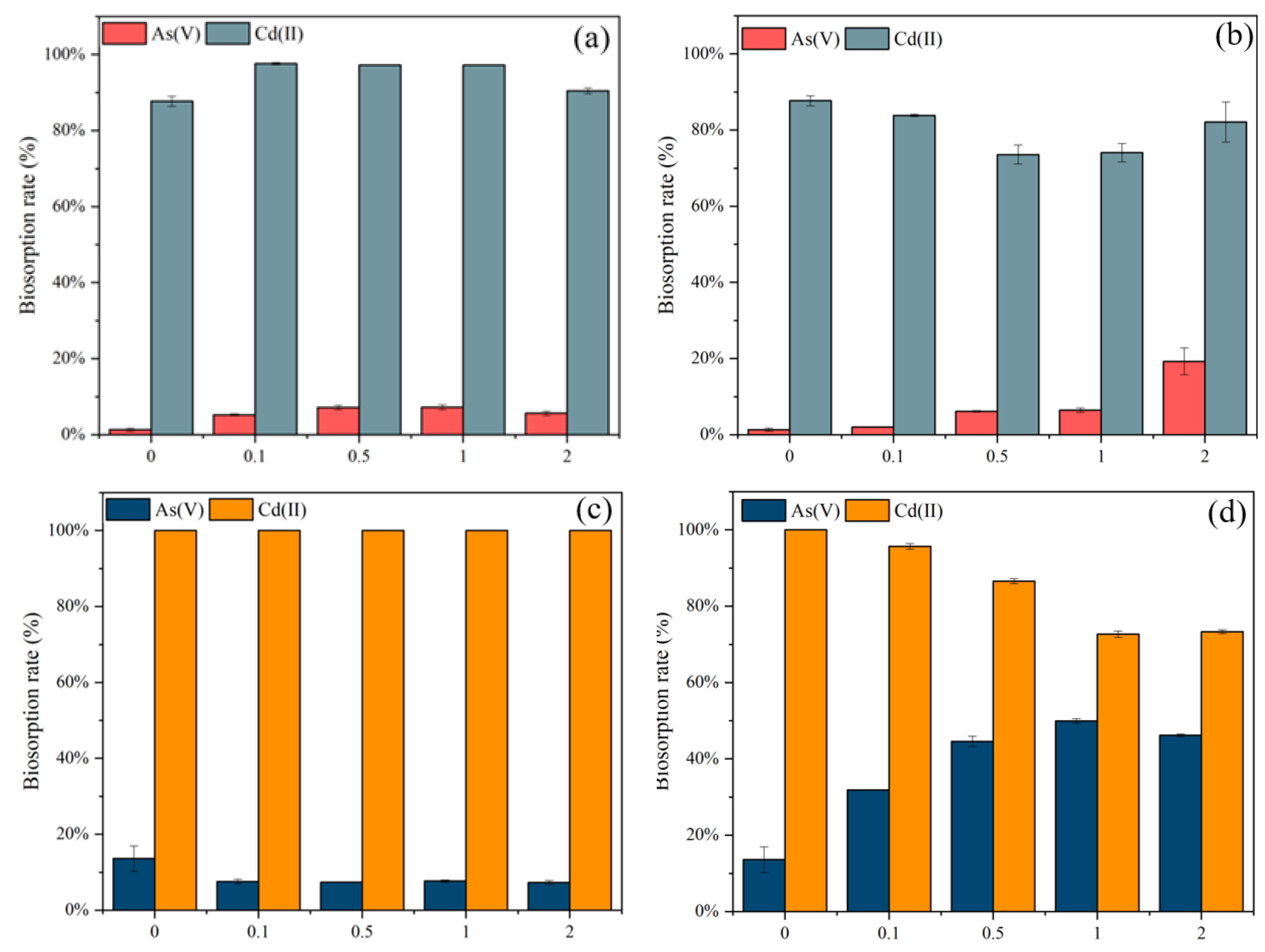
Different modification reagents were employed for the modification of the algae biomasses and the most effective reagents were chosen for subsequent studies. The modification by NaCl and ZnCl2 modification recorded the maximum effect on the removal of As(V) and Cd(II) by C. vulgaris and S. platensis, correspondingly. Therefore, the concentration of the two modification reagents was varied as follows 0, 0.1, 0.5, 1.0, and 2.0 M and the results were shown in Figure 3.
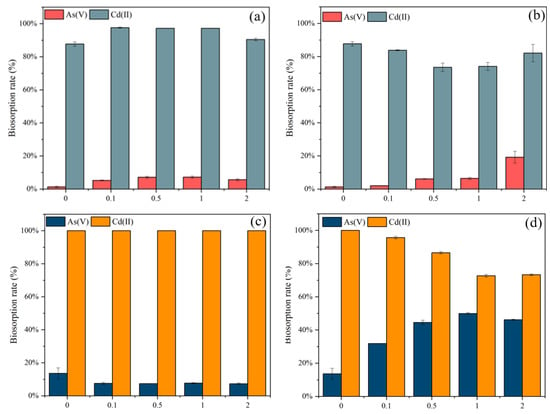
Figure 3.
Biosorption rates (%) for As(V) and Cd(II) by different concentrations of (a) NaCl and (b) ZnCl2 modified C. vulgaris, and (c) NaCl and (d) ZnCl2 modified S. platensis.
In Figure 3a, an increase in the biosorption rate of As(V) and Cd(II) was observed with NaCl-modified C. vulgaris. With the rise in NaCl concentration to 0.1 M, the As(V) removal of NaCl modified C. vulgaris increased from 1.3% to 5.2% and Cd(II) increased from 92.7% to 97.6%. This increasing trend was maintained up to 1.0 mol/L but dropped at 2.0 mol/L. Therefore, 1.0 mol/L was the optimum modified concentration for C. vulgaris. A higher concentration of NaCl could negatively affect the biosorption of As(V) and Cd(II). In Figure 3b, the rise in the concentration of ZnCl2 from 0 to 2 mol/L, led to a slight decrease in the removal of Cd(II), while the removal of As(V) increased marginally.
As for S. platensis (Figure 3c), the modification of NaCl had no remarkable effect on the biosorption of As(V) and Cd(II), the difference between the 0.1 to 2 M concentrations was not significant. However, in Figure 3d, the maximum As(V) removal efficiency onto S. platensis was achieved using 1 M ZnCl2 for modification. The biosorption rate of Cd(II) decreased from 100% to 73% with the increasing concentration, while the biosorption rate of As(V) increased from 13% to 50%. At a higher ZnCl2 concentration (2 M), the As(V) biosorption rate decreased to 46%. The reason may be that the modification improved the porosity development of the adsorbent. Additionally, As(V) interacted more easily with the various functionalities on the cell walls of the adsorbent, while the removal of Cd(II) decreased, attributable to the nonexistence of the available biosorption sites.
To achieve a balance in the removal efficiency of As(V) and Cd(II) and to maximize the total biosorption rate, 0.5 mol/L ZnCl2 modified S. platensis and 1 mol/L NaCl modified C. vulgaris were selected for the follow-up experiments.
3.4. Biosorption Kinetic Analysis
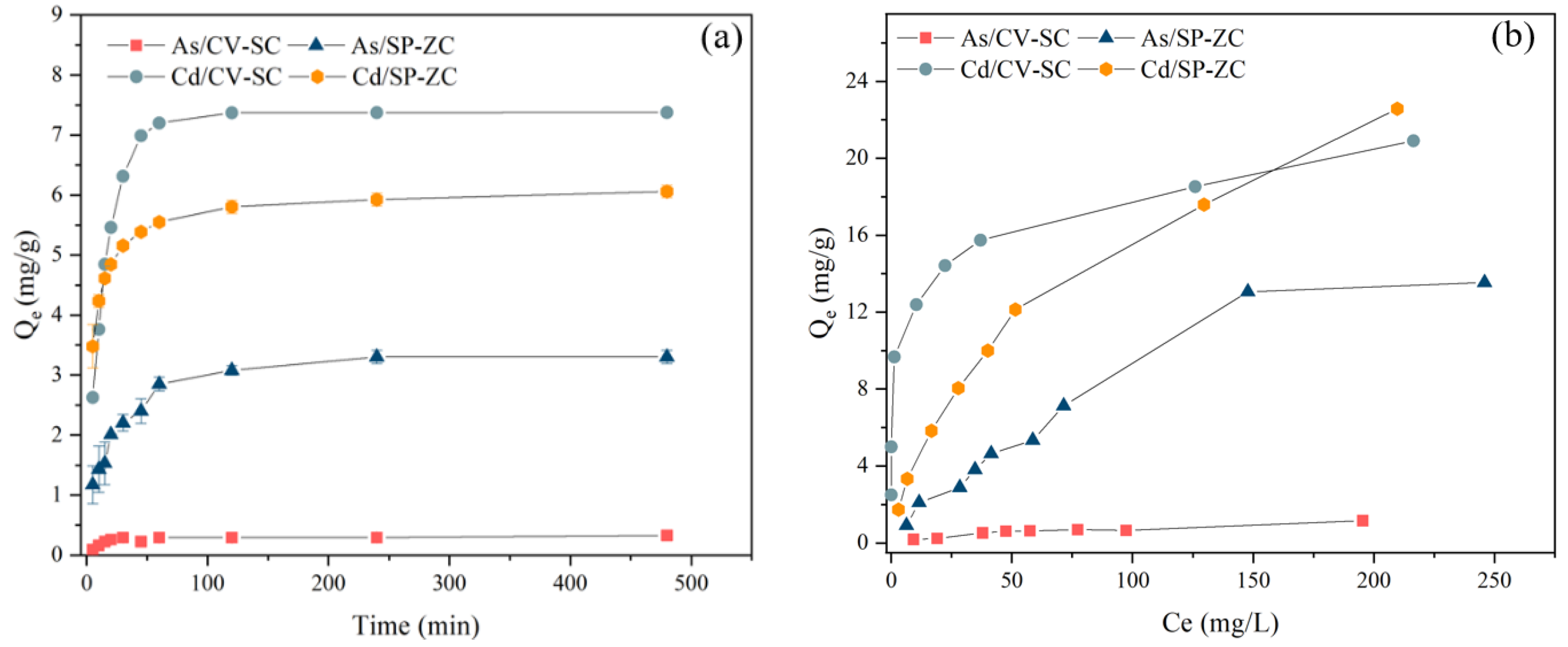
The comparison for the evaluation of the kinetic parameters between the two algae samples is presented in Figure 4a. The biosorption capacity of As(V) and Cd(II) reached about 90% of the equilibrium biosorption capacity at the contact time of 2 h. Thereafter, as a result of the gradual decrease in the available active sites, the uptake of the ions by the adsorbent gradually reduced with the increasing contact time, and at 4 h, the biosorption reached equilibrium.
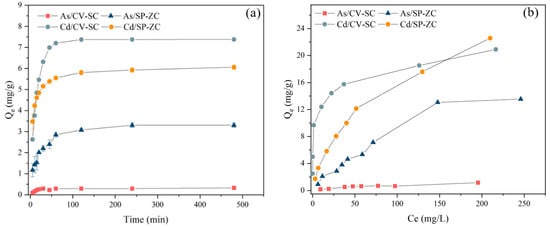
Figure 4.
Effects of (a) contact time and (b) initial concentration on biosorption for As(V) and Cd(II) by NaCl modified C. vulgaris and ZnCl2 modified S. platensis.
To analyze the kinetics of the biosorption kinetics of As(V) and Cd(II) onto NaCl modified C. vulgaris and ZnCl2 modified S. platensis, the experimental data were fitted with both the pseudo-first-order and pseudo-second-order models. The values for the kinetic constant parameters for As(V) and Cd(II) biosorption are listed in Table 1. From Table 1, the values of the R2 for As(V) and Cd(II) obtained from the evaluation of the pseudo-second-order model were much higher (over 0.99) compared to the values obtained from the pseudo-first-order kinetic model for the NaCl modified C. vulgaris and ZnCl2 modified S. platensis. The qe values (calculated) for As(V) and Cd(II) obtained from the pseudo-second-order model using the two adsorbents corroborated with the experimental qe values (0.33 and 7.51 mg/g for the NaCl modified C. vulgaris, respectively, and 3.41 and 6.11 mg/g for the ZnCl2 modified S. platensis, correspondingly). Based on these results, the PSO model best described the biosorption which was largely influenced by a chemical process [34].

Table 1.
Kinetic model parameters of As(V) and Cd(II) biosorption onto the NaCl modified C. vulgaris and S. platensis.
3.5. Biosorption Isotherm
The biosorption capacities for As(V) and Cd(II) for the various initial concentrations (10–300 mg/L) were investigated individually by biosorption isotherm experiments. As shown in Figure 4b, under the experimental condition, the highest biosorption capacities recorded for As(V) and Cd(II) were found to be 1.16 and 20.91 mg/g for the NaCl modified C. vulgaris, respectively, and 13.55 and 22.58 mg/g were for the ZnCl2 modified S. platensis, respectively.
Additionally, the effectiveness of the NaCl modified C. vulgaris and ZnCl2 modified S. platensis for the efficient removal of As(V) and Cd(II), was examined using the Langmuir and Freundlich isotherm models. The various correlation coefficients are presented in Table 2. As shown in Table 2, the As(V) and Cd(II) biosorption data fitted the Freundlich isotherm model (R2, Cd = 0.990, As = 0.936) well for the NaCl modified C. vulgaris, based on the regression coefficients compared to the Langmuir isotherm model. These results suggested that the biosorption of Cd(II) by the NaCl modified C. vulgaris occurs on a heterogeneous surface via multilayer sorption [13].

Table 2.
Isotherm model parameters for As(V) and Cd(II) biosorption onto NaCl modified C. vulgaris and ZnCl2 modified S. platensis.
As for the ZnCl2 modified S. platensis, the As(V) biosorption behaviour fitted the Langmuir isotherm (R2, As = 0.986) better, which suggested that the nature of the biosorption was homogenous via the monolayer biosorption [13]. In contrast, the Cd(II) biosorption behaviour fitted the Freundlich isotherm (R2, Cd = 0.992) better, which was indicated by the higher R2 obtained for the Langmuir isotherm (Table 2). This result could be due to the following reasons: (1) The modification of ZnCl2 might have resulted in an even distribution of the binding sites on the ZnCl2 modified S. platensis surface [38]; (2) there is a possibility that two metal ions might have formed different complexes with the functional groups on the surface of the ZnCl2 modified S. platensis [39].
The maximum biosorption capacity obtained from the Langmuir for the ZnCl2 modified S. platensis for As(V) and Cd(II) was 24.8 and 29.4 mg/g, correspondingly, which were much greater than the NaCl modified C. vulgaris. This suggested that the ZnCl2 modified S. platensis could be effectively used to eliminate As(V) and Cd(II) from wastewater simultaneously.
3.6. Adsorbents Characterization
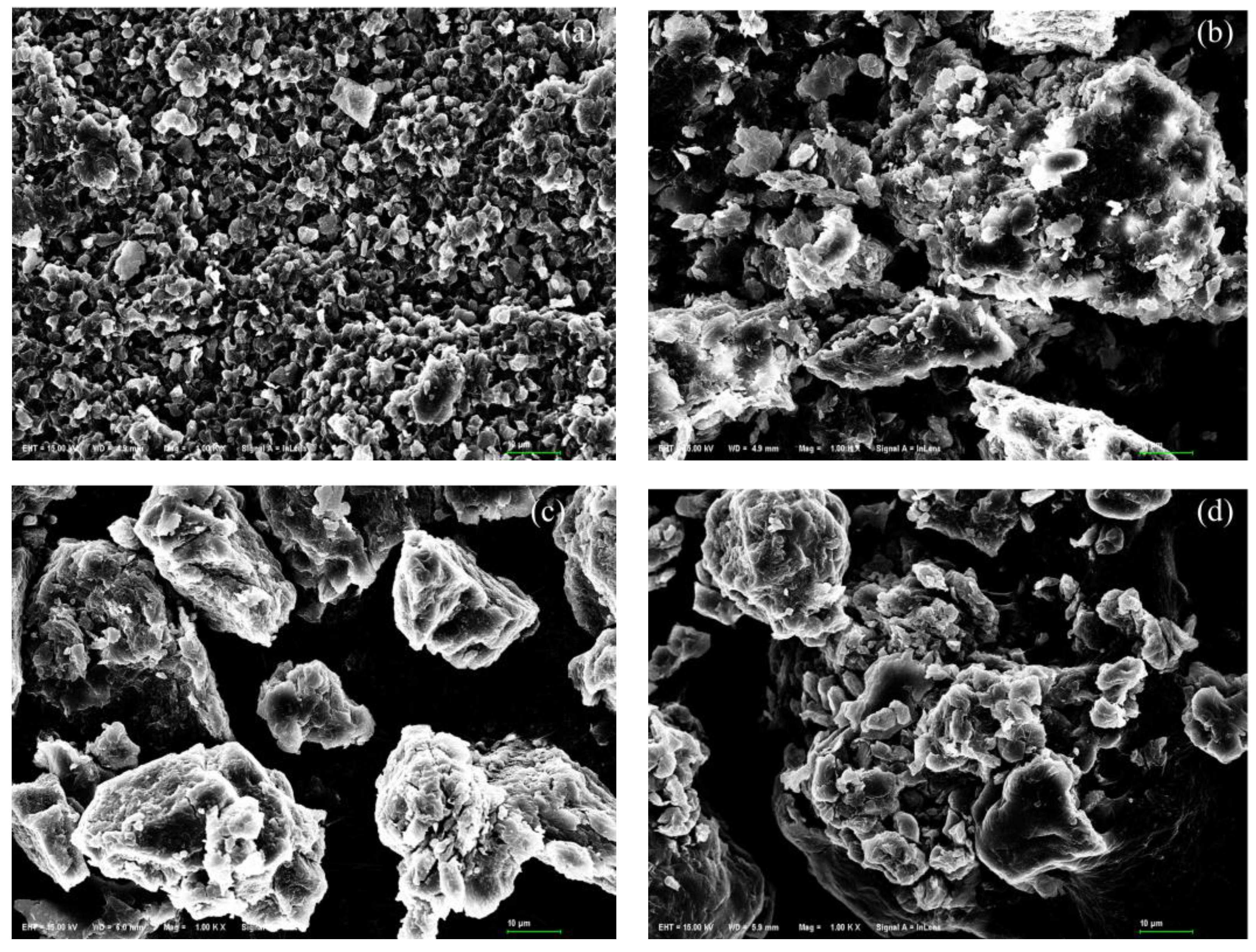
The distinctly different morphological features of unmodified common C. vulgaris and S. platensis is shown in Figure 5. The surface of C. vulgaris is relatively flat and uniformly distributed with many round particles (Figure 5a), with many tiny pores and cavities between these particles. In contrast, S. platensis particles have an irregular shape, with many elongated grooves and larger cavities on the surface (Figure 5c). This structure of S. platensis may allow heavy metal molecules to penetrate more easily into the internal structure and interact with the functional groups on its surface.
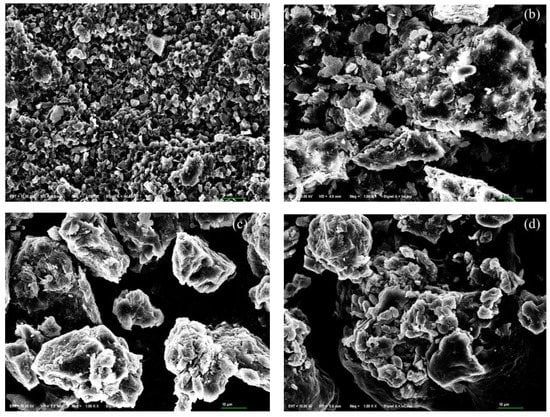
Figure 5.
SEM image of (a) unmodified and (b) ZnCl2 modified C. vulgaris, and (c) unmodified and (d) ZnCl2 modified S. platensis.
The scanning electron microscopy images showed that the uniform pore structure of the surface of C. vulgaris disappeared after modification by ZnCl2 (Figure 5b). The particles were agglomerated, showing more bumps, larger cavities, and rougher surface structures. The sizes of the particles were also reduced in terms of appearance. A similar phenomenon was observed from ZnCl2 modified S. platensis (Figure 5d), where the elongated surface grooves turned into larger cavities and presented a rougher morphology. Generally, the ZnCl2 modification may have increased the specific surface area of both green algae particles, thus enhancing their biosorption capacity for heavy metals.
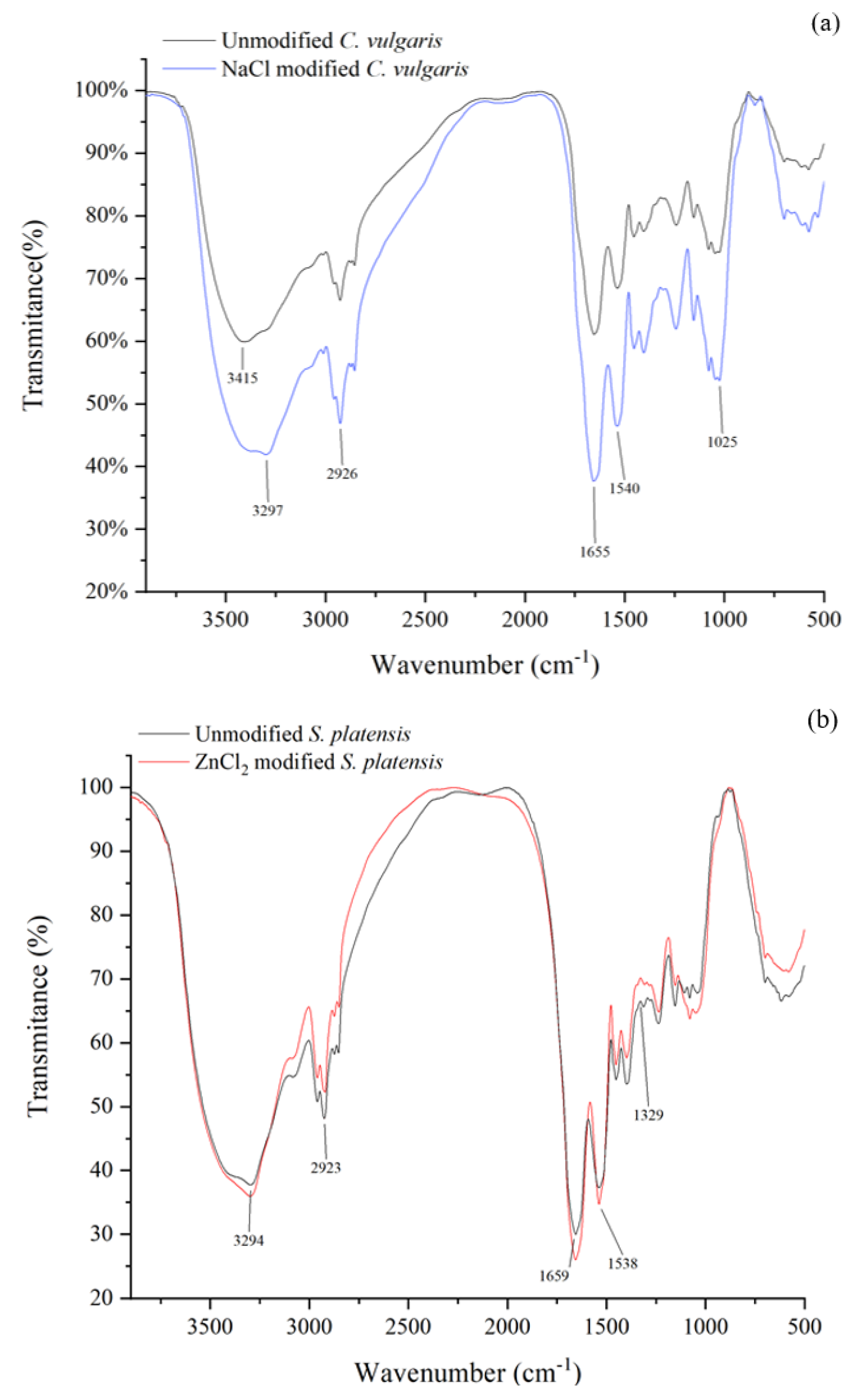
The identification of the various functional groups present on the algae surface was carried out via FTIR spectroscopy. Figure 6 shows the FTIR graph of unmodified and chemically modified algae samples. The FTIR spectroscopic graph indicated an intensity peak at around 3294 cm−1 in all the samples, suggesting the presence of O–H and N–H. The peaks around 2926 cm−1 were ascribed to the C–H stretching vibrations, which could be found in cellulose and lignin [40]. Additionally, the peaks near 1660 cm−1 were ascribed to the C=O asymmetric stretching. Compared to unmodified C. vulgaris, the strength of the O–H, C–H, and C=O stretching band for NaCl modified C. vulgaris increased (Figure 6a), indicating the increase of these functional groups. Similarly, the C–H and C=O functionalities in the ZnCl2 modified S. platensis exhibited an increased intensity (Figure 6b), demonstrating that the ZnCl2 modification increased the surface area and the functional groups. The FTIR graph showed that the C. vulgaris and S. platensis surfaces were occupied by the hydroxyl, carboxylate, amino, and amide groups, which had a remarkable role during the biosorption process [41,42].
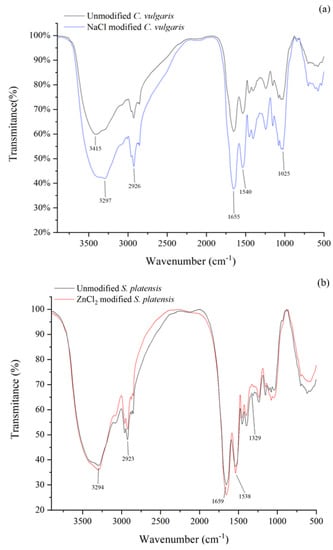
Figure 6.
FTIR spectra of (a) C. vulgaris and (b) S. platensis before and after modification.
4. Conclusions
In this study, the effects of different chemical modifications on the simultaneous biosorption of As(V) and Cd(II) by two green algae were investigated. The results revealed that the effect of modification was related to the type of algae, the chemical reagents, and the reagent concentration. The biosorption rate of S. platensis for As(V) and Cd(II) (13.58% and 100%) was higher than that for C. vulgaris (1.32% and 92.68%), which may be related to its rougher surface structure and larger specific surface area. Moreover, 1 M NaCl and 0.5 M ZnCl2 were found to be the optimal chemical reagents to improve the biosorption performance of C. vulgaris and S. platensis, correspondingly. The biosorption rate of NaCl modified C. vulgaris and ZnCl2 modified S. platensis for As(V) and Cd(II) were 7.22% and 97.23%, 44.57%, and 86.58%, respectively. The biosorption of As(V) and Cd(II) at 30 mg/L exhibited competitive effects in the binary system. The modification improved the biosorption efficiency of the adsorbent for As(V) and Cd(II) but did not change this competitive effect. Overall, in the low concentration range, this work provided an effective and low-cost biosorption technology to simultaneously remove As(V) and Cd(II).
Author Contributions
Conceptualization, L.P.; methodology, W.L. and L.P.; validation, Y.X., C.L. and L.P.; formal analysis, W.L.; investigation, W.L.; resources, L.P. and Y.X.; data curation, W.L.; writing—original draft preparation, W.L. and B.I.M.; writing—review and editing, L.P., Y.X. and C.L.; visualization, W.L.; supervision, L.P., Y.X., and C.L.; funding acquisition, L.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Natural Science Foundation of Hubei Province (No. 2020CFB517), the Natural Science Foundation of Guangdong Province (No. 2019A1515110350), and the National Natural Science Foundation of China (No. 51908436).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All the data generated during this study are included in this article.
Acknowledgments
The authors are grateful for the research collaboration.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gogoi, A.; Biswas, S.; Bora, J.; Bhattacharya, S.S.; Kumar, M. Effect of vermicomposting on copper and zinc removal in activated sludge with special emphasis on temporal variation. Ecohydrol. Hydrobiol. 2015, 15, 101–107. [Google Scholar] [CrossRef]
- Gräfe, M.; Nachtegaal, M.; Sparks, D.L. Formation of Metal−Arsenate Precipitates at the Goethite−Water Interface. Environ. Sci. Technol. 2004, 38, 6561–6570. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Wang, W.; Li, X.; Duan, J.; Jing, C. Evaluating adsorption media for simultaneous removal of arsenate and cadmium from metallurgical wastewater. J. Environ. Chem. Eng. 2016, 4, 2795–2801. [Google Scholar] [CrossRef]
- Yoon, K.; Cho, D.-W.; Tsang, D.; Bolan, N.; Rinklebe, J.; Song, H. Fabrication of engineered biochar from paper mill sludge and its application into removal of arsenic and cadmium in acidic water. Bioresour. Technol. 2017, 246, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Lv, J.; Luo, L.; Yang, K.; Lin, Y.; Hu, F.; Zhang, J.; Zhang, S. Arsenate and cadmium co-adsorption and co-precipitation on goethite. J. Hazard. Mater. 2013, 262, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Nath, S.G.; Debsarkar, A.; Dutta, A. Technology alternatives for decontamination of arsenic-rich groundwater—A critical review. Environ. Technol. Innov. 2018, 13, 277–303. [Google Scholar] [CrossRef]
- Nishijo, M.; Nakagawa, H.; Suwazono, Y.; Nogawa, K.; Kido, T. Causes of death in patients with Itai-itai disease suffering from severe chronic cadmium poisoning: A nested case–control analysis of a follow-up study in Japan. BMJ Open 2017, 7, e015694. [Google Scholar] [CrossRef] [Green Version]
- Sahmoune, M.N. The role of biosorbents in the removal of arsenic from water. Chem. Eng. Technol. 2016, 39, 1617–1628. [Google Scholar] [CrossRef]
- Ghosh, D.; Saha, R.; Ghosh, A.; Nandi, R.; Saha, B. A review on toxic cadmium biosorption from contaminated wastewater. Desalination Water Treat. 2013, 53, 413–420. [Google Scholar] [CrossRef]
- Du, L.; Yu, R.; Wang, H.-y.; LU Yun; Zheng, L. T. Pollution and toxicity of cadmium: A review of recent studies. J. Environ. Health 2013, 30, 167–174. [Google Scholar] [CrossRef]
- Cheng, S.Y.; Show, P.-L.; Lau, B.F.; Chang, J.-S.; Ling, T.C. New Prospects for Modified Algae in Heavy Metal Adsorption. Trends Biotechnol. 2019, 37, 1255–1268. [Google Scholar] [CrossRef]
- Liao, Q.; Tu, G.; Yang, Z.; Wang, H.; He, L.; Tang, J.; Yang, W. Simultaneous adsorption of As(III), Cd(II) and Pb(II) by hybrid bio-nanocomposites of nano hydroxy ferric phosphate and hydroxy ferric sulfate particles coating on Aspergillus niger. Chemosphere 2019, 223, 551–559. [Google Scholar] [CrossRef]
- He, J.; Chen, J.P. A comprehensive review on biosorption of heavy metals by algal biomass: Materials, performances, chemistry, and modeling simulation tools. Bioresour. Technol. 2014, 160, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Lebron, Y.; Moreira, V.; Santos, L. Studies on dye biosorption enhancement by chemically modified Fucus vesiculosus, Spirulina maxima and Chlorella pyrenoidosa algae. J. Clean. Prod. 2019, 240, 118197. [Google Scholar] [CrossRef]
- Pereira, L.; Alves, M. Dyes—Environmental Impact and Remediation. In Environmental Protection Strategies for Sustainable Development; Malik, A., Grohmann, E., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 111–162. [Google Scholar]
- Lebron, Y.; Moreira, V.; Santos, L.; Jacob, R. Remediation of methylene blue from aqueous solution by Chlorella pyrenoidosa and Spirulina maxima biosorption: Equilibrium, kinetics, thermodynamics and optimization studies. J. Environ. Chem. Eng. 2018, 6, 6680–6690. [Google Scholar] [CrossRef]
- Huq, E.; Fahad, S.; Shao, Z.; Sarven, M.S.; Khan, I.A.; Alam, M.; Saeed, M.; Ullah, H.; Adnan, M.; Saud, S.; et al. Arsenic in a groundwater environment in Bangladesh: Occurrence and mobilization. J. Environ. Manag. 2020, 262, 110318. [Google Scholar] [CrossRef]
- Kousha, M.; Daneshvar, E.; Sohrabi, M.S.; Jokar, M.; Bhatnagar, A. Adsorption of acid orange II dye by raw and chemically modified brown macroalga Stoechospermum marginatum. Chem. Eng. J. 2012, 192, 67–76. [Google Scholar] [CrossRef]
- Daneshvar, E.; Vazirzadeh, A.; Niazi, A.; Sillanpää, M.; Bhatnagar, A. A comparative study of methylene blue biosorption using different modified brown, red and green macroalgae—Effect of pretreatment. Chem. Eng. J. 2017, 307, 435–446. [Google Scholar] [CrossRef]
- Feng, N.; Guo, X.; Liang, S.; Zhu, Y.; Liu, J. Biosorption of heavy metals from aqueous solutions by chemically modified orange peel. J. Hazard. Mater. 2011, 185, 49–54. [Google Scholar] [CrossRef] [PubMed]
- García-Rosales, G.; Olguín, M.T.; Colin-Cruz, A.; Romero-Guzmán, E.T. Effect of the pH and temperature on the biosorption of lead(II) and cadmium(II) by sodium-modified stalk sponge of Zea mays. Environ. Sci. Pollut. Res. 2011, 19, 177–185. [Google Scholar] [CrossRef]
- Wassie, A.B.; Srivastava, V.C. Chemical treatment of teff straw by sodium hydroxide, phosphoric acid and zinc chloride: Adsorptive removal of chromium. Int. J. Environ. Sci. Technol. 2016, 13, 2415–2426. [Google Scholar] [CrossRef] [Green Version]
- Guo, X.; Yang, Z.; Dong, H.; Guan, X.; Ren, Q.; Lv, X.; Jin, X. Simple combination of oxidants with zero-valent-iron (ZVI) achieved very rapid and highly efficient removal of heavy metals from water. Water Res. 2015, 88, 671–680. [Google Scholar] [CrossRef]
- Bulgariu, L.; Bulgariu, D. Enhancing Biosorption Characteristics of Marine Green Algae (Ulva lactuca) for Heavy Metals Removal by Alkaline Treatment. J. Bioprocess. Biotech. 2014, 4, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Yu, Q.; Matheickal, J.T.; Yin, P.; Kaewsarn, P. Heavy metal uptake capacities of common marine macro algal biomass. Water Res. 1999, 33, 1534–1537. [Google Scholar] [CrossRef]
- Teodoro, F.S.; Ramos, S.N.D.C.; Elias, M.M.C.; Mageste, A.B.; Ferreira, G.M.D.; Da Silva, L.H.M.; Gil, L.F.; Gurgel, L.V.A. Synthesis and application of a new carboxylated cellulose derivative. Part I: Removal of Co2+, Cu2+ and Ni2+ from monocomponent spiked aqueous solution. J. Colloid Interface Sci. 2016, 483, 185–200. [Google Scholar] [CrossRef]
- Moreira, V.; Lebron, Y.; Freire, S.; Santos, L.; Palladino, F.; Jacob, R. Biosorption of copper ions from aqueous solution using Chlorella pyrenoidosa: Optimization, equilibrium and kinetics studies. Microchem. J. 2018, 145, 119–129. [Google Scholar] [CrossRef]
- Asere, T.G.; Stevens, C.V.; Du Laing, G. Use of (modified) natural adsorbents for arsenic remediation: A review. Sci. Total. Environ. 2019, 676, 706–720. [Google Scholar] [CrossRef]
- Choi, H.-J.; Lee, S.-M. Heavy metal removal from acid mine drainage by calcined eggshell and microalgae hybrid system. Environ. Sci. Pollut. Res. 2015, 22, 13404–13411. [Google Scholar] [CrossRef] [PubMed]
- Dhar, R.; Zheng, Y.; Rubenstone, J.; van Geen, A. A rapid colorimetric method for measuring arsenic concentrations in groundwater. Anal. Chim. Acta 2004, 526, 203–209. [Google Scholar] [CrossRef]
- Kataria, N.; Garg, V. Green synthesis of Fe3O4 nanoparticles loaded sawdust carbon for cadmium (II) removal from water: Regeneration and mechanism. Chemosphere 2018, 208, 818–828. [Google Scholar] [CrossRef]
- Lee, J.S.; Nriagu, J.O. Stability constants for metal arsenates. Environ. Chem. 2007, 4, 123–133. [Google Scholar] [CrossRef]
- Sun, X.; Huang, H.; Zhu, Y.; Du, Y.; Yao, L.; Jiang, X.; Gao, P. Adsorption of Pb2+ and Cd2+ onto Spirulina platensis harvested by polyacrylamide in single and binary solution systems. Colloids Surf. A Physicochem. Eng. Asp. 2019, 583, 123926. [Google Scholar] [CrossRef]
- Anwar, J.; Shafique, U.; Zaman, W.U.; Salman, M.; Dar, A.; Anwar, S. Removal of Pb(II) and Cd(II) from water by adsorption on peels of banana. Bioresour. Technol. 2010, 101, 1752–1755. [Google Scholar] [CrossRef]
- Gupta, V.K.; Rastogi, A.; Nayak, A. Biosorption of nickel onto treated alga (Oedogonium hatei): Application of isotherm and kinetic models. J. Colloid Interface Sci. 2010, 342, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Vatutsina, O.; Soldatov, V.; Sokolova, V.; Johann, J.; Bissen, M.; Weissenbacher, A. A new hybrid (polymer/inorganic) fibrous sorbent for arsenic removal from drinking water. React. Funct. Polym. 2007, 67, 184–201. [Google Scholar] [CrossRef]
- Batzias, F.; Sidiras, D. Simulation of methylene blue adsorption by salts-treated beech sawdust in batch and fixed-bed systems. J. Hazard. Mater. 2007, 149, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Ozdes, D.; Duran, C.; Senturk, H.B. Adsorptive removal of Cd(II) and Pb(II) ions from aqueous solutions by using Turkish illitic clay. J. Environ. Manag. 2011, 92, 3082–3090. [Google Scholar] [CrossRef]
- Paulino, A.T.; Belfiore, L.A.; Kubota, L.T.; Muniz, E.; Almeida, V.C.; Tambourgi, E.B. Effect of magnetite on the adsorption behavior of Pb(II), Cd(II), and Cu(II) in chitosan-based hydrogels. Desalination 2011, 275, 187–196. [Google Scholar] [CrossRef]
- Segovia-Sandoval, S.J.; Ocampo-Pérez, R.; Berber-Mendoza, M.S.; Leyva-Ramos, R.; Jacobo-Azuara, A.; Medellín-Castillo, N.A. Walnut shell treated with citric acid and its application as biosorbent in the removal of Zn(II). J. Water Process. Eng. 2018, 25, 45–53. [Google Scholar] [CrossRef]
- Sheng, P.X.; Ting, Y.-P.; Chen, J.; Hong, L. Sorption of lead, copper, cadmium, zinc, and nickel by marine algal biomass: Characterization of biosorptive capacity and investigation of mechanisms. J. Colloid Interface Sci. 2004, 275, 131–141. [Google Scholar] [CrossRef]
- Gupta, V.; Rastogi, A. Equilibrium and kinetic modelling of cadmium(II) biosorption by nonliving algal biomass Oedogonium sp. from aqueous phase. J. Hazard. Mater. 2008, 153, 759–766. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).