Source, Partition and Ecological Risk of Polycyclic Aromatic Hydrocarbons in Karst Underground River Environment, Southern China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sample Collection
2.3. Sample Pretreatment and PAHs Analysis
2.4. Quality Control and Quality Assurance
2.5. Data Processing
2.6. Method for Source Analysis of PAHs
2.7. Calculation of Distribution Coefficient
2.8. Methods for Ecological Risk Assessment of PAHs
3. Results and Discussions
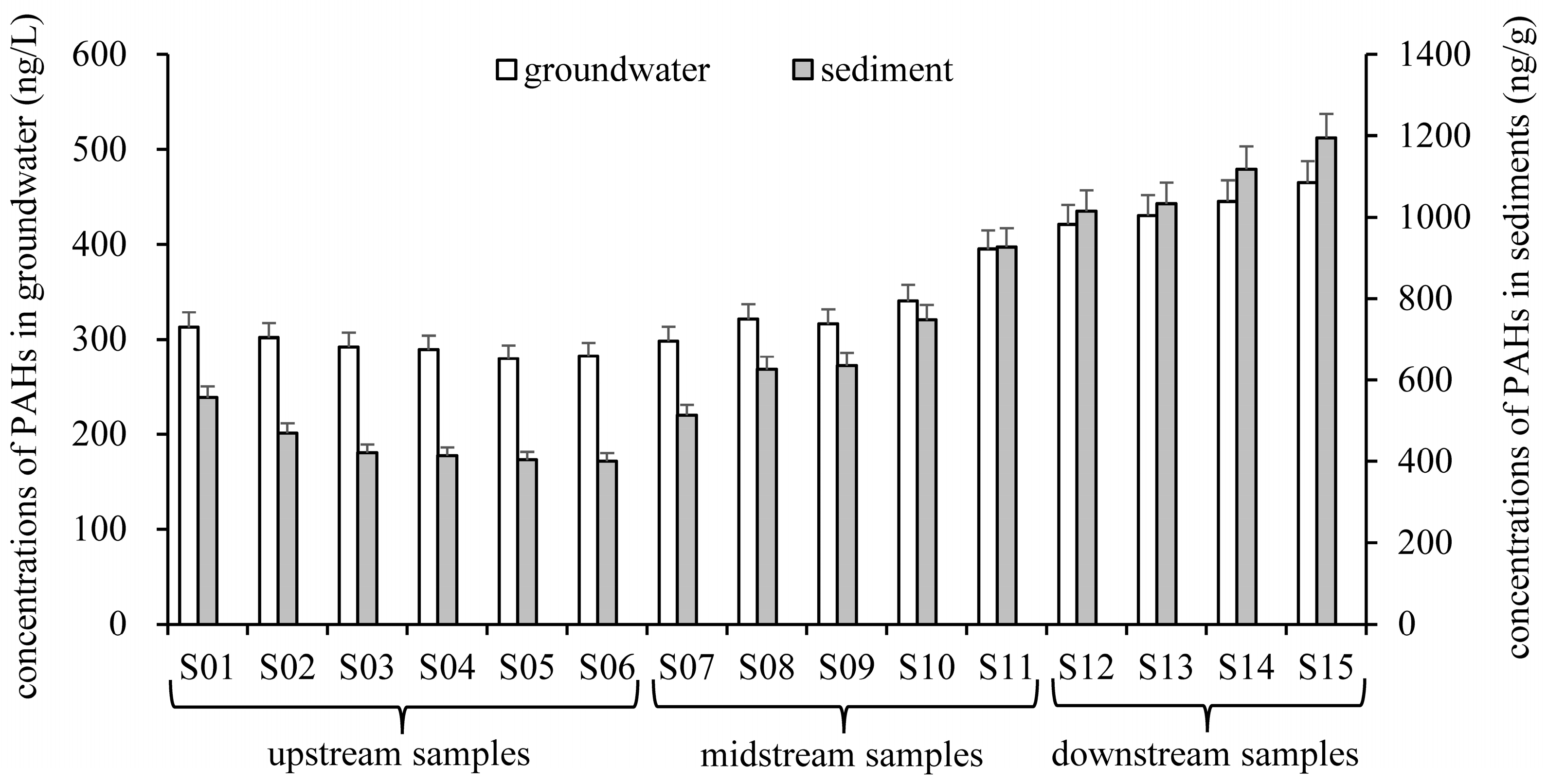
3.1. Contamination Characteristics of PAHs in Groundwater and Sediments
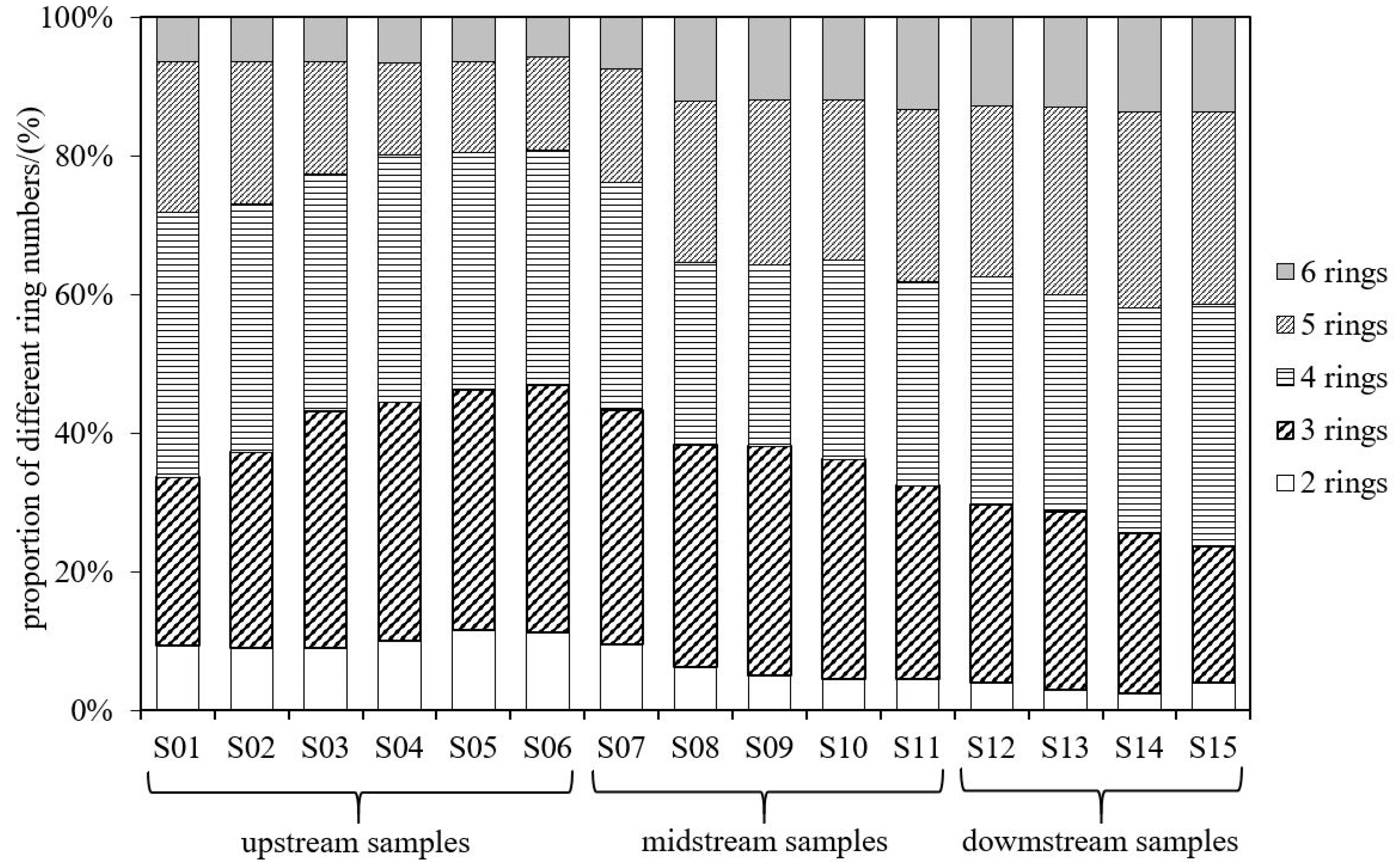
3.1.1. Residue Levels and Composition Characteristics
3.1.2. Concentration and Distribution Characteristics of PAHs
3.2. Source Analysis of PAHs in Groundwater and Sediments
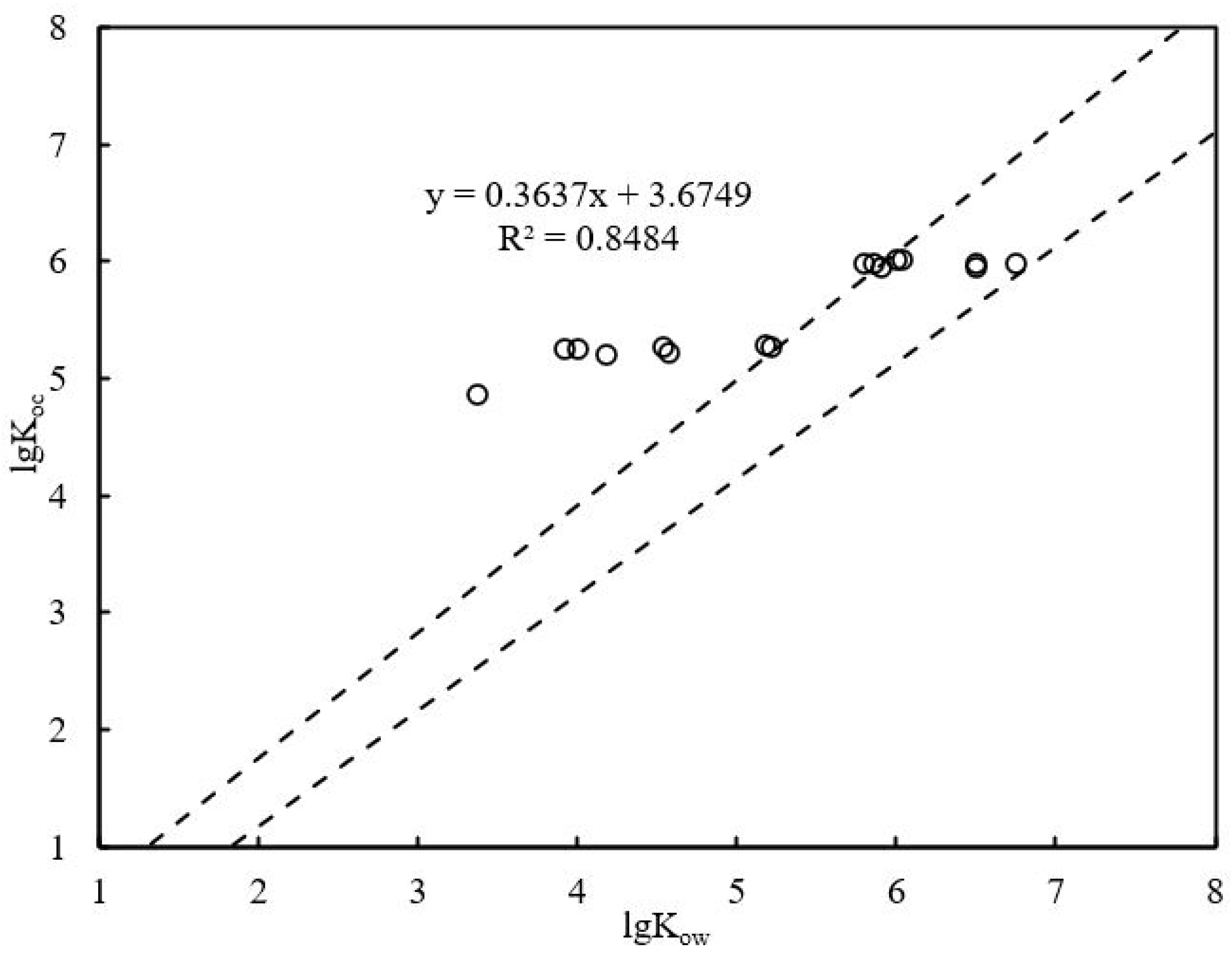
3.3. Partition of PAHs in Groundwater and Sediments
3.4. Ecological Risk Assessment of PAHs in Groundwater and Sediments
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Du, L.L.; Ding, T.T.; Dong, H.J.; Liu, X.X.; Zhang, Y.H.; Zeng, H.H.; He, L.S. Pollution and ecological risk assessment of polycyclic aromatic hydrocarbons in the water environment of Shaying River Basin, China. J. Agro-Environ.Sci. 2020, 39, 601–611. [Google Scholar]
- Wang, C.L.; Zou, Q.X.; Zhao, Y.F.; Li, B.J. Source Apportionment and Ecological Risk Assessment of Polycyclic Aromatic Hydrocarbons in Surface Water from Yangtze River, China: Based on PMF Model. Chin. J. Environ. Sci. 2016, 37, 3789–3797. [Google Scholar]
- Zhang, M.; Tang, F.L.; Zhang, W.; Ma, Q.; Wang, J.P.; Xu, J.F.; Chen, F.; Xu, Z.B. Vertical Profile, Source and Potential Ecological Risk of Polycyclic Aromatic Hydrocarbons in Sediment Cores from the Qingshan Reservoir of Hangzhou. Earth Environ. 2020, 48, 405–412. [Google Scholar]
- Wu, Y.L.; Wang, X.H.; Ya, M.L.; Li, Y.Y.; Hong, H.S. Seasonal variation and spatial transport of polycyclic aromatic hydrocarbons in water of the subtropical Jiulong River watershed and estuary, Southeast China. Chemosphere 2019, 234, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Khumana, S.N.; Chakraborty, P.; Cincinellic, A.; Sonw, D.; Kumar, B. Polycyclic aromatic hydrocarbons in surface waters and riverine sediments of the Hooghly and Brahmaputra Rivers in the Eastern and Northeastern India. Sci. Total Environ. 2019, 636, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Xiong, L.J.; Wu, J.; Sha, C.Y.; Hao, T.; Lin, K.F.; Li, K.F.; Li, D.Y.; Shen, C. Comparison and Source Apportionment of PAHs Pollution of Runoff from Roads in Suburb and Urban Areas of Shanghai. Chin. J. Environ. Sci. 2019, 40, 2240–2248. [Google Scholar]
- Han, R.L.Y.; Rao, R.C.; Dong, Y.C.; Liu, Y.C.; Wang, Q.; Li, J.Y.; Yin, J. Distribution and Health Risk Assessment of Polycyclic Aromatic Hydro-carbons in Surface Water, Sediments and Organisms of Dishui Lake and Its Surrounding Water System. Asian J. Ecotoxicol. 2020, 15, 240–251. [Google Scholar]
- Yin, S.; Bai, J.H.; Wen, X.J.; Zhang, G.L.; Han, L.; Hu, X.Y. Effects of polycyclic aromatic hydrocarbon(phenanthrene)addition on soil nitrogen mineralization processes in rural and urban river wetlands of the Pearl River Estuary, China. J. Agro-Environ. Sci. 2019, 38, 600–608. [Google Scholar]
- Liu, Q.; Gao, J.H.; Shi, Y.; Ai, Q.; Sheng, H.; Zhang, D.L.; Li, J.; Bai, F.L. Distribution characteristics and controlling factors of PAHs in surface sediments in north of North Yellow sea. Mar. Environ. Sci. 2020, 39, 53–58. [Google Scholar]
- Hu, J.; Liu, C.Q.; Guo, Q.J.; Yang, J.; Okoli, C.P.; Lang, Y.C.; Zhao, Z.Q.; Li, S.L.; Liu, B.J.; Song, G.W. Characteristics, source, and potential ecological risk assessment of polycyclic aromatic hydrocarbons (PAHs) in the Songhua River Basin, Northeast China. Environ. Pollut. Res. 2017, 24, 17090–17102. [Google Scholar] [CrossRef]
- Mahler, B.J.; Van Metre, P.C.; Foreman, W.T. Concentrations of polycyclic aromatic hydrocarbons (PAHs) and azaarenes in runoff from coal-tar- and asphalt-sealcoated pavement. Environ. Pollut. 2014, 188, 81–87. [Google Scholar] [CrossRef]
- Yuan, H.Z.; Liu, E.F.; Zhang, E.L.; Luo, W.L.; Chen, L.; Wang, C.; Lin, Q. Historical records and sources of polycyclic aromatic hydrocarbons (PAHs) and organochlorine pesticides (OCPs) in sediment from a representative plateau lake, China. Chemosphere 2017, 173, 78–88. [Google Scholar] [CrossRef]
- Yu, W.W.; Liu, R.M.; Xu, F.; Men, C.; Shen, Z.Y. Identifications and seasonal variations of sources of polycyclic aromatic hydrocarbons (PAHs) in the Yangtze River Estuary, China. Mar. Poll. Bull. 2016, 104, 347–354. [Google Scholar] [CrossRef]
- Lu, L.; Wang, Z.; Pei, J.G. Pollution Characteristics of Polycyclic Aromatic Hydrocarbons in Surface Sediments from Typical Underground River in Southwest China. Environ. Sci. Technol. 2019, 42, 51–56. [Google Scholar]
- Lan, J.C.; Sun, Y.C.; Hu, N. Changes of the concentration and composition of polycyclic aromatic hydrocarbons and their ecological risk assessment during the scale of rainfall events in a karst underground river. Environ. Chem. 2018, 37, 2049–2059. [Google Scholar]
- Song, Y.M.; Wang, C.; Liu, S.; Pan, J.C.; Guo, P.R. Distribution, Sources, and Health Risk Assessment of PAHs in Water Supply Source Regions of Guangzhou. Chin. J. Environ. Sci. 2019, 40, 3489–3500. [Google Scholar]
- Tian, D.N.; Dang, H.L.; Ding, R.M.; Cai, Q.; Zhang, P.J.; Wang, L.; Yang, H.F. Distribution, Sources, and Ecological Risk Assessment of Polycyclic Aromatic Hydrocarbons in the Surface Waters of the Yinchuan Wetlands. Chin. J. Environ. Sci. 2019, 40, 3068–3077. [Google Scholar]
- Karickhoff, S.W.; Brown, D.S.; Scott, T.A. Sorption of hydrophobic pollutants on natural sediments. Water. Res. 1979, 13, 241–248. [Google Scholar] [CrossRef]
- McGeoddy, S.E.; Farrington, J.W. Sediment porewater partitioning of polycyclic aromatic hydrocarbons in three cores from Boston Harbor, Massachusetts. Environ. Sci. Technol. 1995, 29, 1542–1550. [Google Scholar] [CrossRef] [PubMed]
- Kalf, D.F.; Crommentuijn, T.; Plassche, E.J. Environmental quality objectives for 10 polycyclic aromatic hydrocarbons (PAHs). Ecotoxicol. Environ. Saf. 1997, 36, 89–97. [Google Scholar] [CrossRef]
- Cao, Z.G.; Liu, J.L.; Luan, Y.; Ma, M.Y.; Xu, J.; Han, S.L. Distribution and ecosystem risk assessment of polycyclic aromatic hydrocarbons in the Luan River, China. Ecotoxicology 2010, 19, 827–837. [Google Scholar] [CrossRef]
- Long, E.R.; Macdonald, D.D.; Smith, S.L.; Calder, F.D. Incidence of adverse biological effects within ranges of chemical concentrations in marine and estuarine sediments. Environ. Manag. 1995, 19, 81–97. [Google Scholar] [CrossRef]
- Shao, Y.X. A Dissertation Submitted to China University of Geosciences for the Doctor Degree of Environmental Science and Engineering. Ph.D. Thesis, China University of Geosciences, Wuhan, China, 2014. [Google Scholar]
- Zhang, Q.; Hu, W.; Wu, X.; Pan, E.C.; Xu, Q.J.; Liang, C.Z.; He, Y.; Wang, C. Polycyclic Aromatic Hydrocarbons Levels in Drinking Water Sources in Rural Areas in a City of Jiangsu Province. J. Environ. Occupat. Med. 2016, 33, 691–694. [Google Scholar]
- SUN, Y.C.; Zhang, S.Y.; Lan, J.C.; Xie, Z.L.; Pu, J.B.; Yuan, D.X.; Yang, H.; Xing, B.S. Vertical migration from surface soils to groundwater and source appointment of polycyclic aromatic hydrocarbons in epikarst spring systems, southwest China. Chemosphere 2019, 230, 616–627. [Google Scholar] [CrossRef] [PubMed]
- Lan, J.C.; SUN, Y.C.; Yuan, D.X. Transport of polycyclic aromatic hydrocarbons in a highly vulnerable karst underground river system of southwest China. Environ. Sci. Pollut. Res. Int. 2018, 25, 34519–34530. [Google Scholar] [CrossRef]
- Wang, W.Y.; Zhang, J.; Li, X.F.; Li, Y.X.; Hou, H.; Wang, L.Q.; Li, F.S. Characteristics and sources of polycyclic aromatic hydrocarbons pollution in water of Xi’an outskirt regions. Agric. Res. Arid Areas 2015, 33, 201–206. [Google Scholar]
- Xie, Z.L.; Sun, Y.C.; Zhang, M.; Liao, Y.; Wang, Z.B.; Liang, Z.B. Distribution Characteristics and Source Identification of Polycyclic Aromatic Hydrocarbons, Fatty Acids in Water of Karst Underground River. Chin. J. Environ. Sci. 2016, 37, 2547–2555. [Google Scholar]
- Miao, Y.; Kong, X.S.; Li, C.Z. Distribution and Sources of Polycyclic Aromatic Hydrocarbons in a Karst Groundwater System in a Strongly Industrial City. Chin. J. Environ. Sci. 2019, 40, 239–247. [Google Scholar]
- Jia, X.Q.; Wang, H.T.; Zhou, H. Investigation of Organochlorine Pesticides and Polycyclic Aromatic Hydrocarbons in Water Environment of Karst Agricultural Region—Taking the Huangliang Area of Hubei Province as an Example. Saf. Environ. Eng. 2018, 25, 61–66. [Google Scholar]
- Geng, M.J.; Chang, S.; Liu, Y.; Wang, S.J.; Han, X.Y. Pollution status and health risks of drinking water of the polycyclic aromatic hydrocarbons and phthalate esters in the deep shallow pore water of Hutuo River Pluvial Fan. Chin. J. Environ. Sci. 2016, 36, 3824–3830. [Google Scholar]
- Gong, X.Y.; He, Y.Z.; Sun, Y.L. Distribution and source of polycyclic aromatic hydrocarbons in groundwater in the upper region of Sihu Lake Basin from Jianghan Plain. Acta Sci. Circumst. 2015, 35, 789–796. [Google Scholar]
- Zhao, Y.; Dang, J.H.; Wang, F. Spatial Variety and Ecological Risk of Polycyclic Aromatic Hydrocarbons (PAHs) in Water and Surface Sediments of Fenhe River Basin, China. Asian J. Ecotoxicol. 2017, 12, 579–596. [Google Scholar]
- Liu, S.P.; Li, X.G.; Zhang, D.H.; Li, J.F.; Han, B. Distribution, source and ecological risk assessment of polycyclic aromatic hydrocarbons (PAHs) in surface sediments from Moshui River. Environ. Chem. 2018, 37, 843–850. [Google Scholar]
- Li, S.; Liu, Y.Z.; Liu, R.Y.; Xiang, X.X.; Zhang, S.C.; Lu, Y.T. The pollution characteristics and risk assessment of polycyclic aromatic hydrocarbons of Shen-Fu section of the Hun River Basin. China Environ. Sci. 2019, 39, 1551–1559. [Google Scholar]
- Yang, Y.Y.; Wang, H.Y.; Chang, Y.; Yan, G.K.; Chu, Z.S.; Zhao, Z.J.; Li, L.; Li, Z.W.; Wu, T. Distributions, compositions, and ecological risk assessment of polycyclic aromatic hydrocarbons and phthalic acid esters in surface sediment of Songhua river, China. Mar. Poll. Bull. 2019, 152, 110923. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.S.; Sun, X.P.; Zheng, X.; Sun, J.; Zhang, X.L.; Liu, K. Analysis of the contamination characteristics and the eco-risk assessment of PAHs in sediments from the Three Gorges section in Chongqing area. J. Saf. Environ. 2017, 17, 1548–1553. [Google Scholar]
- Han, X.Y.; Chang, S.; Fu, Q.; Wang, S.J.; Yang, G.; Zhao, X.R.; Geng, M.J. Pollution Characteristics and Ecological Risk Assessment of Polycyclic Aromatic Hydrocarbons in Qingyuan section of Beijiang River in Flood Season. Res. Environ. Sci. 2018, 31, 61–69. [Google Scholar]
- Lan, J.C.; Sun, Y.C.; Xiao, S.Z. Water-Sediment Partition of Polycyclic Aromatic Hydrocarbons in Karst Underground River. Chin. J. Environ. Sci. 2015, 11, 4081–4087. [Google Scholar]
- He, S.H.; Cao, X.C.; Li, T.Y.; Zhang, M.S.; Liang, Y.; Chen, B.J. Distribution, source and ecological risk assessment of polycyclic aromatic hydrocarbons (PAHs) in surface sediments from Sanya River. Environ. Chem. 2019, 4, 967–970. [Google Scholar]
- Dong, L.; Tang, X.Q.; Lin, L.; Li, C.; Li, R.; Wu, M. Pollution Characteristics and Source Identification of Polycyclic Aromatic Hydrocarbons and Phthalic Acid Esters During High Water Level Periods in the Wuhan Section of the Yangtze River, China. Chin. J. Environ. Sci. 2018, 6, 2588–2899. [Google Scholar]
- Kong, X.S.; Luan, R.J.; Miao, Y.; Qi, S.H.; Li, F. Polycyclic aromatic hydrocarbons in sediment cores from the Dashiwei Tiankeng reach in the Bailang underground river, South China. Environ. Earth Sci. 2015, 73, 5535–5543. [Google Scholar] [CrossRef]
- Hu, Y.Q.; Sun, Y.C.; Li, X.L.; Cao, M.; Yuan, D.X. Spatial Distribution and Ecological Risk Assessment of PAHs in Different Karst Underground Rivers Based on Species Sensitivity Distribution. Earth Environ. 2021. online. [Google Scholar]


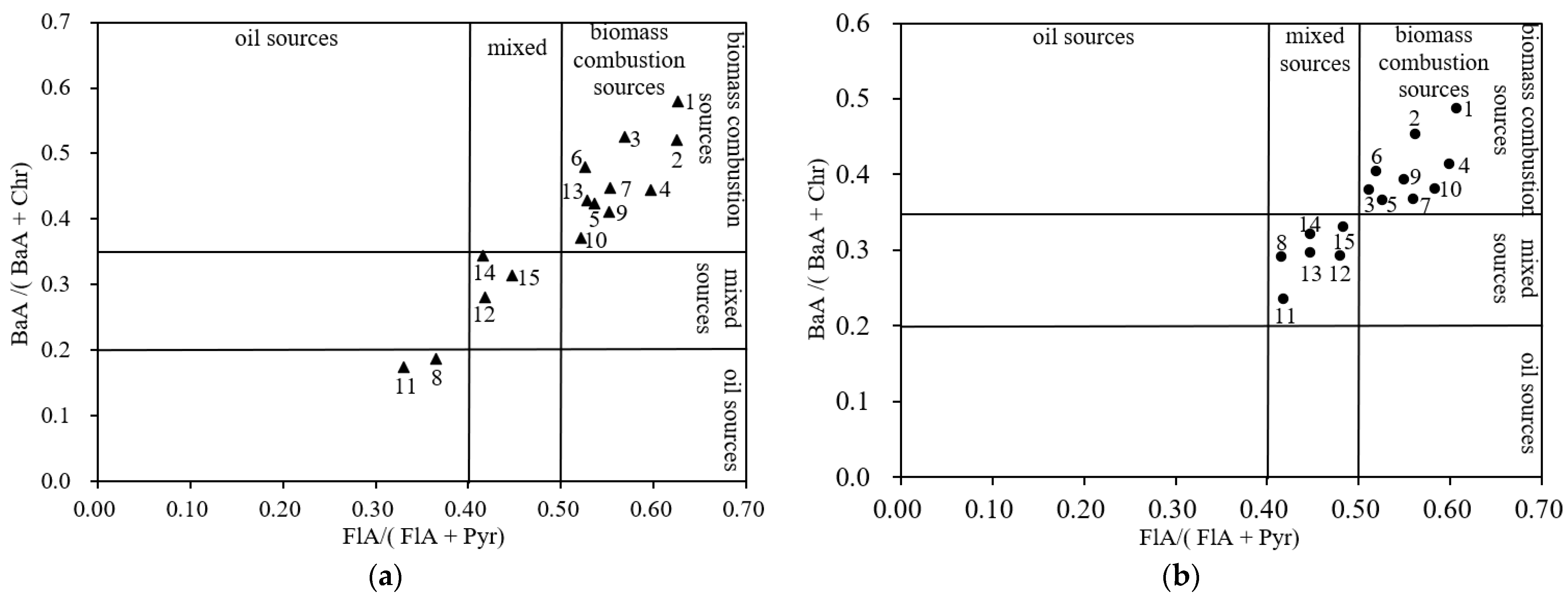

| PAHs Ratio | PAHs Source | ||
|---|---|---|---|
| FlA/(FlA + Pyr) | Oil sources | Combustion sources of petroleum | Combustion sources of grass, wood, coal |
| <0.4 | 0.4–0.5 | >0.5 | |
| BaA/(BaA + Chr) | Oil sources | Mixed sources of oil and combustion | Combustion source |
| <0.2 | 0.2–0.35 | >0.35 | |
| PAHs | Groundwater (ng/L) | Sediments (ng/g) | ||||
|---|---|---|---|---|---|---|
| Minimum | Maximum | Average | Minimum | Maximum | Average | |
| Nap | 31.02 | 55.98 | 45.24 | 25.48 | 47.41 | 32.98 |
| Acy | 5.15 | 14.43 | 12.87 | 17.74 | 57.93 | 22.61 |
| Ace | 6.75 | 13.72 | 12.87 | 21.56 | 61.49 | 23.20 |
| Flu | 7.18 | 31.32 | 18.89 | 20.60 | 58.68 | 29.78 |
| Phe | 25.98 | 50.59 | 36.55 | 40.53 | 109.98 | 61.15 |
| Ant | 3.93 | 7.98 | 6.14 | 7.71 | 24.47 | 11.27 |
| FlA | 24.22 | 49.86 | 32.45 | 32.91 | 116.53 | 60.71 |
| Pyr | 20.13 | 70.20 | 43.31 | 35.89 | 119.61 | 82.64 |
| BaA | 9.77 | 45.14 | 15.89 | 21.93 | 61.25 | 35.63 |
| Chr | 17.22 | 75.12 | 28.35 | 37.58 | 118.71 | 67.90 |
| BbF | 9.24 | 29.54 | 21.21 | 12.75 | 106.19 | 52.07 |
| BkF | 7.70 | 29.21 | 13.87 | 10.74 | 87.30 | 36.45 |
| BaP | 6.67 | 38.50 | 22.49 | 12.57 | 87.14 | 48.39 |
| InP | 2.79 | 19.14 | 8.55 | 15.75 | 77.23 | 30.42 |
| DaA | 5.91 | 25.15 | 13.17 | 10.69 | 78.46 | 49.62 |
| BgP | 6.28 | 27.75 | 14.20 | 11.46 | 89.76 | 54.71 |
| 2–3 ring | 113.04 | 140.44 | 132.56 | 135.63 | 338.31 | 180.99 |
| 4 ring | 94.21 | 212.07 | 120.00 | 132.67 | 390.61 | 246.87 |
| 5–6 ring | 42.38 | 135.01 | 93.49 | 80.91 | 505.66 | 271.67 |
| ∑16PAHs | 282.42 | 464.88 | 346.05 | 400.14 | 1194.69 | 699.53 |
| Research Area | ∑16PAHs Range (Average)/(ng/L) | Place | Number of Main Rings | Sampling Time |
|---|---|---|---|---|
| Guozhuang karst spring | 2137–9037 (5020) | Linfen City, Shanxi Province, China | 2–3 | 2012 |
| Huaian drinking water source | 2700.9 | Huai an City, Jiangsu Province, China | 2 | 2012 |
| Epikarst spring of Nanchuan, Chongqing | 341–4968 (1772) | Nanchuan District, Chongqing City, China | 2–3 | 2010–2011 |
| Laolongdong underground river | 211.7–970.4 (442.2) | Banan District, Chongqing City, China | 2–3 | 2013 |
| Groundwater on the outskirts of Xi’an | 247.05–1867.18 (415.79) | Xi’an City, Shaanxi Province, China | 5–6 | 2013 |
| Qingmuguan underground river | 77.3–702 (398) | Shapingba District, Chongqing City, China | 2–3 | 2013 |
| Groundwater in Liuzhou industrial zone of Guangxi Province | Dry season: 128.61–562.66 (302.02) Wet season: 61.19–899.39 (367.13) | Liuzhou City, Guangxi Province, China | 4 | 2015 |
| Groundwater in the Huang liang area of Hubei Province | 84.79–263.74 | Yichang City, Hubei Province, China | 2–3 | 2016 |
| Deep shallow pore water of Hutuo River Pluvial Fan | 34.4–598.5 (249.7) | Shijiazhuang City, Hebei Province, China | 2–3 | 2015 |
| Groundwater in the upper region of Sihu Lake Basin from Jianghan Plain | Dry season: 62.74–224.63 (129.77) Wet season: 55.86–115.15 (82.85) | Qianjiang City, Hubei Province, China | 2–3 | 2011–2012 |
| Qing-shui Spring underground river | 279.62–464.88 (346.05) | Nanning City, Guangxi Province, China | 2–3 | 2018 |
| Research Area | ∑16PAHs Range (Average)/(ng/g) | Place | Number of Main Rings | Sampling Time |
|---|---|---|---|---|
| Fenhe River | 1290–8440 (3774) | Shanxi Province, China | 3 | 2013 |
| Moshui River | 196.51–8549.33 (3320.03) | Qingdao City, Shandong Province, China | 5–6 | 2015 |
| Hun River | 95.19–8444.21 (2518.42) | Shenyang City, Liaoning Province, China | 3–5 | 2015–2016 |
| Songhua River | 226.70–7086.62 (1179.62) | Heilongjiang Province, China | 4 | 2016 |
| Three Gorges reservoir area (Chongqing Section) | 68.6–4226 (685) | Chongqing City, China | 5–6 | 2014 |
| Beijiang | 54.4–819.8 (424.9) | Guangdong Province, China | 4–6 | 2016 |
| Laolongdong underground river | 58.2–1051 (367.9) | Chongqing City, China | 2–3 | 2012–2013 |
| Sanya River | 3.23–49 3(211) | Sanya City, Hainan Province, China | 4 | 2016 |
| Yangtze River (Wuhan section) | 46.1–424.0 (191.8) | Wuhan City, Hubei Province, China | 2–3 | 2016 |
| Dashiwei Bailang underground river | 35.34–280.17 (107.65) | Leye County, Guangxi Province, China | 5 | 2007 |
| Qing-shui Spring underground river | 405.78–1194.69 (699.53) | Nanning City, Guangxi Province, China | 5–6 | 2018 |
| PAHs | NCs/(ng/L) | MPCs/ (ng/L) | Overall RQNCs | Overall RQMPCs | Upstream RQNCs | Upstream RQMPCs | Midstream RQNCs | Midstream RQMPCs | Downstream RQNCs | Downstream RQMPCs |
|---|---|---|---|---|---|---|---|---|---|---|
| Nap | 12 | 1200 | 3.77 | 0.04 | 4.11 | 0.04 | 3.16 | 0.03 | 4.02 | 0.04 |
| Acy | 0.7 | 70 | 18.39 | 0.18 | 19.60 | 0.20 | 20.64 | 0.21 | 13.76 | 0.14 |
| Ace | 0.7 | 70 | 18.38 | 0.18 | 20.25 | 0.20 | 17.01 | 0.17 | 17.29 | 0.17 |
| Flu | 0.7 | 70 | 26.98 | 0.27 | 33.34 | 0.33 | 33.44 | 0.33 | 9.37 | 0.09 |
| Phe | 3 | 300 | 12.18 | 0.12 | 10.20 | 0.10 | 14.30 | 0.14 | 12.51 | 0.13 |
| Ant | 0.7 | 70 | 8.78 | 0.09 | 9.31 | 0.09 | 8.48 | 0.08 | 8.36 | 0.08 |
| FlA | 3 | 300 | 10.82 | 0.11 | 10.68 | 0.11 | 8.86 | 0.09 | 13.47 | 0.13 |
| Pyr | 0.7 | 70 | 61.87 | 0.62 | 42.33 | 0.42 | 62.30 | 0.62 | 90.63 | 0.91 |
| BaA | 0.1 | 10 | 158.90 | 1.59 | 136.77 | 1.37 | 130.54 | 1.31 | 227.56 | 2.28 |
| Chr | 3.4 | 340 | 8.34 | 0.08 | 4.24 | 0.04 | 9.03 | 0.09 | 13.61 | 0.14 |
| BbF | 0.1 | 10 | 212.11 | 2.12 | 126.35 | 0.96 | 200.05 | 2.20 | 355.82 | 3.76 |
| BkF | 0.4 | 40 | 34.68 | 0.35 | 25.98 | 0.26 | 35.20 | 0.35 | 47.08 | 0.47 |
| BaP | 0.5 | 50 | 44.98 | 0.45 | 31.79 | 0.32 | 38.57 | 0.39 | 72.77 | 0.73 |
| InP | 0.4 | 40 | 21.38 | 0.21 | 18.41 | 0.18 | 13.28 | 0.13 | 35.97 | 0.36 |
| DaA | 0.5 | 50 | 26.34 | 0.26 | 20.66 | 0.21 | 21.90 | 0.22 | 40.41 | 0.40 |
| BgP | 0.3 | 30 | 47.33 | 0.47 | 31.24 | 0.31 | 42.81 | 0.43 | 77.11 | 0.77 |
| ∑16PAHs | 27.2 | 2720 | 12.72 | 0.13 | 10.77 | 0.11 | 12.29 | 0.12 | 16.19 | 0.16 |
| PAHs | ERL | ERM | Overall Average Value | Upstream Average Value | Midstream Average Value | Downstream Average Value |
|---|---|---|---|---|---|---|
| Nap | 160 | 2100 | 32.98 | 34.59 | 29.14 | 35.37 |
| Acy | 16 | 500 | 22.61 | 15.27 | 23.56 | 32.44 |
| Ace | 44 | 640 | 23.20 | 11.23 | 18.63 | 46.86 |
| Flu | 19 | 540 | 29.78 | 18.78 | 29.16 | 47.07 |
| Phe | 240 | 1500 | 61.15 | 51.37 | 75.01 | 58.49 |
| Ant | 85.3 | 1100 | 11.27 | 8.64 | 9.92 | 16.90 |
| FlA | 600 | 5100 | 60.71 | 47.34 | 49.37 | 94.91 |
| Pyr | 665 | 2600 | 82.64 | 51.18 | 71.57 | 143.66 |
| BaA | 261 | 1600 | 35.63 | 30.61 | 30.98 | 48.97 |
| Chr | 384 | 2800 | 67.90 | 42.17 | 66.27 | 108.54 |
| BbF | 52.07 | 23.86 | 48.74 | 98.53 | ||
| BkF | 36.45 | 16.41 | 37.65 | 65.01 | ||
| BaP | 430 | 1600 | 48.39 | 22.79 | 47.37 | 88.08 |
| InP | 30.42 | 13.47 | 32.32 | 53.47 | ||
| DaA | 63.4 | 260 | 49.62 | 26.77 | 48.12 | 85.76 |
| BgP | 54.71 | 20.08 | 59.95 | 100.12 | ||
| ∑16PAHs | 4022 | 40792 | 699.53 | 446.90 | 689.78 | 1090.67 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Li, J.; Lu, L.; Cao, J.; Zhao, L.; Luan, S. Source, Partition and Ecological Risk of Polycyclic Aromatic Hydrocarbons in Karst Underground River Environment, Southern China. Water 2021, 13, 2655. https://doi.org/10.3390/w13192655
Wang Z, Li J, Lu L, Cao J, Zhao L, Luan S. Source, Partition and Ecological Risk of Polycyclic Aromatic Hydrocarbons in Karst Underground River Environment, Southern China. Water. 2021; 13(19):2655. https://doi.org/10.3390/w13192655
Chicago/Turabian StyleWang, Zhe, Jiang Li, Li Lu, Jianwen Cao, Liangjie Zhao, and Song Luan. 2021. "Source, Partition and Ecological Risk of Polycyclic Aromatic Hydrocarbons in Karst Underground River Environment, Southern China" Water 13, no. 19: 2655. https://doi.org/10.3390/w13192655





