Various Natural and Anthropogenic Factors Responsible for Water Quality Degradation: A Review
Abstract
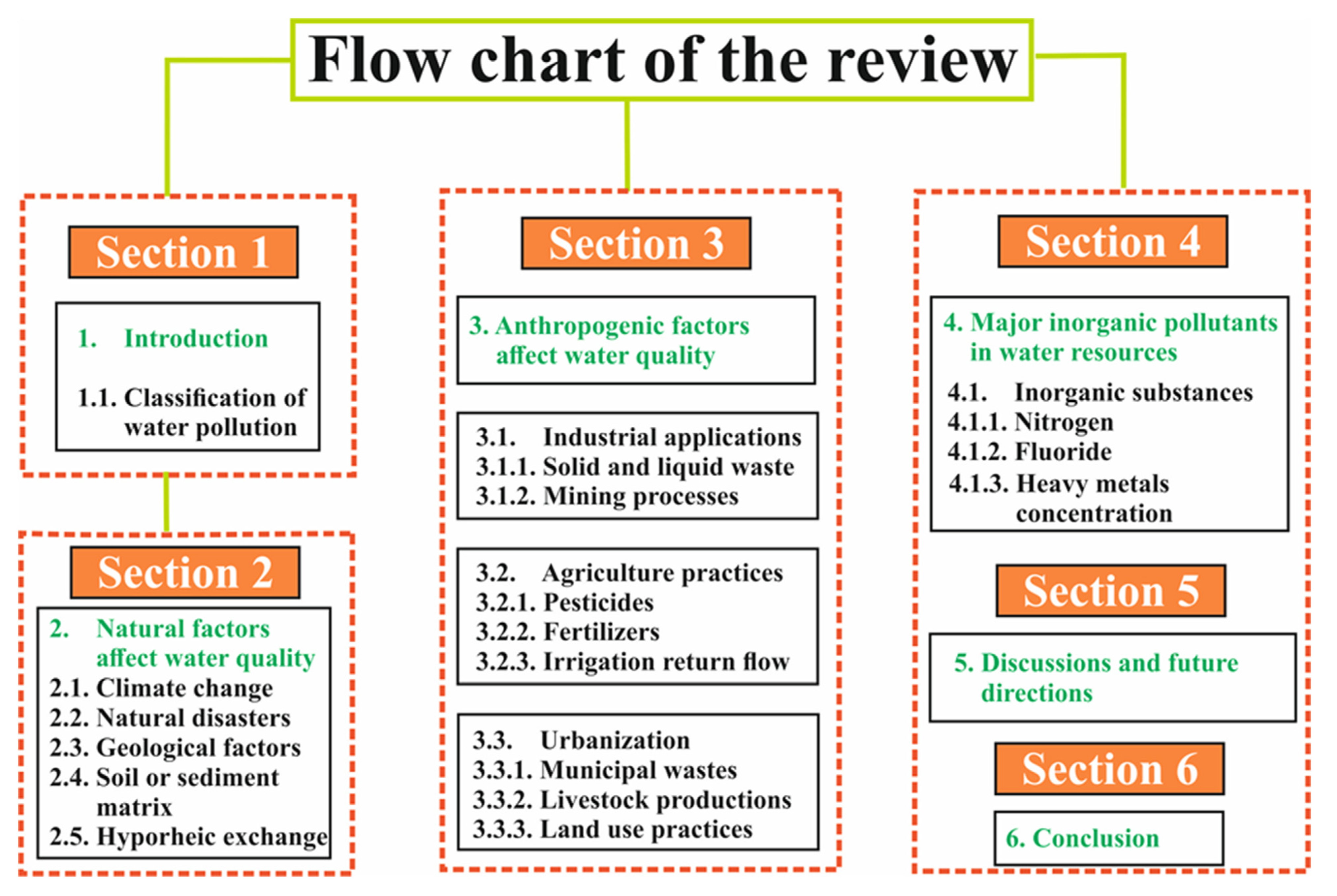
:1. Introduction
Classification of Water Pollution
- I.
- Sources discharging substances because of other planned activities
- II.
- Sources providing conduit or inducing discharge by altered flow patterns
- III.
- Naturally occurring sources; where the discharge is created and/or exacerbated by human activity
- IV.
- Sources designed to retain substances during transport or transmission; discharge by accident or negligence
- V.
- Sources designed to store, treat, and/or dispose of substances; discharge through the unplanned release
- VI.
- Sources designed to discharge substances
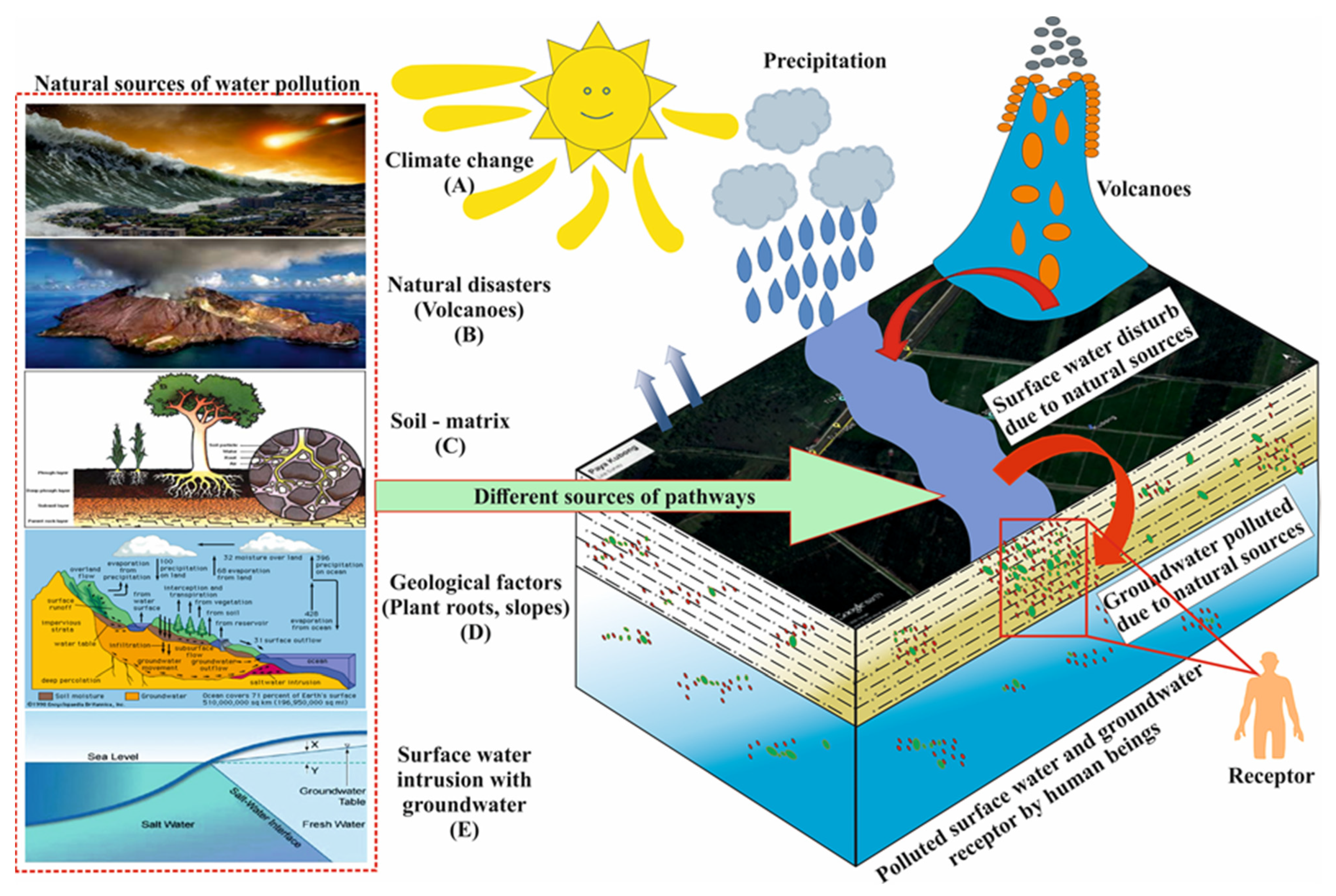
2. Effect of Natural Factors to Water Quality
2.1. Climate Change
2.2. Natural Disasters
2.3. Geological Factors
2.4. Soil or Sediment Matrix
2.5. Hyporheic Exchange
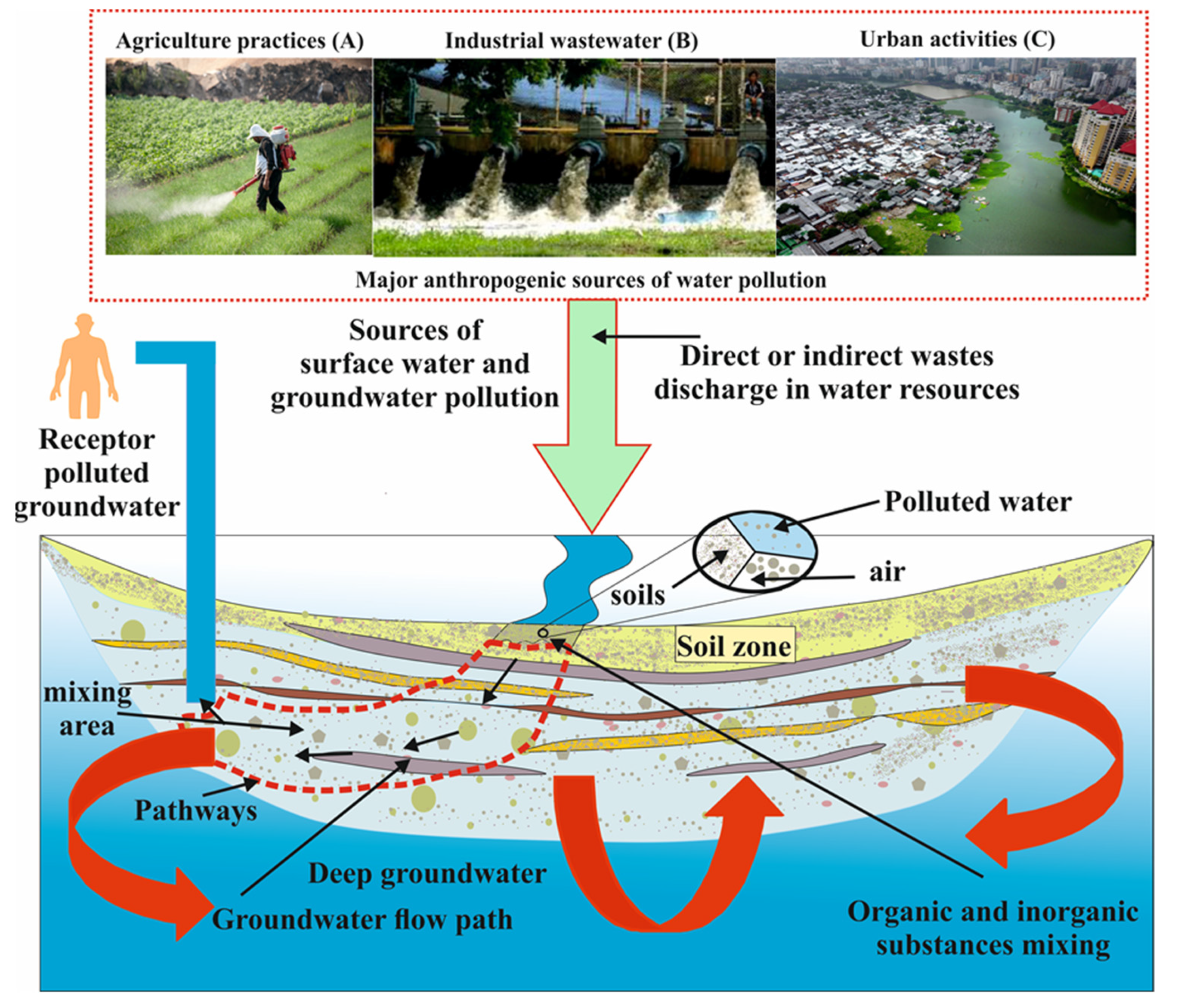
3. Effects of Anthropogenic Factors to Water Quality
3.1. Industrial Applications
3.1.1. Solid and Liquid Waste
3.1.2. Mining Processes
3.2. Agriculture Practices
3.2.1. Pesticides
3.2.2. Fertilizers
3.3. Urbanization
3.3.1. Municipal Wastes
- The construction and maintenance of roads, including impervious surfaces, can adversely influence water quality because of higher rushes, lower groundwater recharge rates, and increased erosion.
- Pollutants, including oil, vehicle exhaust, dirt, and de-icing chemicals, are deposited into roadways and streams’ dehydration.
- Oil spills, especially on the marine side, affect the water quality of inland waterways and coastal regions.
- Leaking subsurface storage tanks release petroleum into groundwater.
3.3.2. Livestock Productions
3.3.3. Land Use Practices
4. Major Pollutants of Water Resources
4.1. Inorganic Substances
4.1.1. Nitrogen
4.1.2. Fluoride
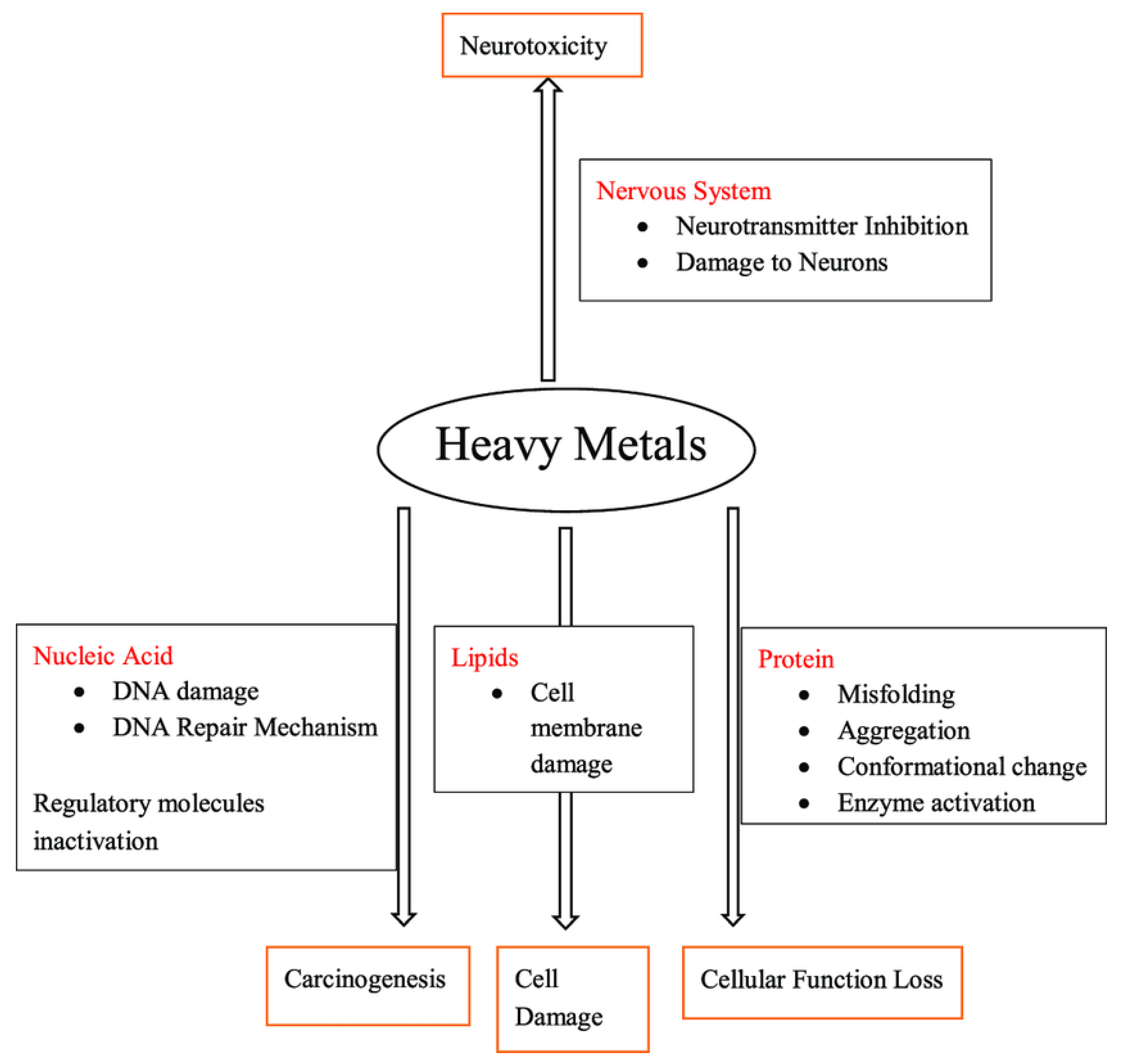
4.2. Sources of Heavy Metals
4.2.1. Heavy Metals Risk in the Environment
4.2.2. Heavy Metals Risk on the Plant
4.2.3. Heavy Metals Risk on the People Health
| Heavy Metals | Source | Pollution Type | Regions/Countries | GW Maximum Concentration | References |
|---|---|---|---|---|---|
| Fluoride | Indusrial | Wastewater | Roopnagar, Delhi, India | 7.4 mg/L | [165] |
| Agriculture fertilizers | Infiltration | Pampa, Argentina | 21.1 mg/L | [166] | |
| Municipal | Waste material | Taiwan | 1.81 mg/L | [167] | |
| Power plant | Thermal Water | China | 50 mg/L | [143] | |
| Nitrate | Agriculture fertilizers | Infiltration | Jharkhand, India | 319.1 mg/L | [168] |
| Livestock farms and landfill | Wastematerial | Beijing, China | 1736 mg/L | [131] | |
| Industrial hazardous | Wastematerial | Liaohe River, China | 175 mg/L | [133] | |
| Anthropogenic activities | Chemical fertilizer | Sicily, Italy | 225 mg/L | [135] |
5. Discussion and Future Directions
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Akhtar, N.; Ishak, M.; Ahmad, M.; Umar, K.; Yusuff, M.M.; Anees, M.; Qadir, A.; Almanasir, Y.A. Modification of the Water Quality Index (WQI) Processfor Simple Calculation Using the Multi-Criteria Decision-Making(MCDM) Method: A Review. Water 2021, 13, 905. [Google Scholar] [CrossRef]
- Khatri, N.; Tyagi, S. Influences of natural and anthropogenic factors on surface and groundwater quality in rural and urban areas. Front. Life Sci. 2014, 8, 23–39. [Google Scholar] [CrossRef]
- Akhtar, N.; Syakir, M.I.; Rai, S.P.; Saini, R.; Pant, N.; Anees, M.T.; Qadir, A.; Khan, U. Multivariate Investigation of Heavy Metals in the Groundwater for Irrigation and Drinking in Garautha Tehsil, Jhansi District, India. Anal. Lett. 2019, 53, 774–794. [Google Scholar] [CrossRef]
- Nagaraju, A.; Thejaswi, A.; Sreedhar, Y. Assessment of Groundwater Quality of Udayagiri area, Nellore District, Andhra Pradesh, South India Using Multivariate Statistical Techniques. Earth Sci. Res. J. 2016, 20, 1. [Google Scholar] [CrossRef] [Green Version]
- Macdonald, A.M.; Davies, J.; Dochartaigh, B.E.O. Simple methods for assessing groundwater resources in low permeability areas of Africa. British Geological Survey Commissioned Report, CR/01/168N. South Africa. Br. Geol. Surv. 2002, 71. [Google Scholar] [CrossRef]
- Trabelsi, R.; Zouari, K. Coupled geochemical modeling and multivariate statistical analysis approach for the assessment of groundwater quality in irrigated areas: A study from North Eastern of Tunisia. Groundw. Sustain. Dev. 2019, 8, 413–427. [Google Scholar] [CrossRef]
- Akhtar, N.; Rai, S.P. Heavy Metals Concentrations in Drinking Water and Their Effect on Public Health around Moth Block of Jhansi District, Uttar Pradesh, India. Indian J. Environ. Prot. 2019, 39, 945–953. [Google Scholar]
- Sasakova, N.; Gregova, G.; Takacova, D.; Mojzisova, J.; Papajová, I.; Venglovsky, J.; Szaboova, T.; Kovacova, S. Pollution of Surface and Ground Water by Sources Related to Agricultural Activities. Front. Sustain. Food Syst. 2018, 2. [Google Scholar] [CrossRef] [Green Version]
- Manjunatha, S.; Bobade, K.; Kudale, M. Pre-cooling Technique for a Thermal Discharge from the Coastal Thermal Power Plant. Procedia Eng. 2015, 116, 358–365. [Google Scholar] [CrossRef] [Green Version]
- Issakhov, A. Numerical Study of the Discharged Heat Water Effect on the Aquatic Environment from Thermal Power Plant by using Two Water Discharged Pipes. Int. J. Nonlinear Sci. Numer. Simul. 2017, 18, 469–483. [Google Scholar] [CrossRef]
- USEPA. Protecting Water Quality from Agricultural Runoff; United State Enviromental Protection Agency (USEPA): Washington, DC, USA, 2005.
- Parris, K. Impact of Agriculture on Water Pollution in OECD Countries: Recent Trends and Future Prospects. Int. J. Water Resour. Dev. 2011, 27, 33–52. [Google Scholar] [CrossRef] [Green Version]
- Varol, M.; Şen, B. Assessment of nutrient and heavy metal contamination in surface water and sediments of the upper Tigris River, Turkey. CATENA 2012, 92, 1–10. [Google Scholar] [CrossRef]
- Vardhan, K.H.; Kumar, P.S.; Panda, R.C. A review on heavy metal pollution, toxicity and remedial measures: Current trends and future perspectives. J. Mol. Liq. 2019, 290, 111197. [Google Scholar] [CrossRef]
- Bhardwaj, R.; Gupta, A.; Garg, J. Evaluation of heavy metal contamination using environmetrics and indexing approach for River Yamuna, Delhi stretch, India. Water Sci. 2017, 31, 52–66. [Google Scholar] [CrossRef] [Green Version]
- Coelho, L.M.; Rezende, H.C.; Coelho, L.M.; Sousa, P.A.R.; Melo, D.F.O.; Coelho, N.M.M. Bioremediation of Polluted Waters Using Microorganisms. In Advances in Bioremediation of Wastewater and Polluted Soil; Shiomi, N., Ed.; IntechOpen: London, UK, 2015; pp. 1–22. [Google Scholar]
- Galindo-Miranda, J.M.; Guízar-González, C.; Becerril-Bravo, E.J.; Moeller-Chávez, G.; León-Becerril, E.; Vallejo-Rodríguez, R. Occurrence of emerging contaminants in environmental surface waters and their analytical methodology. Water Supply 2019, 19, 1871–1884. [Google Scholar] [CrossRef]
- Shahabudin, M.M.; Musa, S. Occurrence of Surface Water Contaminations: An Overview. IOP Conf. Ser. Earth Environ. Sci. 2018, 140, 012058. [Google Scholar] [CrossRef]
- Li, H.; Yu, X.; Zhang, W.; Huan, Y. Risk Assessment of Groundwater Organic Pollution Using Hazard, Intrinsic Vulnerability, and Groundwater Value, Suzhou City in China. Expo. Health 2017, 10, 99–115. [Google Scholar] [CrossRef]
- Lyon, S.W.; Grabs, T.; Laudon, H.; Bishop, K.H.; Seibert, J. Variability of groundwater levels and total organic carbon in the riparian zone of a boreal catchment. J. Geophys. Res. Space Phys. 2011, 116. [Google Scholar] [CrossRef] [Green Version]
- Bellin, A.; Fiori, A.; Dagan, G. Equivalent and effective conductivities of heterogeneous aquifers for steady source flow, with illustration for hydraulic tomography. Adv. Water Resour. 2020, 142, 103632. [Google Scholar] [CrossRef]
- Cabral, J.P.S. Water Microbiology. Bacterial Pathogens and Water. Int. J. Environ. Res. Public Health 2010, 7, 3657–3703. [Google Scholar] [CrossRef]
- OECD. Pharmaceutical Residues in Freshwater: Hazards and Policy Responses. In OECD Studies on Water; Organisation for Economic Cooperation and Development: Paris, France, 2019. [Google Scholar]
- Shwetank; Suhas; Chaudhary, J.K. A Comparative Study of Fuzzy Logic and WQI for Groundwater Quality Assessment. Procedia Comput. Sci. 2020, 171, 1194–1203. [Google Scholar] [CrossRef]
- Akhila, J.S.; Shyamjith, D.; Alwar, C.M. Acute Toxicity Studies and Determination of Median Lethal Dose. Curr. Sci. 2007, 93, 917–920. [Google Scholar]
- Singh, T.; Wu, L.; Gomez-Velez, J.D.; Lewandowski, J.; Hannah, D.M.; Krause, S. Dynamic Hyporheic Zones: Exploring the Role of Peak Flow Events on Bedform-Induced Hyporheic Exchange. Water Resour. Res. 2019, 55, 218–235. [Google Scholar] [CrossRef]
- Varol, M. Use of water quality index and multivariate statistical methods for the evaluation of water quality of a stream affected by multiple stressors: A case study. Environ. Pollut. 2020, 266, 115417. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Das, A.; Das, N.; Goswami, R.; Singh, U.K. Co-occurrence perspective of arsenic and fluoride in the groundwater of Diphu, Assam, Northeastern India. Chemosphere 2016, 150, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Ntanganedzeni, B.; Elumalai, V.; Rajmohan, N. Coastal Aquifer Contamination and Geochemical Processes Evaluation in Tugela Catchment, South Africa—Geochemical and Statistical Approaches. Water 2018, 10, 687. [Google Scholar] [CrossRef] [Green Version]
- Verlicchi, P.; Grillini, V. Surface Water and Groundwater Quality in South Africa and Mozambique—Analysis of the Most Critical Pollutants for Drinking Purposes and Challenges in Water Treatment Selection. Water 2020, 12, 305. [Google Scholar] [CrossRef] [Green Version]
- Burri, N.M.; Weatherl, R.; Moeck, C.; Schirmer, M. A review of threats to groundwater quality in the anthropocene. Sci. Total. Environ. 2019, 684, 136–154. [Google Scholar] [CrossRef]
- Ben Alaya, M.; Saidi, S.; Zemni, T.; Zargouni, F. Suitability assessment of deep groundwater for drinking and irrigation use in the Djeffara aquifers (Northern Gabes, south-eastern Tunisia). Environ. Earth Sci. 2013, 71, 3387–3421. [Google Scholar] [CrossRef] [Green Version]
- Bhaskar, A.S.; Beesley, L.; Burns, M.J.; Fletcher, T.D.; Hamel, P.; Oldham, C.E.; Roy, A.H. Will it rise or will it fall? Managing the complex effects of urbanization on base flow. Freshw. Sci. 2016, 35, 293–310. [Google Scholar] [CrossRef] [Green Version]
- McInnes, R.J. Sustainable Development Goals. In The Wetland Book; Springer: Berlin/Heidelberg, Germany, 2018; pp. 631–636. [Google Scholar] [CrossRef]
- USEPA. The Report to Congress: Waste Disposal Practices and Their Effects on Water; United State Enviromental Protection Agency: Washington, DC, USA, 1977.
- OTA. Protecting the Nation’s Groundwater from Contamination; U.S. Congress Office of Technology Assessment: Washington, DC, USA, 1984; Volume I–II, OTA-0-233. [Google Scholar]
- USEPA. Wellhead Protection Programs: Tools for Local Governments; United State Enviromental Protection Agency: Washington, DC, USA, 1989; 440/6-89-002.
- EPA. Point and Non-Point Sources of Water Pollution; United States Environmental Protection Agency: Washington, DC, USA, 1996.
- Nan, Y.; Bao-Hui, M.; Chun-Kun, L. Impact Analysis of Climate Change on Water Resources. Procedia Eng. 2011, 24, 643–648. [Google Scholar] [CrossRef] [Green Version]
- Kammoun, S.; Trabelsi, R.; Re, V.; Zouari, K. Coastal Aquifer Salinization in Semi-Arid Regions: The Case of Grombalia (Tunisia). Water 2021, 13, 129. [Google Scholar] [CrossRef]
- Anders, I.; Stagl, J.; Auer, I.; Pavlik, D. Climate Change in Central and Eastern Europe. In Managing Protected Areas in Central and Eastern Europe under Climate Change; Rannow, S., Neubert, M., Eds.; Springer: New York, NY, USA, 2014; Chapter 23; pp. 17–30. [Google Scholar] [CrossRef] [Green Version]
- Ching, Y.C.; Lee, Y.H.; Toriman, M.E.; Abdullah, M.; Bin Yatim, B. Effect of the big flood events on the water quality of the Muar River, Malaysia. Sustain. Water Resour. Manag. 2015, 1, 97–110. [Google Scholar] [CrossRef] [Green Version]
- Scardina, P. Effects of Dissolved Gas Supersaturation and Bubble Formation on Water Treatment Plant Performance. Master’s Thesis, Faculty of the Virginia Polytechnic Institute and State University, Blacksburg, VN, USA, 2004. [Google Scholar]
- Payus, C.; Huey, L.A.; Adnan, F.; Rimba, A.B.; Mohan, G.; Chapagain, S.K.; Roder, G.; Gasparatos, A.; Fukushi, K. Impact of Extreme Drought Climate on Water Security in North Borneo: Case Study of Sabah. Water 2020, 12, 1135. [Google Scholar] [CrossRef]
- PAHO. Natural Disaster Mitigation in Drinking Water and Sewerage Systems; World Health Organization: Washington, DC, USA, 1998. [Google Scholar]
- Lee, J.; Perera, D.; Glickman, T.; Taing, L. Water-related disasters and their health impacts: A global review. Prog. Disaster Sci. 2020, 8, 100123. [Google Scholar] [CrossRef]
- Knap, A.H.; Rusyn, I. Environmental exposures due to natural disasters. Rev. Environ. Health 2016, 31, 89–92. [Google Scholar] [CrossRef] [Green Version]
- Sholihah, Q.; Kuncoro, W.; Wahyuni, S.; Suwandi, S.P.; Feditasari, E.D. The analysis of the causes of flood disasters and their impacts in the perspective of environmental law. IOP Conf. Ser. Earth Environ. Sci. 2020, 437, 012056. [Google Scholar] [CrossRef]
- Euripidou, E.; Murray, V. Public health impacts of floods and chemical contamination. J. Public Health 2004, 26, 376–383. [Google Scholar] [CrossRef]
- Schoonover, J.E.; Crim, J.F. An Introduction to Soil Concepts and the Role of Soils in Watershed Management. J. Contemp. Water Res. Educ. 2015, 154, 21–47. [Google Scholar] [CrossRef]
- Winter, T.C.; Harvey, J.W.; Franke, O.L.; Alley, W.M. Ground Water Surface Water and A Single Resource; U.S. Geological Survey Circular 1139: Denver, CO, USA, 1998. [Google Scholar]
- Liu, J.; Zhang, C.; Kou, L.; Zhou, Q. Effects of Climate and Land Use Changes on Water Resources in the Taoer River. Adv. Meteorol. 2017, 2017, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Riedel, T.; Weber, T.K.D. Review: The influence of global change on Europe’s water cycle and groundwater recharge. Hydrogeol. J. 2020, 28, 1939–1959. [Google Scholar] [CrossRef]
- Zaharescu, D.G.; Burghelea, C.I.; Dontsova, K.; Presler, J.K.; Hunt, E.A.; Domanik, K.J.; Amistadi, M.K.; Sandhaus, S.; Munoz, E.N.; Gaddis, E.E.; et al. Ecosystem-bedrock interaction changes nutrient compartmentalization during early oxidative weathering. Sci. Rep. 2019, 9, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, S.; Bhattacharya, A. Drinking water contamination and treatment techniques. Appl. Water Sci. 2016, 7, 1043–1067. [Google Scholar] [CrossRef] [Green Version]
- Wirt, L. Radioactivity in the Environment A Case Study of the Puerco and Little Colorado River Basins, Arizona and New Mexico; U.S. Geological Survey, Water Resources Division: Tucson, AZ, USA, 1994.
- Rejah, B.K.; Alameer, N.K.A.; Kadim, W.H.; Murad, S.T.M. Estimate Level of Radon Concentration for Drinking Water in Some Regions of Baghdad City. Arab. J. Sci. Eng. 2018, 43, 3831–3835. [Google Scholar] [CrossRef] [Green Version]
- Aarkrog, A. Disposal of radioactive wastes into marine and fresh waters: IAEA bibliographical series No. 5 (Vienna, 1962. 368p. $3.00). Nucl. Phys. 1962, 37, 693. [Google Scholar] [CrossRef]
- Alam, I.; Rehman, J.U.; Ahmad, N.; Nazir, A.; Hameed, A.; Hussain, A. An overview on the concentration of radioactive elements and physiochemical analysis of soil and water in Iraq. Rev. Environ. Health 2020, 35, 147–155. [Google Scholar] [CrossRef]
- Al-Alawy, I.T.; Mohammed, R.S.; Fadhil, H.R.; Hasan, A.A. Determination of Radioactivity Levels, Hazard, Cancer Risk and Radon Concentrations of Water and Sediment Samples in Al-Husseiniya River (Karbala, Iraq). J. Phys. Conf. Ser. 2018, 1032, 012012. [Google Scholar] [CrossRef]
- Ahmad, N.; Jaafar, M.S.; Bakhash, M.; Rahim, M. An overview on measurements of natural radioactivity in Malaysia. J. Radiat. Res. Appl. Sci. 2015, 8, 136–141. [Google Scholar] [CrossRef] [Green Version]
- Hussein, Z.A. Assessment of Natural Radioactivity Levels and Radiation Hazards for Soil Samples Used in Erbil Governorate, Iraqi Kurdistan. Aro-Sci. J. Koya Univ. 2019, 7, 34–39. [Google Scholar] [CrossRef] [Green Version]
- Fookes, P.G. Geology for Engineers: The Geological Model, Prediction and Performance. Q. J. Eng. Geol. Hydrogeol. 1997, 30, 293–424. [Google Scholar] [CrossRef]
- Wuana, R.A.; Okieimen, F.E. Heavy Metals in Contaminated Soils: A Review of Sources, Chemistry, Risks and Best Available Strategies for Remediation. ISRN Ecol. 2011, 2011, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Cumberland, S.A.; Douglas, G.; Grice, K.; Moreau, J.W. Uranium mobility in organic matter-rich sediments: A review of geological and geochemical processes. Earth-Sci. Rev. 2016, 159, 160–185. [Google Scholar] [CrossRef] [Green Version]
- Wali, S.U.; Umar, S.W.; Abubakar, S.D.; Ifabiyi, I.P.; Dankani, I.M.; Shera, I.M.; Yauri, S.G. Hydrochemical characterization of shallow and deep groundwater in Basement Complex areas of southern Kebbi State, Sokoto Basin, Nigeria. Appl. Water Sci. 2019, 9, 169. [Google Scholar] [CrossRef] [Green Version]
- Brunner, P.; Cook, P.; Simmons, C.T. Disconnected Surface Water and Groundwater: From Theory to Practice. Groundwater 2010, 49, 460–467. [Google Scholar] [CrossRef] [Green Version]
- Turnadge, C.; Smerdon, B.D. A review of methods for modelling environmental tracers in groundwater: Advantages of tracer concentration simulation. J. Hydrol. 2014, 519, 3674–3689. [Google Scholar] [CrossRef]
- Winter, T.C. Recent advances in understanding the interaction of groundwater and surface water. Rev. Geophys. 1995, 33, 985–994. [Google Scholar] [CrossRef]
- Sophocleous, M. Interactions between groundwater and surface water: The state of the science. Hydrogeol. J. 2002, 10, 52–67. [Google Scholar] [CrossRef]
- Williams, D.D. Nutrient and flow vector dynamics at the hyporheic/groundwater interface and their effects on the interstitial fauna. Hydrobiologia 1993, 251, 185–198. [Google Scholar] [CrossRef] [Green Version]
- Cardenas, M.B. Hyporheic zone hydrologic science: A historical account of its emergence and a prospectus. Water Resour. Res. 2015, 51, 3601–3616. [Google Scholar] [CrossRef]
- Brunner, P.; Therrien, R.; Renard, P.; Simmons, C.T.; Franssen, H.-J.H. Advances in understanding river-groundwater interactions. Rev. Geophys. 2017, 55, 818–854. [Google Scholar] [CrossRef]
- Schmadel, N.M.; Ward, A.S.; Lowry, C.S.; Malzone, J.M. Hyporheic exchange controlled by dynamic hydrologic boundary conditions. Geophys. Res. Lett. 2016, 43, 4408–4417. [Google Scholar] [CrossRef] [Green Version]
- Alfarrah, N.; Walraevens, K. Groundwater Overexploitation and Seawater Intrusion in Coastal Areas of Arid and Semi-Arid Regions. Water 2018, 10, 143. [Google Scholar] [CrossRef] [Green Version]
- Kumar, P.J.S. Deciphering the groundwater–saline water interaction in a complex coastal aquifer in South India using statistical and hydrochemical mixing models. Model. Earth Syst. Environ. 2016, 2, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Doble, R.C.; Crosbie, R. Review: Current and emerging methods for catchment-scale modelling of recharge and evapotranspiration from shallow groundwater. Hydrogeol. J. 2016, 25, 3–23. [Google Scholar] [CrossRef]
- Sagasta, J.M.; Zadeh, S.M.; Turral, H. Water Pollution from Agriculture: A Global Review; Food and Agriculture Organization of the United Nations (FAO) and the International Water Management Institute (IWMI): Colombo, Sri Lanka, 2017; Available online: http://www.fao.org/documents/card/en/c/a9598c47-0ca1-4c77-8d9d-1c2708050ba0/ (accessed on 19 July 2021).
- Masi, F.; Rizzo, A.; Regelsberger, M. The role of constructed wetlands in a new circular economy, resource oriented, and ecosystem services paradigm. J. Environ. Manag. 2017, 216, 275–284. [Google Scholar] [CrossRef]
- USEPA. Defining Hazardous Waste: Listed, Characteristic and Mixed Radiological Wastes; United State Environmental Protection Agency: Washington, DC, USA, 2020.
- Han, D.; Tong, X.; Currell, M.J.; Cao, G.; Jin, M.; Tong, C. Evaluation of the impact of an uncontrolled landfill on surrounding groundwater quality, Zhoukou, China. J. Geochem. Explor. 2014, 136, 24–39. [Google Scholar] [CrossRef]
- Ferronato, N.; Torretta, V. Waste Mismanagement in Developing Countries: A Review of Global Issues. Int. J. Environ. Res. Public Health 2019, 16, 1060. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Shafy, H.; Mansour, M.S. Solid waste issue: Sources, composition, disposal, recycling, and valorization. Egypt. J. Pet. 2018, 27, 1275–1290. [Google Scholar] [CrossRef]
- Elnasri, R.A.A. Assessment of Lndustrial Liquid Waste Management in Omdurman Lndustrial AreaBy, University of Khartoum, Sudan. April 2003. Available online: https://www.osti.gov/etdeweb/biblio/20943506 (accessed on 19 July 2021).
- Marszelewski, W.; Piasecki, A. Changes in Water and Sewage Management after Communism: Example of the Oder River Basin (Central Europe). Sci. Rep. 2020, 10, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jackson, R.; Gorody, A.; Mayer, B.; Roy, J.; Ryan, M.C.; Van Stempvoort, D. Groundwater Protection and Unconventional Gas Extraction: The Critical Need for Field-Based Hydrogeological Research. Groundwater 2013, 51, 488–510. [Google Scholar] [CrossRef] [PubMed]
- Corapcioglu, M.Y.; Baehr, A.L. A compositional multiphase model for groundwater contamination by petroleum products: 1. Theoretical considerations. Water Resour. Res. 1987, 23, 191–200. [Google Scholar] [CrossRef]
- Holt, M. Sources of chemical contaminants and routes into the freshwater environment. Food Chem. Toxicol. 2000, 38, S21–S27. [Google Scholar] [CrossRef]
- Pichtel, J. Oil and Gas. Production Wastewater. Soil Contam. Pollut. Prev. 2020. [Google Scholar] [CrossRef]
- Van Der Gun, J.; Aureli, A.; Merla, A. Enhancing Groundwater Governance by Making the Linkage with Multiple Uses of the Subsurface Space and Other Subsurface Resources. Water 2016, 8, 222. [Google Scholar] [CrossRef] [Green Version]
- Michael, H.A.; Voss, C.I. Estimation of regional-scale groundwater flow properties in the Bengal Basin of India and Bangladesh. Hydrogeol. J. 2009, 17, 1329–1346. [Google Scholar] [CrossRef]
- Zhang, Z.; Xiao, C.; Adeyeye, O.; Yang, W.; Liang, X. Source and Mobilization Mechanism of Iron, Manganese and Arsenic in Groundwater of Shuangliao City, Northeast China. Water 2020, 12, 534. [Google Scholar] [CrossRef] [Green Version]
- Ayilara, M.S.; Olanrewaju, O.S.; Babalola, O.O.; Odeyemi, O. Waste Management through Composting: Challenges and Potentials. Sustainability 2020, 12, 4456. [Google Scholar] [CrossRef]
- Sheng, Z. An aquifer storage and recovery system with reclaimed wastewater to preserve native groundwater resources in El Paso, Texas. J. Environ. Manag. 2005, 75, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Usta, C. Microorganisms in Biological Pest Control a Review (Bacterial Toxin Application and Effect of Environmental Factors). In Current Progress in Biological Research; Silva, M., Ed.; IntechOpen: London, UK, 2013; Chapter 13; pp. 287–317. [Google Scholar] [CrossRef] [Green Version]
- Hensen, B.; Lange, J.; Jackisch, N.; Zieger, F.; Olsson, O.; Kümmerer, K. Entry of biocides and their transformation products into groundwater via urban stormwater infiltration systems. Water Res. 2018, 144, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Agboola, O.; Babatunde, D.E.; Fayomi, O.S.I.; Sadiku, E.R.; Popoola, P.; Moropeng, L.; Yahaya, A.; Mamudu, O.A. A review on the impact of mining operation: Monitoring, assessment and management. Results Eng. 2020, 8, 100181. [Google Scholar] [CrossRef]
- Jain, M.K.; Das, A. Impact of Mine Waste Leachates on Aquatic Environment: A Review. Curr. Pollut. Rep. 2017, 3, 31–37. [Google Scholar] [CrossRef]
- Rybicki, C.; Solecki, T.; Winid, B. Threats to the environment in the areas of abandoned extraction of hydrocarbon deposits. Drill. Oil Gas 2015, 32, 103. [Google Scholar] [CrossRef]
- Anawar, H.M. Sustainable rehabilitation of mining waste and acid mine drainage using geochemistry, mine type, mineralogy, texture, ore extraction and climate knowledge. J. Environ. Manag. 2015, 158, 111–121. [Google Scholar] [CrossRef]
- Schwartz, M.O.; Kgomanyane, J. Modelling natural attenuation of heavy-metal groundwater contamination in the Selebi-Phikwe mining area, Botswana. Environ. Earth Sci. 2007, 54, 819–830. [Google Scholar] [CrossRef]
- Hassaan, M.A.; El Nemr, A. Pesticides pollution: Classifications, human health impact, extraction and treatment techniques. Egypt. J. Aquat. Res. 2020, 46, 207–220. [Google Scholar] [CrossRef]
- European Commission. Groundwater Protection in Europe: The New Groundwater Directive Consolidating the EU Regulatory Framework; Publications Office of the European Union: Luxembourg, 2008. [Google Scholar] [CrossRef]
- Kim, K.-H.; Kabir, E.; Jahan, S.A. Exposure to pesticides and the associated human health effects. Sci. Total. Environ. 2017, 575, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.; Pandey, R.S.; Sharma, B. Water Pollution with Special Reference to Pesticide Contamination in India. J. Water Resour. Prot. 2010, 2, 432–448. [Google Scholar] [CrossRef] [Green Version]
- Šperl, J.; Trčková, J. Permeability and Porosity of Rocks and Their Relationship Based on Laboratory Testing. Acta Geodyn. Geomater. 2008, 5, 41–47. [Google Scholar]
- Smith, L.; Siciliano, G. A comprehensive review of constraints to improved management of fertilizers in China and mitigation of diffuse water pollution from agriculture. Agric. Ecosyst. Environ. 2015, 209, 15–25. [Google Scholar] [CrossRef] [Green Version]
- Hallberg, G.R. The impacts of agricultural chemicals on ground water quality. GeoJournal 1987, 15, 283–295. [Google Scholar] [CrossRef]
- Briški, M.; Stroj, A.; Kosović, I.; Borović, S. Characterization of Aquifers in Metamorphic Rocks by Combined Use of Electrical Resistivity Tomography and Monitoring of Spring Hydrodynamics. Geosciences 2020, 10, 137. [Google Scholar] [CrossRef] [Green Version]
- Akhter, G.; Hasan, M. Determination of aquifer parameters using geoelectrical sounding and pumping test data in Khanewal District, Pakistan. Open Geosci. 2016, 8, 630–638. [Google Scholar] [CrossRef]
- López-Pacheco, I.Y.; Silva-Núñez, A.; Salinas-Salazar, C.; Arévalo-Gallegos, A.; Lizarazo-Holguin, L.A.; Barceló, D.; Iqbal, H.M.; Parra-Saldívar, R. Anthropogenic contaminants of high concern: Existence in water resources and their adverse effects. Sci. Total. Environ. 2019, 690, 1068–1088. [Google Scholar] [CrossRef]
- USEPA. 2018 Edition of the Drinking Water Standards and Health Advisories Tables; United State Enviromental Protection Agency: Washington, DC, USA, 2018.
- Müller, A.; Österlund, H.; Marsalek, J.; Viklander, M. The pollution conveyed by urban runoff: A review of sources. Sci. Total. Environ. 2019, 709, 136125. [Google Scholar] [CrossRef] [PubMed]
- McGrane, S.J. Impacts of urbanisation on hydrological and water quality dynamics, and urban water management: A review. Hydrol. Sci. J. 2016, 61, 2295–2311. [Google Scholar] [CrossRef]
- Milla, O.V.; Company, N.W.; Salvador, E.; Huang, W. Relationship between Solid Waste Pollution and Polluted Drinking Water in El Relationship between Solid Waste Pollution and Polluted Drinking Water in El Salvador. Int. Coop. Dev. Fund. 2012, 7, 37–60. [Google Scholar]
- Stuart, M.; Gooddy, D.; Bloomfield, J.; Williams, A. A review of the impact of climate change on future nitrate concentrations in groundwater of the UK. Sci. Total. Environ. 2011, 409, 2859–2873. [Google Scholar] [CrossRef] [Green Version]
- Żychowski, J.; Bryndal, T. Impact of cemeteries on groundwater contamination by bacteria and viruses a review. J. Water Health 2014, 13, 285–301. [Google Scholar] [CrossRef] [PubMed]
- Akinbile, C.; Erazua, A.; Babalola, T.; Ajibade, F. Environmental implications of animal wastes pollution on agricultural soil and water quality. Soil Water Res. 2016, 11, 172–180. [Google Scholar] [CrossRef] [Green Version]
- Polat, H.E.; Olgun, M. Water Pollution from Livestock Wastes and Required Strategies in Efforts to Adapt to European Union. Int. Water Assoc. 2018, 18, 1–9. [Google Scholar]
- Camara, M.; Jamil, N.R.; Bin Abdullah, A.F. Impact of land uses on water quality in Malaysia: A review. Ecol. Process. 2019, 8, 10. [Google Scholar] [CrossRef]
- EPA. Monitoring Site Information; United States Environmental Protection Agency: Washington DC, USA, 2011.
- World Bank. Investing in Opportunity, Ending Poverty; World International Bank for Reconstruction and Development (IBRD): Washington, DC, USA, 2020; p. 319. Available online: https://www.worldbank.org/en/about/annual-report (accessed on 19 July 2021).
- Wu, Z.; Wang, X.; Chen, Y.; Cai, Y.; Deng, J. Assessing river water quality using water quality index in Lake Taihu Basin, China. Sci. Total. Environ. 2018, 612, 914–922. [Google Scholar] [CrossRef] [PubMed]
- Singhal, B.B.S.; Gupta, R.P.; Singhal, B.B.S.; Gupta, R.P. Introduction and basic concepts. In Applied Hydrogeology of Fracture Rocks; Springer: London, UK; New York, NY, USA, 2010; Chapter 1. [Google Scholar] [CrossRef] [Green Version]
- UNESCO. The United Nations World Water Development Report 2018: Nature-Based Solutions for Water; United State Enviromental Protection Agency: Paris, France, 2018.
- WHO. Guidelines for Drinking Water Quality; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- BIS. Indian Standards Drinking Water Specifications IS 10500:2012; Bahadur Shah Zafar Marg: New Delhi, India, 2012. [Google Scholar]
- Abiye, T.A.; Bhattacharya, P. Arsenic concentration in groundwater: Archetypal study from South Africa. Groundw. Sustain. Dev. 2019, 9. [Google Scholar] [CrossRef]
- Hepburn, E.; Northway, A.; Bekele, D.; Liu, G.-J.; Currell, M. A method for separation of heavy metal sources in urban groundwater using multiple lines of evidence. Environ. Pollut. 2018, 241, 787–799. [Google Scholar] [CrossRef] [PubMed]
- Qu, L.; Huang, H.; Xia, F.; Liu, Y.; Dahlgren, R.; Zhang, M.; Mei, K. Risk analysis of heavy metal concentration in surface waters across the rural-urban interface of the Wen-Rui Tang River, China. Environ. Pollut. 2018, 237, 639–649. [Google Scholar] [CrossRef] [Green Version]
- Huan, H.; Hu, L.; Yang, Y.; Jia, Y.; Lian, X.; Ma, X.; Jiang, Y.; Xi, B. Groundwater nitrate pollution risk assessment of the groundwater source field based on the integrated numerical simulations in the unsaturated zone and saturated aquifer. Environ. Int. 2020, 137, 105532. [Google Scholar] [CrossRef] [PubMed]
- Schwarzenbach, R.P.; Egli, T.; Hofstetter, T.; Von Gunten, U.; Wehrli, B. Global Water Pollution and Human Health. Annu. Rev. Environ. Resour. 2010, 35, 109–136. [Google Scholar] [CrossRef]
- Teng, Y.; Zuo, R.; Xiong, Y.; Wu, J.; Zhai, Y.; Su, J. Risk assessment framework for nitrate contamination in groundwater for regional management. Sci. Total Environ. 2019, 697, 134102. [Google Scholar] [CrossRef]
- Suvarna, B.; Sunitha, V.; Reddy, Y.S.; Reddy, N.R. Data health risk assessment of nitrate contamination in groundwater of rural region in the Yerraguntla Mandal, South India. Data Brief. 2020, 30, 105374. [Google Scholar] [CrossRef] [PubMed]
- Pisciotta, A.; Cusimano, G.; Favara, R. Groundwater nitrate risk assessment using intrinsic vulnerability methods: A comparative study of environmental impact by intensive farming in the Mediterranean region of Sicily, Italy. J. Geochem. Explor. 2015, 156, 89–100. [Google Scholar] [CrossRef]
- Green, C.T.; Fisher, L.H.; Bekins, B.A. Nitrogen Fluxes through Unsaturated Zones in Five Agricultural Settings across the United States. J. Environ. Qual. 2008, 37, 1073–1085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galán, M.J.G.; Garrido, T.; Fraile, J.; Ginebreda, A.; Díaz-Cruz, M.S.; Barceló, D. Simultaneous occurrence of nitrates and sulfonamide antibiotics in two ground water bodies of Catalonia (Spain). J. Hydrol. 2009, 383, 93–101. [Google Scholar] [CrossRef]
- McMahon, P.B.; Dennehy, K.F.; Bruce, B.W.; Bohlke, J.K.; Michel, R.L.; Gurdak, J.J.; Hurlbut, D.B. Storage and transit time of chemicals in thick unsaturated zones under rangeland and irrigated cropland, High Plains, United States. Water Resour. Res. 2006, 42. [Google Scholar] [CrossRef] [Green Version]
- Ayoob, S.; Gupta, A.K. Fluoride in Drinking Water: A Review on the Status and Stress Effects. Crit. Rev. Environ. Sci. Technol. 2006, 36, 433–487. [Google Scholar] [CrossRef]
- Rafique, T.; Naseem, S.; Usmani, T.H.; Bashir, E.; Khan, F.A.; Bhanger, M.I. Geochemical factors controlling the occurrence of high fluoride groundwater in the Nagar Parkar area, Sindh, Pakistan. J. Hazard. Mater. 2009, 171, 424–430. [Google Scholar] [CrossRef]
- Mohanta, V.L.; Singh, S.; Mishra, B.K. Human health risk assessment of fluoride-rich groundwater using fuzzy-analytical process over the conventional technique. Groundw. Sustain. Dev. 2019, 10, 100291. [Google Scholar] [CrossRef]
- Haldar, D.; Duarah, P.; Purkait, M.K. MOFs for the treatment of arsenic, fluoride and iron contaminated drinking water: A review. Chemosphere 2020, 251, 126388. [Google Scholar] [CrossRef]
- Nõmmik, H. Fluorine in Swedish Agricultural Products, Soil and Drinking Water; Swedish National Institute of Public Health: Stockholm, Sweden, 1953. [Google Scholar]
- Latimer, G. The Health and Environmental Impacts of Hazardous Wastes; The Department of the Environment, and Ascend Waste and Environment Pty Ltd.: Melbourne, Australia, 2015. Available online: https://www.environment.gov.au/protection/publications/hazardous-waste-impacts (accessed on 19 July 2021).
- Saipudin, N.A.; Omar, F.M. International Conference on Environmental Research and Technology (ICERT 2017). In International Conference on Environmental Research and Technology (ICERT 2017); Universiti Sains Malaysia: Penang, Malaysia, 2001; pp. 61–66. [Google Scholar]
- Kabir, M.M.; Fakhruddin, A.N.M.; Chowdhury, M.A.Z.; Fardous, Z.; Islam, R. Characterization of Tannery Effluents of Hazaribagh Area, Dhaka, Bangladesh. Pollution 2017, 3, 395–406. [Google Scholar] [CrossRef]
- Valdez-Alegría, C.J.; Fuentes-Rivas, R.M.; Garcia-Rivas, J.-L.; De Oca, R.M.G.F.-M.; García-Gaitán, B. Presence and Distribution of Fluoride Ions in Groundwater for Human in a Semiconfined Volcanic Aquifer. Resources 2019, 8, 116. [Google Scholar] [CrossRef] [Green Version]
- Sankhla, M.S. Contaminant of Heavy Metals in Groundwater & its Toxic Effects on Human Health & Environment. Int. J. Environ. Sci. Nat. Resour. 2019, 18, 1–5. [Google Scholar] [CrossRef]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy Metal Toxicity and the Environment. In Molecular, Clinical and Environmental Toxicology; Springer: Chem, Switzerland, 2012; Chapter 4; pp. 133–164. [Google Scholar]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6. [Google Scholar] [CrossRef] [PubMed]
- Mandich, M. Ranked effects of heavy metals on marine bivalves in laboratory mesocosms: A meta-analysis. Mar. Pollut. Bull. 2018, 131, 773–781. [Google Scholar] [CrossRef] [PubMed]
- Kapahi, M.; Sachdeva, S. Bioremediation Options for Heavy Metal Pollution. J. Health Pollut. 2019, 9, 191203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gurung, S.B.; Geronimo, F.K.; Hong, J.; Kim, L.-H. Application of indices to evaluate LID facilities for sediment and heavy metal removal. Chemosphere 2018, 206, 693–700. [Google Scholar] [CrossRef]
- Yunus, K.; Zuraidah, M.; John, A. A review on the accumulation of heavy metals in coastal sediment of Peninsular Malaysia. Ecofeminism Clim. Chang. 2020, 1, 21–35. [Google Scholar] [CrossRef]
- Saha, P.; Paul, B. Assessment of Heavy Metal Pollution in Water Resources and Their Impacts: A Review. J. Basic Appl. Eng. Res. 2016, 3, 671–675. [Google Scholar]
- Ali, H.; Khan, E.; Ilahi, I. Environmental Chemistry and Ecotoxicology of Hazardous Heavy Metals: Environmental Persistence, Toxicity, and Bioaccumulation. J. Chem. 2019, 2019, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Chibuike, G.U.; Obiora, S.C.; Chibuike, G.U.; Obiora, S.C. Heavy Metal Polluted Soils: Effect on Plants and Bioremediation Methods. Appl. Environ. Soil Sci. 2014, 2014, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Tangahu, B.; Abdullah, S.R.S.; Basri, H.; Idris, M.; Anuar, N.; Mukhlisin, M. A Review on Heavy Metals (As, Pb, and Hg) Uptake by Plants through Phytoremediation. Int. J. Chem. Eng. 2011, 2011, 1–31. [Google Scholar] [CrossRef]
- Razak, N.H.A.; Praveena, S.M.; Aris, A.Z.; Hashim, Z. Drinking water studies: A review on heavy metal, application of biomarker and health risk assessment (a special focus in Malaysia). J. Epidemiol. Glob. Health 2015, 5, 297–310. [Google Scholar] [CrossRef] [Green Version]
- Alidadi, H.; Belin, S.; Sany, T.; Zarif, B.; Oftadeh, G.; Mohamad, T. Health Risk Assessments of Arsenicand Toxic Heavy Metal Exposure in Drinking Waterin Northeast Iran. Environ. Health Prev. Med. 2019, 24, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, L.; Rad, S.; Xu, L.; Gui, L.; Song, X.; Li, Y.; Wu, Z.; Chen, Z. Heavy Metals Distribution, Sources, and Ecological Risk Assessment in Huixian Wetland, South China. Water 2020, 12, 431. [Google Scholar] [CrossRef] [Green Version]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Molecular, Clinicaland Environmental Toxicology. NIH Public Access 2012, 101, 1–30. [Google Scholar] [CrossRef]
- Kinuthia, G.K.; Ngure, V.; Beti, D.; Lugalia, R.; Wangila, A.; Kamau, L. Levels of heavy metals in wastewater and soil samples from open drainage channels in Nairobi, Kenya: Community health implication. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef]
- Shekhar, S.; Sarkar, A. Hydrogeological characterization and assessment of groundwater quality in shallow aquifers in vicinity of Najafgarh drain of NCT Delhi. J. Earth Syst. Sci. 2013, 122, 43–54. [Google Scholar] [CrossRef] [Green Version]
- Smedley, P.; Nicolli, H.; Macdonald, D.; Barros, A.; Tullio, J. Hydrogeochemistry of arsenic and other inorganic constituents in groundwaters from La Pampa, Argentina. Appl. Geochem. 2002, 17, 259–284. [Google Scholar] [CrossRef]
- Ali, S.; Thakur, S.K.; Sarkar, A.; Shekhar, S. Worldwide contamination of water by fluoride. Environ. Chem. Lett. 2016, 14, 291–315. [Google Scholar] [CrossRef]
- Thapa, R.; Gupta, S.; Kaur, H.; Baski, R. Assessment of groundwater quality scenario in respect of fluoride and nitrate contamination in and around Gharbar village, Jharkhand, India. HydroResearch 2019, 2, 60–68. [Google Scholar] [CrossRef]
- Popugaeva, D.; Kreyman, K.; Ray, A.K. Study of aluminium in groundwater using chemometric methods. Environ. Technol. 2018, 41, 1691–1699. [Google Scholar] [CrossRef]
- Blais, J.-F.; Mercier, G. Transformation of red mud from aluminium industry into a coagulant for wastewater treatment. Hydrometallurgy 2008, 92, 16–25. [Google Scholar] [CrossRef]
- Lei, L.-Q.; Song, C.-A.; Xie, X.-L.; Li, Y.-H.; Wang, F. Acid mine drainage and heavy metal contamination in groundwater of metal sulfide mine at arid territory (BS mine, Western Australia). Trans. Nonferrous Met. Soc. China 2010, 20, 1488–1493. [Google Scholar] [CrossRef]
- Fahmi, A.; Radjagukguk, B.; Purwanto, B.H.; Hanudin, E. The Influnece of Peat Layer on Hidrogen and Aluminium Concentration Originating from the Substratum Sulphidic Materials. J. Tanah Trop. (J. Trop. Soils) 2012, 17, 197–202. [Google Scholar] [CrossRef]
- Williams, M.; Fordyce, F.; Paijitprapapon, A.; Charoenchaisri, P. Arsenic Contamination in Surface Drainage and Groundwater in Part of the Southeast Asian Tin Belt, Nakhon Si Thammarat Province, Southern Thailand. Environ. Geol. 1996, 27, 16–33. [Google Scholar] [CrossRef]
- Oluwatosin, Q.I.; Anthony, I.A. Determination of Heavy Metal Contents in Some Industrial Effluents from Ondo State, Nigeria. J. Environ. Chem. Ecotoxicol. 2013, 5, 216–219. [Google Scholar] [CrossRef]
- Matisoff, G.; Khourey, C.J.; Hall, J.F.; Varnes, A.W.; Strain, W.H. The Nature and Source of Arsenic in Northeastern Ohio Ground Watera. Groundwater 1982, 20, 446–456. [Google Scholar] [CrossRef]
- Chatterjee, A.; Das, D.; Chakraborti, D. A study of ground water contamination by arsenic in the residential area of behala, calcutta due to industrial pollution. Environ. Pollut. 1993, 80, 57–65. [Google Scholar] [CrossRef]
- Shallari, S.; Schwartz, C.; Hasko, A.; Morel, J. Heavy metals in soils and plants of serpentine and industrial sites of Albania. Sci. Total. Environ. 1998, 209, 133–142. [Google Scholar] [CrossRef]
- Deepali, K.K.; Gangwar, K. Metals Concentration in Textile and Tannery Effluents, Associated Soils and Ground Water. N. Y. Sci. J. 2010, 3, 82–89. [Google Scholar]
- Ololade, I.A.; Adewunmi, A.; Ologundudu, A.; Adeleye, A. Effects of Household Wastes on Surface and Underground Waters. Int. J. Phys. Sci. 2009, 4, 22–29. [Google Scholar]
- Mirlean, N.; Roisenberg, A. The effect of emissions of fertilizer production on the environment contamination by cadmium and arsenic in southern Brazil. Environ. Pollut. 2006, 143, 335–340. [Google Scholar] [CrossRef]
- Duru, M.K.C.; Nwanekwu, K.E.; Adindu, E.A.; Odika, P.C. Heavy Metal and Bioload Levels of Otamiri River, Owerri, Imo State, Nigeria. Arch. Appl. Sci. Res. 2012, 4, 1002–1006. [Google Scholar]
- Zamani, A.A.; Yaftian, M.R.; Parizanganeh, A. Multivariate statistical assessment of heavy metal pollution sources of groundwater around a lead and zinc plant. Iran. J. Environ. Health Sci. Eng. 2012, 9, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sridhar, S.G.D.; Sakthivel, A.M.; Sangunathan, U.; Balasubramanian, M.; Jenefer, S.; Rafik, M.M.; Kanagaraj, G. Heavy metal concentration in groundwater from Besant Nagar to Sathankuppam, South Chennai, Tamil Nadu, India. Appl. Water Sci. 2017, 7, 4651–4662. [Google Scholar] [CrossRef] [Green Version]
- Atashi, H.; Shahemabadi, M.S.; Mansoorkiai, R.; Spaili, F.A. Cobalt in Zahedan Drinking Water. J. Appl. Sci. Res. 2009, 5, 2203–2207. [Google Scholar]
- Li, F.; Qiu, Z.; Zhang, J.; Liu, W.; Liu, C.; Zeng, G. Investigation, Pollution Mapping and Simulative Leakage Health Risk Assessment for Heavy Metals and Metalloids in Groundwater from a Typical Brownfield, Middle China. Int. J. Environ. Res. Public Health 2017, 14, 768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inácio, M.; Neves, M.O.; Pereira, V.; da Silva, E.F. Levels of selected potential harmful elements (PHEs) in soils and vegetables used in diet of the population living in the surroundings of the Estarreja Chemical Complex (Portugal). Appl. Geochem. 2014, 44, 38–44. [Google Scholar] [CrossRef]
- Dabai, M.U.; Bagudo, B.U.; Jodi, L.M.; Ocheni, L. Evaluation of Some Trace Metal Levels in the Water, Fish and Aquatic Plant in River Sokoto, North-Western Nigeria. Asian J. Appl. Sci. 2013, 1. Available online: https://ajouronline.com/index.php/AJAS/article/view/601 (accessed on 19 July 2021).
- Izbicki, J.A.; Wright, M.; Seymour, W.A.; McCleskey, R.B.; Fram, M.S.; Belitz, K.; Esser, B.K.; Izbicki, J.A.; Wright, M.; Seymour, W.A.; et al. Cr(VI) occurrence and geochemistry in water from public-supply wells in California. Appl. Geochem. 2015, 63, 203–217. [Google Scholar] [CrossRef] [Green Version]
- Wei, W.; Ma, R.; Sun, Z.; Zhou, A.; Bu, J.; Long, X.; Liu, Y. Effects of Mining Activities on the Release of Heavy Metals (HMs) in a Typical Mountain Headwater Region, the Qinghai-Tibet Plateau in China. Int. J. Environ. Res. Public Health 2018, 15, 1987. [Google Scholar] [CrossRef] [Green Version]
- Małecki, J.J.; Kadzikiewicz-Schoeneich, M.; Eckstein, Y.; Szostakiewicz-Hołownia, M.; Gruszczyński, T. Mobility of copper and zinc in near-surface groundwater as a function of the hypergenic zone lithology at the Kampinos National Park (Central Poland). Environ. Earth Sci. 2017, 76, 276. [Google Scholar] [CrossRef]
- Souza, A.M.; Salviano, A.M.; Melo, J.F.B.; Felix, W.P.; Belém, C.S.; Ramos, P.N. Seasonal study of concentration of heavy metals in waters from lower São Francisco River basin, Brazil. Braz. J. Biol. 2016, 76, 967–974. [Google Scholar] [CrossRef] [Green Version]
- Ongen, A.; Dokmeci, H.; Celik, S.O.; Sabudak, T.; Kaykioglu, G.; Dokmeci, I. Copper and Cadmium Contents in Ground and Surface Water in Corlu. Turk. J. Environ. Prot. Ecol. 2008, 9, 753–762. [Google Scholar]
- Li, T.; Li, L.; Song, H.; Meng, L.; Zhang, S.; Huang, G. Evaluation of groundwater pollution in a mining area using analytical solution: A case study of the Yimin open-pit mine in China. SpringerPlus 2016, 5, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bougherira, N.; Hani, A.; Djabri, L.; Toumi, F.; Chaffai, H.; Haied, N.; Nechem, D.; Sedrati, N. Impact of the Urban and Industrial Waste Water on Surface and Groundwater, in the Region of Annaba, (Algeria). Energy Procedia 2014, 50, 692–701. [Google Scholar] [CrossRef] [Green Version]
- Hossain, D.; Islam, M.; Sultana, N.; Tusher, T. Assessment of Iron Contamination in Groundwater at Tangail Municipality, Bangladesh. J. Environ. Sci. Nat. Resour. 2015, 6, 117–121. [Google Scholar] [CrossRef]
- Vetrimurugan, E.; Brindha, K.; Elango, L.; Ndwandwe, O.M. Human exposure risk to heavy metals through groundwater used for drinking in an intensively irrigated river delta. Appl. Water Sci. 2016, 7, 3267–3280. [Google Scholar] [CrossRef] [Green Version]
- Afzal, M.S. Characterization of industrial effluents and groundwater of Hattar industrial estate, Haripur. Adv. Agric. Environ. Sci. Open Access 2018, 1, 70–77. [Google Scholar] [CrossRef]
- Dwivedi, A.K.; Vankar, P.S. Source identification study of heavy metal contamination in the industrial hub of Unnao, India. Environ. Monit. Assess. 2014, 186, 3531–3539. [Google Scholar] [CrossRef]
- Affum, A.O.; Osae, S.D.; Nyarko, B.J.B.; Afful, S.; Fianko, J.R.; Akiti, T.T.; Adomako, D.; Acquaah, S.O.; Dorleku, M.; Antoh, E.; et al. Total coliforms, arsenic and cadmium exposure through drinking water in the Western Region of Ghana: Application of multivariate statistical technique to groundwater quality. Environ. Monit. Assess. 2015, 187, 1–23. [Google Scholar] [CrossRef]
- Anirudhan, T.; Sreekumari, S. Adsorptive removal of heavy metal ions from industrial effluents using activated carbon derived from waste coconut buttons. J. Environ. Sci. 2011, 23, 1989–1998. [Google Scholar] [CrossRef]
- Khattak, S.A.; Rashid, A.; Tariq, M.; Ali, L.; Gao, X.; Ayub, M.; Javed, A. Potential risk and source distribution of groundwater contamination by mercury in district Swabi, Pakistan: Application of multivariate study. Environ. Dev. Sustain. 2020, 23, 2279–2297. [Google Scholar] [CrossRef]
- Panwar, R.M.; Ahmed, S. Assessment of Contamination of Soil and Groundwater Due to e-Waste Handling. Curr. Sci. 2018, 114. [Google Scholar] [CrossRef]
- Hsu, L.-C.; Chuang, Y.-H.; Chen, H.-W.; Chan, Y.-T.; Teah, H.Y.; Chen, T.-Y.; Chang, C.-F.; Liu, Y.-T.; Tzou, Y.-M. Accumulation of heavy metals and trace elements in fluvial sediments received effluents from traditional and semiconductor industries. Sci. Rep. 2016, 6, 34250. [Google Scholar] [CrossRef] [PubMed]
- Eiswirth, M.; Hotzl, H. The Impact of Leaking Sewers on Urban Groundwater. In Urban Groundwater Management and Sustainability; Springer: Cham, Switzerland, 2006; pp. 399–404. [Google Scholar]
- Elumalai, V.; Brindha, K.; Lakshmanan, E. Human Exposure Risk Assessment Due to Heavy Metals in Groundwater by Pollution Index and Multivariate Statistical Methods: A Case Study from South Africa. Water 2017, 9, 234. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.-M.; Liu, M.-C. Ecological risk assessment on a cadmium contaminated soil landfill—a preliminary evaluation based on toxicity tests on local species and site-specific information. Sci. Total. Environ. 2006, 359, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Oreščanin, V.; Mikelic, L.; Lulic, S.; Nad, K.; Mikulić, N.; Rubčić, M.; Pavlović, G. Purification of Electroplating Wastewaters Utilizing Waste By-Product Ferrous Sulfate and Wood Fly Ash. J. Environ. Sci. Health Part. A 2004, 39, 2437–2446. [Google Scholar] [CrossRef] [PubMed]
- Rösner, U. Effects of historical mining activities on surface water and groundwater an example from northwest Arizona. Environ. Earth Sci. 1998, 33, 224–230. [Google Scholar] [CrossRef]
- Paulson, A.J. The transport and fate of Fe, Mn, Cu, Zn, Cd, Pb and SO4 in a groundwater plume and in downstream surface waters in the Coeur d’Alene Mining District, Idaho, U.S.A. Appl. Geochem. 1997, 12, 447–464. [Google Scholar] [CrossRef]
- Frank, V.; Harangozó, M. Heavy metals in industrial wastewater determined by radionuclide X-ray fluorescence analysis and their effects onAllium cepa root tip cells. J. Radioanal. Nucl. Chem. 1994, 187, 137–141. [Google Scholar] [CrossRef]
- Kocher, B.; Wessolek, G. Verlagerung Straßenverkehrsbedingter Stoffe Mit Dem Sickerwasser. 99 S. Straßenbau Straßenverkehrstechnik 2003, 864, 1–15. [Google Scholar]
- Pedroli, G.M.; Maasdam, W.A.; Verstraten, J.M. Zinc in poor sandy soils and associated groundwater. A case study. Sci. Total. Environ. 1990, 91, 59–77. [Google Scholar] [CrossRef]
- Ritter, K.S.L. Sources, Pathways, and relative risks of contaminants in surface water and groundwater: A perspective prepared for the walkerton inquiry. J. Toxicol. Environ. Health Part. A 2002, 65, 1–142. [Google Scholar] [CrossRef]
- Kumar, M.; Goswami, R.; Patel, A.K.; Srivastava, M.; Das, N. Scenario, perspectives and mechanism of arsenic and fluoride Co-occurrence in the groundwater: A review. Chemosphere 2020, 249, 126126. [Google Scholar] [CrossRef] [PubMed]
- K’Oreje, K.; Vergeynst, L.; Ombaka, D.; De Wispelaere, P.; Okoth, M.; Van Langenhove, H.; Demeestere, K. Occurrence patterns of pharmaceutical residues in wastewater, surface water and groundwater of Nairobi and Kisumu city, Kenya. Chemosphere 2016, 149, 238–244. [Google Scholar] [CrossRef]
- Meffe, R.; de Bustamante, I. Emerging organic contaminants in surface water and groundwater: A first overview of the situation in Italy. Sci. Total. Environ. 2014, 481, 280–295. [Google Scholar] [CrossRef]
- Rehman, M.S.U.; Rashid, N.; Ashfaq, M.; Saif, A.; Ahmad, N.; Han, J.-I. Global risk of pharmaceutical contamination from highly populated developing countries. Chemosphere 2015, 138, 1045–1055. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Yin, M.; Yang, W.; Li, H.; Zhong, Y.; Mo, L.; Liang, Y.; Ma, X.; Sun, X. Emerging pollutants in water environment: Occurrence, monitoring, fate, and risk assessment. Water Environ. Res. 2019, 91, 984–991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
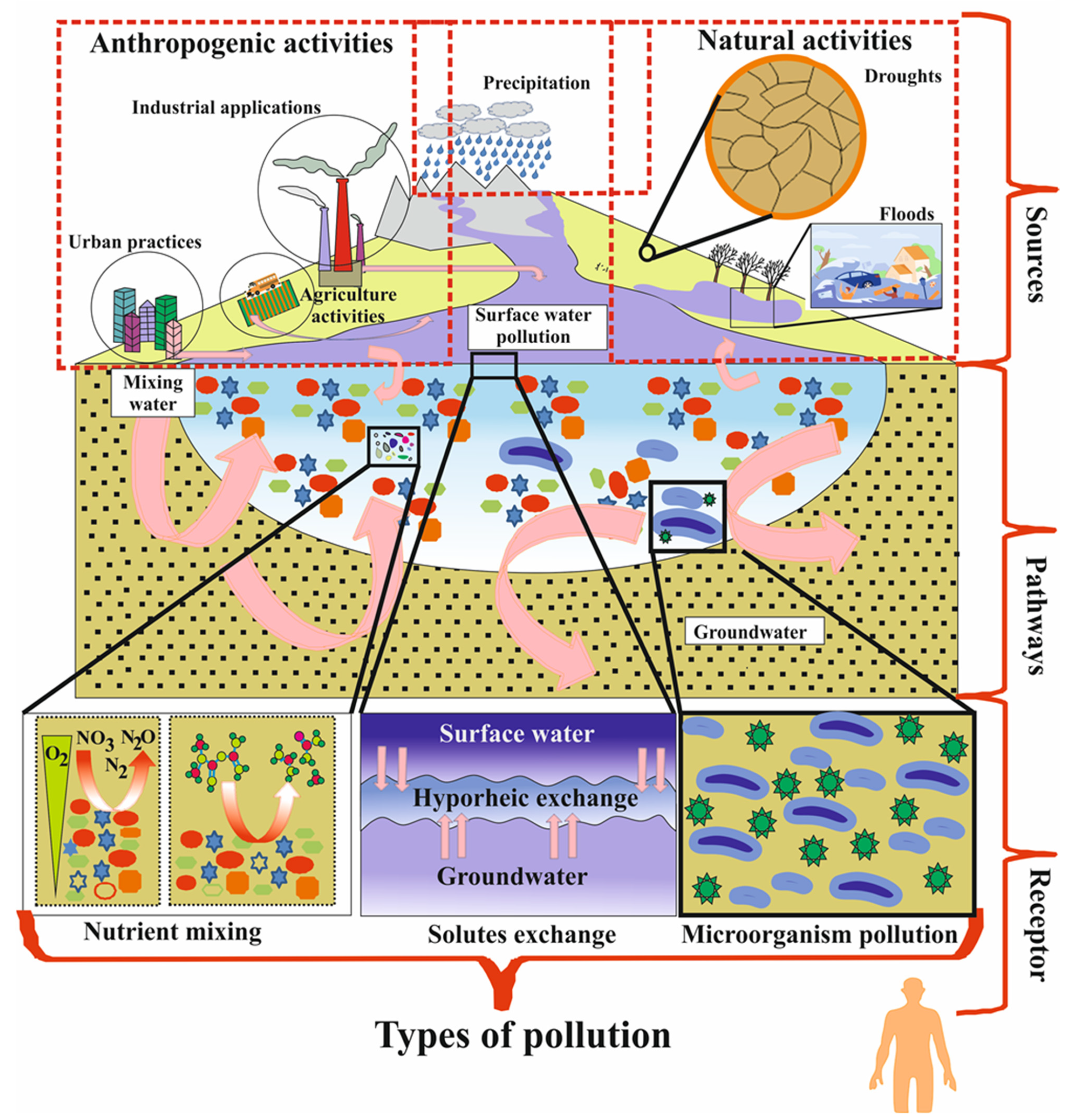

| Main Sources | Pollution Categories | Types of Pollutant Factors | Important Processes or Pathways of Water Contamination |
|---|---|---|---|
| Natural processes | Climate change | Precipitation, humidity and evapotranspiration | Due to high gas solubility, high water viscosity, and wind dynamics, evapotranspiration (heat exchange, soil-humidity radiation), and dilution of water by heavy rainfall and acid precipitation flows into surface water directly or indirectly and affects the groundwater quality. |
| Natural disasters | Droughts, floods, and landslides | Increased drought periods and higher temperatures rates are projected to affect the distribution of rainfall that produces flooding, as well as landslides which are high quantities of earth, rock, or mudthat flow quickly down mountainsides and have an enormous effect on the water resources. | |
| Geological factors | Plant roots, and topography slope | Plant roots absorb contamination or hazardous chemicals via preferred flow pathways and these pollutants infiltrate through soil particles into the groundwater. Further, flat terrains have lower surface runoff to accommodate higher infiltration rates, while steep slopes have tended to raise surface runoff and reduce the residence time of groundwater. | |
| Mineral dissolution and radioactive decay | Mineral dissolution is a slow process that takes several days, years, or decades, depending on the mineral solubility. Radiation material is due to emissions in the atmosphere of toxic ionizing radiation (beta-alpha particles, gamma rays, or neurons) and the radioactive decay of minerals that affects the water resources. | ||
| Soil-matrix | Grain size and pore spaces | Soil type or matrix (sand, clay, and silt) can control pollution with variable recharge or discharge rates; redox reactions are usually retarded in inorganic sediments or soils, whereas organic compounds or microorganisms bacteria tend to accelerate the rate of reactions in soil-matrix strata. | |
| Hyporheic exchange | Solutes exchange, pathogen exchange, and SW and GW interaction | Further, the availability of dissolved substances, solutes, organic-rich matter, and oxygen are highly reactive in the hyporheic exchange zone, and with the addition of microorganisms (viruses, bacteria, and protozoa) can lead to the death of animals under aerobic and anaerobic conditions, through both slow and quick flow routes in the groundwater. Further, the physical, chemical, and biological properties of water can change due to these elements mixing, and the intrusion of seawater makes the coastal groundwater system vulnerable to salinization. | |
| Anthropogenic processes | Industrial waste | Solid/liquid waste and chemical compounds | Landfills (including tailings facilities) are the most frequent places of disposal of solid waste globally and landfill leachate from waste disposal, as well as the presence of organic liquid compounds in industries (proteins, lipids, and carbohydrates) and dissolved inorganic contaminants is a source of water resources pollutants. Further, chemical materials in the industrialization sectors are utilized both outdoors (susceptible to photolysis destruction which is accompanied by soil biodegradation) and indoors (distinct routes of degradation which move through a wastewater plant). |
| Accidental spills and leaks | Spills and leaks in manufacturing products such as tanks and pipelines can also impact water resources, including manufacturing of environment products and chemical waste (benzene, methylbenzene, toluene, xylene) which get into surface water and contaminate groundwater | ||
| Mining processes | Mining practices have effects on the groundwater and surface water by excavating solid waste due to sinkholes, erosion, coal exploration, and chemicals released from mining processes and heavy utilisation of water in mineral processing. The groundwater pumped out of the mine disperse on the Earth’s surface or drain into streamswhere it penetrates the subsurface water, releasing dissolved, disintegrated, oxidized, and leached minerals, causing groundwater pollution. | ||
| Agriculture | Pesticides | Pest chemicals (herbicides, insecticides, rodenticides, and fungicides) can runoff from the surface and enter groundwater systems for a considerable time with their degradation products. | |
| Fertilizers | When the nutrient concentration (nitrates and phosphates) supassesthe plant absorption capability, it can lead to surface runoff and percolate into the groundwater. | ||
| Urban activities | Municipal waste | Solid garbage (wood, plastics, metals, food waste, papers, inert materials, etc.) is dumped and transported to the waste processing plant until it reaches rivers and pollutes the groundwater. Further, liquid wastewater can penetrate groundwater by way of sewage sanitary leaks connected to a storage tank or faulty structure, disturbing the water quality. | |
| Cemeteries | Water pollution from cemeteries was a historical issue, as 0.4–0.6 litres of leachate with a density of 1.23 g·cm−3 per 1 kg body weight are released during the decomposition process of the human body and can pollute aquifers. | ||
| Transportation | Transportation produces air pollution and can directly contribute to water pollution, thus storm events; precipitation extracts air pollution from the land surface, absorbs road deposits, and flows into water bodies. | ||
| Livestock productions | Livestock and poultry farms create animal waste which may be transferred to surrounding lakes, streams, and groundwater across the agricultural land surface, as well as animal manure, which can be used on farms to fertilize plants and add/recover nutrients to the soil. | ||
| Land use practices | The impact of land use activities on the water system from infrastructure, which includes construction, pipelines, and highways roads. |
| Water Resources | Processes/Factors | Important Processes |
|---|---|---|
| Surface water | Hydrological process | Evaporation, suspension, and setting |
| Groundwater | Transpiration, infiltration, and leaching | |
| All water resources | Dilution | |
| All water resources | Physical process | Adsorption and desorption, diffusion |
| Mainly river and lakes | Heating and cooling, vitalization, gas exchange with the atmosphere | |
| Groundwater | Chemical process | Ionic exchange |
| All water resources | Acid-base reactions, redox reactions, Precipitation of minerals, photo degradation, Dissolution of particles | |
| Surface water | Biological process | Primary production |
| All water resources | Microbial die-off and growth | |
| Mainly rivers and | Bioaccumulation, decomposition of organic matter, biomagnifications |
| Heavy Metals | Source | Pollution Type | Regions/Countries | GW Maximum Concentration | References |
|---|---|---|---|---|---|
| Aluminium (Al) | Natural source | Hydrological alkaline massif | Imandra, Kola Peninsula | 1.81 mg/L | [169] |
| Aluminium industry | Waste material | Canada | 12.5 mg/L | [170] | |
| Ni-SO4 mining | Waste material | Western, Australia | 11 mg/L | [171] | |
| Natural source | Peaty acid sulphate soil | Kalimantan, Indonesia | 180 mg/L | [172] | |
| Arsenic (As) | Mining Activity | Deepwater | Thammarat, Thailand | 503 μg/L | [173] |
| Industrial | Wastewater | Ondo, Nigeria | 1.23 mg/L | [174] | |
| Natural source | Arsenic bearing mineral | NE Ohio, USA | 200 μg/L | [175] | |
| Pesticide Production Plant | Infiltration | Kolkata, India | 23,050 μg/L | [176] | |
| Cadmium (Cd) | Fe-Ni-Co Mining | Waste material | Albania, several sites | 185 μg/L | [177] |
| Textile Industry | Wastewater | Haridwar, India | 40 μg/L | [178] | |
| Household waste | Wastewater | Ikare, Nigeria | 580 μg/L | [179] | |
| Fertilizer production | Atmospheric deposition | Rio, Brazil | 3 μg/L | [180] | |
| Cobalt (Co) | Natural source | weathering | Imo, Nigeria | 2 mg/L | [181] |
| Bonab Industrial Estate waste material | Waste material | Zanjan, Iran | 308 μg/L | [182] | |
| Industrial effluents | Waste material | Tamil Nadu, India | 0.5 mg/L | [183] | |
| Household waste | Wastewater | Zahedan City, Iran | 0.204 mg/L | [184] | |
| Chromium (Cr) | Brownfield | Wastewater | Xiangjiang River, China | 94.4 mg/L | [185] |
| Industrial | Wastewater | Spain | 25 mg/L | [186] | |
| Natural source | Biological activity | North-western Nigeria | 2.2 mg/L | [187] | |
| Urban land use/agriculture | wastewater/infiltration | California | 10 μg/L | [188] | |
| Copper (Cu) | Qilan Mountain Mining | Waste material | Qinghai-Tibet Plateau, China | 11.3 mg/L | [189] |
| Natural | Dissolution of Cu-weathering | Kampinos, Poland | 0.59 mg/L | [190] | |
| Urbanization/industrialization | Wastewater | Bahia, Brazil | 1.596 mg/L | [191] | |
| Roadways | Waste material infiltrates | Corlu, Turkey | 554.45 μg/L | [192] | |
| Iron (Fe) | Yimin open pit mine | Waste material | Inner Mongolia, China | [193] | |
| El-Hadjar Industrial | Wastewater | Annaba, (Algeria) | 32 mg/L | [194] | |
| Natural source | Dissolution of Fe-minerals | Shuangliao, China | 46.3 mg/L | [92] | |
| Household waste | Wastewater | Tangail, Bangladesh | 25 mg/L | [195] | |
| Manganese (Mn) | P fertilizer application | Infiltration | Cauvery River basin, India | 7 mg/L | [196] |
| Hattar industrial estate | Wastewater | Haripur, Pakistan | 2 mg/L | [197] | |
| Textile Industry | Atmospheric deposition | Unnao, India | 2.72 mg/L | [198] | |
| Natural source | Dissolution of pyrite | Coode Island, Australia | 0.9 mg/L | [129] | |
| Mercury (Hg) | Household waste | Wastewater | Sekondi-Takoradi Metropolis, Ghana | 90 μg/L | [199] |
| Chloro-alkali Industry | Wastewater | Kerala, India | 9.9 mg/L | [200] | |
| Natural source | Marine sediment intrusion | Zhoushan Island, China | 1 μg/L | [92] | |
| Municipal Waste | Wastewater | Swabi, Pakistan | 2 μg/L | [201] | |
| Nickel (Ni) | Electronically waste recycling | Wastewater | Krishna Vihar, India | 2.9 mg/L | [202] |
| Taichung industrial | Wastewater | Taiwan | 1.022 mg/L | [203] | |
| Sewerage | Leakage | Rastatt, Germany | 0.02 mg/L | [204] | |
| Mining Activity | Wastewater | KwaZulu-Natal Province, South Africa | 2 mg/L | [205] | |
| Lead (Pb) | Landfill | Leachate | Taiwan Alexandria, Egypt | 51 μg/L | [206] |
| Electro planting | Wastewater | Zagreb, Croatia | 8.6 mg/L | [207] | |
| Au-Ag-Pb-Zn mining | Wastewater | Chloride, Arizona USA | 19 μg/L | [208] | |
| Natural source | Oxidation reactions, leaching | South Africa | 1 mg/L | [30] | |
| Zinc (Zn) | Pb-Zn mining | Wastewater | Coeur d’Alene basin, Idaho, USA | 389 μg/L | [209] |
| Engineering plant | Waste material | China | 505 mg/L | [210] | |
| Road Traffic | Infiltration | Celle, Germany | 2.34 mg/L | [211] | |
| Natural source | Atmospheric deposition | Strijer, Netherlands | More than 15 mg/L | [212] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akhtar, N.; Syakir Ishak, M.I.; Bhawani, S.A.; Umar, K. Various Natural and Anthropogenic Factors Responsible for Water Quality Degradation: A Review. Water 2021, 13, 2660. https://doi.org/10.3390/w13192660
Akhtar N, Syakir Ishak MI, Bhawani SA, Umar K. Various Natural and Anthropogenic Factors Responsible for Water Quality Degradation: A Review. Water. 2021; 13(19):2660. https://doi.org/10.3390/w13192660
Chicago/Turabian StyleAkhtar, Naseem, Muhammad Izzuddin Syakir Ishak, Showkat Ahmad Bhawani, and Khalid Umar. 2021. "Various Natural and Anthropogenic Factors Responsible for Water Quality Degradation: A Review" Water 13, no. 19: 2660. https://doi.org/10.3390/w13192660
APA StyleAkhtar, N., Syakir Ishak, M. I., Bhawani, S. A., & Umar, K. (2021). Various Natural and Anthropogenic Factors Responsible for Water Quality Degradation: A Review. Water, 13(19), 2660. https://doi.org/10.3390/w13192660









