Abstract
Lack of knowledge about distribution of charophyte fructifications and importance of environmental conditions in the Baltic Sea coastal waters fostered us to assess the spatial-temporal patterns of oospore bank in relationship with environmental factors in the Curonian Lagoon (Lithuanian part). We mapped the distribution of oospores in 2017–2019. The importance of environmental factors was determined by the cluster analysis and boosted regression trees. Four oospores species were recorded up to 4 m depth. The highest mean densities (58,000 ind·m−2) of viable fructifications were found along the eastern shore, where the densest charophyte stands were recorded. Viable fructifications showed a clear pattern of filling the oospore bank after the vegetation season and a depletion during the summer as they germinated. The distance from charophyte stands, salinity, bottom slope aspect, and wave exposure were the most important environmental variables. Full fructifications mostly occurred within <0.5 km distance from the charophyte stands restricted to flat and sheltered areas exposed to the northern and eastern slopes. Empty fructifications were mostly found within <2 km distance from the charophyte stands but their high density was limited to <1 km distance from the charophyte stands and on the northeastern bottom slopes and >1.5 salinity.
1. Introduction
A propagule bank of charophytes consists of sexual propagules oospores and vegetative propagules [1]. Fertilized oogonia form oospores, which for many species continue their development into calcified gyrogonites. After a decay of charophyte thalli, sexual propagules (fructifications) can remain in sediments for a long period and stay viable up to 300 years [2]. Therefore, for many charophyte species, dispersion, colonization, and maintenance of populations depend entirely on sexual reproduction [1].
Despite the widely recognized importance of oospore bank for charophyte ecology and life cycle, there are few studies that have been focused on distribution of oospores. In the Baltic Sea, the studies on oospore bank have been conducted only along the German coastline [3,4,5], where in contrast to the recent vegetation (angiosperm dominated), more oospores than seeds of angiosperms have been found in the sediment samples. Extensive studies on the distribution of charophytes have been performed in Swedish, Polish, and Estonian coastal waters [6,7,8,9], but obtained results have not been related to an oospore bank.
Mechanisms of spatial variation in potential diaspore banks were also only considered in a few studies [1]. Charophyte stands are considered as a primary source of oospores [10]. Although the density of oospores correlated with the coverage of charophytes in the shallow lakes [11], the oospore density in the shallow brackish lagoon of the Baltic Sea did not correspond to the cover of charophyte vegetation [10]. Another important factor for a spread of oospores is hydrodynamics, which transports oospores to other places and causes their resuspension or borrowing. Water birds are recognized as responsible for a long-distance dispersal of oospores, which if passed through a digestive system are viable and able to germinate [1].
Regarding the water framework directive (2000/60/EC), an understanding of a distribution of oospores in estuarine lagoons is important for an ecological status assessment and a development of macrophythobenthos indicators. For the transitional waters (estuaries and lagoons), about one-third of macrophythobenthos indicators have not been tested for pressure–impact relationships, being the least validated [12]. Therefore, a knowledge development on the ecology of charophytes and other macrophytes in transitional waters of the Baltic Sea is at an early stage compared to coastal and inland waters. There are no data about a status of a charophyte oospore bank and factors determining an oospore distribution in the biggest estuarine lagoons of the Baltic Sea. In the Curonian Lagoon, only the long-term changes of charophyte habitats have been investigated [13], and the empirical relationships between the distribution of charophytes and environmental factors have been assessed previously [14]. The relatively low importance in explaining the variation in the distribution (<28%) of charophytes by environmental factors highlights the need to look into other important factors such as spatial and temporal patterns of charophyte fructifications.
Thus, the aim of the present study was to determine distribution and seasonal patterns of a charophyte oospore bank and its relationship with charophyte stands and abiotic factors in the Curonian Lagoon. We estimated ripening stages of oospores in sediments, hypothesizing that an absence of charophytes in some areas of the lagoon can be explained by a lack of viable fructifications and by a strong hydrodynamic forcing, with both being the most important factors for a distribution of oospores in the estuarine part of the lagoon. We also tried to assess the oospore densities required to maintain charophyte stands for a next generation.
2. Study Area
The Curonian Lagoon is the largest estuarine lagoon in the Baltic Sea, with an area of 1.584 km2. The northern (estuarine) part of the lagoon belongs to the state of Lithuania (area 413 km2) and extends from the Nemunas River inflow to the Baltic Sea via the Klaipeda Strait. The mean depth of the lagoon is 3.8 m, and only the Klaipeda Straight has a maximum 14.5 m depth [15]. The lagoon is almost freshwater, with a salinity range of 0.1–7, depending on an inflow of brackish waters from the Baltic Sea through the narrow and 10 km long strait during cyclonic periods [16]. The inflow of brackish waters mainly occurs along the deeper western shore of the lagoon, whereas freshwater masses move along the eastern shore [15,17].
These water masses strongly effect the water clarity in the lagoon; moreover, the water transparency is largely determined by an algal density and a periodic sediment resuspension during periods of high winds and wave activity [18]. The Curonian Lagoon is considered as eutrophic or hyper-eutrophic with recurring spring diatom blooms followed by summer cyanobacteria blooms, a phenomenon that has been reported for several decades [19,20]. Bottom sediments in the lagoon up to the depth of 2.7 m consist mainly of fine sand, whereas coarse silt and fine silty mud prevail at the depth over 3 m [21].
The diversity of charophytes is relatively low (seven species) in the study area, with Chara contraria being the most frequent, followed by Chara aspera, Nitellopsis obtusa, Chara baltica, Tolypella nidifica, Chara globularis, and Chara canescens [13]. The charophytes form stands shallower than the 2 m depth together with the dominant angiosperms such as Potamogeton perfoliatus, Potamogeton rutilus, and Stuckenia pectinata [14].
3. Materials and Methods
3.1. Field Sampling
An oospore bank was assessed in the Lithuanian part of the Curonian Lagoon in the period of 2017–2019 (Figure 1). The locations of the sampling sites were mainly based on the previous studies of the distribution of charophytes [14], i.e., to sample different depths across the whole study area. In November 2017, a preliminary survey was carried out along the eastern and western shores of the study area in order to estimate a general extent of charophyte fructifications in the upper littoral part (<1 m depth), where most of the charophyte stands are restricted [13]. The depth extent and distribution of charophyte fructifications were surveyed along the depth gradient (0.5, 1, 1.5, 2, 3, and 4 m) in the 7 major sections (profiles) between the western and the eastern shores of the study area (Figure 1). In several profiles (1, 3, and 6), surveys were performed in June 2018 (after the start of the vegetation season), in October 2018 (after the end of the vegetation season), and in May 2019 (before the vegetation season), attempting to represent the main seasonal patterns of a charophyte life cycle.
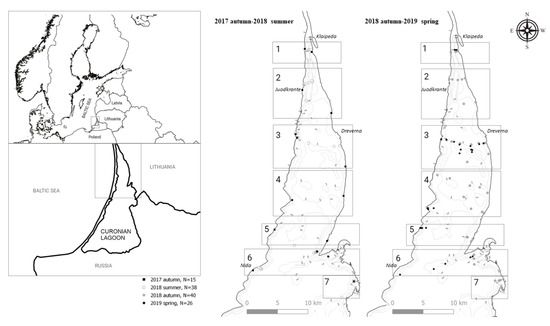
Figure 1.
The study area with the sampling sites (indicated by symbols) and profiles (indicated by squares with numbers) in the Curonian Lagoon for the period of 2017–2019. N—number of the study sites.
In each study site, 3 sediment cores (replicates) were sampled within <5 m distance from each other using a Plexiglass tube (the diameter of 4.5 cm; with a plastic valve on top), which was operated with a push rod (extending to 6 m long) from a boat. The upper 5 cm sediment layer was collected from each core and the samples were stored in separate plastic containers in the dark at 5 °C. In the laboratory, the sampled sediments were sieved (at the mesh sizes of 1, 0.5, and 0.2 mm), and charophyte oospores were collected from the latter 2 mesh sizes. Oospores in each sample were counted and identified using the determination keys [22,23,24] and compared with live specimens under a microscope. A viability of oospores was tested by clicking them with a needle. Fructifications were classified to the 4 types: empty oospores and empty gyrogonites (empty fructifications), and full oospores and full gyrogonites as viable fructifications (see detailed description of oospores and gyrogonites provided by [25]). In each sample, a density of the 4 types of fructifications was calculated (expressed as ind. m−2).
3.2. Data Analysis
For the assessment of the importance of environmental factors explaining the distribution of charophyte fructifications, we tested 1 biotic and 6 abiotic variables: a distance from charophyte stands (biotic), a wave exposure (relative exposure index—REI), a bottom slope, its aspect, a topographic position index (TPI), a depth, and a water salinity. Using QGIS 3.4.1 [26], the distance from charophyte stands was assessed by Euclidean distance from the sites where charophytes were recorded in 2014–2015 [14]. REI was estimated for 16 sectors (fetch rays at increment of 11.25°) according to Malhotra and Fonseca [27]: , where Fj = length for the jth direction fetch ray after clipping to shoreline and interrogating bathymetry (the maximum length of each ray was fixed at 10 km), θj = angle between the ith fetch ray and the jth ray, Vi = wind speed for the ith direction, and Di = wind duration for the ith direction. The REI value of 0 indicated absolutely sheltered from wave effect, REI < 50 corresponded to very sheltered areas in the study area (in the shallow northeastern shore), while REI > 300 showed the most exposed study sites in the southern part of the study area. The bathymetric data were obtained from the hydrological model [17]. The model was coupled with the wind data (velocity and direction) for the vegetation period (May–September) during 2000–2017, which was measured in the coastal hydrometeorological stations located in Nida and Klaipeda. REI was calculated in R 4.0.3 [28] applying the “raster” [29], the “rgdal” [30], the “geosphere” [31], and the “rgeos” [32] packages. In this study, the seafloor geomorphological parameters (the bottom slope, its aspect, and TPI) were considered as proxies for effects of water currents. The bottom slope and its aspect were derived from the bathymetric data (the spatial resolution of 48 × 48 m) using terrain function with the “raster” package in R. Using the same data, TPI was calculated by the tpi function and the focal window size of 59 (in order to represent large-scale topographic patterns) with the “spatialEco” package [33] in R. The TPI value of 0 indicates flat relief (plain), positive TPI values show elevated relief forms (convexities), while negative values represent concave surfaces. The depth was considered as a proxy of a light and wave gradient. The mean near bottom salinity data for the vegetation period during 2013–2017 was obtained from the same hydrological model developed for the Curonian Lagoon [17]. For each sediment sampling site in 2019, we extracted all environmental data from their raster layers in QGIS or R.
For the analysis of seasonal dynamics of charophyte fructifications, we selected the third profile, where the similar locations of study sites were sampled in the main seasons (summer, autumn, and spring).
3.3. Statistical Analysis
The Bray–Curtis dissimilarity [34] was applied to quantify differences in the densities of the fructifications between the profiles (the averaged density of each type of fructifications at three depths: 1, 2, and 3 m), which was inspected by the hierarchical cluster analysis using the “vegan” package [35] in R. The one factor analysis-of-similarities was used to test differences between groups of clusters.
The importance of 7 environmental factors was assessed by indirect and direct approaches. The indirect approach was based on a comparison of means of each environmental factor among several groups of the study sites, i.e., the sites with and without charophyte fructifications and delineated clusters (groups of the profiles) in the cluster analysis. The Welch ANOVA with the Games–Howell post hoc test in the “userfriendlyscience” package [36] in R were used for the comparisons of means due to the heterogeneity of variance and the unequal sample size between the groups. The same tests were applied for the comparisons of mean densities of charophyte fructifications among the different seasons and depths (1 and 2 m).
The statistical modelling and machine learning methods were chosen as the direct approach for the assessment of the importance of 7 environmental factors (explanatory variables) on the distribution (occurrence) and the density of charophyte fructifications (4 response variables). Firstly, the generalized additive models were fitted for each response variable; however, the residuals did not meet the assumption of homoscedasticity of variance and multicollinearity of several explanatory variables, e.g., between TPI and REI (rSpearman = −0.62), between the salinity and the distance to the charophyte stands (rSpearman = −0.61). Therefore, the boosted regression trees (BRT) method was selected. BRT was originally developed to predict data in computer science [37], and has recently been used for predicting species density, distribution, and diversity [38]. This method does not assume that an explanatory variable has the same relationship with a response across the entire range of the environmental factor, as BRT uses decision trees to classify explanatory variables and predict the response by minimizing a loss function [39]. BRT produces a partial dependence plot to illustrate the relationship between an explanatory variable and a response, after controlling for all other explanatory variables in a model. This technique improves a prediction accuracy because it adjusts weights on the basis of explanatory variables through a stagewise learning of the data. Moreover, BRT addresses a multicollinearity issue because it considers interactions among explanatory variables—a response to an explanatory variable depends on the values of other explanatory variables at the higher levels of trees.
BRT was performed using the package “H2O” [40] in R. Two models with the Bernoulli distribution were performed for the occurrence of full and empty fructifications, and two models with the Poisson distribution for the density of full and empty fructifications. For each response, multiple models were run varying either the model learning rate (between 0.1 and 0.001) and the number of trees (between 50 and 5000). Then, the optimum model parameters were selected on the basis of the model performance (the area under the receiver operating characteristic curve for the models with the Bernoulli distribution, and R2 for the models with the Poisson distribution): the learning rate of 0.005, the number of trees at 100, and the bag fraction of 0.75. The tree complexity was set to 2 in order to represent two-way interactions between environmental factors. For each model, the explained deviance with all environmental factors was assessed by . The relative importance of explanatory variables in predicting the response variable (i.e., the empirical improvement in reducing a squared error relative to other explanatory variables) was estimated. Partial dependence plots were derived for each model, which showed a marginal effect of explanatory variables on the predicted outcome of a model [36].
4. Results
4.1. Structure and Distribution of Oospore Bank
Fructifications (oospores and gyrogonites) of four charophyte species were identified in the study area. In some cases, C. contraria and C. aspera calcified gyrogonites were hardly distinguished from each other, and also there were many oospores damaged by abrasion which were determined as the Chara contraria/aspera group. The species composition was similar among the second and seventh profiles, where oospores of C. contraria/aspera dominated (Figure 2). The oospores of Chara baltica and Tolypella nidifica were only found in the first profile and accounted for 0.5% of the total density.
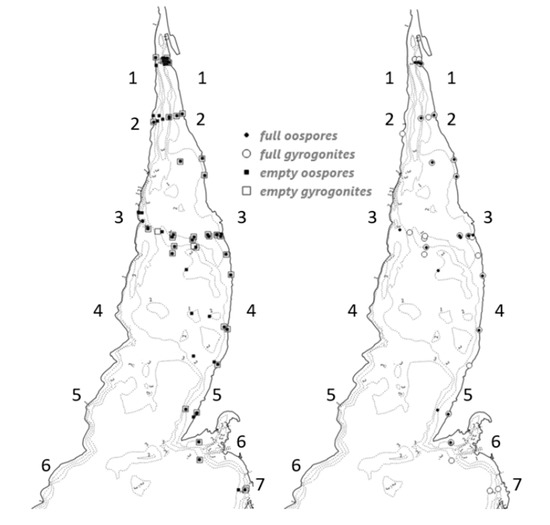
Figure 2.
The distribution of charophyte fructifications in the study area: empty oospores and gyrogonites (left), viable (full) oospores, and gyrogonites (right). Numbers along the shores indicate the profiles.
Occurrence of viable (full) and empty fructifications together were recorded in 59% of sampled sites during the study period (Figure 2), while full fructifications were found in only 33%. Viable fructifications were recorded in all sites along the eastern shore, whereas they were recorded only at the second and third profiles along the western shore. Empty fructifications were distributed further from the eastern shore than viable ones, except for the second and the third profiles, where fructifications occurred across both shores.
The highest density of empty oospores was observed in the fourth profile at 1 m depth (up to 88,000 ind. m−2 near the eastern shore) and in the third profile near the western shore at 2.5 m depth at the beginning of June 2018. Empty gyrogonites had the highest density (20,000 ind. m−2) in the third profile at 1.5 m depth. The maximum number of full oospores (11,875 ind. m−2) was recorded in the first profile at 1 m depth. Viable gyrogonites were most abundant in the profiles 1–3 at depths of 1–2 m (up to 78,000 ind. m−2) in October 2018.
The empty oospores made up most of the oospore bank, and their percentage increased with a depth (Table 1). The percentage of other fructifications decreased with depth, and there were no records of full fructifications deeper than 2.5 m.

Table 1.
The oospore bank structure in different depths.
The two groups (clusters) of the profiles were identified (R = 0.98, p < 0.05) after the cluster analysis (Figure 3). The first group consisted of the profiles 1–4, where charophyte fructifications were found on both the eastern and western shores (Figure 4A). The second group consisted of the profiles 5–7, where fructifications were found only on the eastern shore (Figure 4B). The mean density of fructifications in the first cluster was >80% higher than in the second one.
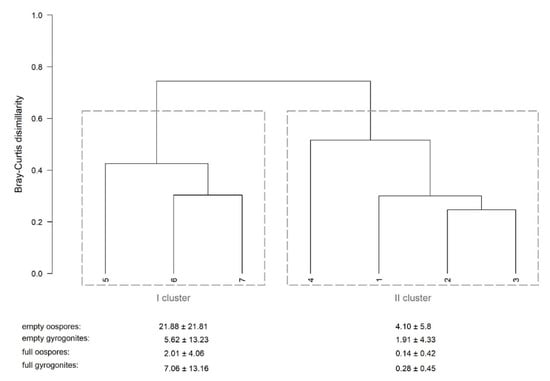
Figure 3.
The dendrogram derived from the density of charophyte fructifications found in the seven profiles in the study area. The two main clusters (marked with Roman numerals) of the profiles (marked with Arabic numerals), indicated by dashed lines, and the mean (±standard deviation) density (103 ind. m−2) of empty and full fructifications are provided below.
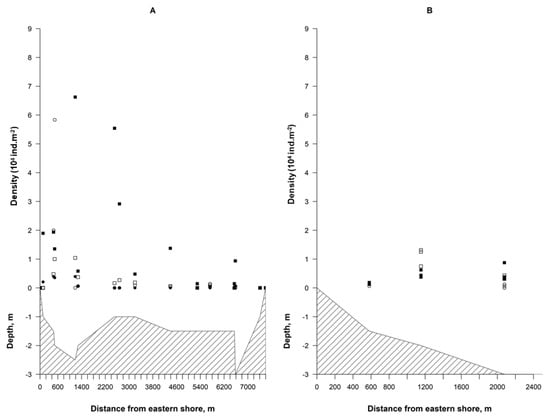
Figure 4.
The distribution of the density of charophyte fructifications in the most representative profiles (third and sixth) of the first cluster (A) and the second cluster (B) during the period of 2017–2019. Full circles—full oospores, empty circles—full gyrogonites, full squares—empty oospores, and empty squares—empty gyrogonites.
4.2. Seasonal Patterns
There were significant seasonal differences in the density of C. contraria/aspera fructifications (Figure 5), and they were dependent on the shore and the depth. On the eastern shore at the 1 m depth, the mean density of empty gyrogonites was statistically significantly (Games–Howell post hoc test, p < 0.05) higher in the summer of 2018 than in the spring of 2019. There was no significant (p > 0.05) difference in the mean density of empty or full oospores. The mean density of full gyrogonites in the autumn of 2018 was significantly (p < 0.05) higher than in the summer of 2018 and the spring of 2019.
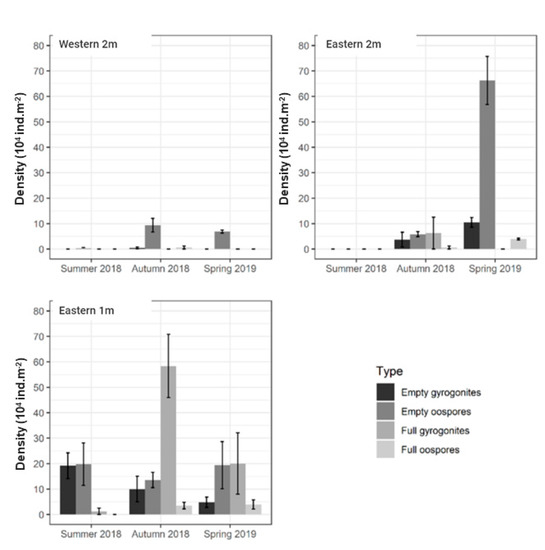
Figure 5.
The seasonal dynamics of the mean (±standard deviation) density of Chara contraria/contraria group fructifications in the third profile of the study area. For the comparison, the profile was divided into sections according to the side of the lagoon shore (eastern and western) and the depth zone (1 and 2 m).
We did not find charophyte fructifications on the western shore at the 1 m depth during all seasons. Empty fructifications prevailed at the 2 m depth, but no significant differences (p > 0.05) were observed in the mean density between the seasons for any type of fructification. At the same depth on the eastern shore, the highest mean density of empty oospores and gyrogonites were estimated in the spring 2019, while no fructifications were recorded in the summer 2018. The mean density of full gyrogonites in the autumn of 2018 was significantly (p < 0.05) higher than in the spring of 2019. The mean density of full oospores in the spring of 2019 was significantly (p < 0.05) higher than in the autumn of 2018.
4.3. Environmental Factors Explaining Patterns in Oospore Bank
The magnitude of the environmental variables assessed varied throughout the study area (Table 2). Charophyte fructifications were found in the depth from 0.5 to 4 m, where the relative wave exposure index (REI) varied from 44 to 335. The highest distance between the fructification presence and the charophyte stands was determined at the level of >6.5 km. The maximum near bottom salinity of 1.8 indicated occasional intrusions of brackish Baltic Sea waters to the investigation area. Fructifications occurred on 0.003–0.907° slopes with 0.4–351.8° aspect and bottom places where topographic position index (TPI) ranged from −0.339 to 0.626.

Table 2.
The statistics of environmental variables in the sites with presence and absence of charophyte fructifications, and in the two clusters delineated in the cluster analysis (Figure 3).
REI, the distance from the charophyte stands, the salinity, and partly the aspect of bottom slopes were the most important environmental factors for characterizing differences between the cluster groups and the areas with presence and absence of charophyte fructifications. The statistically significant differences in the mean REI were determined between the sites with the presence and absence of fructifications (the mean ± standard deviation, respectively, 150 ± 67 and 205 ± 82; p < 0.01), between the first and the second cluster groups (respectively, 144 ± 80 and 202 ± 76; p < 0.01), between the first cluster group and the sites with absence of fructifications (p < 0.05), and between the presence of fructifications and the second cluster group (p < 0.001). There were significant differences in the mean distance from the charophyte stands among the same groups—between the sites with the presence and absence of fructifications (respectively, 1.2 ± 1.5 km and 5.2 ± 3.9 km; p < 0.001), between the first and the second cluster groups (respectively, 1.2 ± 1.4 km and 6.1 ± 3.6 km; p < 0.001), between the sites with the presence of fructifications and the second cluster (p < 0.001), between the first cluster and the sites with the absence of fructifications (p < 0.001), and between the sites with the absence of fructifications and the second cluster (p < 0.01). The statistical differences in the mean salinity were determined among these groups—between the sites with the presence and absence of fructifications (respectively, 0.7 ± 0.6 and 0.3 ± 0.4; p < 0.001), between the first and the second cluster groups (respectively, 0.8 ± 0.6 and 0.2 ± 0.05; p < 0.001), between the first cluster group and the sites with the absence of fructifications (p < 0.001), between the sites with the presence of fructifications and the second cluster group (p < 0.001), and between the second cluster group and the sites with the absence of fructifications (p < 0.05). The statistical differences in the mean aspect of bottom slopes were determined only between the sites with the presence and absence of fructifications (respectively, 134.8 ± 102.3° and 203.4 ± 109.9°; p < 0.01).
In the BRT models, the explained variance (deviation) by the used environmental factors was relatively low for the occurrence of full and empty charophyte fructifications (respectively, 14% and 20%). The distance to the charophyte stands and the aspect of bottom slopes were the most important factors (relative importance > 30%) in explaining the presence/absence of full fructifications (Figure 6). The highest probability (≥40%) to find viable fructifications was less than 0.5 km away from the charophyte stands and on the northern, eastern, and southern bottom slopes (aspect of 0–225°). Less important were the wave exposure and the bottom slope (relative importance, respectively, 21% and 11%), where the highest probability to find full fructifications was up to 150 REI and relatively flat bottom areas (slope < 0.05°). The importance of TPI, the depth, and the salinity was minor (≤5%). The highest probability to find viable fructifications was on shallow (<1.2 m) and elevated areas (TPI > 0.1) affected by brackish waters (salinity > 0.1).
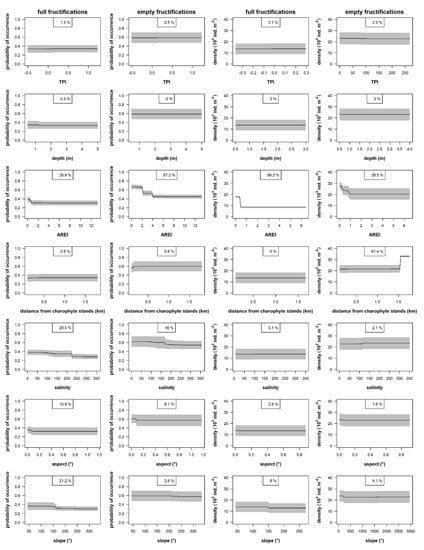
Figure 6.
The partial dependence curves between the occurrence and density of full (viable) charophyte fructifications and empty charophyte fructifications and the environmental factors. The relative importance of environmental factors is provided in the top legend for each plot.
The distance to the charophyte stands was the most important factor (relative importance = 67%) in explaining the occurrence of empty fructifications (Figure 6). The distance from the charophytes stands had negative relationship with the response variable. Empty fructifications were mostly found (probability > 60%) less than 2 km away from the charophyte stands. The bottom aspect and slope were less important (relative importance, respectively, 16% and 8%), indicating the highest probability (>60%) of empty fructifications on flat bottom slopes (<0.15°) exposed to the northern, eastern, or southern directions (aspect of 0–160°). The other factors were of less importance (<6%). The higher probability of empty fructifications was up to 200 REI and salinity > 0.31. The effect of TPI and depth was not important for the occurrence of empty fructifications.
The explained variance by the used environmental factors was relatively low for the density of full and empty charophyte fructifications (respectively, 23% and 21%). The distance to the charophyte stands was the most important for the full fructifications (relative importance = 89%), where their high density (>15,000 ind. m−2) was in less than 0.5 km away from the charophyte stands (Figure 6). Less important (relative importance = 6%) was the wave exposure, where the high density (>15,000 ind. m−2) was in less than 150 REI. The other factors were even less important (<3%), where the density of fructifications was higher on relatively flat (slope < 0.1°) or elevated (TPI ≥ 0) areas. The effect of the bottom aspect, the salinity, and the depth was not important for the density of full fructifications.
The salinity was the most important factor (relative importance = 61%) in explaining the density of empty fructifications (Figure 6), where their highest density (>30,000 ind. m2) was from the salinity >1.5. The second important (29%) factor was the distance from the charophyte stands. Relatively high density (>20,000 ind. m−2) of empty fructifications was in the distance of <1 km from the charophyte stands. The importance of other factors was minor (<5%). The density of empty fructifications was higher in sheltered areas (REI < 200) on relatively flat slopes (<0.1°) or elevated (TPI of 0–100) areas exposed to the northern, southern, and western directions (120–360°). The effect of the depth was not important for the density of empty fructifications.
5. Discussion
5.1. Species Composition
The current paper describes spatial patterns of generative reproduction of charophytes and the potential viability of charophyte habitats in the Curonian Lagoon. We determined the oospores of four species of charophytes, whereas in total seven charophyte species have been recorded in the study area previously [13], and a total of 24 species have been recorded in the Baltic Sea [41].
Oospores of C. contraria and C. aspera were the most abundant, and these species are dominant in the study area [14]. The production of oospores is important for a long-distance dispersal [42]; therefore, a generative reproduction strategy may explain the spread of oospores of C. contraria in deeper areas and away from primary habitats in the upper littoral part of the Curonian Lagoon [13]. Oospores of brackish charophyte species (C. baltica and T. nidifica) were very rare in the study area and restricted to the northeastern part, which is frequently affected by inflows of the Baltic Sea waters. According to Doege et al. [43], T. nidifica is common in the Baltic Sea with salinity range of 8–25; however, salinities in the northern part of our study area during the summer can reach only 7.5, with the average salinity of 2 [17].
5.2. Density and Distribution
The differences in the density and distribution of charophyte fructifications were found between the eastern and western shores of the Curonian Lagoon. The highest mean densities of all oospore types were found on the eastern shore (the profiles 1–4), where the dense charophyte stands were recorded [14]. The mean density of viable fructifications at the 1 m depth was 17,500 ind. m−2, which corresponds to the mean density of oospores (about 20,000 ind. m−2) reported in the Westrugensche Bodden lagoon system at the same depth [4]. Although some differences in the oospore density between these waterbodies and the Curonian Lagoon could be due to species composition (C. aspera, C. baltica, and C. canescens were recorded in the Grieben Bay), one common pattern was observed for both lagoons—the highest densities of oospores were observed in the areas with >90% of charophyte cover at 1–1.5 m depth.
Along the western shore of the Curonian Lagoon, the distribution of fructifications was restricted to the littoral from Klaipeda to Juodkrante (Figure 1), where their mean density was the highest in vicinity of Juodkrante. This corresponded to the presence of charophyte stands [14]. We found empty fructifications down to the 2 m depth, where charophyte stands have not been recorded before. The reason of these findings could be that fructifications are carried from charophyte stands along steep bottom slopes towards the deepest part (i.e., navigation channel) by waves and currents; however, environmental conditions (mainly a light climate and sedimentation) most likely are unfavorable for germination, development, and growth there.
A colonization of unvegetated areas by charophytes may take several years and may require the sufficient number of a production and accumulation of oospores [11]. Other studies also confirm high densities of fructifications in densely vegetated areas, for example, about 20,000 ind. m−2 for C. contraria in the Grieben Bay and for C. aspera in the Windebyer Noor at the 0.5 m depth [44]. The mean density of full fructifications was relatively low (<10,000 ind. m−2) along the western shore of the study area, as the mean coverage of charophytes was ≤30% [14]. This density of oospores seems to be too low to establish dense charophyte stands in this area and in the southern part of the study area along the eastern shore (the profiles 5–7).
We found the seasonality of generative reproduction in our study corresponding to a well-documented pattern [45]. In the Curonian Lagoon, viable fructifications (especially gyrogonites) showed a clear pattern of filling the oospore bank in the autumn (after vegetation season) and depletion during the summer as they germinate. Such seasonality is strongly expressed along the eastern shore at the 1 m depth. Empty gyrogonites, prevailing in the 2 m depth along both shores, had an opposite trend. The explanation for this pattern could be that empty fructifications in shallow zones are transported after a germination to deeper zones by currents and waves. Several viable fructifications were found up to the 2.5 m depth, but an insufficient light regime might have a negative impact on a charophyte development, growth, and reproduction [46,47]. We hypothesize that charophytes in the 2 m depth germinate, but they cannot produce a sufficient number of viable fructifications limiting a spread of charophyte stands to deeper areas.
Its apparent that seasonal measurements of viable and empty gyrogonites indicate a status of the charophyte habitats. On the basis of results of this study, we conclude that the mean density of full gyrogonites in autumn ≥61,000 ind. m−2 should be sufficient for the establishment and development of C. contraria in incoming spring. Certainly, the threshold requires testing under experimental conditions in order to develop the indicator of biological status for transitional waters.
5.3. Importance of Environmental Factors
In this study, the distance from the charophyte stands, the salinity, the aspect of bottom slope, and the wave exposure (REI) were revealed as statistically significant important environmental factors for explaining the distribution of charophyte fructifications. The significance of these factors was confirmed by the two approaches, enhancing the confidence that this observation was not random.
The explained deviance by the tested environmental variables was relatively low for the occurrence and the density (respectively, ≤20% and ≤23%) of fructifications in the BRT models. There could be multiple reasons for this, where most likely the main issue was the difference in the spatial and temporal scale of the measurements of the environmental factors and the charophyte fructifications. For instance, the estimates of the distance from the charophyte stands were based on the charophyte mapping data in 2014–2015 [14]. The indirectly measured factors also could affect the poor fit of the models, where REI was a surrogate of a wave impact and the bottom topographic variables were proxies for an effect of water movement. Another possible reason for the relatively low correlation between the distribution of charophyte fructifications and the environmental factors is an interaction of environmental factors in transitional type of waterbodies such as estuaries and lagoons. Due to the estuarine quality paradox [48], it is more difficult to distinguish pure effects of environmental factors than in lakes. It has been suggested that a large part of the variation in abundance models can be explained by including interactions of biotic and abiotic parameters [49]. However, data of biological parameters is usually spatially and temporally limited, and models cannot accurately predict outside the range of their sampling [50].
5.3.1. Distance from Charophyte Stands
Charophyte habitats are the main source of the oospore bank [1], and were therefore the one of the most important factors explaining the distribution of oospores in our study. The widest stands of charophytes were located in the northeastern part of the study area (the profiles 1–4) [14], where oospores were abundant. Whereas, in the remaining part of the study area (the profiles 5–7), the narrower spread of oospores and charophytes was restricted only to the eastern shore. The spatial-temporal patterns of oospores suggest that their transport to uncolonized areas was relatively low and resulted in a sharp decrease of oospore densities at the border of dense charophyte vegetated areas (from 30,000 ind. m−2 at the highly vegetated area in the eastern shore to 2500 ind. m−2 at the 2 km distance to the west). Similar distribution was observed for the colonization of C. aspera in the shallow lake, where no oospores were recorded in the >3 km distance [11]. As it was mentioned in the previous chapter, the relatively high number of empty oospores found in the charophyte stands indicates an intensive reproduction within this habitat.
5.3.2. Hydrodynamics and Transport of Fructification
The wave exposure was important explanatory variable for the occurrence and partly for the density of full fructifications. In general, hydrodynamics is highlighted as an important factor for the forming of macrophyte communities [8,10]. Waves also affect a distribution and spread of charophyte fructifications by a mechanical disturbance (resuspension) of fructifications [51], and by reducing water transparency (i.e., light availability) for a germination of charophytes [44].
In this study, the mean wave exposure was higher in the southeastern part (the profiles 5–7) than in the northeastern part (the profiles 1–4), which corresponds to the distribution of denser stands of charophytes in the first part than in the second [14]. It is most likely that the wave exposure limits the distribution of charophyte stands and consequently affects the density of their fructifications. There is some research performed on the seed dispersal of angiosperms, e.g., [52]; however, mechanisms of transport of oospores is poorly studied. In the Curonian Lagoon, the cyclonic storms prevail [16], and therefore wave-induced water movements should transport oospores towards the eastern shore. This could be confirmed by the observed high amount of detached charophytes with oospores on the shore after stormy periods (personal observation). It also explains why nearshore zones are relatively abundant in fructifications. However, there are no data showing at which depths charophyte thalli are usually teared and what amount of their vegetation and fructifications is transported towards a shore and to deeper areas. Thus, sheltered areas may have a higher generative recolonization potential than exposed areas, but this hypothesis was not addressed in this study.
Environment of the Curonian Lagoon is hydrodynamically active, and the water turbidity can increase during windy periods [15]. Several full fructifications were found in the wave-exposed areas (REI > 150), located in the delta part of the lagoon and along the western shore, where a concentration of suspended sediments can increase in spring and summer up to 25 mg L−1. Such high turbidity may reduce a light penetration near the bottom, which may restrict an establishment of charophytes deeper as they use more resources for an elongation and less for a reproduction under low light conditions [46].
5.3.3. Salinity
Salinity is considered a major factor limiting a distribution of many macrophyte species in estuarine ecosystems [53]. However, the salinity gradient in the Curonian Lagoon did not correlate with the distribution of C. contraria and C. aspera [14]. It was demonstrated that the salinity influenced the production of C. contraria oospores, where its reproductive effort decreased in the salinities higher than 2, while C. aspera was tolerant [54]. Our results show a relative high importance (61%) of the salinity only for the density of empty fructifications, which increased in the northern part of study area. This could be explained as indirect effect of salinity, i.e., the dominant direction of riverine waters flow by the eastern shore towards the Baltic Sea [15,17], and may transport high amounts of oospores to the northern part of the lagoon. The direct effect of salinity was found only on the distribution of brackish water species (T. nidifica and C. baltica), but their fructifications were only 0.5% from the total oospore density and were restricted to the first profile.
5.3.4. Depth
The importance of water depth was relatively low (<5%) for the occurrence and distribution of charophyte fructifications, whereas other studies mention that a distribution of oospores along a depth gradient can reflect former zonation of charophytes [4]. Charophytes can be found up to the 4 m depth in the coastal waters of the Baltic Sea [6,9,10], but there are no records of maximum depth distribution of their fructifications. In the Curonian Lagoon, the maximum depth limit of full fructifications of charophytes was described at the 2.5 m depth, which is deeper than the recorded one in 2014–2015 [14]. However, we found empty fructifications down to the depth of 4 m, and their density was higher from the 1.5 to 2 m depth than in the shallower part, most likely due to a transport of oospores by waves and currents.
5.3.5. Topography of the Seafloor (Proxy of Intensity of Water Movement)
In this study, we did not directly assess the effect of water currents, which hypothetically should be important for the spread and accumulation of charophyte fructifications. However, we analyzed the importance of currents by surrogates quantitative geomorphological seafloor parameters (i.e., the slope, aspect, and TPI). The relative importance of geomorphological bottom parameters was not high (for the bottom aspect < 30%, slope < 11%, for the TPI < 3%). Nevertheless, we think that they indirectly revealed the effect of water currents since the high density of empty fructifications corresponded to the relatively flat western slopes. This can be explained by downwards transport of empty oospores to deeper areas, i.e., navigation channel, which is situated along the western shore of the study area. With respect to the TPI, the presence of full fructifications was related to lower seafloor locations (valleys) rather than to higher places (ridges). Viable oospores or gyrogonites are heavier than empty ones and probably are more often accumulated in seafloor depressions.
5.3.6. Waterfowl
We found several viable fructifications in the areas where charophytes were not recorded before, which could also be transported by waterfowl since they have a high potential in the dispersal of aquatic macrophytes [55]. In the Curonian Lagoon, the highest total bird number (30,000–48,000 ind.) was registered during the June–September 2018 [56], wherein up to 25% of them belonged to herbivorous waterfowl (coots, mute swans, and mallards). The data from transmitters attached to mute swans has provided preliminary information that the main feeding, nesting, and molting areas correspond to the areas vegetated by charophytes in the lagoon (Morkūnė et al., unpublished). These observations are useful for the assessment of a potential role of herbivorous birds on a dispersion of oospores in the study area, which requires an additional research.
6. Conclusions
The mayor number of charophyte oospores found in the estuarine part of the Curonian Lagoon belong to the dominant charophyte species (C. contraria and C. aspera). The highest mean density of all fructification types (full and empty oospores and gyrogonites) was found on the eastern shore, where the dense charophyte stands are established. Viable fructifications extended up to the 2.5 m depth, showing the improving status of charophyte stands. The temporal changes of oospore density followed the seasonal pattern—the density in autumn represented the quantity of matured oospores during summer, while the density in spring indicated how many viable fructifications remained after winter and how many had an opportunity to germinate.
The explained variation (deviance) in the occurrence and density of charophyte fructifications by the environmental factors was relatively low. The main important environmental variables were the distance to the charophyte stands, the salinity, the aspect, and the wave exposure, which have also been determined as significant factors in other studies in the Baltic Sea. In the lagoon, full fructifications were abundant in sheltered nearshore areas in a proximity of around 1 km from the charophyte stands. The relatively lower importance of the salinity and geomorphological seafloor parameters probably indirectly revealed the effect of water currents. All these findings confirm our hypothesis that absence of charophytes in some areas (especially western and southern parts of the study area) are due to unfavorable environmental conditions (mainly due to the wave exposure and water transparency) and lack of viable oospores. The mean density of viable fructifications in spring was similar to the results recorded in other studies in the Baltic Sea and can be considered as sufficient to maintain charophyte stands in the Curonian Lagoon.
Author Contributions
Conceptualization, V.S. and M.B.; methodology, M.B. and. V.S.; software, V.S. and M.B.; validation, V.S. and M.B.; formal analysis, V.S. and M.B.; investigation, V.S. and M.B.; resources, V.S. and M.B.; data curation, V.S., M.B., and G.M.; writing—original draft preparation, V.S. and M.B.; writing—review and editing, V.S., M.B., and G.M.; visualization, V.S. and M.B.; supervision, M.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Research Council of Lithuania (no. S-MIP-19-29). Work was supported by the Doctorate Study program in Ecology and Environmental Sciences, Klaipeda University (for V.S.).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to the thesis that is being prepared from these data.
Acknowledgments
We are grateful to J. Gintauskas and E. Tiškus for assistance in sediment collection, and J. Mėžinė for providing hydrometeorological data for analysis. Valuable comments of the two anonymous reviewers improved the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bonis, A.; Grillas, P. Deposition, germination and spatio-temporal patterns of charophyte propagule banks: A review. Aquat. Bot. 2002, 72, 235–248. [Google Scholar] [CrossRef]
- Stobbe, A.; Gregor, T.; Röpke, A. Long-lived banks of oospores in lake sediments from the Trans-Urals (Russia) indicated by germination in over 300 years old radiocarbondated sediments. Aquat. Bot. 2014, 119, 84–90. [Google Scholar] [CrossRef]
- Steinhardt, T.; Selig, U. Comparison of recent vegetation and diaspore banks along abiotic gradients in brackish coastal lagoons. Aquat. Bot. 2009, 91, 20–26. [Google Scholar] [CrossRef]
- Blindow, I.; Dahlke, S.; Dewart, A.; Flügge, S.; Hendreschke, M.; Kerkow, A.; Meyer, J. Long-term and interannual changes of submerged macrophytes and their associated diaspore reservoir in a shallow southern Baltic Sea bay: Influence of eutrophication and climate. Hydrobiologia 2016, 778, 121–136. [Google Scholar] [CrossRef]
- Nowak, P.; Steinhardt, T.; von Ammon, U.; Rohde, H.; Schoor, A.; Holzhausen, A.; Schaible, R.; Schubert, H. Diaspore bank analysis of Baltic coastal waters. Bot. Lett. 2018, 165, 159–173. [Google Scholar] [CrossRef]
- Blindow, I. Distribution of charophytes along the Swedish coast in relation to salinity and eutrophication. Int. Rev. Hydrobiol. 2000, 85, 707–717. [Google Scholar] [CrossRef]
- Brzeska, P.; Woźniczka, A.; Pełechaty, M.; Blindow, I. New records of Chara connivens P. Salzmann ex A. Braun1835—An extremely rare and protected species in Polish brackish waters. Acta Soc. Bot. Pol. 2015, 84, 143–146. [Google Scholar] [CrossRef][Green Version]
- Torn, K.; Kovtun-Kante, A.; Herkül, K.; Martin, G.; Mäemets, H. Distribution and predictive occurrence model of charophytes in Estonian waters. Aquat. Bot. 2015, 120, 142–149. [Google Scholar] [CrossRef]
- Kovtun, A.; Torn, K.; Martin, G.; Kullas, T.; Kotta, J.; Suursaar, Ü. Influence of abiotic environmental conditions on spatial distribution of charophytes in the coastal waters of West Estonian Archipelago, Baltic Sea. J. Coast. Res. 2011, 412–416. [Google Scholar] [CrossRef]
- Steinhardt, T.; Selig, U. Spatial distribution patterns and relationship between recent vegetation and diaspore bank of a brackish coastal lagoon on the southern Baltic Sea. Estuar. Coast. Shelf Sci. 2007, 74, 205–214. [Google Scholar] [CrossRef]
- Van den Berg, M.S.; Coops, H.; Simons, J. Propagule bank buildup of Chara aspera and its significance for colonization of a shallow lake. Hydrobiologia 2001, 462, 9–17. [Google Scholar] [CrossRef]
- Birk, S.; Bonne, W.; Borja, A.; Brucet, S.; Courrat, A.; Poikane, S.; Solimini, A.; Van De Bund, W.; Zampoukas, N.; Hering, D. Three hundred ways to assess Europe’s surface waters: An almost complete overview of biological methods to implement the Water Framework Directive. Ecol. Indic. 2012, 18, 31–41. [Google Scholar] [CrossRef]
- Sinkevičienė, Z.; Bučas, M.; Ilginė, R.; Vaičiūtė, D.; Kataržytė, M.; Petkuvienė, J. Charophytes in the estuarine Curonian Lagoon: Have the changes in diversity, abundance and distribution occurred since the late 1940s? Oceanol. Hydrobiol. Stud. 2017, 46, 186. [Google Scholar] [CrossRef]
- Bučas, M.; Sinkevičienė, Z.; Kataržytė, M.; Vaičiūtė, D.; Petkuvienė, J.; Stragauskaitė, V.; Ilginė, R. How much can the occurrence and coverage of charophytes in an estuarine lagoon (Curonian Lagoon) be explained by environmental factors? Estuar. Coast. Shelf Sci. 2019, 216, 128–138. [Google Scholar] [CrossRef]
- Christian, F.; Arturas, R.; Saulius, G.; Georg, U.; Lina, B. Hydraulic regime-based zonation scheme of the Curonian Lagoon. Hydrobiologia 2008, 611, 133–146. [Google Scholar] [CrossRef]
- Dailidienė, I.; Davulienė, L. Long-term mean salinity in the Curonian Lagoon in 1993–2005. Acta Zool Lit. 2007, 17, 172–181. [Google Scholar] [CrossRef]
- Zemlys, P.; Ferrarin, C.; Umgiesser, G.; Gulbinskas, S.; Bellafiore, D. Investigation of saline water intrusions into the Curonian Lagoon (Lithuania) and two-layer flow in the Klaipėda Strait using finite element hydrodynamic model. Ocean Sci. 2013, 9. [Google Scholar] [CrossRef]
- Lesutiene, J.; Bukaveckas, P.A.; Gasiunaite, Z.R.; Pilkaityte, R.; Razinkovas-Baziukas, A. Tracing the isotopic signal of a cyanobacteria bloom through the food web of a Baltic Sea coastal lagoon. Estuar. Coast. Shelf Sci. 2014, 138, 47–56. [Google Scholar] [CrossRef]
- Bresciani, M.; Adamo, M.; De Carolis, G.; Matta, E.; Pasquariello, G.; Vaičiūtė, D.; Giardino, C. Monitoring blooms and surface accumulation of cyanobacteria in the Curonian Lagoon by combining MERIS and ASAR data. Remote Sens. Environ. 2014, 146, 124–135. [Google Scholar] [CrossRef]
- Vaičiūtė, D.; Bresciani, M.; Bartoli, M.; Giardino, C.; Bučas, M. Spatial and temporal distribution of coloured dissolved organic matter in a hypertrophic freshwater lagoon. J. Limnol. 2015, 74, 572–583. [Google Scholar] [CrossRef]
- Trimonis, E.; Gulbinskas, S.; Kuzavinis, M. The Curonian Lagoon bottom sediments in the Lithuanian water area. Baltica 2003, 16, 13–20. [Google Scholar]
- Krause, W. Zur Bestimmungsmöglichkeit subfossiler Characeen-Oosporen an Beispielen aus Schweizer Seen. Vierteljahrsschr. Naturforsch. Ges. Zürich. 1986, 141, 295–313. [Google Scholar]
- Haas, J.N. First identification key for charophyte oospores from central Europe. Pflugers. Arch. 1994, 29, 227–235. [Google Scholar] [CrossRef]
- Vedder, F. Morphologie und Taxonomie rezenter und subfossiler Characeen-Oosporen aus der Ostsee. Rostock. Meeresbiolog. Beitr. 2004, 13, 43–54. [Google Scholar]
- Soulié-Märsche, I.; García, A. Gyrogonites and oospores, complementary viewpoints to improve the study of the charophytes (Charales). Aquat. Bot. 2015, 120, 7–17. [Google Scholar] [CrossRef]
- QGIS Development Team. QGIS Geographic Information System. v 2.18.12- Las Palmas. Open Source Geospatial Found. Proj. 2020. Available online: https://doi.org/http://www.qgis.org/ (accessed on 18 April 2020).
- Malhotra, A.; Fonseca, M.S. WEMo (Wave Exposure Model): Formulation, Procedures and Validation; NOAA Technical Memorandum NOS NCCOS 65: Beaufort, NC, USA, 2007. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 20 June 2020).
- Hijmans, R.J. Raster: Geographic Data Analysis and Modeling; R Package Version 3.3-13; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://CRAN.R-project.org/package=raster (accessed on 20 June 2020).
- Bivand, R.; Keitt, T.; Rowlingson, B. rgdal: Bindings for the ‘Geospatial’ Data Abstraction Library; R Package Version 1.5-17; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://CRAN.R-project.org/package=rgdal (accessed on 20 June 2020).
- Hijmans, R.J.; Williams, E.; Vennes, C. Geosphere: Spherical Trigonometry; R Package Version 1.3-11; R Package Version 1.5-17; R Foundation for Statistical Computing: Vienna, Austria, 2014; Available online: https://cran.r-project.org/web/packages/geosphere (accessed on 20 June 2020).
- Bivand, R.; Rundel, C. Rgeos: Interface to Geometry Engine—Open Source (GEOS); R Package Version 0.3-11; R Package Version 1.5-17; R Foundation for Statistical Computing: Vienna, Austria, 2015; Available online: https://cran.r-project.org/web/packages/rgeos/i (accessed on 20 June 2020).
- Evans, J.S. SpatialEco; R Package Version 1.3-1; R Package Version 1.5-17; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://github.com/jeffreyevans/spatialEco (accessed on 20 June 2020).
- Zuur, A.F.; Ieno, E.N.; Smith, G.M. Ordination—First encounter. Analysing Ecological Data. 2007, 189–192. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package; R Package Version 2.4-3; R Foundation for Statistical Computing: Vienna, Austria, 2016; Available online: https://cran.r-project.org/web/packages/vegan (accessed on 20 June 2020).
- Peters, G. Userfriendlyscience: Quantitative Analysis made Accessible; R Package Version 0.7.0; R Foundation for Statistical Computing: Vienna, Austria, 2017; Available online: https://cran.r-project.org/web/packages/userfriendlyscience (accessed on 20 June 2020).
- Friedman, J.H. Greedy function approximation: A gradient boosting machine. Ann. Stat. 2001, 1189–1232. [Google Scholar] [CrossRef]
- Torn, K.; Peterson, A.; Herkül, K.; Suursaar, U. Effects of climate change on the occurrence of charophytes and angiosperms in a brackish environment. Webbia. 2019, 74, 167–177. [Google Scholar] [CrossRef]
- Elith, J.; Leathwick, J.R.; Hastie, T. A working guide to boosted regression trees. J Anim. Ecol. 2008, 77, 802–813. [Google Scholar] [CrossRef]
- LeDell, E.; Gill, N.; Aiello, S.; Fu, A.; Candel, A.; Click, C.; Kraljevic, T.; Nykodym, T.; Aboyoun, P.; Kurka, M.; et al. h2o: R Interface for the ‘H2O’ Scalable Machine Learning Platform; R Package Version 3.30.1.3; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://cran.r-project.org/web/packages/h2o/h2o.pdf (accessed on 20 June 2020).
- Schubert, H.; Blindow, I. Charophyte of the Baltic Sea; The Baltic Marine Biologists Publication, No. 19; A.R.G. Gantner Verlag Kommanditgesellschaft: Ruggell, Liechtenstein, 2003; pp. 27–36. [Google Scholar]
- Santamaría, L. Why are most aquatic plants widely distributed? Dispersal, clonal growth and small-scale heterogeneity in a stressful environment. Acta oecol. 2002, 23, 137–154. [Google Scholar] [CrossRef]
- Doege, A.K.; van de Weyer, K.; Becker, R.; Schubert, H. Bioindikation mit Characeen [Bioindication with Characeae]. In Armleuchteralgen. Die Characeen Deutschlands [Stoneworts. Characeae of Germany]; Chara, A.G., Ed.; Springer: Berlin/Heidelberg, Germany, 2016; Volume 8, pp. 97–138. [Google Scholar]
- Steinhardt, T.; Selig, U. Influence of salinity and sediment resuspension on macrophyte germination in coastal lakes. J. Limnol. 2011, 70, 11–20. [Google Scholar] [CrossRef]
- Casanova, M.T.; Brock, M.A. Life histories of charophytes from permanent and temporary wetlands in eastern Australia. Aust. J. Bot. 1999, 47, 383–397. [Google Scholar] [CrossRef]
- De Winton, M.D.; Casanova, M.T.; Clayton, J.S. Charophyte germination and establishment under low irradiance. Aquat. Bot. 2004, 79, 175–187. [Google Scholar] [CrossRef]
- Sanjuan, J.; Vicente, A.; Flor-Arnau, N.; Monleón, T.; Cambra, J.; Martín-Closas, C. Effects of light and temperature on Chara vulgaris (Charophyceae) gyrogonite productivity and polymorphism–palaeoenvironmental implications. Phycologia 2017, 56, 204–212. [Google Scholar] [CrossRef]
- Elliott, M.; Quintino, V. The estuarine quality paradox, environmental homeostasis and the difficulty of detecting anthropogenic stress in naturally stressed areas. Mar. Pollut. Bull. 2007, 54, 640–645. [Google Scholar] [CrossRef]
- Elith, J.; Leathwick, J.R. Species distribution models: Ecological explanation and prediction across space and time. Annu. Rev. Ecol. Evol.System. 2009, 40, 677–697. [Google Scholar] [CrossRef]
- Bertrin, V.; Boutry, S.; Alard, D.; Haury, J.; Jan, G.; Moreira, S.; Ribaudo, C. Prediction of macrophyte distribution: The role of natural versus anthropogenic physical disturbances. Appl. Veg. Sci. 2018, 21, 395–410. [Google Scholar] [CrossRef]
- Van Zuidam, B.G.; Peeters, E.T. Wave forces limit the establishment of submerged macrophytes in large shallow lakes. Limnol. Oceanogr. 2015, 60, 1536–1549. [Google Scholar] [CrossRef]
- Ruiz-Montoya, L.; Lowe, R.J.; Kendrick, G.A. Contemporary connectivity is sustained by wind-and current-driven seed dispersal among seagrass meadows. Mov. Ecol. 2015, 3, 9. [Google Scholar] [CrossRef]
- Schubert, H.; Feuerpfeil, P.; Marquardt, R.; Telesh, I.; Skarlato, S. Macroalgal diversity along the Baltic Sea salinity gradient challenges Remane’s species-minimum concept. Mar. Pollut. Bull. 2011, 62, 1948–1956. [Google Scholar] [CrossRef]
- Bonis, A.; Grillas, P.; van Wijck, C.; Lepart, J. The effect of salinity on the reproduction of coastal submerged macrophytes in experimental communities. J. Veg. Sci. 1993, 4, 461–468. [Google Scholar] [CrossRef]
- Van Leeuwen, C.H.; Van der Velde, G.; van Groenendael, J.M.; Klaassen, M. Gut travellers: Internal dispersal of aquatic organisms by waterfowl. J. Biogeogr. 2012, 39, 2031–2040. [Google Scholar] [CrossRef]
- Morkūnė, R.; Petkuvienė, J.; Bružas, M.; Morkūnas, J.; Bartoli, M. Monthly Abundance Patterns and the Potential Role of Waterbirds as Phosphorus Sources to a Hypertrophic Baltic Lagoon. Water 2020, 12, 1392. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).