Effects of Hypoxia on the Distribution of Calanoid Copepod Eggs in the Seabed Sediments of the Eutrophic Masan Bay, Korea
Abstract
:1. Introduction
2. Materials and Methods
3. Results
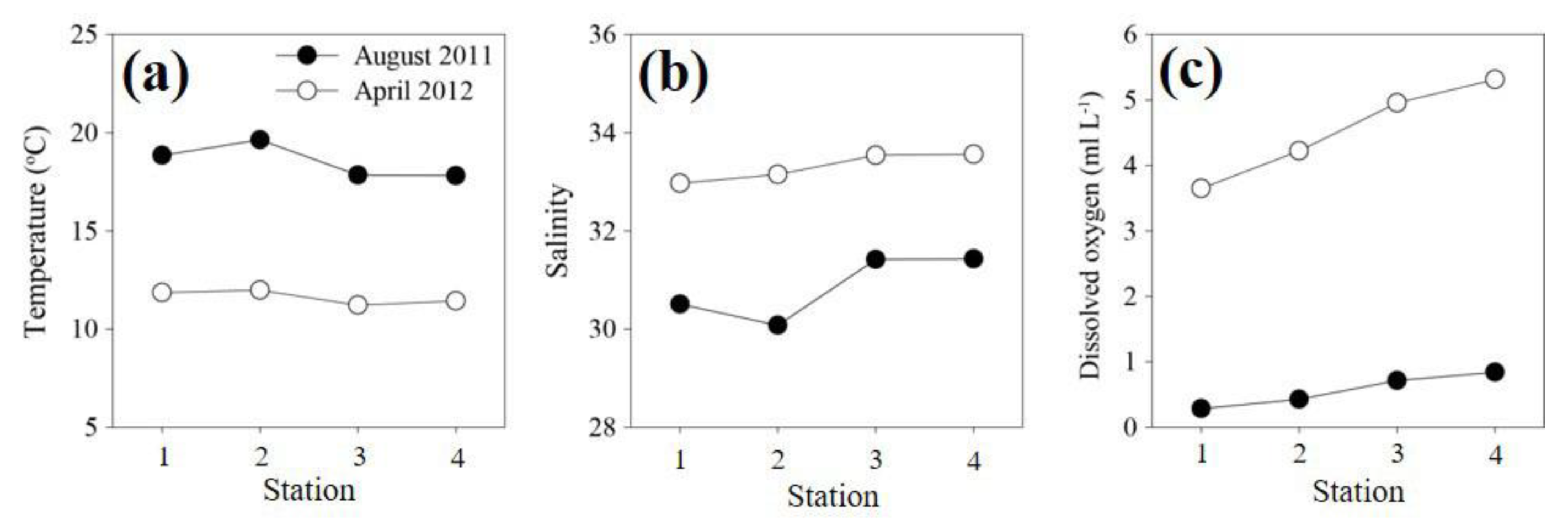
3.1. Environmental Variation
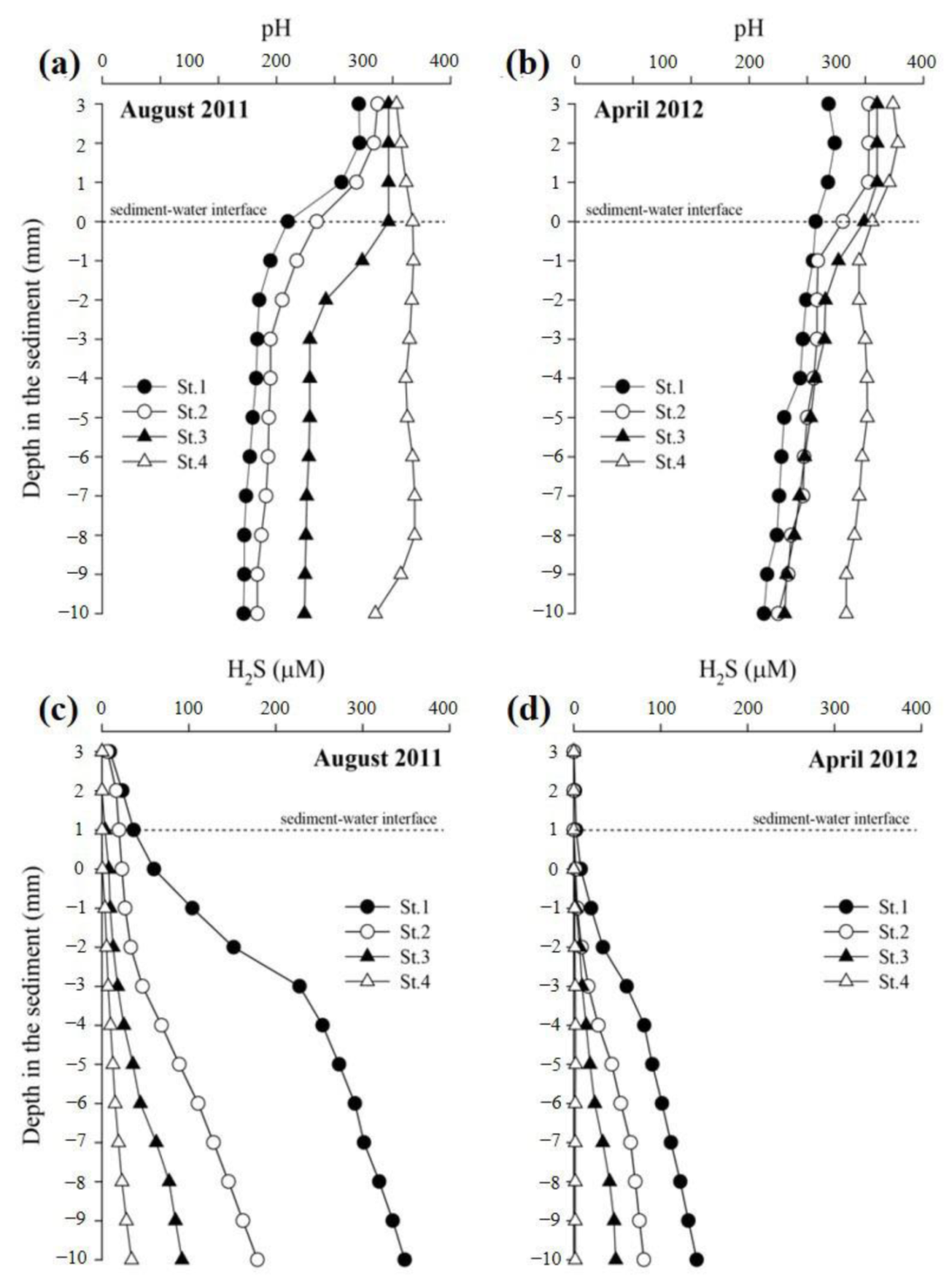
3.2. Sediment pH and H2S Concentration
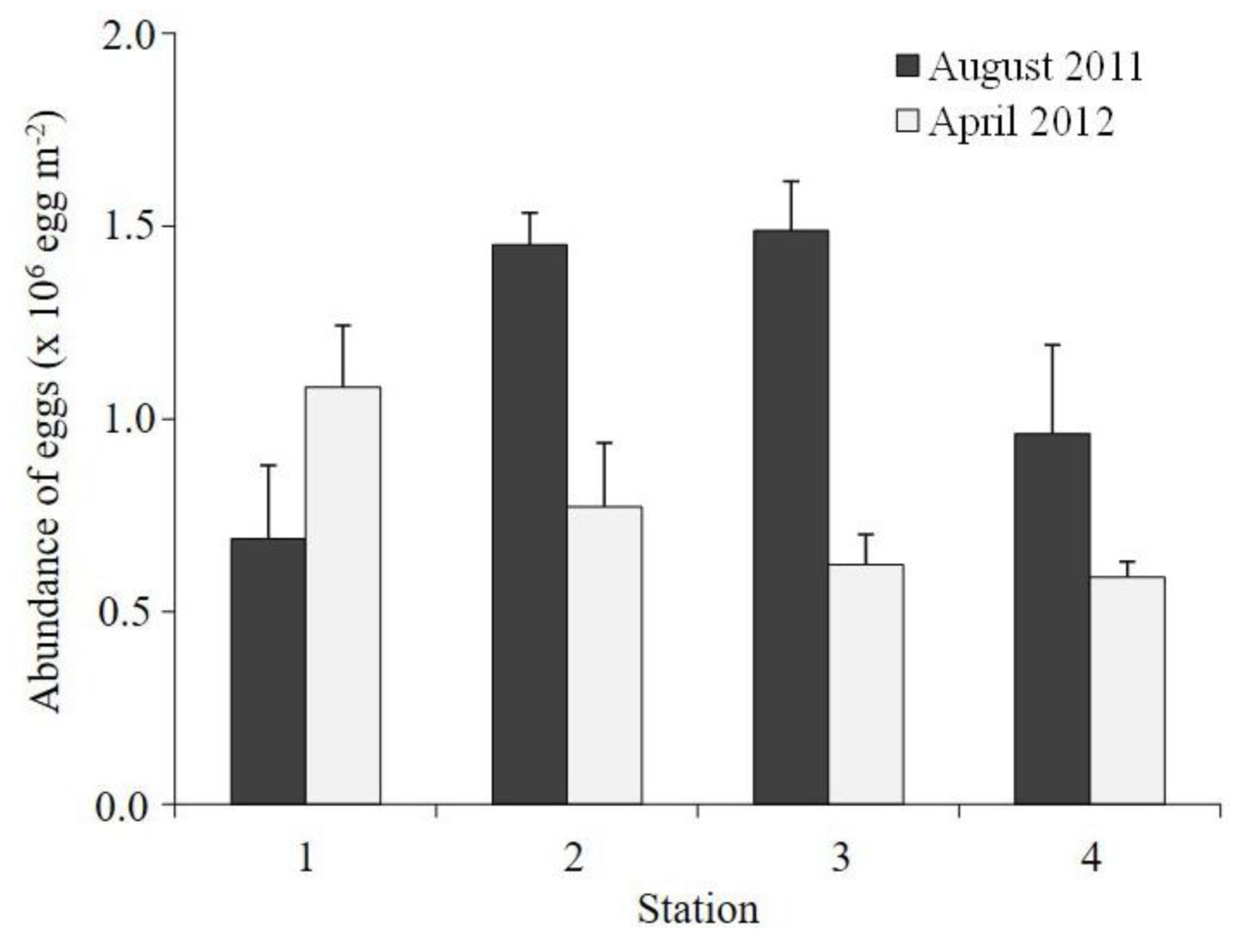
3.3. Egg Abundance and Ratio According to Morphology (Normal and Abnormal)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Park, J.S.; Cho, B.C. Active heterotrophic nanoflagellates in the hypoxic water-column of the eutrophic Masan Bay, Korea. Mar. Ecol. Prog. Ser. 2002, 230, 35–45. [Google Scholar] [CrossRef] [Green Version]
- Diaz, R.J.; Rosenberg, R. Marine benthic hypoxia: A review of its ecological effects and the behavioral responses of benthic macrofauna. Oceanogr. Mar. Biol. Annu. Rev. 1955, 33, 245–303. [Google Scholar]
- Bagarinao, T. Sulfide as an environmental factor and toxicant: Tolerance and adaptations in aquatic organisms. Aquat. Toxicol. 1992, 24, 21–62. [Google Scholar] [CrossRef]
- Vismann, B. Sulfide species and total sulfide toxicity in the shrimp Crangon crangon. J. Exp. Mar. Biol. Ecol. 1996, 204, 141–154. [Google Scholar] [CrossRef]
- Wang, F.; Chapman, P.M. Biological implications of sulfide in sediment—A review focusing on sediment toxicity. Environ. Toxicol. Chem. 1999, 18, 2526–2532. [Google Scholar] [CrossRef]
- Tang, K.W.; Dam, H.G.; Feinberg, L.R. The relative importance of egg production rate, hatching success, hatching duration and egg sinking in population recruitment of two species of marine copepods. J. Plankton Res. 1998, 20, 1971–1987. [Google Scholar] [CrossRef]
- Grice, G.D.; Marcus, N.H. Dormant eggs of marine copepods. Oceanogr. Mar. Biol. Annu. Rev. 1981, 19, 125–140. [Google Scholar]
- Marcus, N.H.; Lutz, R.V. Effects of anoxia on the viability of subitaneous eggs of planktonic copepods. Mar. Biol. 1994, 121, 83–87. [Google Scholar] [CrossRef]
- Masero, R.; Villate, F. Composition, vertical distribution and age of zooplankton benthic eggs in the sediments of two contrasting estuaries of the Bay of Biscay. Hydrobiologia 2004, 518, 201–212. [Google Scholar] [CrossRef]
- Marcus, N.H. Ecological and evolutionary significance of resting eggs in marine copepods: Past, present, and future studies. Hydrobiologia 1996, 320, 141–152. [Google Scholar] [CrossRef]
- Marcus, N.H. Recruitment of copepod nauplii into the plankton: Importance of diapause eggs and benthic processes. Mar. Ecol. Prog. Ser. 1984, 15, 47–54. [Google Scholar] [CrossRef]
- De Stasio, B.T. The seed bank of a freshwater crustacean: Copepodology for the plant ecologist. Ecology 1989, 70, 1377–1389. [Google Scholar] [CrossRef]
- Marcus, N.H.; Lutz, R.V.; Burnett, W.; Cable, P. Age, viability, and vertical distribution of zooplankton resting eggs from an anoxic basin: Evidence of an egg bank. Limnol. Oceanogr. 1994, 39, 154–158. [Google Scholar] [CrossRef]
- Marcus, N.H.; Boero, F. Minireview: The importance of benthic-pelagic coupling and the forgotten role of life cycles in coastal aquatic systems. Limnol. Oceanogr. 1998, 43, 763–768. [Google Scholar] [CrossRef]
- Marcus, N.H.; Lutz, R.V.; Chanton, J.P. Impact of anoxia and sulfide on the viability of eggs of three planktonic copepods. Mar. Ecol. Prog. Ser. 1997, 146, 291–295. [Google Scholar] [CrossRef]
- Katajisto, T.; Viitasalo, M.; Koski, M. Seasonal occurrence and hatching of calanoid eggs in sediments of the northern Baltic Sea. Mar. Ecol. Prog. Ser. 1998, 163, 133–143. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, P.; Mortensen, J.; Vismann, B.; Hansen, B.W. Physiological tolerance of marine calanoid copepod eggs to sulphide. Mar. Ecol. Prog. Ser. 2006, 328, 171–182. [Google Scholar] [CrossRef]
- Invidia, M.; Sei, S.; Gorbi, G. Survival of the copepod Acartia tonsa following egg exposure to near anoxia and to sulfide at different pH values. Mar. Ecol. Prog. Ser. 2004, 276, 187–196. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.-W.; Min, B.-Y. Pollution in Masan Bay, a matter of concern in South Korea. Mar. Pollut. Bull. 1990, 21, 226–229. [Google Scholar] [CrossRef]
- Lee, M.-O.; Kim, J.-K. Characteristics of algal blooms in the southern coastal waters of Korea. Mar. Environ. Res. 2008, 65, 128–147. [Google Scholar] [CrossRef]
- Lee, C.; Lim, W. Variation of harmful algal blooms in Masan–Chinhae Bay. Sci. Asia 2006, 32, 51–56. [Google Scholar] [CrossRef]
- Hong, J.-S.; Lee, J.H. Effects of the pollution on the benthic macrofauna in Masan Bay, Korea. J. Korean Soc. Oceanogr. 1983, 18, 169–173. [Google Scholar]
- Yoo, K.I. Population dynamics of dinoflagellate community in Masan Bay with a note on the impact of environmental parameters. Mar. Pollut. Bull. 1991, 23, 185–188. [Google Scholar] [CrossRef]
- Lim, H.-S.; Diaz, R.J.; Hong, J.-S.; Schaffner, L.C. Hypoxia and benthic community recovery in Korea coastal waters. Mar. Pollut. Bull. 2006, 52, 1517–1526. [Google Scholar] [CrossRef]
- Jung, J.-H.; Kim, S.-J.; Lee, T.-K.; Shim, W.J.; Woo, S.; Kim, D.-J.; Han, C.-H. Biomarker responses in caged rockfish (Sebastes schlegeli) from Masan Bay and Haegeumgang, South Korea. Mar. Pollut. Bull. 2008, 57, 599–606. [Google Scholar] [CrossRef]
- Kim, S.-Y.; Lee, Y.-H.; Kim, Y.-S.; Shim, J.-H.; Ye, M.-J.; Jeon, J.-W.; Hwang, J.-R.; Jun, S.-H. Characteristics of marine environmental in the hypoxic season at Jinhae Bay in 2010. Korean J. Nat. Conserv. 2012, 6, 115–129. [Google Scholar] [CrossRef]
- Cline, J.D. Spectrophotometric determination of hydrogen sulfide in natural waters 1. Limnol. Oceanogr. 1969, 14, 454–458. [Google Scholar] [CrossRef]
- Blades-Eckelbarger, P.I.; Marcus, N.H. The origin of cortical vesicles and their role in egg envelope formation in the “spiny” eggs of a calanoid copepod, Centropages velificatus. Biol. Bull. 1992, 182, 41–53. [Google Scholar] [CrossRef]
- Dharani, G.; Altaff, K. Ultra structure of subitaneous and diapausing eggs of planktonic copepod Sinodiaptomus (Rhinediaptomus) indicus. Curr. Sci. 2004, 87, 109–112. [Google Scholar]
- Briski, E.; Cristescu, M.E.; Bailey, S.A.; MacIsaac, H.J. Use of DNA barcoding to detect invertebrate invasive species from diapausing eggs. Biol. Invasions 2011, 13, 1325–1340. [Google Scholar] [CrossRef]
- Belmonte, G.; Castello, P.; Piccinni, M.R.; Quarta, S.; Rubino, F.; Geraci, S.; Boero, F. Resting stages in marine sediments off the Italian coast. In Biology and Ecology of Shallow Coastal Waters; Olsen and Olsen: Fredensborg, Denmark, 1995; pp. 53–58. [Google Scholar]
- Viitasalo, M.; Katajisto, T. Mesozooplankton resting eggs in the Baltic sea: Identification and vertical distribution in laminated and mixed sediments. Mar. Biol. 1994, 120, 455–466. [Google Scholar] [CrossRef]
- Jiang, X.D.; Wang, G.Z.; Li, S.J. Age, distribution and abundance of viable resting eggs of Acartia pacifica (Copepoda: Calanoida) in Xiamen Bay, China. J. Exp. Mar. Biol. Ecol. 2004, 312, 89–100. [Google Scholar] [CrossRef] [Green Version]
- Uriarte, I.; Villate, F. First evidences of Acartia bifilosa resting eggs in sediments of the Urdaibai estuary (Bay of Biscay): Abundance and hatching success. Sci. Mar. 2006, 70, 565–572. [Google Scholar] [CrossRef] [Green Version]
- Glippa, O.; Souissi, S.; Denis, L.; Lesourd, S. Calanoid copepod resting egg abundance and hatching success in the sediment of the Seine estuary (France). Estuar. Coast. Shelf Sci. 2011, 92, 255–262. [Google Scholar] [CrossRef]
- Soh, H.Y.; Choi, S.D. Species composition and occurrence patterns of zooplankton in Jinhae Bay. Korean J. Environ. Biol. 2004, 22, 43–56. [Google Scholar]
- Kim, J.S.; Jeong, H.J.; Yoo, Y.D.; Kang, N.S.; Kim, S.K.; Song, J.Y.; Lee, M.J.; Kim, S.T.; Kang, J.H.; Seong, K.A.; et al. Red tides in Masan Bay, Korea, in 2004–2005, III: Daily variations in the abundance of mesozooplankton and their grazing impacts on red-tide organisms. Harmful Algae 2013, 30, S102–S113. [Google Scholar] [CrossRef]
- Jang, M.-C.; Shin, K.; Jang, P.G.; Lee, W.-J.; Choi, K.-H. Mesozooplankton community in a seasonally hypoxic and highly eutrophic bay. Mar. Freshw. Res. 2015, 66, 719–729. [Google Scholar] [CrossRef]
- Marcus, N.H. Seasonal study of planktonic copepods and their benthic resting eggs in northern California coastal waters. Mar. Biol. 1995, 123, 459–465. [Google Scholar] [CrossRef]
- Marcus, N.H. Planktonic copepods in a sub-tropical estuary: Seasonal patterns in the abundance of adults, copepodites, nauplii, and eggs in the sea bed. Biol. Bull. 1991, 181, 269–274. [Google Scholar] [CrossRef]
- Næss, T. Benthic resting eggs of calanoid copepods in Norwegian enclosures used in mariculture: Abundance, species composition and hatching. Hydrobiologia 1996, 320, 161–168. [Google Scholar] [CrossRef]
- Hirose, E.; Toda, H.; Saito, Y.; Watanabe, H. Formation of the multiple-layered fertilization envelope in the embryo of Calanus sinicus Brodsky (Copepoda: Calanoida). J. Crust. Biol. 1992, 12, 186–192. [Google Scholar] [CrossRef]
- Kang, S.W. Circulation and pollutant dispersion in Masan-Jinhae Bay of Korea. Mar. Pollut. Bull. 1991, 23, 37–40. [Google Scholar] [CrossRef]
- Lee, Y.-W.; Hwang, D.-W.; Kim, G.; Lee, W.-C.; Oh, H.-T. Nutrient inputs from submarine groundwater discharge (SGD) in Masan Bay, an embayment surrounded by heavily industrialized cities, Korea. Sci. Total Environ. 2009, 407, 3181–3188. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.U. Study on the Benthic Foraminiferal Assemblage as a Coastal Pollution Proxy: Case Studies in Busan North Port and Masan Bay. Ph.D. Thesis, Busan University, Busan, Korea, 2015. [Google Scholar]
- Knutsen, T.; Melle, W.; Calise, L. Determining the mass density of marine copepods and their eggs with a critical focus on some of the previously used methods. J. Plankton Res. 2001, 23, 859–873. [Google Scholar] [CrossRef]
- Jiang, X.D.; Wang, G.H.; Li, S.J. Population dynamics of Acartia pacifica (Copepoda: Calanoida) the importance of benthic–pelagic coupling. Acta Oceanol. Sin. 2006, 25, 88–98. [Google Scholar]
- Kasahara, S.; Uye, S.-I.; Onbé, T. Calanoid copepod eggs in sea-bottom muds. Mar. Biol. 1975, 31, 25–29. [Google Scholar] [CrossRef]
- Guerrero, F.; Rodriguez, V. Existence and significance of Acartia grani resting eggs (Copepoda: Calanoida) in sediments of a coastal station in the Alboran Sea (SE Spain). J. Plankton Res. 1998, 20, 305–314. [Google Scholar] [CrossRef] [Green Version]
- Lutz, R.V.; Marcus, N.H.; Chanton, J.P. Effects of low oxygen concentrations on the hatching and viability of eggs of marine calanoid copepods. Mar. Biol. 1992, 114, 241–247. [Google Scholar] [CrossRef]
- Lutz, R.V.; Marcus, N.H.; Chanton, J.P. Hatching and viability of copepod eggs at two stages of embryological development: Anoxic/hypoxic effect. Mar. Biol. 1994, 119, 199–204. [Google Scholar] [CrossRef]
- Katajisto, T. Effects of anoxia and hypoxia on the dormancy and survival of subitaneous eggs of Acartia bifilosa (Copepoda: Calanoida). Mar. Biol. 2004, 145, 751–757. [Google Scholar] [CrossRef]
- Guelorget, O.; Perthuisot, J.P. Paralic ecosystems—Biological organization and functionning. Vie Milieu 1992, 42, 215–251. [Google Scholar]
- Belmonte, G.; Rubino, F. Resting cysts from coastal marine plankton. Oceanogr. Mar. Biol. Annu. Rev. 2019, 57, 1–88. [Google Scholar] [CrossRef]
- Belmonte, G. Resting eggs in the life cycle of Acartia italica and A. adriatica (Copepoda, Calanoida, Acartiidae). Crustaceana 1997, 70, 114–117. [Google Scholar] [CrossRef]
- Hansen, B.W.; Drillet, G.; Kristensen, R.M.; Sørensen, T.F.; Tøttrup, M.T. Production, hatching success and surface ornamentation of eggs of calanoid copepods during a winter at 57° N. Mar. Biol. 2010, 157, 59–68. [Google Scholar] [CrossRef]
- Posi, M.E.; Belmonte, G. Ritmi di produzione di uova di diapausa in Paracartia latisetosa (Copepoda, Calanoida). Thalassia Salentina 2011, 33, 83–94. [Google Scholar] [CrossRef]
- Choi, K.H.; Jang, M.C.; Shin, H.H.; Lee, W.J.; Shin, K. In situ hatching success of calanoid copepod eggs in hypoxic sediments of a Coastal Bay. J. Coast. Res. 2016, 32, 333–338. [Google Scholar] [CrossRef]
- Jónasdóttir, S.H.; Kiørboe, T. Copepod recruitment and food composition: Do diatoms affect hatching success? Mar. Biol. 1996, 125, 743–750. [Google Scholar] [CrossRef]
- Santhanam, P.; Jeyaraj, N.; Jothiraj, K. Effect of temperature and algal food on egg production and hatching of copepod, Paracalanus parvus. J. Environ. Biol. 2013, 34, 243–246. [Google Scholar]
- Huntley, M.E.; Ciminiello, P.; Lopez, M.D.G. Importance of food quality in determining development and survival of Calanus pacificus (Copepoda: Calanoida). Mar. Biol. 1987, 95, 103–113. [Google Scholar] [CrossRef]
- Baek, S.H.; Shin, H.H.; Kim, D.S.; Kim, Y.O. Relationship between Distributional Characteristics of Heterotrophic Dinoflagellate Noctiluca scintillans and Environmental Factors in Gwangyang Bay and Jinhae Bay. Korean J. Environ. Biol. 2011, 29, 81–91. [Google Scholar]



| Survey Area | Egg Abundance (Agg m−2) | References |
|---|---|---|
| Inland Sea of Japan | 3–10 × 106 | Kasahara et al., (1975) [48] |
| Northern California coastal waters | 0.12–0.19 × 106 | Marcus (1995) [39] |
| Tyrrhenian Sea | 0.0016–0.012 × 106 | Belmonte et al., (1995) [31] |
| Ionian Sea | 0.031–1.07 × 106 | Belmonte et al., (1995) [31] |
| Adriatic Sea | 0.15–1.19 × 106 | Belmonte et al., (1995) [31] |
| Màlaga Harbor, Spain | 0.19–6.6 × 106 | Guerrero and Rodríguez (1998) [49] |
| Estuary of Mundaka, Bay of Biscay | 0.019–0.16 × 106 | Masero and Villate (2004) [9] |
| Estuary of Bilbao, Bay of Biscay | 0.008–0.009 × 106 | Masero and Villate (2004) [9] |
| Sällvik, Baltic Sea | 1.03 × 106 | Viitasalo and Katajisto (1994) [32] |
| Seine estuary, France | 1.42 × 106 | Glippa et al., (2011) [35] |
| Gamak Bay, Korea | 0.033–3.3 × 106 | Jang (2012) (unpublished data) |
| Masan Bay, Korea | 0.59–1.49 × 106 | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, S.Y.; Hyun, B.; Jang, P.-G.; Shin, K.; Soh, H.Y.; Kang, J.-H.; Jang, M.-C. Effects of Hypoxia on the Distribution of Calanoid Copepod Eggs in the Seabed Sediments of the Eutrophic Masan Bay, Korea. Water 2021, 13, 3116. https://doi.org/10.3390/w13213116
Choi SY, Hyun B, Jang P-G, Shin K, Soh HY, Kang J-H, Jang M-C. Effects of Hypoxia on the Distribution of Calanoid Copepod Eggs in the Seabed Sediments of the Eutrophic Masan Bay, Korea. Water. 2021; 13(21):3116. https://doi.org/10.3390/w13213116
Chicago/Turabian StyleChoi, Seo Yeol, Bonggil Hyun, Pung-Guk Jang, Kyoungsoon Shin, Ho Young Soh, Jung-Hoon Kang, and Min-Chul Jang. 2021. "Effects of Hypoxia on the Distribution of Calanoid Copepod Eggs in the Seabed Sediments of the Eutrophic Masan Bay, Korea" Water 13, no. 21: 3116. https://doi.org/10.3390/w13213116






