Multi-Indices Assessment of Origin and Controlling Factors of Trace Metals in River Sediments from a Semi-Arid Carbonated Basin (the Sebou Basin, Morocco)
Abstract
:1. Introduction
2. Materials and Methods
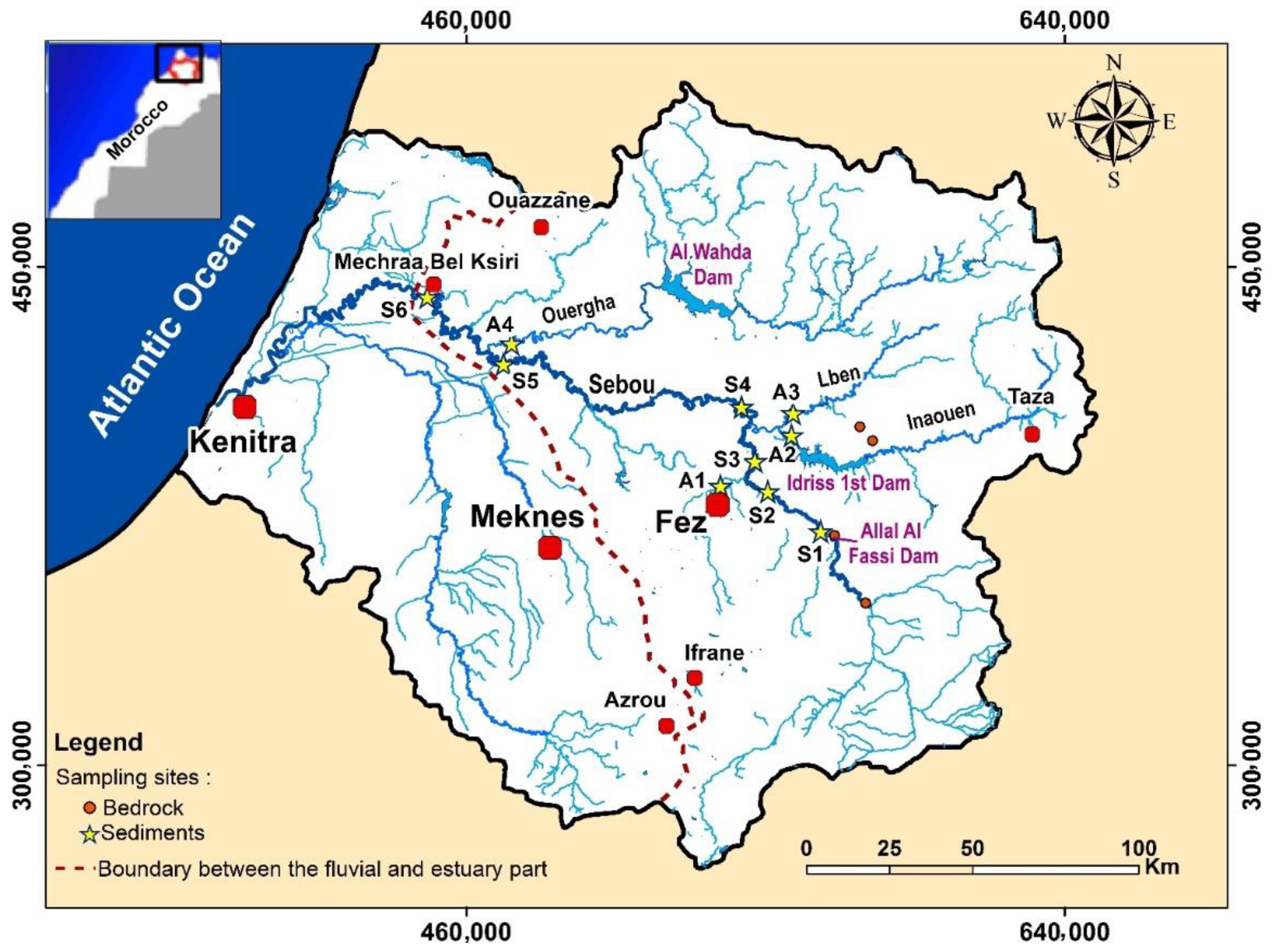
2.1. Description of the Sampling Area
2.2. Sampling and Pre-Treatment of the Samples
2.3. Physico-Chemical Treatments and Analysis
2.4. Data Treatment
2.4.1. Contamination Indices
Enrichment Factor (EF)
Geo-Accumulation Index (Igeo)
Contamination Degree (CD)
Potential Ecological Risk Index (RI)
Sediment Quality Guidelines (SQG)
2.4.2. Statistical Analysis
3. Results
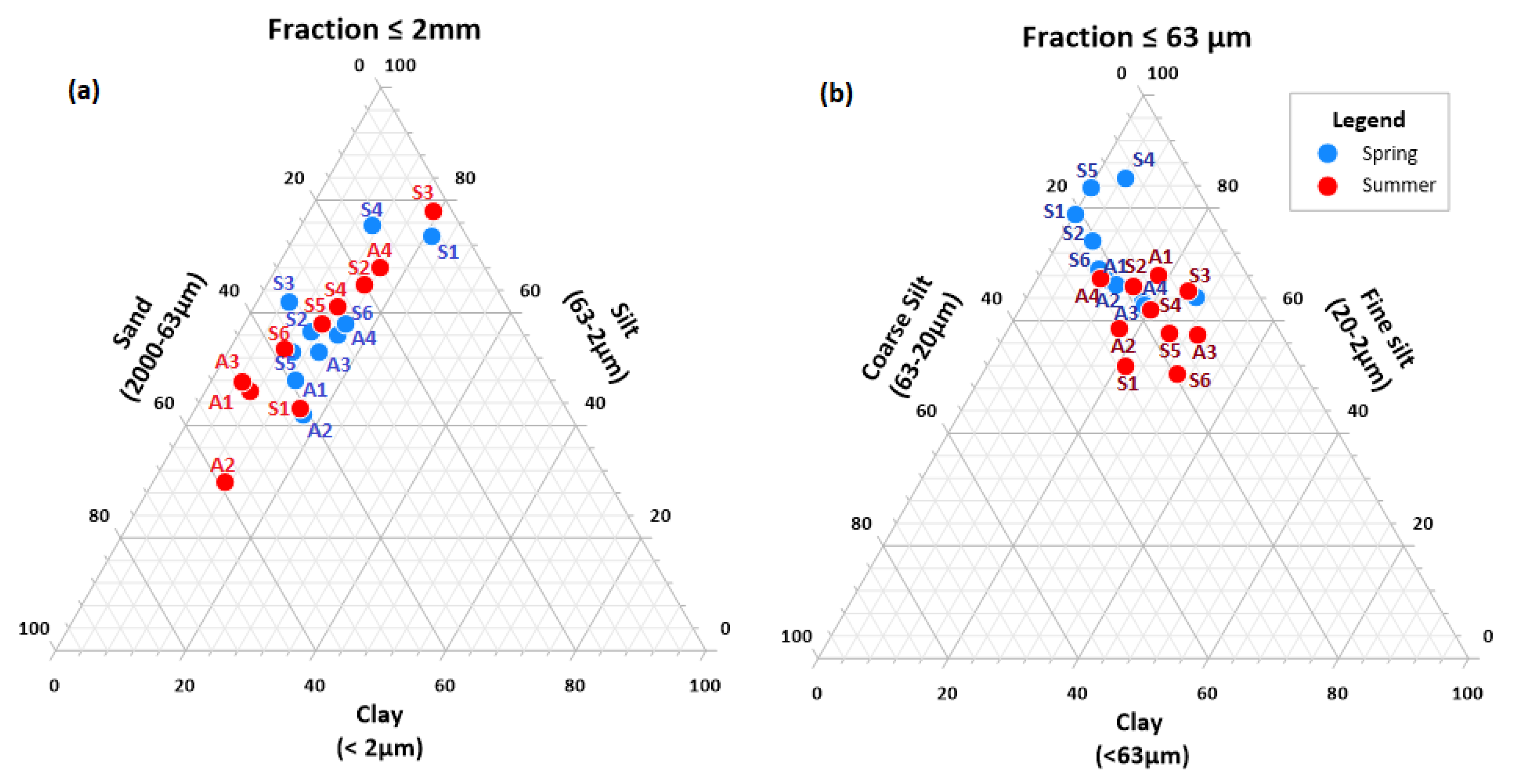
3.1. Sediment Texture and Particular Organic Carbon Content
3.2. Major and Trace Metal Concentrations in Sediments
3.3. Spatial and Temporal Variation of Trace Metal Concentrations
3.4. Assessment of Sediment Contamination
3.4.1. With the Enrichment Factor (EF)
3.4.2. With Other Contamination Indices
3.4.3. With Metal Fractionation
3.5. PCA and Relationships between Element Concentration and Main Parameters
4. Discussion
4.1. Origin of Trace Metals and Controlling Factors
4.2. Intensity and Location of Trace Metal Contamination
4.3. Availability of Trace Metal and Environmental Risk
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barton, C.; Schmitz, S. Chapter 26—Environmental Toxicology: Terrestrial. In Information Resources in Toxicology, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2009; pp. 247–250. [Google Scholar] [CrossRef]
- Mantei, E.J.; Foster, M.V. Heavy metals in stream sediments: Effects of human activities. Environ. Earth Sci. 1991, 18, 95–104. [Google Scholar] [CrossRef]
- Benamar, M.; Toumert, I.; Tobbeche, S.; Tchantchane, A.; Chalabi, A. Assessment of the state of pollution by heavy metals in the surficial sediments of Algiers Bay. Appl. Radiat. Isot. 1999, 50, 975–980. [Google Scholar] [CrossRef]
- Volpe, M.; Lacara, F.; Volpe, F.; De Mattia, A.; Serino, V.; Petitto, F.; Zavalloni, C.; Limone, F.; Pellecchia, R.; De Prisco, P.; et al. Heavy metal uptake in the enological food chain. Food Chem. 2009, 117, 553–560. [Google Scholar] [CrossRef]
- Hayzoun, H.; Garnier, C.; Durrieu, G.; Lenoble, V.; Le Poupon, C.; Angeletti, B.; Ouammou, A.; Mounier, S. Organic carbon, and major and trace element dynamic and fate in a large river subjected to poorly-regulated urban and industrial pressures (Sebou River, Morocco). Sci. Total Environ. 2015, 502, 296–308. [Google Scholar] [CrossRef] [PubMed]
- N’Guessan, Y.; Probst, J.; Bur, T.; Probst, A. Trace elements in stream bed sediments from agricultural catchments (Gascogne region, S-W France): Where do they come from? Sci. Total Environ. 2009, 407, 2939–2952. [Google Scholar] [CrossRef] [PubMed]
- Leleyter, L.; Probst, J.-L. A New Sequential Extraction Procedure for the Speciation of Particulate Trace Elements in River Sediments. Int. J. Environ. Anal. Chem. 1999, 73, 109–128. [Google Scholar] [CrossRef]
- Singh, K.P.; Mohan, D.; Singh, V.K.; Malik, A. Studies on distribution and fractionation of heavy metals in Gomti river sediments—A tributary of the Ganges, India. J. Hydrol. 2005, 312, 14–27. [Google Scholar] [CrossRef]
- Birch, G. Determination of sediment metal background concentrations and enrichment in marine environments—A critical review. Sci. Total Environ. 2017, 580, 813–831. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, M.; Zhang, R.; Zhuang, C.; Liu, Y.; Saito, Y.; Xie, J.; Zhao, B. Impacts of human activity on the late-Holocene development of the subaqueous Yangtze delta, China, as shown by magnetic properties and sediment accumulation rates. Holocene 2011, 21, 393–407. [Google Scholar] [CrossRef]
- Rodrigues, M.L.K.; Formoso, M.L.L. Geochemical Distribution of Selected Heavy Metals in Stream Sediments Affected by Tannery Activities. Water Air Soil Pollut. 2006, 169, 167–184. [Google Scholar] [CrossRef]
- Bur, T.; Probst, J.; N’Guessan, M.; Probst, A. Distribution and origin of lead in stream sediments from small agricultural catchments draining Miocene molassic deposits (SW France). Appl. Geochem. 2009, 24, 1324–1338. [Google Scholar] [CrossRef] [Green Version]
- Sundaray, S.K.; Nayak, B.B.; Lin, S.; Bhatta, D. Geochemical speciation and risk assessment of heavy metals in the river estuarine sediments—A case study: Mahanadi basin, India. J. Hazard. Mater. 2011, 186, 1837–1846. [Google Scholar] [CrossRef] [PubMed]
- Charriau, A.; Lesven, L.; Gao, Y.; Leermakers, M.; Baeyens, W.; Ouddane, B.; Billon, G. Trace metal behaviour in riverine sediments: Role of organic matter and sulfides. Appl. Geochem. 2011, 26, 80–90. [Google Scholar] [CrossRef] [Green Version]
- Hassimi, H.; Taleb, A.; Bouezmarni, M.; Karzazi, O.; Taleb, M.; Kherbeche, A.; Debbaut, V. The effect of the physicochemical conditions variations on the behavior of heavy metals trapped in polluted fluvial system sediments: The case of Oued Sebou, Morocco. Appl. Water Sci. 2019, 9, 17. [Google Scholar] [CrossRef] [Green Version]
- Benabdelkader, A.; Taleb, A.; Probst, J.; Belaidi, N.; Probst, A. Anthropogenic contribution and influencing factors on metal features in fluvial sediments from a semi-arid Mediterranean river basin (Tafna River, Algeria): A multi-indices approach. Sci. Total Environ. 2018, 626, 899–914. [Google Scholar] [CrossRef] [Green Version]
- Fovet, O.; Belemtougri, A.; Boithias, L.; Braud, I.; Charlier, J.; Cottet, M.; Daudin, K.; Dramais, G.; Ducharne, A.; Folton, N.; et al. Intermittent rivers and ephemeral streams: Perspectives for critical zone science and research on socio-ecosystems. Wiley Interdiscip. Rev. Water 2021, 8, e1523. [Google Scholar] [CrossRef]
- Rozemeijer, J.; Noordhuis, R.; Ouwerkerk, K.; Pires, M.D.; Blauw, A.; Hooijboer, A.; van Oldenborgh, G.J. Climate variability effects on eutrophication of groundwater, lakes, rivers, and coastal waters in the Netherlands. Sci. Total Environ. 2021, 771, 145366. [Google Scholar] [CrossRef]
- Huttunen, I.; Hyytiäinen, K.; Huttunen, M.; Sihvonen, M.; Veijalainen, N.; Korppoo, M.; Heiskanen, A.-S. Agricultural nutrient loading under alternative climate, societal and manure recycling scenarios. Sci. Total Environ. 2021, 783, 146871. [Google Scholar] [CrossRef] [PubMed]
- Lamhasni, N.; Chillasse, L.; Abba, H.; Haouat, S.E.L.; El, M. Typologie des eaux de surface du bassin du Sebou par multi-approche : Corrélation entre indice biologique global des réseaux de contrôle et de surveillance (IBG-RCS) et l’approche physicochimique et microbiologique. Afr. Sci. Rev. Int. Des Sci. Technol. 2013, 9, 35–49. [Google Scholar]
- Perrin, J.; Rais, N.; Chahinian, N.; Moulin, P.; Ijjaali, M. Water quality assessment of highly polluted rivers in a semi-arid Mediterranean zone Oued Fez and Sebou River (Morocco). J. Hydrol. 2014, 510, 26–34. [Google Scholar] [CrossRef]
- ABHS. Inventaire du degré de pollution des ressources en eau dans le bassin du Sebou. In Mission 2 Rapp. l’inventaire Du Degré Pollut. Sebou; ABHS: Rabat, Morocco, 2014; 174p. [Google Scholar]
- De Waele, J.; Di Gregorio, F.; El Wartiti, M.; Fadli, D.; Follesa, R.; Marini, A.; Melis, M. Geo-environmental risk in the upper valley of the Oued Sebou (Fès, Central Morocco): A preliminary approach. J. Afr. Earth Sci. 2004, 39, 491–500. [Google Scholar] [CrossRef]
- Koukal, B.; Dominik, J.; Vignati, D.; Arpagaus, P.; Santiago, S.; Ouddane, B.; Benaabidate, L. Assessment of water quality and toxicity of polluted Rivers Fez and Sebou in the region of Fez (Morocco). Environ. Pollut. 2004, 131, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Hayzoun, H.; Garnier, C.; Durrieu, G.; Lenoble, V.; Bancon-Montigny, C.; Ouammou, A.; Mounier, S.J.L. Impact of rapid urbanisation and industrialisation on river sediment metal contamination. Environ. Monit. Assess. 2014, 186, 2851–2865. [Google Scholar] [CrossRef]
- Dra, A.; El Gaidoumi, A.; Tanji, K.; Benabdallah, A.C.; Taleb, A.; Kherbeche, A. Characterization and Quantification of Heavy Metals in Oued Sebou Sediments. Sci. World J. 2019, 2019, 7496576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Snoussi, M.; Haïda, S.; Imassi, S. Effects of the construction of dams on the water and sediment fluxes of the Moulouya and the Sebou Rivers, Morocco. Reg. Environ. Chang. 2002, 3, 5–12. [Google Scholar] [CrossRef]
- Arthurton, R.; Le Tissier, M.; Snoussi, M.; Kitheka, J.; Shaghude, Y.; Kane, A.; Flöser, G.; Kremer, H. AfriCat: LOICZ—Global Change Assessment and Synthesis of River Catchment—Coastal Sea Interactions and Human Dimensions in Africa; LOICZ Reports & Studies; LOICZ IPO: Geesthacht, Germany, 2008; 30p. [Google Scholar]
- Haida, S. Transport de Matière et Bilan de L’érosion Mécanique et de L’altération Chimique Dans un Bassin Versant de Zone Semi-Aride: Le Sebou. Impacts des Variations Climatiques et des Activités Humaines. Ph.D. Thesis, Ibn Tofail University, Kenitra, Morocco, 2000; 268p. [Google Scholar]
- ABHS. Inventaire du Degré de Pollution des Ressources en Eau dans le Bassin du Sebou. Rapport de l’Agence du Bassin Hydraulique du Sebou-Maroc; ABHS: Rabat, Morocco, 2015. [Google Scholar]
- ABHS. Etude D’actualisation du Plan Directeur D’aménagement Intégré des Ressources en Eau du Bassin du Sebou; PDAIRE ABHS, MISSION I; ABHS: Rabat, Morocco, 2007. [Google Scholar]
- Probst, J.-L.; Messaïtfa, A.; Krempp, G.; Behra, P. Fluvial transports of Mercury pollution in the Ill river basin (Northeastern France): Partitioning into aqueous phases, suspended matter and bottom sediments. Environ. Sci. Mercur. Contam. 1999, 501–520. [Google Scholar] [CrossRef] [Green Version]
- Carignan, J.; Hild, P.; Mevelle, G.; Morel, J.; Yeghicheyan, D. Routine Analyses of Trace Elements in Geological Samples using Flow Injection and Low Pressure On-Line Liquid Chromatography Coupled to ICP-MS: A Study of Geochemical Reference Materials BR, DR-N., UB-N., AN-G and GH. Geostand. Newsl. 2001, 25, 187–198. [Google Scholar] [CrossRef]
- Sahuquillo, A. Overview of the use of leaching/extraction tests for risk assessment of trace metals in contaminated soils and sediments. TrAC Trends Anal. Chem. 2003, 22, 152–159. [Google Scholar] [CrossRef]
- Leleyter, L.; Rousseau, C.; Biree, L.; Baraud, F. Comparison of EDTA, HCl and sequential extraction procedures, for selected metals (Cu, Mn, Pb, Zn), in soils, riverine and marine sediments. J. Geochem. Explor. 2012, 116–117, 51–59. [Google Scholar] [CrossRef]
- Kuo, S.; Lai, M.; Lin, C. Influence of solution acidity and CaCl2 concentration on the removal of heavy metals from metal-contaminated rice soils. Environ. Pollut. 2006, 144, 918–925. [Google Scholar] [CrossRef]
- Chester, R.; Stoner, J.H. Pb in Particulates from the Lower Atmosphere of the Eastern Atlantic. Nat. Cell Biol. 1973, 245, 27–28. [Google Scholar] [CrossRef]
- Reimann, C.; de Caritat, P. Distinguishing between natural and anthropogenic sources for elements in the environment: Regional geochemical surveys versus enrichment factors. Sci. Total Environ. 2005, 337, 91–107. [Google Scholar] [CrossRef]
- Aloupi, M.; Angelidis, M. Normalization to lithium for the assessment of metal contamination in coastal sediment cores from the Aegean Sea, Greece. Mar. Environ. Res. 2001, 52, 1–12. [Google Scholar] [CrossRef]
- Loring, D.H. Normalization of heavy-metal data from estuarine and coastal sediments. ICES J. Mar. Sci. 1991, 48, 101–115. [Google Scholar] [CrossRef]
- Ackermann, F. A procedure for correcting the grain size effect in heavy metal analyses of estuarine and coastal sediments. Environ. Technol. Lett. 1980, 1, 518–527. [Google Scholar] [CrossRef]
- Roussiez, V.; Ludwig, W.; Probst, J.-L.; Monaco, A. Background levels of heavy metals in surficial sediments of the Gulf of Lions (NW Mediterranean): An approach based on 133Cs normalization and lead isotope measurements. Environ. Pollut. 2005, 138, 167–177. [Google Scholar] [CrossRef] [Green Version]
- Hernandez, L.; Probst, A.; Probst, J.L.; Ulrich, E. Heavy metal distribution in some French forest soils: Evidence for atmospheric contamination. Sci. Total Environ. 2003, 312, 195–219. [Google Scholar] [CrossRef] [Green Version]
- Larrose, A.; Coynel, A.; Schäfer, J.; Blanc, G.; Massé, L.; Maneux, E. Assessing the current state of the Gironde Estuary by mapping priority contaminant distribution and risk potential in surface sediment. Appl. Geochem. 2010, 25, 1912–1923. [Google Scholar] [CrossRef]
- Zoller, W.H.; Gladney, E.S.; Duce, R.A. Atmospheric Concentrations and Sources of Trace Metals at the South Pole. Science 1974, 183, 198–200. [Google Scholar] [CrossRef]
- Taylor, S.R.; McLennan, S.M. The Continental Crust: Its Composition and Evolution; Blackwell Malden Mass: Hoboken, NJ, USA, 1985; 312p. [Google Scholar]
- Wedepohl, K.H. The composition of the continental crust. Geochim. Cosmochim. Acta 1995, 59, 1217–1232. [Google Scholar] [CrossRef]
- Reimann, A.C.; de Caritat, P. Intrinsic Flaws of Element Enrichment Factors (EFs) in Environmental Geochemistry. Environ. Sci. Technol. 2000, 34, 5084–5091. [Google Scholar] [CrossRef]
- Sutherland, R.A. Depth Variation in Copper, Lead, and Zinc Concentrations and Mass Enrichment Ratios in Soils of an Urban Watershed. J. Environ. Qual. 2000, 29, 1414–1422. [Google Scholar] [CrossRef]
- Muller, G. Index of geoaccumulation in sediments of the Rhine River. GeoJournal 1969, 2, 108–118. [Google Scholar]
- Hakanson, L. An ecological risk index for aquatic pollution control : A sedimentological approach. Water Res. 1980, 14, 975–1001. [Google Scholar] [CrossRef]
- MacDonald, D.D.; Ingersoll, C.G.; Berger, T.A. Development and Evaluation of Consensus-Based Sediment Quality Guidelines for Freshwater Ecosystems. Arch. Environ. Contam. Toxicol. 2000, 39, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.L.; MacDonald, D.D.; Keenleyside, K.A.; Ingersoll, C.G.; Field, L.J. A Preliminary Evaluation of Sediment Quality Assessment Values for Freshwater Ecosystems. J. Great Lakes Res. 1996, 22, 624–638. [Google Scholar] [CrossRef]
- Shepard, F.P. Nomenclature Based on Sand-silt-clay Ratios. J. Sediment. Res. 1954, 24, 151–158. [Google Scholar] [CrossRef]
- Qu, S.; Wu, W.; Nel, W.; Ji, J. The behavior of metals/metalloids during natural weathering: A systematic study of the mono-lithological watersheds in the upper Pearl River Basin, China. Sci. Total Environ. 2020, 708, 134572. [Google Scholar] [CrossRef]
- Bounouira, H. Etude des Qualites Chimiques et Geochimiques du Bassin Versant du Bouregreg. Ph.D. Thesis, Ibn Tofail Kenitra University, Kenitra, Morocco, 2007; 274p. [Google Scholar]
- Barakat, A.; El Baghdadi, M.; Rais, J.; Nadem, S. Assessment of Heavy Metal in Surface Sediments of Day River at Beni-Mellal Region, Morocco. Res. J. Environ. Earth Sci. 2012, 4, 797–806. [Google Scholar]
- Bouzekri, S.; El Hachimi, M.L.; Touach, N.; El Fadili, H.; El Mahi, M.; Lotfi, E.M. The study of metal (As, Cd, Pb, Zn and Cu) contamination in superficial stream sediments around of Zaida mine (High Moulouya-Morocco). J. Afr. Earth Sci. 2019, 154, 49–58. [Google Scholar] [CrossRef]
- Azzaoui, S. Les Métaux Lourds Dans le Bassin Versant du Sebou. Géochimie Source de Pollution et Impact sur la Qualité des Eaux de Surface. Ph.D. Thesis, Ibn Tofail University, Kenitra, Morocco, 1999; 135p. [Google Scholar]
- Amri, N.; Benslimane, M.; Zaoui, H.; Hamedoun, M.; Outiti, B. Evaluation Of The Heavy Metals Accumulate In Samples Of The Sediments, Soils And Plants By Icp-oes With The Average Sebou, Moroccan Stat. Phys. Condens. Matter Soc. 2007, 8, 43–52. [Google Scholar]
- Fan, W.; Xu, Z.; Wang, W.-X. Metal pollution in a contaminated bay: Relationship between metal geochemical fractionation in sediments and accumulation in a polychaete. Environ. Pollut. 2014, 191, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Probst, A. Influence of ponds on hazardous metal distribution in sediments at a catchment scale (agricultural critical zone, S-W France). J. Hazard. Mater. 2021, 411, 125077. [Google Scholar] [CrossRef] [PubMed]
- Tessier, A.; Fortin, D.; Belzile, N.; DeVitre, R.; Leppard, G. Metal sorption to diagenetic iron and manganese oxyhydroxides and associated organic matter: Narrowing the gap between field and laboratory measurements. Geochim. Cosmochim. Acta 1996, 60, 387–404. [Google Scholar] [CrossRef]
- Micó, C.; Recatalá, L.; Peris, M.; Sánchez, J. Assessing heavy metal sources in agricultural soils of an European Mediterranean area by multivariate analysis. Chemosphere 2006, 65, 863–872. [Google Scholar] [CrossRef]
- Benabdelkader, A.; Taleb, A.; Probst, J.; Belaidi, N.; Probst, A. Origin, distribution, and behaviour of rare earth elements in river bed sediments from a carbonate semi-arid basin (Tafna River, Algeria). Appl. Geochem. 2019, 106, 96–111. [Google Scholar] [CrossRef] [Green Version]
- Burton, G.A. Sediment quality criteria in use around the world. Limnology 2002, 3, 65–75. [Google Scholar] [CrossRef]
- Badassan, T.; Avumadi, A.; Ouro-Sama, K.; Gnandi, K.; Jean-Dupuy, S.; Probst, J.-L. Geochemical Composition of the Lomé Lagoon Sediments, Togo: Seasonal and Spatial Variations of Major, Trace and Rare Earth Element Concentrations. Water 2020, 12, 3026. [Google Scholar] [CrossRef]
- Cuvier, A.; Leleyter, L.; Probst, A.; Probst, J.-L.; Prunier, J.; Pourcelot, L.; Le Roux, G.; Lemoine, M.; Reinert, M.; Baraud, F. Why comparison between different chemical extraction procedures is necessary to better assess the metals availability in sediments. J. Geochem. Explor. 2021, 225, 106762. [Google Scholar] [CrossRef]
- Baize, D. Teneurs en Eléments Traces Métalliques dans les sols (France). References et Stratégies D’interprétation; INRA: Paris, France, 1997; p. 408. [Google Scholar]
- Snape, I.; Scouller, R.; Stark, S.; Stark, J.; Riddle, M.; Gore, D. Characterisation of the dilute HCl extraction method for the identification of metal contamination in Antarctic marine sediments. Chemosphere 2004, 57, 491–504. [Google Scholar] [CrossRef]
- Chao, T.T.; Zhou, L. Extraction Techniques for Selective Dissolution of Amorphous Iron Oxides from Soils and Sediments. Soil Sci. Soc. Am. J. 1983, 47, 225–232. [Google Scholar] [CrossRef]
- Mitchell, K.N.; Gómez, M.S.R.; Barrera, A.L.G.; Flores, L.Y.; de la Torre, J.A.F.; González, F.J.A. Evaluation of Environmental Risk of Metal Contaminated Soils and Sediments Near Mining Sites in Aguascalientes, Mexico. Bull. Environ. Contam. Toxicol. 2016, 97, 216–224. [Google Scholar] [CrossRef]
- Sutherland, R.A.; Tack, F.M.; Ziegler, A.D.; Bussen, J.O. Metal extraction from road-deposited sediments using nine partial decomposition procedures. Appl. Geochem. 2004, 19, 947–955. [Google Scholar] [CrossRef]
- Leleyter, L. Speciation Chimique des Elements Majeurs, Traces et des Terres Rares dans les Matieres en Suspension et dans les Sediments de Fond des Cours d’eau : Application aux Fleuves de Patagonie (Argentine), a la Piracicaba (Bresil), a L’oued Sebou (Maroc) et a l’ill (France). Ph.D. Thesis, Louis Pasteur University, Strasbourg, France, 1999; 298p. [Google Scholar]
- Roussiez, V.; Probst, A.; Probst, J.-L. Significance of floods in metal dynamics and export in a small agricultural catchment. J. Hydrol. 2013, 499, 71–81. [Google Scholar] [CrossRef] [Green Version]




| Station | Trace Metals (µg·g−1) | Major Element (mg·g−1. Except POC (%) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Zn | Cr | Cu | Ni | Pb | Co | As | Cd | Si | Al | Fe | Mn | Mg | Ca | Na | K | Ti | P | POC | C/N | |||
| Sediment | S1 | 91.52 | 75.24 | 23.09 | 31.87 | 15.26 | 11.05 | 6.83 | 0.21 | 140.28 | 49.50 | 29.08 | 0.39 | 10.49 | 178.41 | 1.02 | 14.07 | 3.12 | 0.89 | 0.61 | 6.53 | |
| σ | 55.09 | 35.90 | 10.25 | 18.15 | 5.56 | 4.59 | 2.24 | 0.05 | 42.82 | 23.52 | 13.80 | 0.16 | 4.66 | 80.09 | 0.54 | 7.34 | 1.25 | 0.34 | 0.33 | 2.11 | ||
| S2 | 69.79 | 77.46 | 20.21 | 26.13 | 18.03 | 9.52 | 5.76 | 0.19 | 153.64 | 45.34 | 27.13 | 0.39 | 29.45 | 144.70 | 2.40 | 11.06 | 3.13 | 0.65 | 1.01 | 7.64 | ||
| σ | 6.81 | 10.00 | 2.09 | 3.00 | 2.05 | 0.85 | 0.54 | 0.02 | 5.95 | 6.36 | 2.57 | 0.03 | 3.91 | 7.67 | 0.34 | 0.97 | 0.18 | 0.13 | 0.28 | 0.66 | ||
| A1 | 195.67 | 84.57 | 79.21 | 34.90 | 26.74 | 11.66 | 5.60 | 0.22 | 157.06 | 50.68 | 30.76 | 0.32 | 20.92 | 141.34 | 2.45 | 10.99 | 3.28 | 0.84 | 1.77 | 7.48 | ||
| σ | 70.73 | 5.16 | 32.51 | 3.71 | 4.10 | 1.15 | 0.47 | 0.02 | 4.58 | 3.89 | 2.32 | 0.04 | 3.10 | 7.48 | 0.64 | 0.59 | 0.11 | 0.15 | 0.74 | 1.03 | ||
| S3 | 279.67 | 243.98 | 103.20 | 30.17 | 61.07 | 7.15 | 4.82 | 0.39 | 165.55 | 41.74 | 25.47 | 0.31 | 19.95 | 146.94 | 2.68 | 9.68 | 2.86 | 1.47 | 0.87 | 5.87 | ||
| σ | 30.64 | 53.43 | 17.50 | 2.66 | 0.58 | 0.70 | 0.49 | 0.03 | 19.77 | 3.58 | 2.57 | 0.03 | 1.63 | 10.58 | 0.74 | 0.78 | 0.09 | 0.28 | 0.56 | 0.71 | ||
| A2 | 79.27 | 79.64 | 33.20 | 32.11 | 13.02 | 11.22 | 4.78 | 0.19 | 176.62 | 47.00 | 29.98 | 0.60 | 13.65 | 146.21 | 3.59 | 11.56 | 2.95 | 0.73 | 0.38 | 6.12 | ||
| σ | 5.92 | 10.70 | 4.80 | 5.11 | 2.40 | 1.46 | 1.19 | 0.02 | 10.97 | 7.54 | 2.75 | 0.13 | 1.14 | 22.82 | 0.91 | 1.82 | 0.28 | 0.02 | 0.08 | 1.22 | ||
| A3 | 79.57 | 82.82 | 34.46 | 32.03 | 15.13 | 12.46 | 4.33 | 0.18 | 210.24 | 54.84 | 32.75 | 0.48 | 10.88 | 112.20 | 5.74 | 13.13 | 3.50 | 0.68 | 0.45 | 5.95 | ||
| σ | 16.28 | 19.73 | 4.98 | 7.44 | 1.76 | 3.12 | 0.50 | 0.02 | 25.64 | 20.47 | 8.84 | 0.05 | 1.57 | 32.50 | 1.04 | 5.73 | 0.77 | 0.03 | 0.21 | 0.68 | ||
| S4 | 130.66 | 174.58 | 54.30 | 30.79 | 23.83 | 9.92 | 3.97 | 0.26 | 182.22 | 45.82 | 26.85 | 0.42 | 14.84 | 136.23 | 3.67 | 11.13 | 3.01 | 1.33 | 1.42 | 6.69 | ||
| σ | 67.56 | 109.55 | 26.49 | 3.31 | 10.27 | 0.65 | 0.27 | 0.09 | 12.79 | 3.23 | 1.46 | 0.06 | 0.79 | 1.15 | 0.56 | 0.98 | 0.05 | 0.59 | 0.84 | 1.82 | ||
| S5 | 84.68 | 104.36 | 35.27 | 29.04 | 14.86 | 9.80 | 3.96 | 0.22 | 188.24 | 44.87 | 26.26 | 0.47 | 13.59 | 136.50 | 3.81 | 10.67 | 3.03 | 1.05 | 1.04 | 6.17 | ||
| σ | 16.02 | 28.94 | 7.67 | 4.33 | 2.39 | 0.77 | 0.24 | 0.04 | 18.32 | 4.94 | 2.67 | 0.02 | 0.71 | 4.72 | 0.60 | 1.35 | 0.05 | 0.16 | 0.25 | 0.55 | ||
| A4 | 104.15 | 102.13 | 29.22 | 34.58 | 19.42 | 13.07 | 4.70 | 0.17 | 231.72 | 65.12 | 38.88 | 0.52 | 10.38 | 78.87 | 4.90 | 12.44 | 4.07 | 0.80 | 0.95 | 5.83 | ||
| σ | 12.76 | 18.35 | 3.38 | 5.72 | 1.74 | 1.87 | 0.35 | 0.02 | 10.54 | 11.86 | 5.59 | 0.06 | 0.81 | 8.73 | 0.49 | 2.16 | 0.52 | 0.07 | 0.71 | 0.85 | ||
| S6 | 84.80 | 89.86 | 30.11 | 31.02 | 14.91 | 10.96 | 4.44 | 0.20 | 217.74 | 51.86 | 31.09 | 0.47 | 11.79 | 106.27 | 4.47 | 11.01 | 3.50 | 0.84 | 0.52 | 5.73 | ||
| σ | 4.37 | 4.56 | 2.35 | 0.86 | 0.78 | 0.32 | 0.29 | 0.00 | 9.95 | 1.81 | 0.80 | 0.03 | 0.51 | 4.87 | 0.27 | 0.71 | 0.07 | 0.04 | 0.12 | 0.80 | ||
| Sebou basin | 111.83 | 101.23 | 41.05 | 31.40 | 20.27 | 10.95 | 4.95 | 0.21 | 184.64 | 50.25 | 30.23 | 0.45 | 15.56 | 130.44 | 3.58 | 11.63 | 3.28 | 0.89 | 0.88 | 6.43 | ||
| σ | 64.18 | 50.08 | 26.33 | 6.40 | 11.57 | 2.27 | 1.11 | 0.06 | 33.80 | 11.89 | 6.28 | 0.11 | 6.52 | 36.01 | 1.47 | 2.96 | 0.56 | 0.30 | 0.59 | 1.16 | ||
| Min | 37.46 | 45.81 | 13.42 | 18.41 | 8.89 | 6.66 | 3.44 | 0.14 | 92.67 | 31.70 | 19.15 | 0.26 | 7.64 | 68.95 | 0.53 | 7.76 | 1.95 | 0.52 | 0.26 | 4.31 | ||
| Max | 301.34 | 281.76 | 115.57 | 52.51 | 61.48 | 17.06 | 8.59 | 0.40 | 247.25 | 85.46 | 45.93 | 0.79 | 34.63 | 254.97 | 6.54 | 22.13 | 4.62 | 1.75 | 2.50 | 8.96 | ||
| Tafna | 74.31 | 40.83 | 17.51 | 16.63 | 43.37 | 6.61 | 5.13 | 0.19 | 33.40 | 30.72 | 17.63 | 0.33 | 14.43 | 113.97 | 2.21 | 10.26 | - | 0.58 | 2.14 | - | ||
| Upper Pearl | 159.55 | 205.25 | 106.32 | 84.33 | 19.30 | - | 17.36 | 0.41 | - | - | - | - | - | - | - | - | - | - | - | - | ||
| Bouregreg | 139.50 | 73.88 | - | 32.00 | 53.50 | 13.88 | 32.13 | - | - | - | - | - | - | - | - | - | - | - | - | - | ||
| Day | 100.13 | 102.27 | 108.62 | - | 109.01 | - | - | 1.27 | - | - | - | - | - | - | - | - | - | - | - | - | ||
| Ansgumir | 74.00 | - | 23.67 | - | 41.33 | - | 5.71 | 1.11 | - | - | - | - | - | - | - | - | - | - | - | - | ||
| SC guidelines | TEL | 123.00 | 37.30 | 35.70 | 18.00 | 35.00 | - | 5.90 | 0.59 | - | - | - | - | - | - | - | - | - | - | - | - | |
| PEL | 315.00 | 90.00 | 197.00 | 36.00 | 91.30 | - | 17.00 | 3.53 | - | - | - | - | - | - | - | - | - | - | - | - | ||
| Bedrock | Sebou | 50.93 | 20.54 | 11.15 | 16.00 | 5.18 | 3.34 | 2.74 | 0.26 | 86.75 | 12.08 | 8.60 | 0.24 | 31.76 | 259.47 | 1.64 | 3.78 | 1.22 | 0.59 | - | - | |
| Tafna | 22.40 | 15.90 | 10.20 | 11.80 | 8.60 | 2.10 | 6.90 | 0.10 | 177.73 | 16.19 | 8.29 | 0.34 | 17.29 | 194.17 | 5.76 | 7.41 | - | - | - | - | ||
| UCC | 52.00 | 35.00 | 14.30 | 18.60 | 17.00 | 11.60 | 2.00 | 0.10 | 303.48 | 77.44 | 30.89 | 0.53 | 13.51 | 29.45 | 25.67 | 28.65 | 3.12 | 0.67 | - | - | ||
| As | Cd | Co | Cr | Cu | Ni | Pb | Zn | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ppm | % | ppm | % | ppm | % | ppm | % | ppm | % | ppm | % | ppm | % | ppm | % | |||
| EDTA Extraction | S1 | 0.09 | 1.04 | 0.1 | 42.38 | 0.67 | 4.08 | 0.04 | 0.03 | 7.84 | 23.17 | 1.00 | 1.9 | 4.17 | 21.79 | 19.28 | 13.06 | |
| S2 | 0.09 | 1.62 | 0.06 | 32.60 | 0.76 | 8.18 | 0.08 | 0.11 | 4.65 | 24.56 | 1.36 | 5.12 | 4.58 | 27.59 | 3.92 | 5.74 | ||
| A1 | 0.18 | 2.9 | 0.09 | 41.07 | 0.89 | 7.37 | 0.08 | 0.08 | 13.39 | 32.02 | 1.32 | 3.86 | 12.36 | 44.37 | 15.86 | 12.37 | ||
| S3 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | ||
| A2 | 0.02 | 0.71 | 0.05 | 33.36 | 0.58 | 4.98 | 0.07 | 0.08 | 2.33 | 6.78 | 0.84 | 2.55 | 1.24 | 11.25 | 1.32 | 1.69 | ||
| A3 | 0.03 | 0.77 | 0.05 | 26.67 | 0.55 | 4.89 | 0.08 | 0.10 | 3.07 | 9.82 | 0.66 | 2.37 | 2.27 | 15.12 | 1.18 | 1.71 | ||
| S4 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | ||
| S5 | 0.08 | 2.13 | 0.07 | 39.95 | 0.48 | 5.33 | 0.11 | 0.15 | 7.14 | 26.42 | 1.43 | 5.8 | 3.25 | 26.88 | 3.46 | 5.11 | ||
| A4 | 0.05 | 1.08 | 0.08 | 42.80 | 0.65 | 5.70 | 0.06 | 0.07 | 5.37 | 19.58 | 1.03 | 3.43 | 4.7 | 25.54 | 7.06 | 7.76 | ||
| S6 | 0.05 | 1.06 | 0.07 | 35.96 | 0.61 | 5.44 | 0.08 | 0.09 | 6.87 | 22.83 | 1.15 | 3.64 | 3.1 | 21.33 | 2.44 | 2.92 | ||
| Sebou basin | 0.07 | 1.32 | 0.07 | 36.07 | 0.65 | 5.85 | 0.07 | 0.09 | 5.88 | 20.16 | 1.08 | 3.6 | 4.19 | 23.62 | 5.76 | 5.71 | ||
| σ | 0.04 | 0.66 | 0.02 | 7.13 | 0.15 | 1.57 | 0.02 | 0.03 | 3.19 | 8.71 | 0.33 | 1.32 | 2.88 | 9.07 | 6.11 | 4.4 | ||
| HCl Extraction | S1 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| S2 | 0.03 | 0.58 | 0.01 | 3.55 | 0.09 | 0.9 | 0.04 | 0.05 | 0.23 | 1.14 | 0.22 | 0.85 | 0.04 | 0.26 | 1.85 | 2.67 | ||
| A1 | 0.06 | 1.03 | 0.01 | 3.62 | 0.07 | 0.6 | 0.03 | 0.03 | 0.42 | 0.66 | 0.19 | 0.58 | 0.04 | 0.15 | 2.12 | 1.23 | ||
| S3 | 0.04 | 0.8 | 0.02 | 5.77 | 0.12 | 1.74 | 0.03 | 0.01 | 0.55 | 0.58 | 0.58 | 2.00 | 0.03 | 0.05 | 4.27 | 1.60 | ||
| A2 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | ||
| A3 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | ||
| S4 | 0.07 | 1.8 | 0.02 | 5.59 | 0.21 | 2.2 | 0.04 | 0.02 | 0.47 | 0.83 | 0.82 | 2.58 | 0.03 | 0.13 | 3.72 | 2.58 | ||
| S5 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | ||
| A4 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | ||
| S6 | 0.01 | 0.33 | 0.01 | 2.69 | 0.06 | 0.58 | 0.02 | 0.02 | 0.19 | 0.63 | 0.21 | 0.68 | 0.03 | 0.19 | 1.71 | 2.02 | ||
| Sebou basin | 0.04 | 0.80 | 0.01 | 3.90 | 0.1 | 1.04 | 0.03 | 0.03 | 0.33 | 0.79 | 0.34 | 1.13 | 0.03 | 0.17 | 2.44 | 2.05 | ||
| σ | 0.03 | 0.67 | 0.01 | 2.43 | 0.06 | 0.79 | 0.01 | 0.02 | 0.22 | 0.31 | 0.33 | 1.06 | 0.01 | 0.08 | 1.58 | 2.77 | ||
| Elements | Rotated Component Matrix | |||
|---|---|---|---|---|
| PC1 | PC2 | PC3 | PC4 | |
| Zn | 0.96 | 0.05 | −0.15 | 0.16 |
| Cr | 0.89 | −0.25 | 0.15 | 0.04 |
| Cu | 0.95 | −0.01 | −0.05 | 0.16 |
| Ni | 0.15 | 0.88 | −0.09 | −0.11 |
| Pb | 0.95 | −0.11 | −0.07 | 0.07 |
| Co | −0.58 | 0.77 | 0.10 | −0.09 |
| As | −0.16 | 0.13 | −0.91 | 0.09 |
| Cd | 0.92 | −0.35 | −0.11 | −0.01 |
| Si | −0.13 | 0.45 | 0.83 | −0.25 |
| Al | −0.31 | 0.89 | 0.25 | −0.06 |
| Fe | −0.30 | 0.89 | 0.24 | −0.12 |
| Mn | −0.65 | 0.02 | 0.43 | −0.53 |
| Mg | 0.14 | −0.45 | −0.14 | 0.62 |
| Ca | 0.09 | −0.55 | −0.80 | 0.00 |
| Na | −0.11 | 0.30 | 0.89 | −0.26 |
| K | −0.53 | 0.47 | −0.41 | −0.33 |
| Ti | −0.25 | 0.84 | 0.37 | 0.00 |
| P | 0.82 | −0.31 | 0.00 | 0.09 |
| POC | 0.29 | 0.10 | −0.04 | 0.90 |
| CS | 0.58 | −0.00 | 0.56 | −0.46 |
| FS | 0.00 | −0.23 | −0.43 | 0.72 |
| Clays | −0.81 | 0.26 | 0.31 | −0.16 |
| Eigen value | 9.92 | 5.19 | 2.72 | 1.61 |
| % Total variance | 45.10 | 23.57 | 12.36 | 7.33 |
| % Cumulative variance | 45.10 | 68.68 | 81.03 | 88.36 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Mrissani, S.; Haida, S.; Probst, J.-L.; Probst, A. Multi-Indices Assessment of Origin and Controlling Factors of Trace Metals in River Sediments from a Semi-Arid Carbonated Basin (the Sebou Basin, Morocco). Water 2021, 13, 3203. https://doi.org/10.3390/w13223203
El Mrissani S, Haida S, Probst J-L, Probst A. Multi-Indices Assessment of Origin and Controlling Factors of Trace Metals in River Sediments from a Semi-Arid Carbonated Basin (the Sebou Basin, Morocco). Water. 2021; 13(22):3203. https://doi.org/10.3390/w13223203
Chicago/Turabian StyleEl Mrissani, Sara, Souad Haida, Jean-Luc Probst, and Anne Probst. 2021. "Multi-Indices Assessment of Origin and Controlling Factors of Trace Metals in River Sediments from a Semi-Arid Carbonated Basin (the Sebou Basin, Morocco)" Water 13, no. 22: 3203. https://doi.org/10.3390/w13223203
APA StyleEl Mrissani, S., Haida, S., Probst, J.-L., & Probst, A. (2021). Multi-Indices Assessment of Origin and Controlling Factors of Trace Metals in River Sediments from a Semi-Arid Carbonated Basin (the Sebou Basin, Morocco). Water, 13(22), 3203. https://doi.org/10.3390/w13223203








