Simulated Modelling, Design, and Performance Evaluation of a Pilot-Scale Trickling Filter System for Removal of Carbonaceous Pollutants from Domestic Wastewater
Abstract
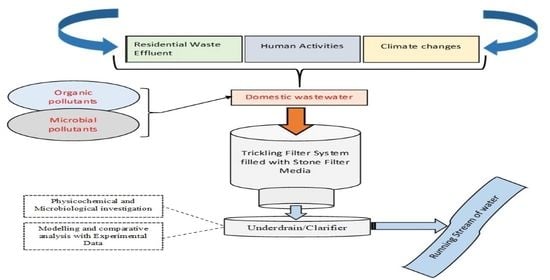
:1. Introduction
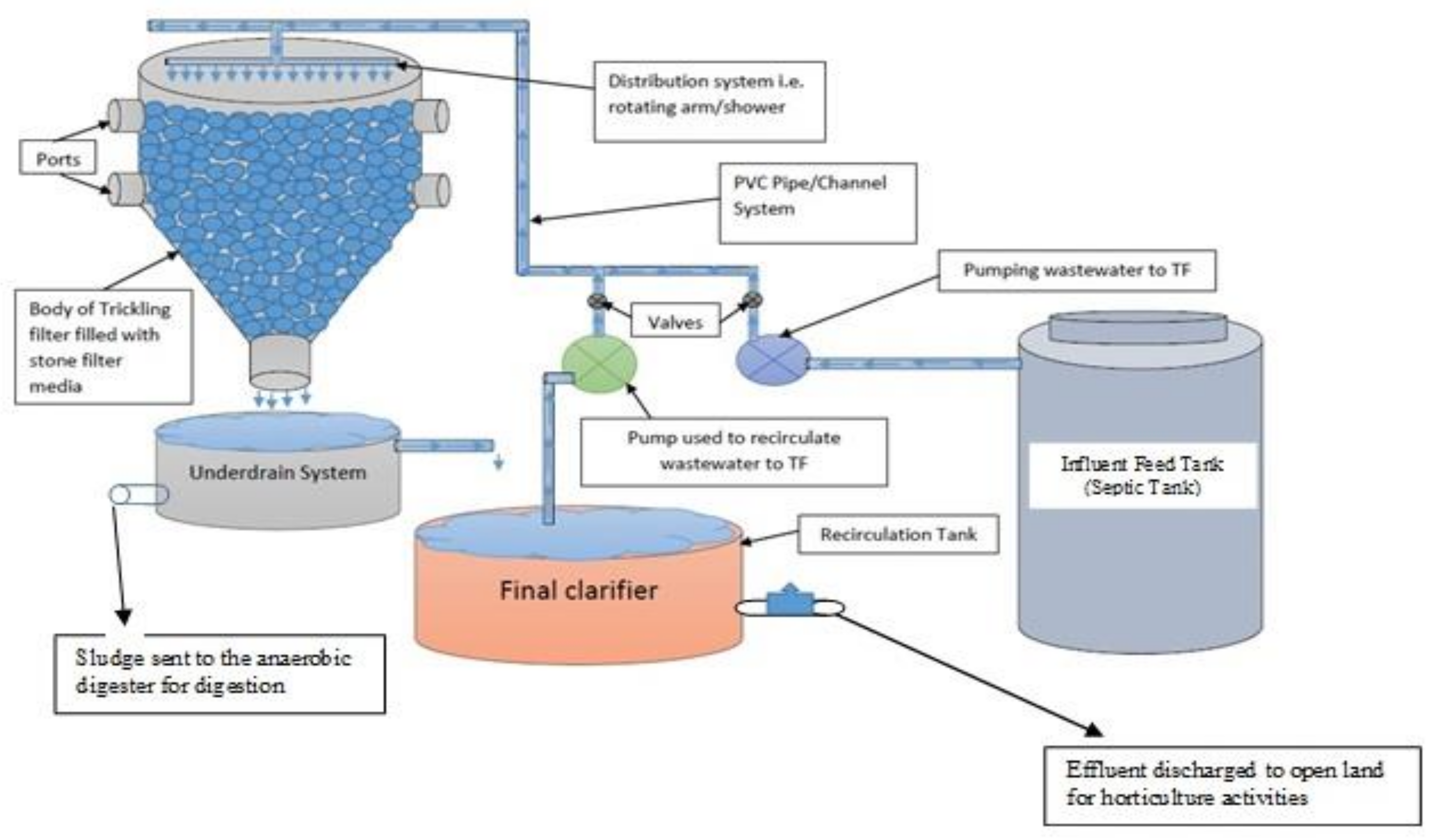
2. Materials and Methods
2.1. Experimental Setup and Operation
2.2. Microbial Profiling of Wastewater
Most Probable Number Test (MPN Index) for Fecal Coliforms
2.3. Determination of Physicochemical Parameters of Influent and Effluent Samples
2.4. Model Expansion and Standardization
3. Results and Discussion
3.1. Efficiency of Pilot-Scale TF for the Removal of Fecal Coliforms
3.2. Evaluation of the Pilot-Scale TF System for the Removal of Carbonaceous Compounds
3.2.1. Pre-Treatment Characterization of Wastewater
3.2.2. Post-Treatment Characterization of Wastewater
3.3. Mathematical Model Applications
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Souissi, M.; Laabidi, R.; Aissa, P.; Pringault, O.; Said, O.B. Influence of Bizerte city wastewater treatment plant (WWTP) on abundance and antibioresistance of culturable heterotrophic and fecal indicator bacteria of Bizerte Lagoon (Tunisia). Ecotoxicol. Environ. Saf. 2018, 148, 201. [Google Scholar] [CrossRef]
- Azizi, S.; Valipour, A.; Sithebe, T. Evaluation of different wastewater treatment processes and development of a modified attached growth bioreactor as a decentralized approach for small communities. Sci. World J. 2013, 2013, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Wang, W.; Xiong, J.; Li, L.; Zhao, B.; Sohail, I.; He, Z. A constructed wetland system with aquatic macrophytes for cleaning contaminated runoff/storm water from urban area in Florida. J. Environ. Manag. 2020, 280, 111794. [Google Scholar] [CrossRef]
- Rehman, A.; Ayub, N.; Naz, I.; Perveen, I.; Ahmed, S. Effects of Hydraulic Retention Time (HRT) on the Performance of a Pilot-Scale Trickling Filter System Treating Low-Strength Domestic Wastewater. Pol. J. Environ. Stud. 2020, 29, 249–259. [Google Scholar] [CrossRef]
- Danish, M.; Hashim, R.; Mohamad Ibrahim, M.N.; Rafatullah, M.; Sulaiman, O.; Ahmad, T.; Shamsuzzoha, M.; Ahmad, A. Sorption of copper (II) and nickel (II) ions from aqueous solutions using calcium oxide activated date (Phoenix dactylifera) stone carbon: Equilibrium, kinetic, and thermodynamic studies. J. Chem. Eng. Data 2011, 56, 3607–3619. [Google Scholar] [CrossRef]
- Bressani-Ribeiro, T.; Almeida, P.G.; Volcke, E.I.; Chernicharo, C.A. Trickling filters following anaerobic sewage treatment: State of the art and perspectives. Environ. Sci. Water Res. Technol. 2018, 4, 1721–1738. [Google Scholar] [CrossRef]
- Chen, Y.; He, H.; Liu, H.; Li, H.; Zeng, G.; Xia, X.; Yang, C. Effect of salinity on removal performance and activated sludge characteristics in sequencing batch reactors. Bioresour. Technol. 2018, 249, 890–899. [Google Scholar] [CrossRef]
- Zhou, Q.; Lin, Y.; Li, X.; Yang, C.; Han, Z.; Zeng, G.; Lu, L.; He, S. Effect of zinc ions on nutrient removal and growth of Lemna aequinoctialis from anaerobically digested swine wastewater. Bioresour. Technol. 2018, 249, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Naz, I.; Ullah, W.; Sehar, S.; Rehman, A.; Khan, Z.U.; Ali, N.; Ahmed, S. Performance evaluation of stone-media pro-type pilot-scale trickling biofilter system for municipal wastewater treatment. Desalin. Water Treat. 2016, 57, 15792–15805. [Google Scholar] [CrossRef]
- Gong, B.; Wang, Y.; Wang, J.; Dou, Y.; Zhou, J. In situ excess sludge reduction in SBBR through uncoupling of metabolism induced by novel aeration modes. RSC Adv. 2017, 7, 29058–29064. [Google Scholar] [CrossRef] [Green Version]
- Sehar, S.; Naeem, S.; Perveen, I.; Ali, N.; Ahmed, S. A comparative study of macrophytes influence on wastewater treatment through subsurface flow hybrid constructed wetland. Ecol. Eng. 2015, 81, 62–69. [Google Scholar] [CrossRef]
- Sehar, S.; Naz, I.; Khan, S.; Naeem, S.; Perveen, I.; Ali, N.; Ahmed, S. Performance Evaluation of Integrated Constructed Wetland for Domestic Wastewater Treatment. Water Environ. Res. 2016, 88, 280–287. [Google Scholar] [CrossRef]
- Li, X.; Yang, W.L.; He, H.; Wu, S.; Zhou, Q.; Yang, C.; Zeng, G.; Luo, L.; Lou, W. Responses of microalgae Coelastrella sp. to stress of cupric ions in treatment of anaerobically digested swine wastewater. Bioresour. Technol. 2018, 251, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Iribarnegaray, M.A.; Rodriguez-Alvarez, M.S.; Moraña, L.B.; Tejerina, W.A.; Seghezzo, L. Management challenges for a more decentralized treatment and reuse of domestic wastewater in metropolitan areas. J. Water Sanit. Hyg. Dev. 2018, 8, 113–122. [Google Scholar] [CrossRef]
- Sopilniak, A.; Elkayam, R.; Rossin, A.V.; Lev, O. Emerging organic pollutants in the vadose zone of a soil aquifer treatment system: Pore water extraction using positive displacement. Chemosphere 2018, 190, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cheng, Y.; Yang, C.; Luo, W.; Zeng, G.; Lu, L. Performance of system consisting of vertical flow trickling filter and horizontal flow multi-soil-layering reactor for treatment of rural wastewater. Bioresour. Technol. 2015, 193, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Iliuta, I.; Larachi, F.; Déry, M.; Baillargeon, S. Liquid residence time distribution in a two-compartment wastewater treatment bioreactor. Can. J. Chem. Eng. 2015, 93, 599–612. [Google Scholar] [CrossRef]
- Zhang, L. Anaerobic Treatment of Municipal Wastewater in a UASB-Digester System. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 2016. Available online: https://edepot.wur.nl/393388 (accessed on 11 November 2021).
- Luo, W.; Yang, C.; He, H.; Zeng, G.; Yan, S.; Cheng, Y. Novel two-stage vertical flow biofilter system for efficient treatment of decentralized domestic wastewater. Ecol. Eng. 2014, 64, 415–423. [Google Scholar] [CrossRef]
- Sehar, S.; Naz, I. Role of the biofilms in wastewater treatment. Microb. Biofilms-Importance Appl. 2016, 121–144. [Google Scholar] [CrossRef] [Green Version]
- Rehman, A.; Naz, I.; Khan, Z.U.; Rafiq, M.; Ali, N.; Ahmad, S. Sequential application of plastic media-trickling filter and sand filter for domestic wastewater treatment at low temperature condition. Biotechnol. J. 2012, 2, 179–191. [Google Scholar] [CrossRef]
- Naz, I.; Saroj, D.P.; Mumtaz, S.; Ali, N.; Ahmed, S. Assessment of biological trickling filter systems with various packing materials for improved wastewater treatment. Environ. Technol. 2015, 36, 424–434. [Google Scholar] [CrossRef]
- Khan, Z.U.; Naz, I.; Rehman, A.; Rafiq, M.; Ali, N.; Ahmed, S. Performance efficiency of an integrated stone media fixed biofilm reactor and sand filter for sewage treatment. Desalin. Water Treat. 2015, 54, 2638–2647. [Google Scholar] [CrossRef]
- Kour, D.; Rana, K.L.; Kaur, T.; Yadav, N.; Yadav, A.N.; Rastegari, A.A.; Saxena, A.K. Microbial biofilms: Functional annotation and potential applications in agriculture and allied sectors. In New and Future Developments in Microbial Biotechnology and Bioengineering: Microb Biofilms; 2020; pp. 283–301. Available online: https://doi.org/10.1016/C2018-0-01771-5 (accessed on 11 November 2021).
- Rasool, T.; Rehman, A.; Naz, I.; Ullah, R.; Ahmed, S. Efficiency of a locally designed pilot-scale trickling biofilter (TBF) system in natural environment for the treatment of domestic wastewater. Environ. Technol. 2018, 39, 1295–1306. [Google Scholar] [CrossRef]
- Mailler, R.; Gasperi, J.; Rocher, V.; Gilbert-Pawlik, S.; Geara-Matta, D.; Moilleron, R.; Chebbo, G. Biofiltration vs. conventional activated sludge plants: What about priority and emerging pollutants removal? Environ. Sci. Pollut. Res. 2014, 21, 5379–5390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hewawasam, C.; Matsuura, N.; Takimoto, Y.; Hatamoto, M.; Yamaguchi, T. Optimization of rotational speed and hydraulic retention time of a rotational sponge reactor for sewage treatment. J. Environ. Manag. 2018, 222, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Khalid, S.; Shahid, M.; Bibi, I.; Sarwar, T.; Shah, A.H.; Niazi, N.K. A review of environmental contamination and health risk assessment of wastewater use for crop irrigation with a focus on low and high-income countries. Int. J. Environ. Res. Public Health 2018, 15, 895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tesfamariam, E.H.; Musazura, W.; Odindo, A.O.; Buckley, C.A. Decentralised wastewater treatment effluent fertigation: Preliminary technical assessment. Water SA 2018, 44, 250–257. [Google Scholar]
- Murtaza, G.; Saqib, M.; Zia-ur-Rehman, M.; Naveed, M.; Ghafoor, A. Mitigation of Climate Change Impacts Through Treatment and Management of Low Quality Water for Irrigation in Pakistan. In Environmental and Agricultural Informatics: Concepts, Methodologies.; Tools, and Applications; 2020; pp. 1181–1198. Available online: https://doi.org/10.4018/978-1-5225-9621-9.ch053 (accessed on 11 November 2021).
- Starkl, M.; Anthony, J.; Aymerich, E.; Brunner, N.; Chubilleau, C.; Das, S.; Ghangrekar, M.M.; Kazmi, A.A.; Philip, L.; Singh, A. Interpreting best available technologies more flexibly: A policy perspective for municipal wastewater management in India and other developing countries. Environ. Impact Asses. Rev. 2018, 71, 132–141. [Google Scholar] [CrossRef]
- Woldetsadik, D.; Drechsel, P.; Keraita, B.; Itanna, F.; Gebrekidan, H. Heavy metals of Addis Ababa, Ethiopia. Int. J. Food Contam. 2017, 4, 9. [Google Scholar] [CrossRef]
- Wang, J.; Tai, Y.; Man, Y.; Wang, R.; Feng, X.; Yang, Y.; Fan, J.; Guo, J.; Tao, R.; Yang, Y.; et al. Capacity of various single-stage constructed wetlands to treat domestic sewage under optimal temperature in Guangzhou City, South China. Ecol. Eng. 2018, 115, 35–44. [Google Scholar] [CrossRef]
- Nnaji, C.C.; Nnam, J.P. Assessment of potability of stored rainwater and impact of environmental conditions on its quality. Int. J. Environ. Sci. Technol. 2019, 16, 8471–8484. [Google Scholar] [CrossRef]
- Angelakis, A.N.; Asano, T.; Bahri, A.; Jimenez, B.E.; Tchobanoglous, G. Water reuse: From ancient to modern times and the future. Front. Environ. Sci. 2018, 6, 26. [Google Scholar] [CrossRef] [Green Version]
- Tang, C.Y.; Yang, Z.; Guo, H.; Wen, J.J.; Nghiem, L.D.; Cornelissen, E. Potable water reuse through advanced membrane technology. Environ. Sci. Technol. 2018, 52, 10215–10223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boyd, C.E. Water Quality: An Introduction; Springer Nature: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Bailey, E.S.; Casanova, L.M.; Simmons, O.D.; Sobsey, M.D. Tertiary treatment and dual disinfection to improve microbial quality of reclaimed water for potable and non-potable reuse: A case study of facilities in North Carolina. Sci. Total Environ. 2018, 630, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Korajkic, A.; Wanjugi, P.; Brooks, L.; Cao, Y.; Harwood, V.J. Persistence and decay of fecal microbiota in aquatic habitats. Microbiol. Mol. Biol. Rev. 2019, 83, e00005-19. [Google Scholar] [CrossRef]
- Latrach, L.; Ouazzani, N.; Hejjaj, A.; Mahi, M.; Masunaga, T.; Mandi, L. Two-stage vertical flow multi-soil-layering (MSL) technology for efficient removal of coliforms and human pathogens from domestic wastewater in rural areas under arid climate. Int. J. Hyg. Environ. Health 2018, 221, 64–80. [Google Scholar] [CrossRef]
- Allegue, L.D.; Puyol, D.; Melero, J.A. Novel approach for the treatment of the organic fraction of municipal solid waste: Coupling thermal hydrolysis with anaerobic digestion and photo-fermentation. Sci. Total Environ. 2020, 714, 136845. [Google Scholar] [CrossRef] [PubMed]
- Jouhara, H.; Czajczyńska, D.; Ghazal, H.; Krzyżyńska, R.; Anguilano, L.; Reynolds, A.J.; Spencer, N. Municipal waste management systems for domestic use. Energy 2017, 139, 485–506. [Google Scholar] [CrossRef]
- Shoushtarian, F.; Negahban-Azar, M. Worldwide regulations and guidelines for agricultural water reuse: A critical review. Water 2020, 12, 971. [Google Scholar] [CrossRef] [Green Version]
- Lekeufack, M.; Fonkou, T.; Tedonkeng, E.P. Growth characteristics of Fuirena umbellata in a surface flow constructed wetland and its influence in nutrients and faecal bacteria removal from domestic wastewater in Cameroon. J. Environ. Protec. 2017, 8, 171. [Google Scholar] [CrossRef] [Green Version]
- Kishimoto, N.; Ohara, T.; Hinobayashi, J.; Hashimoto, T. Roughness and temperature effects on the filter media of a trickling filter for nitrification. Environ. Technol. 2014, 35, 1549–1555. [Google Scholar] [CrossRef]
- Kim, S.H.; Cho, J.S.; Park, J.H.; Heo, J.S.; Ok, Y.S.; Delaune, R.D.; Seo, D.C. Long-term performance of vertical-flow and horizontal-flow constructed wetlands as affected by season, N load, and operating stage for treating nitrogen from domestic sewage. Environ. Sci. Pollut. Res. 2016, 23, 1108–1119. [Google Scholar] [CrossRef]
- EPA. Sediments. In Water: Pollution Prevention & Control; 2014. Available online: http://water.epa.gov/polwaste/sediments/ (accessed on 4 October 2021).
- Ali, I.; Khan, Z.M.; Sultan, M.; Mahmood, M.H.; Farid, H.U.; Ali, M.; Nasir, A. Experimental study on Maize Cob trickling filter-based wastewater treatment system: Design, development, and performance evaluation. Pol. J. Environ. Stud. 2016, 25, 2265–2273. [Google Scholar] [CrossRef]
- Pungrasmi, W.; Chaisri, R.; Malaphol, E.; Powtongsook, S. Efficiency of a hybrid solid digestion-denitrification column in suspended solid and nitrate removal from recirculating aquaculture system. Environ. Eng. Res. 2015, 20, 175–180. [Google Scholar] [CrossRef]
- Boltz, J.P.; Smets, B.F.; Rittmann, B.E.; Van Loosdrecht, M.C.; Morgenroth, E.; Daigger, G.T. From biofilm ecology to reactors: A focused review. Wat. Sci. Technol. 2017, 75, 1753–1760. [Google Scholar] [CrossRef] [PubMed]
- Hvala, N.; Vrečko, D.; Levstek, M.; Bordon, C. The use of dynamic mathematical models for improving the designs of upgraded wastewater treatment plants. J. Sustain. Dev. Energy Water Environ. Syst. 2017, 5, 15–31. [Google Scholar] [CrossRef]
- San-Valero, P.; Dorado, A.D.; Quijano, G.; Álvarez-Hornos, F.J.; Gabaldón, C. Biotrickling filter modeling for styrene abatement. Part 2: Simulating a two-phase partitioning bioreactor. Chemosphere 2018, 191, 1075–1082. [Google Scholar] [CrossRef]
- Oliynyk, O.Y.; Kolpakova, O.A. Modelling and calculations of biological wastewater treatment at drip biofilters. Environ. Saf. Environ. Manag. 2014, 1, 68–86. [Google Scholar]




 denotes experimental data and — denotes model results.
denotes experimental data and — denotes model results.
 denotes experimental data and — denotes model results.
denotes experimental data and — denotes model results.
| Seasons | Months | Average Fecal Coliforms MPN/100 mL | Treatment Efficiency (Average % Reduction) | |||
|---|---|---|---|---|---|---|
| Influent | Effluent at Day 1 | Effluent at Day 6 | At Day 1 | At Day 6 | ||
| Rainy Monsoon | Aug | >1100 | 534.2 | 185.4 | 51.44 | 83.15 |
| Autumn | Sep | >1100 | 516 | 175.2 | 53.10 | 84.07 |
| Oct | >1100 | 563.4 | 181.6 | 48.78 | 83.51 | |
| Winter | Nov | >1100 | 586.8 | 200.6 | 46.65 | 81.8 |
| Dec | >1100 | 607.2 | 447.6 | 44.8 | 59.31 | |
| Jan | >1100 | 620.8 | 464.8 | 43.56 | 57.75 | |
| Feb | >1100 | 591 | 368.2 | 46.273 | 66.53 | |
| Spring | Mar | >1100 | 515.2 | 177.6 | 53.2 | 83.85 |
| Apr | >1100 | 492.8 | 173.2 | 55.2 | 84.32 | |
| Summer | May | >1100 | 499 | 151 | 54.64 | 86.31 |
| June | >1100 | 490 | 145.4 | 55.45 | 86.78 | |
| Season | Months and Temperature (°C) | Parameters (mg/L) | Mean Influent | Mean Effluent at Day 1 | Mean Effluent at Day 6 | Avg. % Reduction at Day 1 | Avg. % Reduction at Day 6 |
|---|---|---|---|---|---|---|---|
| Rainy Monsoon | August (40 ± 4) | BOD5 | 222.4 ± 5.7 | 147.7 ± 9.03 | 69.4 ± 6.7 | 33.58 | 68.84 |
| COD | 327 ± 8.4 | 217.6 ± 13.3 | 102 ± 9.95 | 33.45 | 67.83 | ||
| TDS | 601 ± 7.6 | 249.7 ± 8.83 | 153 ± 12.5 | 58.45 | 74.55 | ||
| TSS | 429.3 ± 10.2 | 228.5 ± 17 | 103.2 ± 13.7 | 46.77 | 76.1 | ||
| Autumn | September (24 ± 3) | BOD5 | 195.3 ± 7.02 | 137.65 ± 7.2 | 69.7 ± 4.3 | 29.51 | 64.34 |
| COD | 287.2 ± 10.3 | 202.4 ± 10.6 | 102.6 ± 6.4 | 29.52 | 63.2 | ||
| TDS | 382.1 ± 10.7 | 304.5 ± 15.35 | 149.27 ± 4 | 20.30 | 60.89 | ||
| TSS | 430.1 ± 3.3 | 259.8 ± 2.75 | 108.37 ± 3.2 | 39.59 | 74.82 | ||
| October (20 ± 1) | BOD5 | 235.5 ± 3.2 | 215.6 ± 3.2 | 112.8 ± 3.1 | 8.45 | 52.1 | |
| COD | 346.36 ± 4.7 | 317 ± 4.67 | 165.89 ± 4.1 | 8.47 | 51.85 | ||
| TDS | 563.5 ± 10.6 | 354.7 ± 16.1 | 143.1 ± 4.25 | 37.05 | 74.6 | ||
| TSS | 419.7 ± 10.1 | 257.53 ± 7.8 | 109.1 ± 6.4 | 38.63 | 74.04 | ||
| Winter | Nov (18 ± 3) | BOD5 | 244.2 ± 4.5 | 194.63 ± 3.3 | 128.55 ± 7.6 | 20.29 | 47.33 |
| COD | 359.1 ± 6.7 | 286.23 ± 4.9 | 189.1 ± 11.2 | 20.29 | 46.77 | ||
| TDS | 626.8 ± 5.5 | 485.05 ± 5.4 | 268.6 ± 11.6 | 22.61 | 57.15 | ||
| TSS | 419.8 ± 7.3 | 178.41 ± 4 | 90.06 ± 5.6 | 57.50 | 78.55 | ||
| December (12 ± 2) | BOD5 | 264.8 ± 4.2 | 241.05 ± 2.1 | 193.22 ± 6.0 | 8.96 | 27.03 | |
| COD | 389.5 ± 6.1 | 354.5 ± 3.1 | 284.2 ± 8.6 | 8.98 | 26.84 | ||
| TDS | 541.3 ± 7.0 | 490.1 ± 3.06 | 309.55 ± 9.2 | 9.45 | 42.8 | ||
| TSS | 478.1 ± 8.2 | 256.84 ± 3.3 | 122.7 ± 3.1 | 46.27 | 74.33 | ||
| January (10 ± 2) | BOD5 | 245.8 ± 3.5 | 231.67 ± 3.3 | 212.78 ± 3.9 | 5.74 | 13.42 | |
| COD | 361.5 ± 5.14 | 340.7 ± 4.85 | 312.9 ± 5.8 | 5.75 | 12.94 | ||
| TDS | 608.1 ± 10.7 | 574.84 ± 10.8 | 467.3 ± 21 | 5.46 | 23.16 | ||
| TSS | 463.5 ± 7.3 | 217.8 ± 8.3 | 107.4 ± 7.3 | 53.0 | 76.82 | ||
| February (15 ± 1) | BOD5 | 196.6 ± 12.5 | 132.5 ± 4.6 | 96.97 ± 2.75 | 32.6 | 50.48 | |
| COD | 289.1 ± 18.2 | 194.85 ± 6.8 | 142.6 ± 4.05 | 32.6 | 49.37 | ||
| TDS | 492.3 ± 13 | 288.62 ± 9.44 | 189.52 ± 6.1 | 41.37 | 61.5 | ||
| TSS | 518.8 ± 4.1 | 220.23 ± 2.1 | 99.8 ± 3.1 | 57.5 | 80.76 | ||
| Spring | March (23 ± 3) | BOD5 | 218.5 ± 4 | 153.13 ± 5.22 | 71.38 ± 3.73 | 29.9 | 67.34 |
| COD | 321.3 ± 5.7 | 225.2 ± 7.7 | 104.9 ± 5.48 | 29.9 | 67.11 | ||
| TDS | 539.4 ± 7.8 | 246.4 ± 13.47 | 103.75 ± 6.5 | 54.3 | 80.77 | ||
| TSS | 484.1 ± 6.7 | 226.35 ± 5.4 | 106.7 ± 3.12 | 53.2 | 77.96 | ||
| April (27 ± 2) | BOD5 | 197.8 ± 4.8 | 110.01 ± 6.8 | 55.75 ± 4.5 | 44.38 | 71.8 | |
| COD | 290.8 ± 7.1 | 161.78 ± 10 | 81.98 ± 6.63 | 44.36 | 72.5 | ||
| TDS | 428.3 ± 4.5 | 234.5 ± 15.5 | 112.2 ± 14.7 | 45.24 | 73.8 | ||
| TSS | 562.5 ± 9.9 | 244.9 ± 21.7 | 64.08 ± 6.8 | 56.46 | 88.62 | ||
| Summer | May (32 ± 2) | BOD5 | 247.8 ± 2.87 | 174.86 ± 9.5 | 74.67 ± 2.72 | 29.43 | 69.88 |
| COD | 364.5 ± 4.2 | 257.15 ± 13.9 | 109.8 ± 4 | 29.45 | 68.76 | ||
| TDS | 536.6 ± 5.7 | 328.57 ± 4.4 | 143.6 ± 4.1 | 38.76 | 73.24 | ||
| TSS | 481.7 ± 3.8 | 239.4 ± 10.8 | 110.84 ± 8.6 | 50.3 | 77.2 | ||
| June (38 ± 3) | BOD5 | 231.5 ± 2.3 | 145.9 ± 2.46 | 61.26 ± 1.45 | 36.9 | 73.54 | |
| COD | 340.5 ± 3.4 | 214.57 ± 3.63 | 90.08 ± 2.14 | 36.98 | 72.63 | ||
| TDS | 590.3 ± 6.2 | 336.7 ± 6.8 | 128.83 ± 4.4 | 42.9 | 78.2 | ||
| TSS | 441.3 ± 6 | 212.1 ± 6.3 | 93.1 ± 3.25 | 51.9 | 79.22 |
| Seasons | Months | Temperature of Environment (°C) | Biomass (X) (mg/m3) | Flow Rate (Q) (m3/d) | Height × Area of Reactor (m3) | kt Value | OLR (Kg/m3/d) |
|---|---|---|---|---|---|---|---|
| Rainy Monsoon | Aug | 40 ± 4 | 44 | 1.9293 | 2.945 | 1.11 | 145.23 |
| Autumn | Sep | 24 ± 3 | 42 | 1.9293 | 2.945 | 0.809 | 127.55 |
| Oct | 20 ± 1 | 32 | 1.9293 | 2.945 | 0.624 | 153.82 | |
| Winter | Nov | 18 ± 3 | 31 | 1.9293 | 2.945 | 0.589 | 159.50 |
| Dec | 12 ± 2 | 21 | 1.9293 | 2.945 | 0.496 | 172.94 | |
| Jan | 10 ± 2 | 19 | 1.9293 | 2.945 | 0.468 | 160.56 | |
| Feb | 15 ± 1 | 34 | 1.9293 | 2.945 | 0.54 | 128.4 | |
| Spring | Mar | 23 ± 3 | 37 | 1.9293 | 2.945 | 0.68 | 142.67 |
| Apr | 27 ± 2 | 42 | 1.9293 | 2.945 | 0.763 | 129.18 | |
| Summer | May | 32 ± 2 | 42 | 1.9293 | 2.945 | 0.882 | 161.84 |
| Jun | 38 ± 3 | 47 | 1.9293 | 2.945 | 1.048 | 151.20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rehman, A.; Anees, M.; Sehar, S.; Alhewairini, S.S.; Saroj, D.P.; Ahmed, S. Simulated Modelling, Design, and Performance Evaluation of a Pilot-Scale Trickling Filter System for Removal of Carbonaceous Pollutants from Domestic Wastewater. Water 2021, 13, 3210. https://doi.org/10.3390/w13223210
Rehman A, Anees M, Sehar S, Alhewairini SS, Saroj DP, Ahmed S. Simulated Modelling, Design, and Performance Evaluation of a Pilot-Scale Trickling Filter System for Removal of Carbonaceous Pollutants from Domestic Wastewater. Water. 2021; 13(22):3210. https://doi.org/10.3390/w13223210
Chicago/Turabian StyleRehman, Abdul, Muhammad Anees, Shama Sehar, Saleh S. Alhewairini, Devendra P. Saroj, and Safia Ahmed. 2021. "Simulated Modelling, Design, and Performance Evaluation of a Pilot-Scale Trickling Filter System for Removal of Carbonaceous Pollutants from Domestic Wastewater" Water 13, no. 22: 3210. https://doi.org/10.3390/w13223210
APA StyleRehman, A., Anees, M., Sehar, S., Alhewairini, S. S., Saroj, D. P., & Ahmed, S. (2021). Simulated Modelling, Design, and Performance Evaluation of a Pilot-Scale Trickling Filter System for Removal of Carbonaceous Pollutants from Domestic Wastewater. Water, 13(22), 3210. https://doi.org/10.3390/w13223210