Openness of Fish Habitat Matters: Lake Pelagic Fish Community Starts Very Close to the Shore
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling Sites
2.2. Gillnet Sampling in General
2.3. Most Lake Sampling Design
2.4. Římov Reservoir Sampling Design
2.5. Data Analyses
3. Results
3.1. Most
3.2. Římov
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kark, S. Ecotones ecotone and Ecological Gradients ecological/ecology gradients. In Encyclopedia of Sustainability Science and Technology; Springer: New York, NY, USA, 2012; pp. 3357–3367. [Google Scholar]
- Clements, F.E. Peculiar Zonal Formations of the Great Plains. Am. Nat. 1897, 31, 968–970. [Google Scholar] [CrossRef] [Green Version]
- Traut, B.H. The Role of Coastal Ecotones: A Case Study of the Salt Marsh/Upland Transition Zone in California. J. Ecol. 2005, 93, 279–290. [Google Scholar] [CrossRef]
- de Morais, L.T.; Lointier, M.; Hoff, M. Extent and role for fish populations of riverine ecotones along the Sinnamary river (French Guiana). In The Importance of Aquatic-Terrestrial Ecotones for Freshwater Fish; Springer: Dordrecht, The Netherlands, 1995; pp. 163–179. [Google Scholar]
- Jowett, I.G.; Richardson, J. Distribution and abundance of freshwater fish in New Zealand rivers. N. Z. J. Mar. Freshw. Res. 1996, 30, 239–255. [Google Scholar] [CrossRef]
- Hayes, J.W.; Leathwick, J.R.; Hanchet, S.M. Fish distribution patterns and their association with environmental factors in the Mokau River catchment, New Zealand. N. Z. J. Mar. Freshw. Res. 1989, 23, 171–180. [Google Scholar] [CrossRef]
- Trigal, C.; Degerman, E. Multiple factors and thresholds explaining fish species distributions in lowland streams. Glob. Ecol. Conserv. 2015, 4, 589–601. [Google Scholar] [CrossRef] [Green Version]
- Prchalová, M.; Kubecka, J.; Vasek, M.; Peterka, J.; Seda, J.; Juza, T.; Riha, M.; Jarolim, O.; Tuser, M.; Kratochvil, M.; et al. Distribution patterns of fishes in a canyon-shaped reservoir. J. Fish Biol. 2008, 73, 54–78. [Google Scholar] [CrossRef]
- Brosse, S.; Lek, S. Relationships between Environmental Characteristics and the Density of Age-0 Eurasian Perch Perca fluviatilis in the Littoral Zone of a Lake: A Nonlinear Approach. Trans. Am. Fish. Soc. 2002, 131, 1033–1043. [Google Scholar] [CrossRef]
- Gido, K.B.; Matthews, W.J. Dynamics of the Offshore Fish Assemblage in a Southwestern Reservoir (Lake Texoma, Oklahoma–Texas). Copeia 2000, 2000, 917–930. [Google Scholar] [CrossRef]
- Prchalová, M.; Kubečka, J.; Čech, M.; Frouzová, J.; Draštík, V.; Hohausová, E.; Jůza, T.; Kratochvíl, M.; Matěna, J.; Peterka, J.; et al. The effect of depth, distance from dam and habitat on spatial distribution of fish in an artificial reservoir. Ecol. Freshw. Fish 2009, 18, 247–260. [Google Scholar] [CrossRef]
- Medeiros, P.R.; Grempel, R.G.; Souza, A.T.; Ilarri, M.I.; Rosa, R.S. Non-random reef use by fishes at two dominant zones in a tropical, algal-dominated coastal reef. Environ. Biol. Fishes 2010, 87, 237–246. [Google Scholar] [CrossRef]
- Negi, R.K.; Mamgain, S. Species Diversity, Abundance and Distribution of Fish Community and Conservation Status of Tons River of Uttarakhand State, India. J. Fish. Aquat. Sci. 2013, 8, 617–626. [Google Scholar] [CrossRef] [Green Version]
- Vašek, M.; Kubečka, J.; Peterka, J.; Čech, M.; Draštík, V.; Hladík, M.; Prchalová, M.; Frouzová, J. Longitudinal and Vertical Spatial Gradients in the Distribution of Fish within a Canyon-shaped Reservoir. Int. Rev. Hydrobiol. 2004, 89, 352–362. [Google Scholar] [CrossRef]
- Cech, M.; Kubečka, J. Sinusoidal cycling swimming pattern of reservoir fishes. J. Fish Biol. 2002, 61, 456–471. [Google Scholar] [CrossRef]
- Blanck, A.; Tedesco, P.A.; Lamouroux, N. Relationships between life-history strategies of European freshwater fish species and their habitat preferences. Freshw. Biol. 2007, 52, 843–859. [Google Scholar] [CrossRef]
- Lewin, W.; Okun, N.; Mehner, T. Determinants of the distribution of juvenile fish in the littoral area of a shallow lake. Freshw. Biol. 2004, 49, 410–424. [Google Scholar] [CrossRef]
- Vašek, M.; Kubečka, J.; Matěna, J.; Seďa, J. Distribution and Diet of 0+ Fish within a Canyon-Shaped European Reservoir in Late Summer. Int. Rev. Hydrobiol. 2006, 91, 178–194. [Google Scholar] [CrossRef]
- Matthews, W.J. Disturbance, Harsh Environments, and Physicochemical Tolerance. In Patterns in Freshwater Fish Ecology; Springer: Boston, MA, USA, 2011; pp. 318–379. [Google Scholar]
- Parker, G.A.; Smith, J.M. Optimality theory in evolutionary biology. Nature 1990, 348, 27–33. [Google Scholar] [CrossRef]
- Paller, M. Estimating Fish Species Richness across Multiple Watersheds. Diversity 2018, 10, 42. [Google Scholar] [CrossRef] [Green Version]
- Stewart, S.D.; Hamilton, D.P.; Baisden, W.T.; Dedual, M.; Verburg, P.; Duggan, I.C.; Hicks, B.J.; Graham, B.S. Variable littoral-pelagic coupling as a food-web response to seasonal changes in pelagic primary production. Freshw. Biol. 2017, 62, 2008–2025. [Google Scholar] [CrossRef]
- Peters, J.A.; Lodge, D.M. Littoral Zone. In Encyclopedia of Inland Waters; Elsevier: Amsterdam, The Netherlands, 2009; pp. 79–87. ISBN 9780123706263. [Google Scholar]
- Fernando, C.H.; Holčík, J. Fish in Reservoirs. Int. Rev. Gesamten Hydrobiol. Hydrogr. 1991, 76, 149–167. [Google Scholar] [CrossRef]
- Jůza, T.; Vašek, M.; Kratochvíl, M.; Blabolil, P.; Čech, M.; Draštík, V.; Frouzová, J.; Muška, M.; Peterka, J.; Prchalová, M.; et al. Chaos and stability of age-0 fish assemblages in a temperate deep reservoir: Unpredictable success and stable habitat use. Hydrobiologia 2014, 724, 217–234. [Google Scholar] [CrossRef]
- Gliwicz, Z.M.; Jachner, A. Diel migrations of juvenile fish: A ghost of predation past or present? Arch. Hydrobiol. 1992, 124, 385–410. [Google Scholar] [CrossRef]
- Gliwicz, M.Z.; Slon, J.; Szynkarczyk, I. Trading safety for food: Evidence from gut contents in roach and bleak captured at different distances offshore from their daytime littoral refuge. Freshw. Biol. 2006, 51, 823–839. [Google Scholar] [CrossRef]
- Muška, M.; Tušer, M.; Balk, H.; Kubečka, J.; Hladík, M. Migrace ryb mezi údolní nádrží Lipno a přítoky na území NP Šumava (Fish migration between Lipno Reservoir and inflowing rivers in Šumava National Park). In Proceedings of the Seminář Zprůchodnění Migračních Překážek Vodních Toků; Agentura Ochrany Přírody a Krajiny České Republiky: Prague, Czech Republic, 2014; pp. 41–43. [Google Scholar]
- Muška, M.; Tušer, M.; Frouzová, J.; Mrkvička, T.; Ricard, D.; Seďa, J.; Morelli, F.; Kubečka, J. Real-time distribution of pelagic fish: Combining hydroacoustics, GIS and spatial modelling at a fine spatial scale. Sci. Rep. 2018, 8, 5381. [Google Scholar] [CrossRef] [PubMed]
- Říha, M.; Ricard, D.; Vašek, M.; Prchalová, M.; Mrkvička, T.; Jůza, T.; Čech, M.; Draštík, V.; Muška, M.; Kratochvíl, M.; et al. Patterns in diel habitat use of fish covering the littoral and pelagic zones in a reservoir. Hydrobiologia 2015, 747, 111–131. [Google Scholar] [CrossRef] [Green Version]
- Mehner, T.; Diekmann, M.; Bramick, U.; Lemcke, R. Composition of fish communities in German lakes as related to lake morphology, trophic state, shore structure and human-use intensity. Freshw. Biol. 2005, 50, 70–85. [Google Scholar] [CrossRef]
- Poikane, S.; Ritterbusch, D.; Argillier, C.; Białokoz, W.; Blabolil, P.; Breine, J.; Jaarsma, N.G.; Krause, T.; Kubečka, J.; Lauridsen, T.L.; et al. Response of fish communities to multiple pressures: Development of a total anthropogenic pressure intensity index. Sci. Total Environ. 2017, 586, 502–511. [Google Scholar] [CrossRef]
- Alexander, T.J.; Vonlanthen, P.; Periat, G.; Degiorgi, F.; Raymond, J.C.; Seehausen, O. Estimating whole-lake fish catch per unit effort. Fish. Res. 2015, 172, 287–302. [Google Scholar] [CrossRef]
- Alexander, T.J.; Vonlanthen, P.; Periat, G.; Degiorgi, F.; Raymond, J.C.; Seehausen, O. Evaluating gillnetting protocols to characterize lacustrine fish communities. Fish. Res. 2015, 161, 320–329. [Google Scholar] [CrossRef]
- Lauridsen, T.L.; Landkildehus, F.; Jeppesen, E.; Jørgensen, T.B.; Søndergaard, M. A comparison of methods for calculating Catch Per Unit Effort (CPUE) of gill net catches in lakes. Fish. Res. 2008, 93, 204–211. [Google Scholar] [CrossRef]
- Kubečka, J.; Prchalová, M.; Cech, M.; Draštík, V.; Frouzová, J.; Hladík, M.; Hohausová, E.; Juza, T.; Ketelaars, H.A.M.; Kratichvíl, M.; et al. Fish (Osteichthyes) in Biesbosch storage reservoirs (the Netherlands): A method for assessing complex stocks of fish. Acta Soc. Zool. Bohemicae 2013, 77, 37–54. [Google Scholar]
- Čtvrtlíková, M.; Kučerová, A.; Rychtecký, P.; Blabolil, P.; Borovec, J. Hydrobotanický průzkum umělých jezer Medard, Most a Milada. Zprav. Hnědé uhlí 2018, 58, 16–24. [Google Scholar]
- Znachor, P.; Rychtecký, P.; Nedoma, J.; Visocká, V. Factors affecting growth and viability of natural diatom populations in the meso-eutrophic Římov Reservoir (Czech Republic). Hydrobiologia 2015, 762, 253–265. [Google Scholar] [CrossRef]
- CEN. Water Quality—Sampling of Fish with Multi-Mesh Gillnets (EN 14757); CEN: Brussels, Belgium, 2015. [Google Scholar]
- Šmejkal, M.; Prchalová, M.; Čech, M.; Vašek, M.; Říha, M.; Jůza, T.; Blabolil, P.; Kubečka, J. Associations of fish with various types of littoral habitats in reservoirs. Ecol. Freshw. Fish 2014, 23, 405–413. [Google Scholar] [CrossRef]
- Prchalová, M.; Mrkvička, T.; Kubečka, J.; Peterka, J.; Čech, M.; Muška, M.; Kratochvíl, M.; Vašek, M. Fish activity as determined by gillnet catch: A comparison of two reservoirs of different turbidity. Fish. Res. 2010, 102, 291–296. [Google Scholar] [CrossRef]
- Vašek, M.; Prchalová, M.; Říha, M.; Blabolil, P.; Čech, M.; Draštík, V.; Frouzová, J.; Jůza, T.; Kratochvíl, M.; Muška, M.; et al. Fish community response to the longitudinal environmental gradient in Czech deep-valley reservoirs: Implications for ecological monitoring and management. Ecol. Indic. 2016, 63, 219–230. [Google Scholar] [CrossRef]
- Canadian Aquatic Biomonitoring Network Field Manual: Wadeable Streams; Environment Canada: Ottawa, ON, Canada, 2012; ISBN 9781100208169.
- Zuur, A.F.; Ieno, E.N.; Walker, N.; Saveliev, A.A.; Smith, G.M. Mixed Effects Models and Extensions in Ecology with R; Statistics for Biology and Health; Springer: New York, NY, USA, 2009; ISBN 978-0-387-87457-9. [Google Scholar]
- Ripley, B.; Venables, B.; Hornik, K.; Gebhardt, A.; Firth, D. Support Functions and Datasets for Venables and Ripley’s MASS; 2013; 170p, Available online: http://cran.wustl.edu/web/packages/MASS/MASS.pdf (accessed on 11 November 2021).
- R Core Team. R: A Language and Environment for Statistical Computing Reference Index The R Core Team; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package, Version 2; R Package, 2017; Available online: https://cran.r-project.org/web/packages/vegan/index.html (accessed on 11 November 2021).
- Sánchez-Botero, J.I.; Araujo-Lima, C.A.R.M.; Garcez, D.S. Effects of types of aquatic macrophyte stands and variations of dissolved oxygen and of temperature on the distribution of fishes in lakes of the amazonian floodplain. Acta Limnológica Bras. 2008, 20, 45–54. [Google Scholar]
- Tonn, W.M.; Magnuson, J.J. Patterns in the species composition and richness of fish assemblages in northern Wisconsin lakes. Ecology 1982, 63, 1149–1166. [Google Scholar] [CrossRef]
- Rodríguez, M.A.; Lewis, W.M. Structure of fish assemblages along environmental gradients in floodplain lakes of the Orinoco River. Ecol. Monogr. 1997, 67, 109–128. [Google Scholar] [CrossRef]
- Jackson, D.A.; Peres-Neto, P.R.; Olden, J.D. What controls who is where in freshwater fish communities—The roles of biotic, abiotic, and spatial factors. Can. J. Fish. Aquat. Sci. 2011, 58, 157–170. [Google Scholar] [CrossRef]
- Meschiatti, A.J.; Arcifa, M.S.; Fenerich-Verani, N. Fish Communities Associated with Macrophytes in Brazilian Floodplain Lakes. Environ. Biol. Fishes 2000, 58, 133–143. [Google Scholar] [CrossRef]
- Grenouillet, G.; Pont, D. Juvenile fishes in macrophyte beds: Influence of food resources, habitat structure and body size. J. Fish Biol. 2001, 59, 939–959. [Google Scholar] [CrossRef]
- Kratochvíl, M.; Mrkvička, T.; Vašek, M.; Peterka, J.; Čech, M.; Draštík, V.; Jůza, T.; Matěna, J.; Muška, M.; Sed’a, J.; et al. Littoral age 0+ fish distribution in relation to multi-scale spatial heterogeneity of a deep-valley reservoir. Hydrobiologia 2012, 696, 185–198. [Google Scholar] [CrossRef]
- Vašek, M.; Kubečka, J.; Seďa, J. Cyprinid predation on zooplankton along the longitudinal profile of a canyon-shaped reservoir. Arch. Hydrobiol. 2003, 156, 535–550. [Google Scholar]
- Žák, J.; Prchalová, M.; Šmejkal, M.; Blabolil, P.; Vašek, M.; Matěna, J.; Říha, M.; Peterka, J.; Seďa, J.; Kubečka, J. Sexual segregation in European cyprinids: Consequence of response to predation risk influenced by sexual size dimorphism. Hydrobiologia 2020, 847, 1439–1451. [Google Scholar] [CrossRef]
- Tušer, M.; Prchalová, M.; Mrkvička, T.; Frouzová, J.; Čech, M.; Peterka, J.; Jůza, T.; Vašek, M.; Kratochvíl, M.; Draštík, V.; et al. A simple method to correct the results of acoustic surveys for fish hidden in the dead zone. J. Appl. Ichthyol. 2013, 29, 358–363. [Google Scholar] [CrossRef]






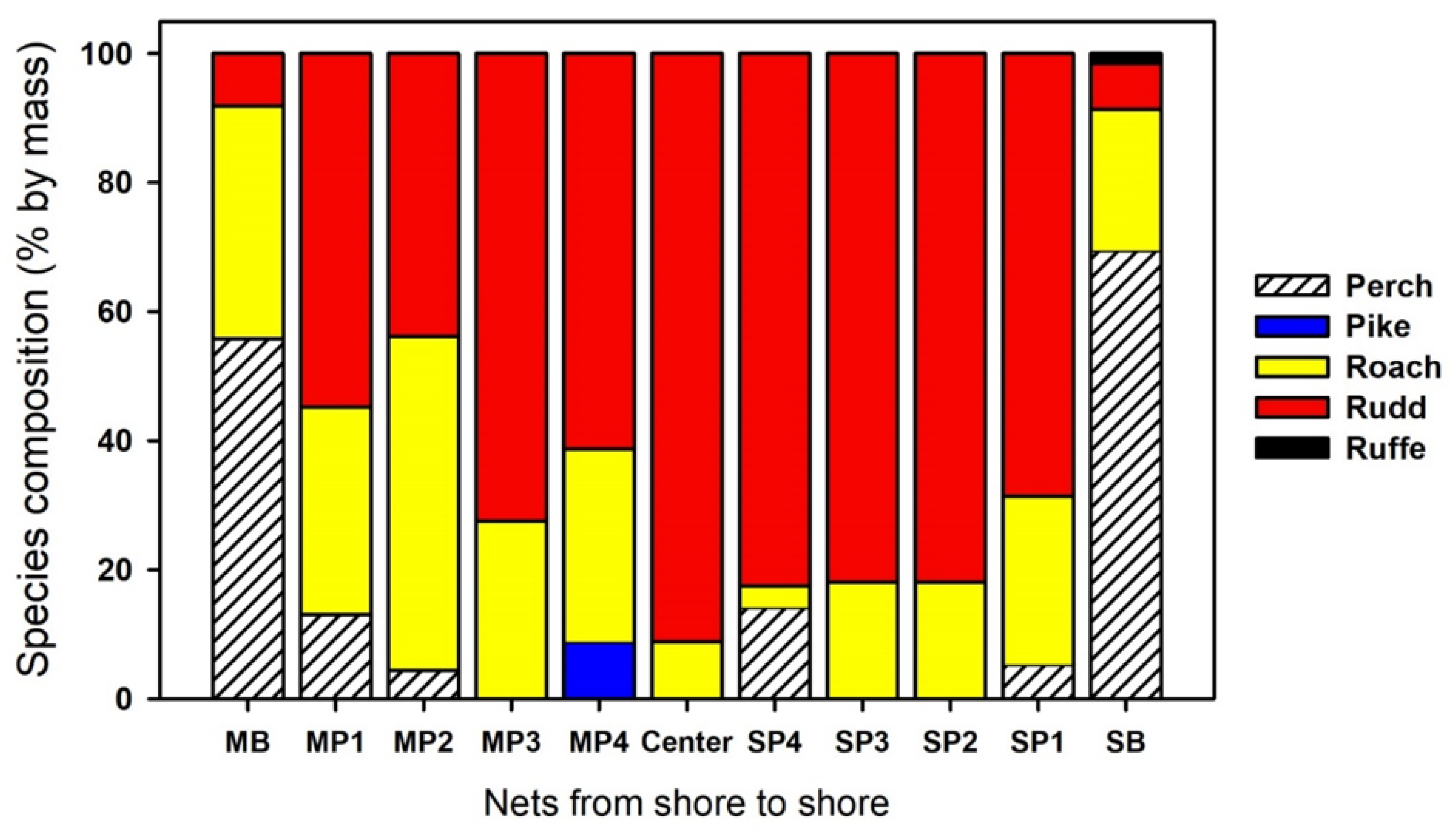




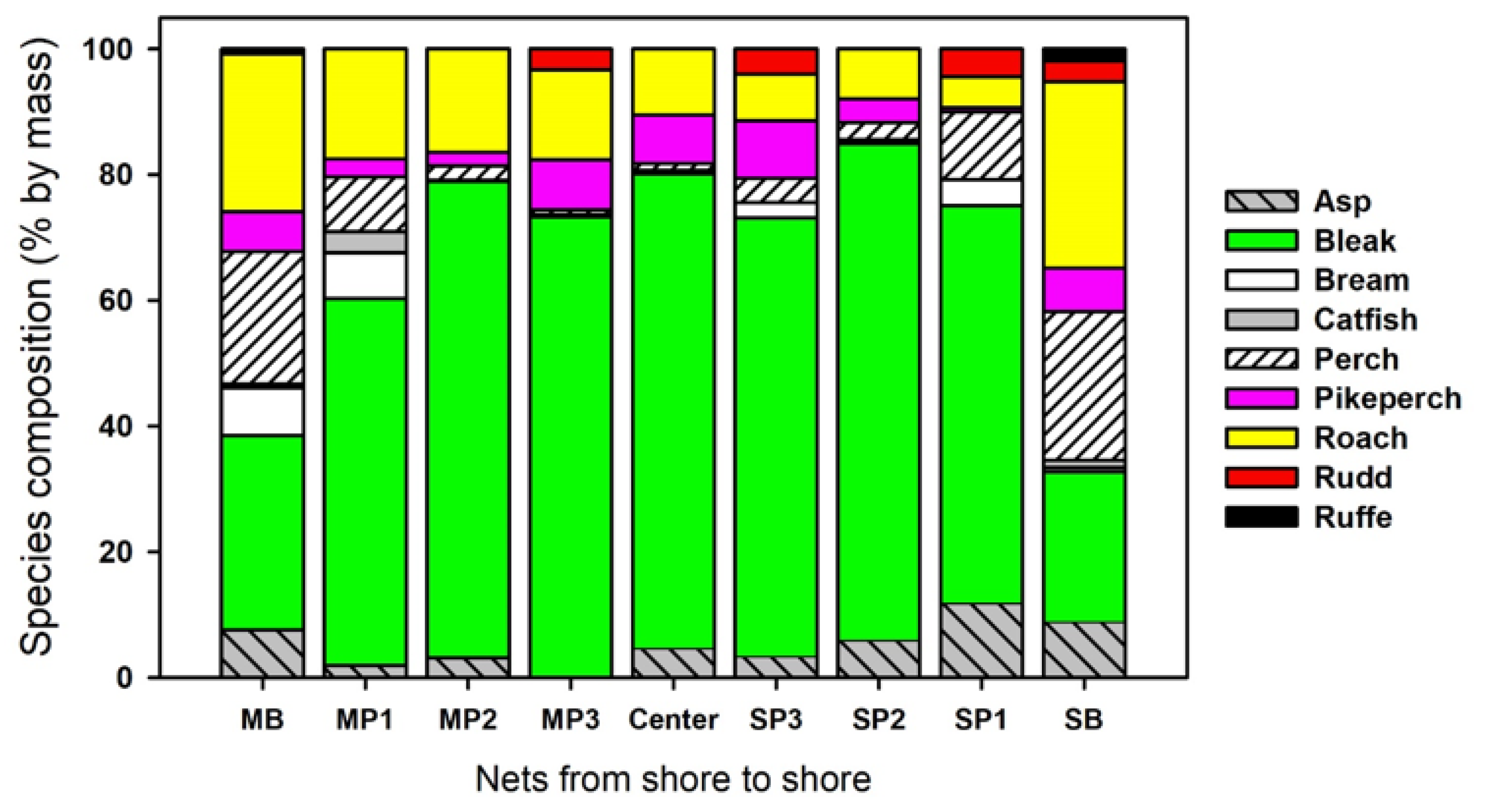




| Species | MB | se | MP1 | se | MP2 | se | MP3 | se | MP4 | se | Center | se | p_value |
| Esox lucius | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3.70 | 3.70 | 0 | 0 | ns |
| Gymnocephalus cernua | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ns |
| Perca fluviatilis | 177.78 | 22.22 | 29.63 | 9.80 | 7.41 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ns |
| Rutilus rutilus | 400.1 | 111.1 | 859.26 | 53.9 | 181.5 | 94.57 | 137.04 | 9.80 | 55.56 | 12.80 | 18.52 | 13.35 | 0.00 |
| Scardinius erythrophthalmus | 81.48 | 45.07 | 285.19 | 53.4 | 66.67 | 27.96 | 103.7 | 3.70 | 48.15 | 9.80 | 37.04 | 9.80 | 0.00 |
| Species | SB | se | SP1 | se | SP2 | se | SP3 | se | SP4 | se | Center | se | p_value |
| Esox lucius | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ns |
| Gymnocephalus cernua | 7.41 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ns |
| Perca fluviatilis | 222.22 | 102.64 | 11.11 | 0 | 0 | 0 | 0 | 0 | 3.70 | 0 | 0 | 0 | ns |
| Rutilus rutilus | 162.96 | 51.85 | 325.93 | 45.1 | 51.85 | 9.80 | 111.1 | 0 | 14.81 | 7.41 | 18.52 | 13.4 | 0.00 |
| Scardinius erythrophthalmus | 81.48 | 60.63 | 148.15 | 16.14 | 96.3 | 18.52 | 40.74 | 13.35 | 59.26 | 20.62 | 37.04 | 9.80 | ns |
| Species | MB | se | MP1 | se | MP2 | se | MP3 | se | MP4 | se | Center | se | p_value |
| Esox lucius | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 607.41 | 0 | 0 | 0 | ns |
| Gymnocephalus cernua | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | - |
| Perca fluviatilis | 11,422 | 1824 | 6526 | 3593 | 700 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.0001 |
| Rutilus rutilus | 7387 | 3283 | 1611 | 2290 | 8170 | 2073 | 5770 | 763 | 2044 | 742 | 1151 | 1089 | 0.0001 |
| Scardinius erythrophthalmus | 1659 | 830 | 27,444 | 8510 | 6911 | 2269 | 15,193 | 1817 | 4207 | 2120 | 11,874 | 4166 | ns |
| Species | SB | SP1 | SP2 | SP3 | SP4 | Center | p_value | ||||||
| Esox lucius | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | - |
| Gymnocephalus cernua | 281 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ns |
| Perca fluviatilis | 12,385 | 4375 | 1233 | 0 | 0 | 0 | 0 | 0 | 955.56 | 0 | 0 | 0 | ns |
| Rutilus rutilus | 3911 | 2410 | 6091 | 1774 | 2733 | 1389 | 2503 | 633.28 | 229.63 | 114.99 | 1150 | 1089 | 0.002 |
| Scardinius erythrophthalmus | 1259 | 1004 | 16,025 | 3609 | 12,381 | 4658 | 11,348 | 6231 | 5577 | 2549 | 11,874 | 4165 | ns |
| Species | MB | se | MP1 | se | MP2 | se | MP3 | se | MP4 | se | Center | se |
| Esox lucius | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 260 | 0 | 0 | 0 |
| Gymnocephalus cernua | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Perca fluviatilis | 138.33 | 6.87 | 196.25 | 28.53 | 167.5 | 22.5 | 0 | 0 | 0 | 0 | 0 | 0 |
| Rutilus rutilus | 85.61 | 2.46 | 89.16 | 1.6 | 109.49 | 6.82 | 108.24 | 7.11 | 110.67 | 9.18 | 126 | 21.35 |
| Scardinius erythrophthalmus | 96.91 | 4.79 | 138.25 | 5.81 | 137.22 | 13.64 | 155 | 11.26 | 128.85 | 14.73 | 198.5 | 23.52 |
| Species | SB | se | SP1 | se | SP2 | se | SP3 | se | SP4 | se | Center | se |
| Esox lucius | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Gymnocephalus cernua | 125 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Perca fluviatilis | 131.5 | 6.96 | 161.67 | 32.19 | 0 | 0 | 0 | 0 | 220 | 0 | 0 | 0 |
| Rutilus rutilus | 85.14 | 7.63 | 91.89 | 2.41 | 106.07 | 15.35 | 94.5 | 4.39 | 90 | 9.13 | 126 | 21.35 |
| Scardinius erythrophthalmus | 86.55 | 4.03 | 145.4 | 8.36 | 136.35 | 13.63 | 195 | 20.83 | 134.69 | 13.58 | 198.5 | 23.52 |
| Species | MB | se | MP1 | se | MP2 | se | MP3 | se | Center | se | p_value |
| Abramis brama | 42.59 | 16.74 | 12.96 | 4.46 | 3.7 | 0 | 1.85 | 0 | 3.7 | 0 | 0.015 |
| Alburnus alburnus | 1220 | 312.11 | 1083 | 50.25 | 924.07 | 77.8 | 903.7 | 89.57 | 775.93 | 91.06 | ns |
| Gymnocephalus cernua | 112.96 | 25.87 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ns |
| Leuciscus aspius | 37.04 | 16.26 | 7.41 | 4.68 | 5.56 | 3.8 | 0 | 0 | 1.85 | 0 | 0.013 |
| Perca fluviatilis | 327.78 | 59.37 | 18.52 | 5.49 | 3.7 | 2.34 | 1.85 | 1.85 | 1.85 | 0 | 0.0001 |
| Rutilus rutilus | 561.11 | 68.45 | 62.96 | 19.6 | 55.56 | 10.34 | 48.15 | 9.37 | 38.89 | 11.74 | 0.0001 |
| Sander lucioperca | 24.07 | 6.95 | 3.7 | 2.34 | 3.7 | 2.34 | 3.7 | 2.34 | 3.7 | 0 | ns |
| Scardinius erythrophthalmus | 0 | 0 | 0 | 0 | 0 | 0 | 1.85 | 0 | 0 | 0 | ns |
| Silurus glanis | 1.85 | 1.85 | 1.85 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ns |
| Species | SB | se | SP1 | se | SP2 | se | SP3 | se | Center | se | p_value |
| Abramis brama | 18.52 | 9 | 1.85 | 0 | 3.7 | 2.34 | 16.6 | 12.75 | 3.7 | 0 | ns |
| Alburnus alburnus | 520.37 | 262.7 | 1200 | 166.27 | 837.04 | 64.26 | 705.56 | 58.36 | 775.93 | 91.06 | ns |
| Gymnocephalus cernua | 122.22 | 28.23 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ns |
| Leuciscus aspius | 22.22 | 7.74 | 18.52 | 6.2 | 7.41 | 4.68 | 5.56 | 3.8 | 1.85 | 0 | ns |
| Perca fluviatilis | 166.67 | 21.1 | 22.22 | 14.05 | 3.7 | 2.34 | 3.7 | 2.34 | 1.85 | 0 | 0.0001 |
| Rutilus rutilus | 529.63 | 107.93 | 96.3 | 24.29 | 44.44 | 11.83 | 40.74 | 6.83 | 38.89 | 11.74 | 0.0001 |
| Sander lucioperca | 7.41 | 4.18 | 1.85 | 0 | 1.85 | 0 | 3.7 | 0 | 3.7 | 3.7 | ns |
| Scardinius erythrophthalmus | 3.7 | 2.5 | 7.41 | 2.34 | 0 | 0 | 1.85 | 0 | 0 | 0 | ns |
| Silurus glanis | 7.41 | 3.16 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ns |
| Species | MB | se | MP1 | se | MP2 | se | MP3 | se | Center | se | p_value |
| Abramis brama | 6050 | 3132 | 3118 | 2085 | 37 | 0 | 70.44 | 0 | 133 | 0 | 0.0026 |
| Alburnus alburnus | 24,742 | 5686 | 24,826 | 1582 | 19,927 | 11,885 | 21,618 | 2746 | 17,156 | 2029 | ns |
| Gymnocephalus cernua | 645 | 145 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ns |
| Leuciscus aspius | 5986 | 2811 | 840 | 589 | 803 | 618 | 0 | 0 | 1065 | 0 | ns |
| Perca fluviatilis | 16,799 | 2945 | 3702 | 1119 | 608 | 420 | 230 | 0 | 249 | 0 | 0.0001 |
| Rutilus rutilus | 19,992 | 2805 | 7441 | 3326 | 4307 | 3116 | 4236 | 1831 | 2391 | 970.4 | 0.0001 |
| Sander lucioperca | 5084 | 1656 | 1235 | 784 | 574 | 571 | 2348 | 2068 | 1749 | 0 | ns |
| Scardinius erythrophthalmus | 0 | 0 | 0 | 0 | 0 | 0 | 972 | 0 | 0 | 0 | ns |
| Silurus glanis | 459 | 0 | 1435 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ns |
| Species | SB | se | SP1 | se | SP2 | se | SP3 | se | Center | se | p_value |
| Abramis brama | 297 | 140 | 1653 | 0 | 143 | 98 | 568 | 364 | 132 | 0 | ns |
| Alburnus alburnus | 9610 | 4590 | 24,794 | 2798 | 19,455 | 1948 | 15,913 | 1347 | 17,156 | 2029 | ns |
| Gymnocephalus cernua | 774 | 138 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ns |
| Leuciscus aspius | 3598 | 2065 | 4690 | 2324 | 1475 | 976 | 787 | 690 | 1065 | 0 | 0.043 |
| Perca fluviatilis | 9545 | 2346 | 4199 | 2069 | 682 | 524 | 891 | 613 | 249 | 0 | 0.0001 |
| Rutilus rutilus | 11,999 | 3441 | 1928 | 737 | 1972 | 822 | 1692 | 1185 | 2391 | 970 | 0.05 |
| Sander lucioperca | 2816 | 2228 | 287 | 0 | 916 | 0 | 2093 | 0 | 1748 | 0 | ns |
| Scardinius erythrophthalmus | 1332 | 921 | 1733 | 937 | 0 | 0 | 913 | 0 | 0 | 0 | ns |
| Silurus glanis | 496 | 301 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ns |
| Species | MB | se | MP1 | se | MP2 | se | MP3 | se | Center | se |
| Abramis brama | 150.13 | 15.24 | 165.14 | 41.07 | 67.5 | 27.5 | 120 | 0 | 116 | 14 |
| Alburnus alburnus | 111.64 | 0.65 | 116.69 | 0.63 | 114.27 | 0.71 | 118.1 | 0.73 | 115.05 | 0.8 |
| Gymnocephalus cernua | 60.02 | 1.93 | - | - | - | - | - | - | - | - |
| Leuciscus aspius | 193.8 | 16.6 | 187.5 | 24.19 | 206.67 | 26.82 | - | - | 340 | 0 |
| Perca fluviatilis | 96 | 5.17 | 214.5 | 7.65 | 200 | 25 | 185 | 0 | 190 | 0 |
| Rutilus rutilus | 104.15 | 2.17 | 136.5 | 13.04 | 113.63 | 12.53 | 121.19 | 13.69 | 99.62 | 14.24 |
| Sander lucioperca | 241.08 | 17.53 | 297.5 | 7.5 | 168 | 122 | 334 | 116 | 332.5 | 27.5 |
| Scardinius erythrophthalmus | - | - | - | - | - | - | 265 | 0 | - | - |
| Silurus glanis | 340 | 0 | 480 | 0 | - | - | - | - | - | - |
| Species | SB | se | SP1 | se | SP2 | se | SP3 | se | Center | se |
| Abramis brama | 88.8 | 3.75 | 340 | 0 | 119 | 14 | 110 | 8.85 | 116 | 14 |
| Alburnus alburnus | 108.79 | 0.89 | 112.24 | 0.67 | 116.79 | 0.79 | 115.07 | 0.93 | 115.05 | 0.8 |
| Gymnocephalus cernua | 63.85 | 1.35 | - | - | - | - | - | - | - | - |
| Leuciscus aspius | 187.83 | 23.18 | 228.1 | 28.02 | 221.25 | 36.08 | 193.33 | 43.43 | 340 | 0 |
| Perca fluviatilis | 108.23 | 6.85 | 196.58 | 18.77 | 202.5 | 42.5 | 227.5 | 27.5 | 190 | 0 |
| Rutilus rutilus | 88.93 | 1.92 | 85.98 | 4.16 | 95.58 | 10.94 | 98.73 | 10.73 | 99.62 | 14.24 |
| Sander lucioperca | 260 | 69.13 | 230 | 0 | 340 | 0 | 341.5 | 71.5 | 332.5 | 27.5 |
| Scardinius erythrophthalmus | 235 | 15 | 188.75 | 33.44 | - | - | 260 | 0 | - | - |
| Silurus glanis | 206.25 | 39.34 | - | - | - | - | - | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moraes, K.; Souza, A.T.; Vašek, M.; Bartoň, D.; Blabolil, P.; Čech, M.; Santos, R.A.d.; Draštík, V.; Holubová, M.; Jůza, T.; et al. Openness of Fish Habitat Matters: Lake Pelagic Fish Community Starts Very Close to the Shore. Water 2021, 13, 3291. https://doi.org/10.3390/w13223291
Moraes K, Souza AT, Vašek M, Bartoň D, Blabolil P, Čech M, Santos RAd, Draštík V, Holubová M, Jůza T, et al. Openness of Fish Habitat Matters: Lake Pelagic Fish Community Starts Very Close to the Shore. Water. 2021; 13(22):3291. https://doi.org/10.3390/w13223291
Chicago/Turabian StyleMoraes, Karlos, Allan T. Souza, Mojmír Vašek, Daniel Bartoň, Petr Blabolil, Martin Čech, Romulo A. dos Santos, Vladislav Draštík, Michaela Holubová, Tomáš Jůza, and et al. 2021. "Openness of Fish Habitat Matters: Lake Pelagic Fish Community Starts Very Close to the Shore" Water 13, no. 22: 3291. https://doi.org/10.3390/w13223291
APA StyleMoraes, K., Souza, A. T., Vašek, M., Bartoň, D., Blabolil, P., Čech, M., Santos, R. A. d., Draštík, V., Holubová, M., Jůza, T., Kočvara, L., Kolářová, K., Matěna, J., Peterka, J., Říha, M., Sajdlová, Z., Šmejkal, M., Tsering, L., & Kubečka, J. (2021). Openness of Fish Habitat Matters: Lake Pelagic Fish Community Starts Very Close to the Shore. Water, 13(22), 3291. https://doi.org/10.3390/w13223291








