Effects of Acute Ammonia Stress on Antioxidant Responses, Histopathology and Ammonia Detoxification Metabolism in Triangle Sail Mussels (Hyriopsis cumingii)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Mussel and Chemicals
2.2. Acute Toxicity Test
2.3. Ammonia Challenge Test
2.3.1. Sampling and Biochemical Analysis
2.3.2. Histological Examination
2.4. Statistical Analysis
3. Results
3.1. Physiological Changes of H. cumingii under Ammonia Stress
3.2. The 96 h LC50 of NH4Cl Exposure
3.3. Effects of Biochemical Parameters in the Hemolymph
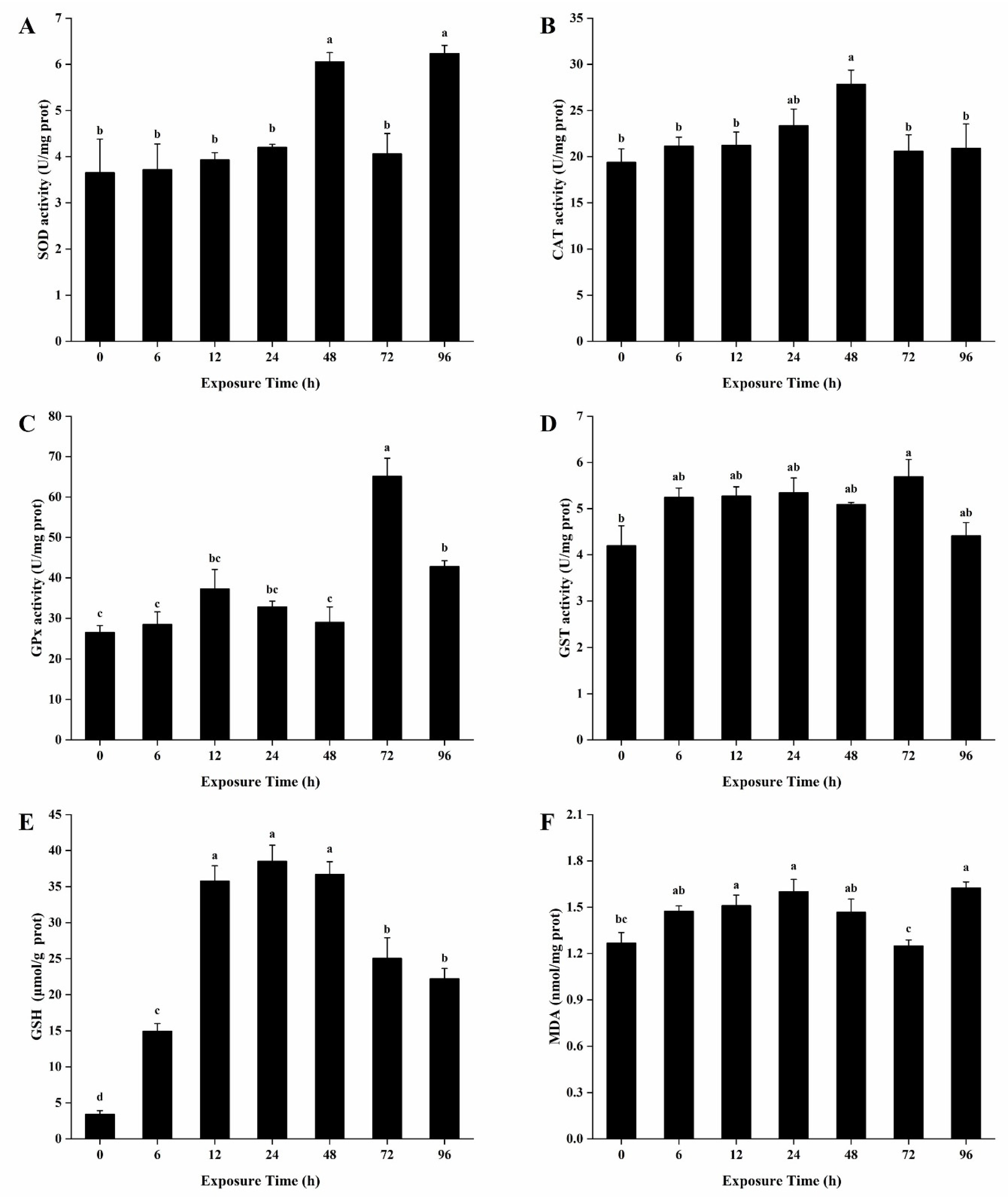
3.4. Antioxidant Enzymes Activities and Contents of GSH and MDA in the Hepatopancreas
3.5. Antioxidant Enzymes Activities and Contents of GSH and MDA in the Gills
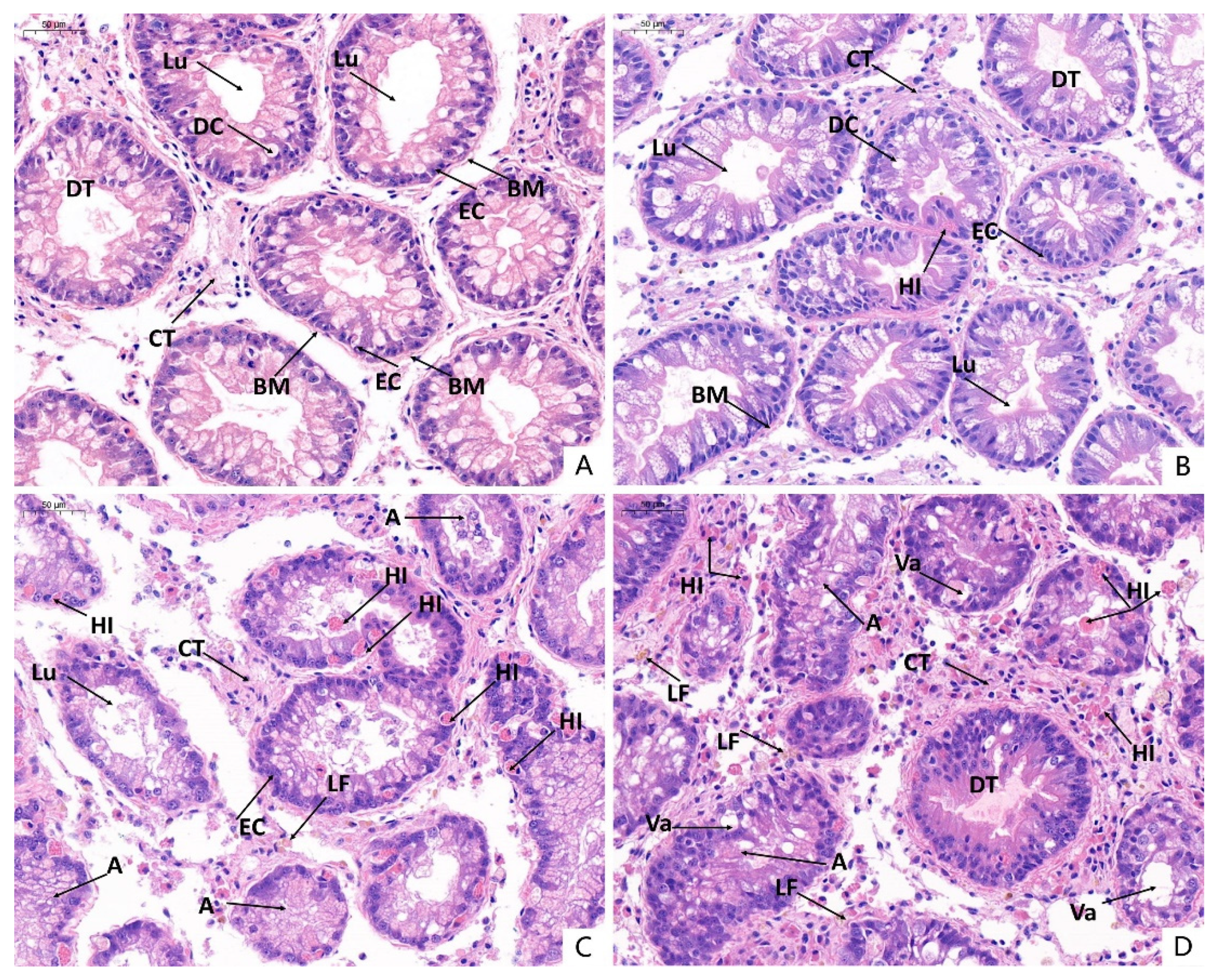
3.6. Histopathological Observations
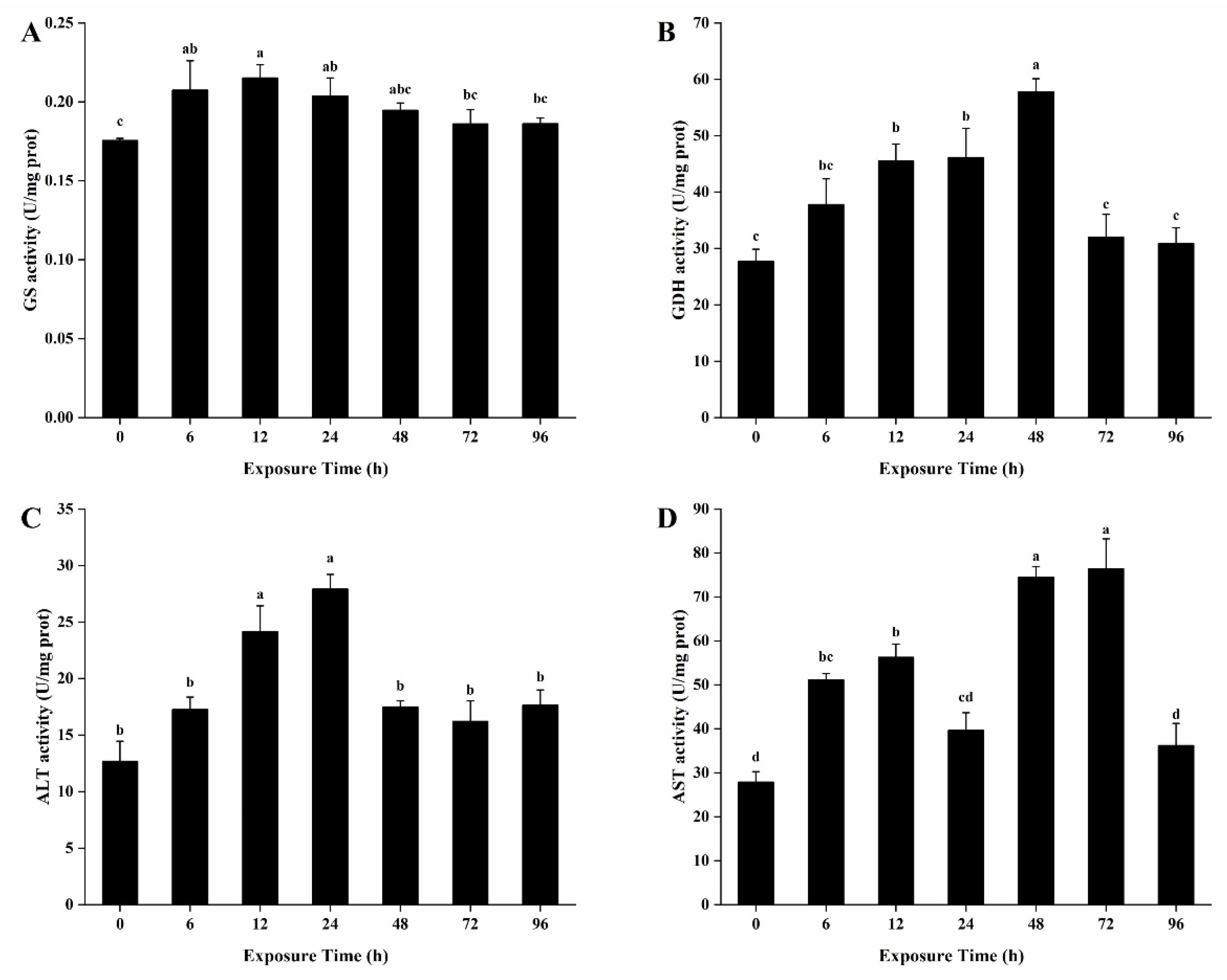
3.7. Activities of Enzymes Related to Ammonia Detoxification Metabolism in the Hepatopancreas
3.8. Activities of Enzymes Related to Ammonia Detoxification Metabolism in the Gills
4. Discussion
5. Conclusions
Author Contributions
Funding
Ethics Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gu, H.; Hu, M.; Wei, S.; Kong, H.; Huang, X.; Bao, Y.; Wang, Y. Combined effects of toxic Microcystis aeruginosa and hypoxia on the digestive enzyme activities of the triangle sail mussel Hyriopsis cumingii. Aquat. Toxicol. 2019, 212, 241–246. [Google Scholar] [CrossRef]
- Wang, L.; Liu, P.; Sun, J.; Zhang, Y.; Zhou, Q.; Wu, Z.; He, F. Comparison and combination of selective grazing on natural seston by benthic bivalves (Hyriopsis cumingii) and pelagic fish (Hypophthalmichthys molitrix). Environ. Sci. Pollut. Res. 2018, 25, 33423–33431. [Google Scholar] [CrossRef]
- Yan, L.-L.; Zhang, G.-F.; Liu, Q.-G.; Li, J.-L. Optimization of culturing the freshwater pearl mussels, Hyriopsis cumingii with filter feeding Chinese carps (bighead carp and silver carp) by orthogonal array design. Aquaculture 2009, 292, 60–66. [Google Scholar] [CrossRef]
- Yang, Z.; Wu, H.; Li, Y. Toxic effect on tissues and differentially expressed genes in hepatopancreas identified by suppression subtractive hybridization of freshwater pearl mussel (Hyriopsis cumingii) following microcystin-LR challenge. Environ. Toxicol. 2010, 27, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Spencer, P.; Pollock, R.; Dubé, M. Effects of un-ionized ammonia on histological, endocrine, and whole organism endpoints in slimy sculpin (Cottus cognatus). Aquat. Toxicol. 2008, 90, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-L.; Zhang, H.-L.; Wang, L.-Y.; Gu, B.-Y.; Fan, Q. Changes of ammonia, urea contents and transaminase activity in the body during aerial exposure and ammonia loading in Chinese loach Paramisgurnus dabryanus. Fish Physiol. Biochem. 2016, 43, 631–640. [Google Scholar] [CrossRef]
- Chen, S.; Yu, Y.; Gao, Y.; Yin, P.; Tian, L.-X.; Niu, J.; Liu, Y. Exposure to acute ammonia stress influences survival, immune response and antioxidant status of pacific white shrimp (Litopenaeus vannamei) pretreated with diverse levels of inositol. Fish Shellfish Immunol. 2019, 89, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Hsiao, I.-S.; Chen, J.-C. Effect of ammonia on the immune response of Taiwan abalone Haliotis diversicolor supertexta and its susceptibility to Vibrio parahaemolyticus. Fish Shellfish Immunol. 2004, 17, 193–202. [Google Scholar] [CrossRef]
- Randall, D.; Tsui, T. Ammonia toxicity in fish. Mar. Pollut. Bull. 2002, 45, 17–23. [Google Scholar] [CrossRef]
- Lemarié, G.; Dosdat, A.; Covès, D.; Dutto, G.; Gasset, E.; Ruyet, J.P.-L. Effect of chronic ammonia exposure on growth of European seabass (Dicentrarchus labrax) juveniles. Aquaculture 2004, 229, 479–491. [Google Scholar] [CrossRef] [Green Version]
- Cheng, C.-H.; Yang, F.-F.; Ling, R.-Z.; Liao, S.-A.; Miao, Y.-T.; Ye, C.-X.; Wang, A.-L. Effects of ammonia exposure on apoptosis, oxidative stress and immune response in pufferfish (Takifugu obscurus). Aquat. Toxicol. 2015, 164, 61–71. [Google Scholar] [CrossRef]
- Sun, H.; Lü, K.; Minter, E.J.; Chen, Y.; Yang, Z.; Montagnes, D.J. Combined effects of ammonia and microcystin on survival, growth, antioxidant responses, and lipid peroxidation of bighead carp Hypophthalmythys nobilis larvae. J. Hazard. Mater. 2012, 221, 213–219. [Google Scholar] [CrossRef]
- Zhang, W.; Jiang, Q.; Yang, J.; Pan, D.; Liu, X.; Yang, Y. The effects of acute ammonia exposure on the immune response of juvenile freshwater prawn, Macrobrachium nipponense. J. Crustac. Biol. 2015, 35, 76–80. [Google Scholar] [CrossRef] [Green Version]
- Benli, A.; Çağlan, K.; Köksal, G.; Özkul, A. Sublethal ammonia exposure of Nile tilapia (Oreochromis niloticus L.): Effects on gill, liver and kidney histology. Chemosphere 2008, 72, 1355–1358. [Google Scholar] [CrossRef]
- Pinto, W.; Aragão, C.; Soares, F.; Dinis, M.T.; Conceição, L.E.C. Growth, stress response and free amino acid levels in Senegalese sole (Solea senegalensis Kaup 1858) chronically exposed to exogenous ammonia. Aquac. Res. 2007, 38, 1198–1204. [Google Scholar] [CrossRef]
- Foss, A.; I Siikavuopio, S.; Sæther, B.-S.; Evensen, T.H. Effect of chronic ammonia exposure on growth in juvenile Atlantic cod. Aquaculture 2004, 237, 179–189. [Google Scholar] [CrossRef]
- Pan, L.; Ren, J.; Liu, J. Responses of antioxidant systems and LPO level to benzo(a)pyrene and benzo(k)fluoranthene in the haemolymph of the scallop Chlamys ferrari. Environ. Pollut. 2006, 141, 443–451. [Google Scholar] [CrossRef]
- Hu, M.; Wu, F.; Yuan, M.; Li, Q.; Gu, Y.; Wang, Y.; Liu, Q. Antioxidant responses of triangle sail mussel Hyriopsis cumingii exposed to harmful algae Microcystis aeruginosa and hypoxia. Chemosphere 2015, 139, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Lushchak, V.I. Environmentally induced oxidative stress in aquatic animals. Aquat. Toxicol. 2011, 101, 13–30. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Liu, R.; Zhao, D.; Wang, L.; Sun, M.; Wang, M.; Song, L. Ammonia exposure induces oxidative stress, endoplasmic reticulum stress and apoptosis in hepatopancreas of pacific white shrimp (Litopenaeus vannamei). Fish Shellfish Immunol. 2016, 54, 523–528. [Google Scholar] [CrossRef]
- Zhao, L.; Cui, C.; Liu, Q.; Sun, J.; He, K.; Adam, A.A.; Luo, J.; Li, Z.; Wang, Y.; Yang, S. Combined exposure to hypoxia and ammonia aggravated biological effects on glucose metabolism, oxidative stress, inflammation and apoptosis in largemouth bass (Micropterus salmoides). Aquat. Toxicol. 2020, 224, 105514. [Google Scholar] [CrossRef] [PubMed]
- Hegazi, M.M.; Attia, Z.I.; Ashour, O.A. Oxidative stress and antioxidant enzymes in liver and white muscle of Nile tilapia juveniles in chronic ammonia exposure. Aquat. Toxicol. 2010, 99, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Sıkdokur, E.; Belivermiş, M.; Sezer, N.; Pekmez, M.; Bulan Ömür, K.; Kılıç, Ö. Effects of microplastics and mercury on manila clam Ruditapes philippinarum: Feeding rate, immunomodulation, histopathology and oxidative stress. Environ. Pollut. 2020, 262, 114247. [Google Scholar] [CrossRef]
- Zhang, Y.-L.; Wang, G.-Y.; Zhang, Z.-H.; Xie, Y.-Y.; Jin, H.; Dong, Z.-R. Partial Amino Acid Metabolism and Glutamine Synthesis as the Ammonia Defensive Strategies during Aerial Exposure in Chinese Loach Paramisgurnus dabryanus. Front. Physiol. 2019, 10, 14. [Google Scholar] [CrossRef] [PubMed]
- Wicks, B.; Randall, D. The effect of sub-lethal ammonia exposure on fed and unfed rainbow trout: The role of glutamine in regulation of ammonia. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2002, 132, 275–285. [Google Scholar] [CrossRef]
- Nakamura, K.; Cañete, J.; Vijuesca, D.; Guillén, N.; Sosa, C.; Mesquita-Joanes, F.; Sousa, R.; Ginés, E.; Sorribas, V. Sensitivity of Pseudunio auricularius to metals and ammonia: First evaluation. Hydrobiologia 2020, 1–16. [Google Scholar] [CrossRef]
- Kleinhenz, L.S.; Humphrey, C.L.; Mooney, T.J.; Trenfield, M.A.; Van Dam, R.A.; Nugegoda, D.; Harford, A.J. Chronic ammonia toxicity to juveniles of 2 tropical Australian freshwater mussels (Velesunio spp.): Toxicity test optimization and implications for water quality guideline values. Environ. Toxicol. Chem. 2019, 38, 841–851. [Google Scholar] [CrossRef]
- Beggel, S.; Hinzmann, M.; Machado, J.; Geist, J. Combined Impact of Acute Exposure to Ammonia and Temperature Stress on the Freshwater Mussel Unio pictorum. Water 2017, 9, 455. [Google Scholar] [CrossRef]
- Zhang, T.; Yan, Z.; Zheng, X.; Wang, S.; Fan, J.; Liu, Z. Effects of acute ammonia toxicity on oxidative stress, DNA damage and apoptosis in digestive gland and gill of Asian clam (Corbicula fluminea). Fish Shellfish Immunol. 2020, 99, 514–525. [Google Scholar] [CrossRef]
- Miao, J.; Barnhart, M.C.; Brunson, E.L.; Hardesty, D.K.; Ingersoll, C.G.; Wang, N. An evaluation of the influence of substrate on the response of juvenile freshwater mussels (fatmucket, Lampsilis siliquoidea) in acute water exposures to ammonia. Environ. Toxicol. Chem. 2010, 29, 2112–2116. [Google Scholar] [CrossRef]
- Wang, N.; Consbrock, R.A.; Ingersoll, C.G.; Barnhart, M. Evaluation of influence of sediment on the sensitivity of a unionid mussel (Lampsilis siliquoidea) to ammonia in 28-day water exposures. Environ. Toxicol. Chem. 2011, 30, 2270–2276. [Google Scholar] [CrossRef]
- Wang, H.; Xiao, X.-C.; Wang, H.; Li, Y.; Yu, Q.; Liang, X.M.; Feng, W.; Shao, J.; Rybicki, M.; Jungmann, D.; et al. Effects of high ammonia concentrations on three cyprinid fish: Acute and whole-ecosystem chronic tests. Sci. Total Environ. 2017, 598, 900–909. [Google Scholar] [CrossRef] [PubMed]
- Goudreau, S.E.; Neves, R.J.; Sheehan, R.J. Effects of wastewater treatment plant effluents on freshwater mollusks in the upper Clinch River, Virginia, USA. Hydrobiologia 1993, 252, 211–230. [Google Scholar] [CrossRef]
- Li, M.; Gong, S.; Li, Q.; Yuan, L.; Meng, F.; Wang, R. Ammonia toxicity induces glutamine accumulation, oxidative stress and immunosuppression in juvenile yellow catfish Pelteobagrus fulvidraco. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2016, 183–184, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Saha, N.; Dutta, S.; Bhattacharjee, A. Role of amino acid metabolism in an air-breathing catfish, Clarias batrachus in response to exposure to a high concentration of exogenous ammonia. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2002, 133, 235–250. [Google Scholar] [CrossRef]
- Hong, M.; Chen, L.; Sun, X.; Gu, S.; Zhang, L.; Chen, Y. Metabolic and immune responses in Chinese mitten-handed crab (Eriocheir sinensis) juveniles exposed to elevated ambient ammonia. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2007, 145, 363–369. [Google Scholar] [CrossRef]
- Farat, O.; Cogun, H.Y.; Yuzereroglu, T.A.; Gok, G.; Firat, O. A comparative study on the effects of a pesticide (cypermethrin) and two metals (copper, lead) to serum biochemistry of Nile tilapia, Oreochromis niloticus. Fish Physiol. Biochem. 2011, 37, 657–666. [Google Scholar] [CrossRef] [Green Version]
- Kong, X.; Wang, S.; Jiang, H.; Nie, G.; Li, X. Responses of acid/alkaline phosphatase, lysozyme, and catalase activities and lipid peroxidation to mercury exposure during the embryonic development of goldfish Carassius auratus. Aquat. Toxicol. 2012, 120–121, 119–125. [Google Scholar] [CrossRef]
- Qin, Q.; Qin, S.; Wang, L.; Lei, W. Immune responses and ultrastructural changes of hemocytes in freshwater crab Sinopotamon henanense exposed to elevated cadmium. Aquat. Toxicol. 2012, 106–107, 140–146. [Google Scholar] [CrossRef]
- Peyghan, R.; Takamy, G.A. Histopathological, serum enzyme, cholesterol and urea changes in experimental acute toxicity of ammonia in common carp Cyprinus carpio and use of natural zeolite for prevention. Aquac. Int. 2002, 10, 317–325. [Google Scholar] [CrossRef]
- Amacher, D.E. Serum Transaminase Elevations as Indicators of Hepatic Injury Following the Administration of Drugs. Regul. Toxicol. Pharmacol. 1998, 27, 119–130. [Google Scholar] [CrossRef] [Green Version]
- Sinha, A.K.; Giblen, T.; AbdelGawad, H.; De Rop, M.; Asard, H.; Blust, R.; De Boeck, G. Regulation of amino acid metabolism as a defensive strategy in the brain of three freshwater teleosts in response to high environmental ammonia exposure. Aquat. Toxicol. 2013, 130–131, 86–96. [Google Scholar] [CrossRef]
- Basha, P.S.; Rani, A.U. Cadmium-induced antioxidant defense mechanism in freshwater teleost Oreochromis mossambicus (Tilapia). Ecotoxicol. Environ. Saf. 2003, 56, 218–221. [Google Scholar] [CrossRef]
- Jia, X.; Zhang, D.; Wang, F.; Dong, S. Immune responses of Litopenaeus vannamei to non-ionic ammonia stress: A comparative study on shrimps in freshwater and seawater conditions. Aquac. Res. 2015, 48, 177–188. [Google Scholar] [CrossRef]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Ni, D.; Song, L.; Zhao, J.; Zhang, H.; Li, L. Molecular cloning and characterization of a catalase gene from Zhikong scallop Chlamys farreri. Fish Shellfish Immunol. 2008, 24, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Ni, D.; Song, L.; Gao, Q.; Wu, L.; Yu, Y.; Zhao, J.; Qiu, L.; Zhang, H.; Shi, F. The cDNA cloning and mRNA expression of cytoplasmic Cu, Zn superoxide dismutase (SOD) gene in scallop Chlamys farreri. Fish Shellfish Immunol. 2007, 23, 1032–1042. [Google Scholar] [CrossRef]
- De Felice, B.; Parolini, M. Effects of single and combined exposure to cocaine and benzoylecgonine on the oxidative status of Mytilus galloprovincialis. Environ. Toxicol. Pharmacol. 2020, 80, 103475. [Google Scholar] [CrossRef] [PubMed]
- Verlecar, X.; Jena, K.; Chainy, G. Biochemical markers of oxidative stress in Perna viridis exposed to mercury and temperature. Chem. Interact. 2007, 167, 219–226. [Google Scholar] [CrossRef]
- Liu, Y.; Li, L.; Zheng, L.; Fu, P.; Wang, Y.; Nguyen, H.; Shen, X.; Sui, Y. Antioxidant responses of triangle sail mussel Hyriopsis cumingii exposed to harmful algae Microcystis aeruginosa and high pH. Chemosphere 2020, 243, 125241. [Google Scholar] [CrossRef]
- Gehringer, M.M. Microcystin-LR and okadaic acid-induced cellular effects: A dualistic response. FEBS Lett. 2003, 557, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Chetty, A.; Indira, K. Alterations in the tissue lipid profiles of Lamellidens marginalis under ambient ammonia stress. Bull. Environ. Contam. Toxicol. 1994, 53, 693–698. [Google Scholar] [CrossRef]
- Aguirre-Martínez, G.V.; DelValls, A.T.; Martín-Díaz, M.L. Yes, caffeine, ibuprofen, carbamazepine, novobiocin and tamoxifen have an effect on Corbicula fluminea (Müller, 1774). Ecotoxicol. Environ. Saf. 2015, 120, 142–154. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, L.; Yao, C.; Qiu, L.; Zhang, H.; Zhi, Z.; Song, L. Alternation of immune parameters and cellular energy allocation of Chlamys farreri under ammonia-N exposure and Vibrio anguillarum challenge. Fish Shellfish Immunol. 2012, 32, 741–749. [Google Scholar] [CrossRef] [PubMed]
- Amachree, D.; Moody, A.J.; Handy, R.D. Comparison of intermittent and continuous exposures to cadmium in the blue mussel, Mytilus edulis: Accumulation and sub-lethal physiological effects. Ecotoxicol. Environ. Saf. 2013, 95, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Sheir, S.K.; Handy, R.D.; Galloway, T.S. Tissue injury and cellular immune responses to mercuric chloride exposure in the common mussel Mytilus edulis: Modulation by lipopolysaccharide. Ecotoxicol. Environ. Saf. 2010, 73, 1338–1344. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Li, W.; Du, C.; Liu, Y.; Ma, S.; Yu, X.; Yao, W.; Wu, Z. Emerging pathogens caused disease and mortality in freshwater mussels, Hyriopsis cumingii, in China. Aquac. Res. 2020, 51, 5096–5105. [Google Scholar] [CrossRef]
- Wu, F.; Kong, H.; Shang, Y.; Zhou, Z.; Gul, Y.; Liu, Q.; Hu, M. Histopathological alterations in triangle sail mussel (Hyriopsis cumingii) exposed to toxic cyanobacteria (Microcystis aeruginosa) under hypoxia. Aquaculture 2017, 467, 182–189. [Google Scholar] [CrossRef]
- Ip, Y.K.; Lee, S.; Wong, W.; Chew, S. Mechanisms of and defense against acute ammonia toxicity in the aquatic Chinese soft-shelled turtle, Pelodiscus sinensis. Aquat. Toxicol. 2008, 86, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Hegazi, M.M.; Attia, Z.I.; Hegazi, M.A.; Hasanein, S.S. Metabolic consequences of chronic sublethal ammonia exposure at cellular and subcellular levels in Nile tilapia brain. Aquaculture 2010, 299, 149–156. [Google Scholar] [CrossRef]
- Peh, W.; Chew, S.; Ching, B.; Loong, A.; Ip, Y.K. Roles of intestinal glutamate dehydrogenase and glutamine synthetase in environmental ammonia detoxification in the euryhaline four-eyed sleeper, Bostrychus sinensis. Aquat. Toxicol. 2010, 98, 91–98. [Google Scholar] [CrossRef] [PubMed]




| Exposure Concentration (mg/L) | Mussels (unit) | Exposure Time (h) | |||
|---|---|---|---|---|---|
| 24 | 48 | 72 | 96 | ||
| 0.00 | 30 | 0% | 0.00% | 0.00% | 0.00% |
| 3.96 ± 0.09 | 30 | 0% | 0.00% | 3.33% | 13.33% |
| 5.46 ± 0.16 | 30 | 0% | 6.67% | 10% | 16.67% |
| 7.53 ± 0.08 | 30 | 0% | 3.33% | 10% | 16.67% |
| 10.39 ± 0.98 | 30 | 0% | 6.67% | 13.30% | 26.67% |
| 14.33 ± 0.25 | 30 | 0% | 16.67% | 33.30% | 63.33% |
| 19.78 ± 0.33 | 30 | 0% | 23.33% | 33.30% | 73.33% |
| 27.29 ± 0.15 | 30 | 3.33% | 33.33% | 46.67% | 100% |
| 37.65 ± 0.20 | 30 | 3.33% | 20.00% | 56.67% | 100% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Q.; Feng, K.; Zhang, L.; Bai, Y.; Yao, W. Effects of Acute Ammonia Stress on Antioxidant Responses, Histopathology and Ammonia Detoxification Metabolism in Triangle Sail Mussels (Hyriopsis cumingii). Water 2021, 13, 425. https://doi.org/10.3390/w13040425
Zhao Q, Feng K, Zhang L, Bai Y, Yao W. Effects of Acute Ammonia Stress on Antioxidant Responses, Histopathology and Ammonia Detoxification Metabolism in Triangle Sail Mussels (Hyriopsis cumingii). Water. 2021; 13(4):425. https://doi.org/10.3390/w13040425
Chicago/Turabian StyleZhao, Qianqian, Ke Feng, Lianbo Zhang, Yunpeng Bai, and Weizhi Yao. 2021. "Effects of Acute Ammonia Stress on Antioxidant Responses, Histopathology and Ammonia Detoxification Metabolism in Triangle Sail Mussels (Hyriopsis cumingii)" Water 13, no. 4: 425. https://doi.org/10.3390/w13040425
APA StyleZhao, Q., Feng, K., Zhang, L., Bai, Y., & Yao, W. (2021). Effects of Acute Ammonia Stress on Antioxidant Responses, Histopathology and Ammonia Detoxification Metabolism in Triangle Sail Mussels (Hyriopsis cumingii). Water, 13(4), 425. https://doi.org/10.3390/w13040425





