Abstract
Anthropogenic activities performed in the Ecuadorian Amazon have released potentially toxic elements (PTEs) into the rivers, causing severe environmental pollution and increasing the risk of exposure to the residents of the surrounding areas. This study aims to carry out a human health risk assessment using deterministic and probabilistic methods to estimate the hazard index (HI) and total cancer risk (TCR) related to multi-pathway human exposure to PTEs in polluted rivers. Concentrations of Al, Cd, Cr, Cu, Hg, Ni, Pb, and Zn in surface water and sediment samples from rivers on the Ecuadorian Amazon were considered to assess the potential adverse human health effects. As a result, deterministic and probabilistic estimations of cancer and non-cancer risk through exposure to surface waters and sediments were above the safety limit. A sensitivity analysis identified the concentration of PTEs and the exposure duration (ED) as the two most important variables for probabilistic health risk assessment. The highest risk for receptors was related to exposure to polluted sediments through incidental ingestion and dermal contact routes. According to the deterministic estimation, the human health risk through ingestion of water was above the threshold in specific locations. This study reveals the potential health risk to which the population is exposed. This information can be used as a baseline to develop public strategies to reduce anthropogenic pollution and exposure to PTEs in Ecuadorian Amazon rivers.
1. Introduction
Potentially toxic elements (PTEs), including trace elements and heavy metals, are naturally occurring substances in the environment; however, the anthropogenic activities in some regions have increased their concentration [1,2]. Due to their bioaccumulation capacity, persistent nature, and toxicity, some PTEs are considered priority pollutants [3]. Potentially toxic elements (PTEs) can enter surface waters from both natural and anthropogenic sources [4,5]. Several studies have reported that exposure to PTEs can cause various acute and chronic health hazards [6,7]. These pollutants are easily released into many environmental media and may enter the human body; consequently, inhabitants of polluted areas, mainly children, are exposed to PTEs from several different sources and pathways [8,9,10]. Among the PTEs of greatest concern are, for example, Cr and Pb, recognized as human carcinogens [11]. In addition, Cd, Cr, Hg, and Pb are systemic non-cancerous, and can produce adverse health effects even at low levels of exposure [12].
The population surrounding polluted areas can be exposed to PTEs through drinking water, groundwater, surface water, sediments, and soils [3,13,14,15,16]. Illnesses, such as an increased cancer risk in the exposed population or even deaths due to poisoning, have been associated with massive environmental pollution [17,18]. Clinical and epidemiological studies are the most reliable instruments for control and intervention in public health [19,20]. However, these studies involve a high economic cost that cannot always be assumed by the evaluators. On the other hand, risk assessment is a useful and simple instrument that allows a quantitative estimation of health problems derived from exposure to pollutants [21].
Health risk assessment (HRA) is widely used to quantify the risk of human exposure to certain pollutants [16,22,23]. HRA can be estimated by both deterministic and probabilistic methods. The deterministic method represents the output health risk as a single point value. In contrast, in probabilistic risk assessment (PRA), the combination of the probability distribution of several input parameters in the risk equation yields the output risk as a range of values [3,24]. PRA is mainly useful when a deterministic outcome of risk is close to the safe exposure thresholds or when there is a need to decrease uncertainties [25]. In addition, probabilistic analysis and sensitivity analysis can be used together to identify the effects of variability and uncertainty of input parameters in the risk calculations output [26]. Overall, HRA supplies information that can contribute to decision-making by providing a quantitative estimation of risk. Furthermore, it can help allocate resources to control exposures to environmental hazards [27].
The Ecuadorian Amazon is an area with considerable biodiversity [28], but anthropogenic activities, mainly petroleum extraction and illegal gold mining, have caused severe environmental pollution and negative impacts on ecosystems [29,30,31,32]. Anthropogenic pollution represents a potential health hazard for the surrounding population [33]; therefore, it is necessary to establish whether contamination by PTEs in the Amazon rivers endangers river users’ health. Previous studies in the Ecuadorian Amazon were focused on assessing the impact of oil activities on human health, mainly related to drinking water, soils, and crops [34,35]. However, there is a lack of information about the health effects of the users of the Amazon Rivers polluted by multiple sources.
This study aims to: (a) estimate the human exposure risks of surface waters and sediments using deterministic and probabilistic (Monte Carlo simulations) methods, (b) identify the sensitive receptors as well as the pollutants of major health risk concern, and (c) identify the key input parameters of health risk by conducting sensitivity analysis. The results of this work provide insight into human health risk levels in the Ecuadorian Amazon. This information could help in risk management decisions to reduce anthropogenic pollution and protect public health.
2. Materials and Methods
2.1. Study Area
The area selected for the case study is located in the upper Napo River tributaries in the Napo province, Northern Ecuadorian Amazon. It corresponds to an approximate area of 200 km2. The site is characterized by an extensive hydrographic network, which flows from the west (from the Andes) to the east (Peruvian Amazon) [36]. The main tributaries and rivers in the studied area are Colonso, Tena, Misahuallí, and Napo (Figure 1). Small-scale gold mining, urban pollution, fish farming, and non-functional municipal landfill areas have been reported as the primary anthropogenic sources of pollution in the studied area [37].
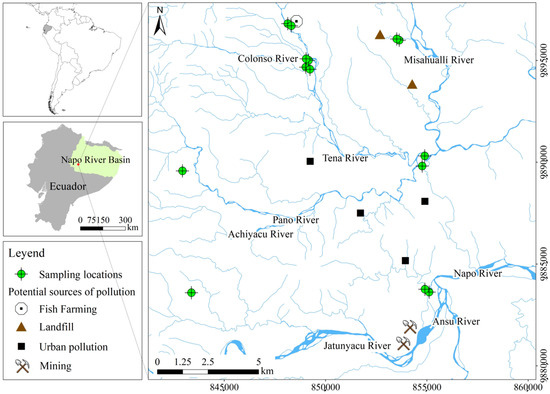
Figure 1.
Location of the study area, anthropogenic activities, and sampling locations.
2.2. Human Health Risk Assessment
2.2.1. Data Collection and Analyses
Concentrations of Al, Cd, Cr, Cu, Hg, Ni, Pb, and Zn in water samples (n = 14) and sediment samples (n = 14) reported by Capparelli et al. [37] were used in this study (Figure 1 and Table 1). Sample treatment and analytical protocols for the mentioned study can be found therein. The concentrations of PTEs were determined using inductively coupled plasma optical emission spectrometry (ICP-OES). Mercury quantification was carried out using a direct mercury Milestone (DMA 80). Quality control was conducted by employing certified reference material for every 10 samples—NIST 1640a for water and NIST 1646a for sediments [37].

Table 1.
Summary statistics of the potentially toxic elements (PTEs) concentration in surface water and sediment samples.
The data were statistically analyzed using the R free software [38]. Descriptive statistics were used to observe trends of the data. Goodness-of-fit (GoF) tests were applied to find the theoretical distributions of the data set. In addition, the Spearman rank order correlation coefficient was employed to the sensitivity analysis. The statistical significance level was set at α < 0.05. The spatial distribution of the PTEs in surface water and sediments was analyzed using the geographic information system software ArcMap 10.8.1.
2.2.2. Exposure Parameters
The human exposure to PTEs was estimated both for adults and children receptors through incidental ingestion of water, dermal contact with water, incidental ingestion of sediments, and dermal contact with sediments (during swimming/recreational activities). The chronic daily intake (CDI: mg/kg-day) was calculated according to Equations (1)–(4) proposed by the United States Environmental Protection Agency (USEPA) [21,26].
where C is the PTE concentration in water (Cw: µg/L) and sediments (Cs: mg/kg), EF is the annual exposure frequency in local rivers (days/year), IRw and IRs are the incidental ingestion rate of water (L/day) and sediments (mg/day), ET is the exposure time in local rivers (hours/day), ED is the lifetime exposure duration (years), AT is the averaging time (days), BW is the body weight (kg), SA is the skin surface area exposed (cm2), AF is the adherence factor (mg/cm2), ABS is the dermal absorption fraction (unitless), kp is the skin permeability constant (cm/hour), and CF is a conversion factor.
2.2.3. Risk Characterization
Carcinogenic and non-carcinogenic risk assessment was conducted to estimate health effects due to exposure to PTEs. The non-carcinogenic risk was quantified in terms of hazard quotients (HQs) for all the elements and exposure routes, according to Equations (5) and (6). The sum of all HQs was likewise expressed as the hazard index (HI). If HQ and HI are > 1, the recommended admissible thresholds are exceeded [39]. Potential carcinogenic health effect (CR) through incidental ingestion of water and sediments was calculated according to Equation (7). The cancer risk was assessed for Cr and Pb, which have slope factors (SForal) reported. The CR values were then summed for each exposure route, expressed as a total cancer risk (TCR), and compared to the acceptable reference values (TCR < 1 × 10−5) [19,26]. Reference dose (RfD) and slope factors (SF) were obtained from the Risk Assessment Information System (RAIS) website [40]. The toxicity values used in this study are given in Table S1. A conservative criterion was used to calculate the exposure to Cr, taking the toxicity value for Cr(VI) since this species is more harmful to health [41] and can persist in aquatic media for long periods [42]. The Hg-inorganic was selected for exposure to water [43], and methylmercury (Me-Hg) was chosen for exposure to sediments since the methylation of Hg takes place mostly on sediments [44]. Furthermore, Al, Cd-water, Cu, Ni-soluble salts, Zn and compounds, and Pb and compounds were selected for exposure to water and sediments [9,10].
2.3. Deterministic Approach
The traditional deterministic (point) approach is based on assigning a single value to each input parameter in the risk assessment model, which leads to an output of a single value of risk [45]. This method is advantageous due to its simplicity and easy understanding [46]; however, variability is not accounted for in input variables [24]. Furthermore, this method is based on a reasonable exposure situation and is relatively conservative [47]. The use point values of input parameters, as well as assumptions, could lead to an unrealistic risk estimation.
Table 2 shows the population exposure parameters and generic values for exposure factors used in the deterministic approach. The equations used to calculate the human health risk deterministically were implemented in R language. Point risk maps were generated using geographic information system software (ArcMap 10.8.1) to identify the sampling locations of major concern.
2.4. Probabilistic Approach: Monte Carlo Simulation (MCS)
Monte Carlo simulation, in which parameters are described by their distribution, is a widely used method for probabilistic risk assessment [10]. MCS employs statistical sampling techniques to obtain a full range of possible outcomes (in the form of probability distributions) [48], considering the inherent randomness and uncertainty associated with the data [24]. The MCS and other probability-based techniques to obtain a range of possible outputs from uncertain inputs have been widely used in the human health risk assessment [3,10,46] since they allow a sensitivity analysis of the input variables in the model [26].
In this study, carcinogenic and non-carcinogenic risks were estimated by applying MCS as probabilistic modeling, using Oracle Crystal Ball [8,15]. The number of 10,000 iterations (for every run) was set to obtain the probabilistic risk distributions [14].
Before the MCS was carried out, the application of GoF tests was performed to select the theoretical distribution that represents the concentration of PTEs in surface waters and sediments. The rriskDistributions package was used to identify the probability distribution that best fitted the data. The GoF was evaluated with Anderson–Darling (AD) tests and Kolmogorov–Smirnov (KS) [3,48]. The statistical significance level was set at α < 0.05. Lastly, Table 2 shows the standard distributions and values for the exposure parameters used for probabilistic assessment.
Sensitivity Analysis
Sensitivity analysis is based on the rank coefficient correlation or contribution to variance to identify the significance of the input variables to cancer and non-cancer risk estimation. It can be performed based on the outcomes of the MCS [10]. This methodology is widely used in risk management actions and decision-making to identify the main contributors to risk outcome [27]. In this study, the sensitivity analysis was estimated using the Spearman rank order correlation coefficient, which measures the strength and direction of the association between the ranks of the values (not the values themselves) of quantitative variables [26]. The sensitivity analysis was performed using 10,000 iterations and a confidence level of 95%.

Table 2.
Parameters and values used for deterministic and probabilistic assessment.
Table 2.
Parameters and values used for deterministic and probabilistic assessment.
| Parameters | Deterministic Approach | Probabilistic Approach | Reference | ||
|---|---|---|---|---|---|
| Point Estimate (RME) | Distribution | Values | |||
| EF | Exposure frequency-adults and children (day/year) | 120 | Triangular | 120 (26–260) | |
| EDa | Exposure duration-adults (year) | 30 | Lognormal | 11.36 ± 13.72 | Israeli et al. [49] |
| EDc | Exposure duration-children (year) | 6 | Uniform | 1–6 | Spence and Walden [50] |
| ET | Exposure time-adults and children (hour/event) | 2.6 | Triangular | 2.6 (0.5–6) | |
| SAa | Skin surface area (swimming)-adults (cm2) | 23,000 | Normal | 18,400 ± 2300 | Anderson et al. [51] |
| SAc | Skin surface area (swimming)-children (cm2) | 7280 | Normal | 6800 ± 600 | Carr [52]; Spence and Walden [50] |
| BWa | Body weight-adults (kg) | 70 | Normal | 72 ± 15.9 | Carr [52] |
| BWc | Body weight-children (kg) | 15 | Normal | 15.6 ± 3.7 | Anderson et al. [51] |
| IRwa | Ingestion rate of water-adults (L/event) | 0.053 | - | 0.053 | USEPA [39] |
| IRwc | Ingestion rate of water-children (L/event) | 0.090 | - | 0.090 | |
| IRsa | Ingestion rate of sediments-adults (mg/event) | 12.5 | - | 12.5 | Goldblum et al. [53] |
| IRsc | Ingestion rate of sediments-children (mg/event) | 50 | - | 50 | |
| ATnc | Averaging time non-carcinogen (day) | 365 × ED | - | 365 × ED | USEPA [21] |
| ATca | Averaging time carcinogen (day) | 365 × 70 | - | 365 × 70 | USEPA [21] |
| AFa | Adherence factor-adults (mg/cm2) | 0.07 | - | 0.07 | USEPA [21] |
| AFc | Adherence factor-children (mg/cm2) | 0.2 | - | 0.2 | USEPA [21] |
| ABS | Dermal absorption factor (unit-less) | 0.001 | - | 0.001 | Wang et al. [9]; USEPA [39] |
| Kp: | Permeability constant (cm/hour) | Al, Cd, Cr, Cu, Hg = 0.001, Pb = 0.0001, Hg, Ni = 0.0002; Zn = 0.0006 | - | Al, Cd, Cr, Cu, Hg = 0.001, Pb = 0.0001, Hg, Ni = 0.0002; Zn = 0.0006 | RAIS [40] |
RME: reasonable maximum exposure.
3. Results
The presence of hazardous elements in surface water and sediment samples showed serious human health implications. The human health risk due to exposure to PTEs was assessed both for adults and children residents. The risk outcomes by deterministic and probabilistic methods were above the safe exposure limit recommended by USEPA [21,26].
3.1. Deterministic Approach
The results estimated by the deterministic method showed unacceptable values of carcinogenic and non-carcinogenic risk. Table 3 summarizes the HI and TCR values resulting from exposure to the PTEs in different media for both age groups.

Table 3.
Deterministic hazard index (HI) and total cancer risk (TCR) from exposure to PTEs in surface waters and sediments for adults and children receptors.
Regarding exposure to polluted waters, the HI through incidental water ingestion was below 1 for adults (in the order of 10−2 and 10−5) but above 1 for dermal contact. For children, the most vulnerable receptors, the HI was almost two times higher than the recommended value through ingestion and dermal contact routes. Cd, Cr, Hg, and Pb were identified as the primary pollutants that risk human health (Figure 2). On the other hand, TCR was above the safe limit through incidental ingestion of water in almost 7% of the sampling locations, with Cr as the pollutant of major concern.
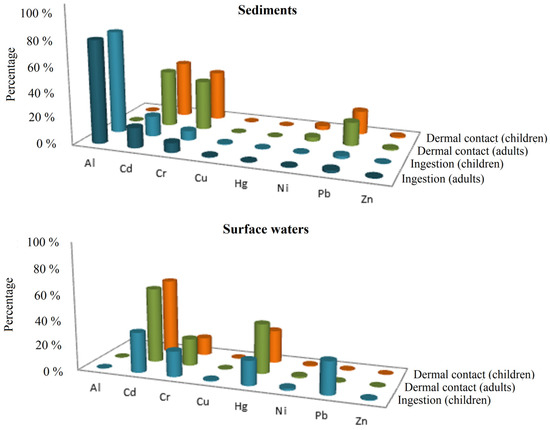
Figure 2.
PTEs and exposure routes of major concern in the hazard index (HI) outcomes estimated for surface waters and sediments.
Exposure to PTEs through the incidental ingestion of polluted sediments was the main contributor to the non-carcinogenic risk for adults and children. While Al, Cd, Cr, and Pb were the pollutants of primary concern, exposure to Cu, Hg, Ni, and Zn was negligible in all cases (HQ values were in the order of 10−1 and 10−3) (Figure 2). Regarding the TCR, the values were above the safe exposure threshold for both adults and children receptors, showing that residents were exposed to an intolerable risk level through incidental ingestion of sediments, with Cr as the main contributor to the overall risk.
Point Risk Maps
Point risk maps were generated to identify the sites of primary concern. For sediments, 100% of the sampling locations showed non-carcinogenic and carcinogenic values above the safe exposure limit (HI > 1 and TCR > 1 × 10−5) for adults and children receptors (Figure S1). Regarding exposure to polluted waters, while the HI values were below 1 in all the sampling locations for adults, one site showed a HI above 1 for children receptors. On the other hand, TCR was above the safe exposure value in one location for adults and four locations for children.
The sites that reported human health risk above the safe exposure threshold are close to mining activities and landfills (Figure 3). These results were to be expected since mining, mainly illegal, has been widely recognized as a polluting activity in the Ecuadorian Amazon [29,31,32,54,55]. Similar results were reported in the Brazilian Amazon, where discharges from gold mining activities still contribute to high concentrations of PTEs in soils, waters, and sediments [6,56,57].
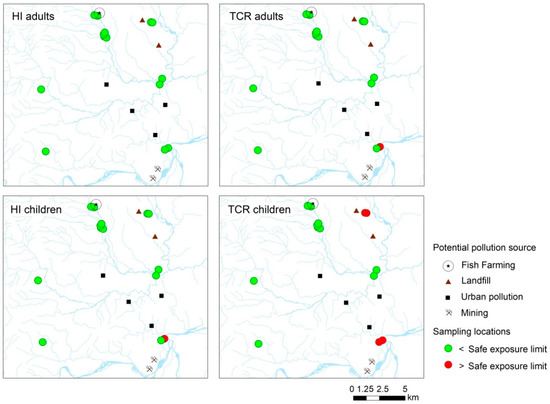
Figure 3.
Point risk map of HI and TCR for both age groups exposed to polluted surface waters.
3.2. Probabilistic Approach: Monte Carlo Simulation (MCS)
Table 4 summarizes the GoF test results of the data of PTEs concentration in surface water and sediments. The distributions representing the observed data were used in the risk models to obtain the HQ and CR for each exposure route and receptor. For Cu and Ni, given the small data set for surface waters, a point estimate value (50th percentile) was used to assess the human health risk. The 95th percentile risk result was obtained (Table 5), and the histograms of the risk assessment by each exposure media and receptor were then represented from the outcomes (Figure 4 and Figure 5).

Table 4.
Fitted distributions for PTEs concentration in surface waters and sediments.

Table 5.
Probabilistic HI and TCR (95th percentile) in surface waters and sediments for adults and children receptors.
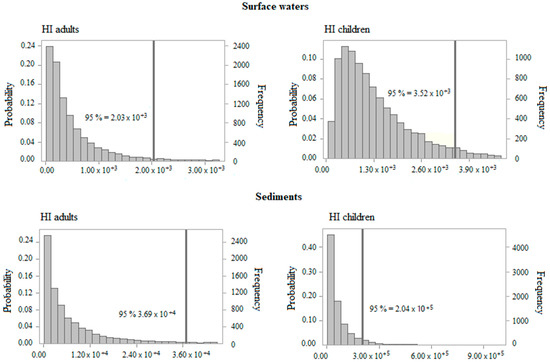
Figure 4.
Histograms of HI for adults and children for exposure to surface waters and sediments.
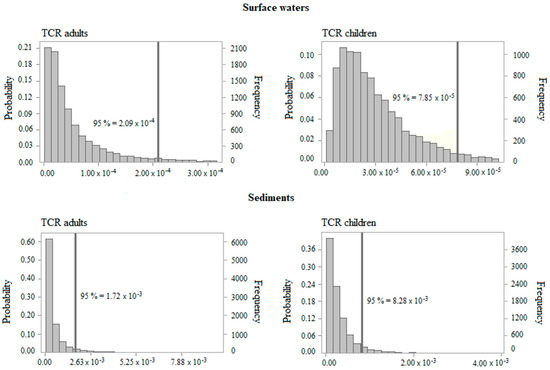
Figure 5.
Histograms of TCR for adults and children for exposure to surface waters and sediments.
3.2.1. Non-Carcinogenic Risk
The non-carcinogenic risk (HI) associated with the combined ingestion and dermal exposure to Al, Cd, Cr, Cu, Hg, Ni, Pb, and Zn exceeded the safe limit (HI > 1) for both receptors in the studied rivers. The HI for adults and children’s exposure to polluted waters was above the safe exposure limit for all the percentiles. Therefore, 100% of the receptors are exposed to an unacceptable risk level. Cadmium displayed the highest values of HQ for adults and children, followed by Cr, Hg, and Pb through incidental ingestion of water. Furthermore, this study found an important contribution of the dermal routes on HI, mainly by Cd, Cr, and Hg. The 95th percentile of HI estimated for children was almost two times higher than adults, demonstrating that children are the most vulnerable receptors (Figure 4).
Regarding exposure to sediments, the HI was greater than 1 for the 1.5th and 1th percentiles, for children and adults, respectively. These results show that almost 99% of receptors present an intolerable non-cancer risk associated with exposure to contaminated sediments. Furthermore, the results indicate that children were more susceptible to non-cancer risk than adults (Figure 4). Overall, the risk outcomes were extremely high— the HI for the 95th percentile was three orders of magnitude above 1 for both age groups exposed to polluted waters. The 95th percentile of HI estimated to sediments was between four and five orders of magnitude above the safe exposure limit for both receptors.
3.2.2. Carcinogenic Risk
The carcinogenic risk (TCR) for exposure to surface waters was above the threshold for the 15th percentile for children and 14th percentile for adults, indicating that almost 85% of exposed receptors presented a cancer risk above the recommended limit (TCR > 1 × 10−5). The 95th percentile TCR values were 2.09 × 10−4 for adults and 7.85 × 10−5 for children (Figure 5), with Cr as the main contributor to the overall cancer risk.
Regarding exposure to polluted sediments, the TCR was above the safe exposure boundary for all the percentiles. Therefore, residents living around the studied rivers are exposed to an intolerable risk level. The 95th percentile TCR values were 1.72 × 10−3 and 8.28 × 10−3 for adults and children, respectively (Figure 5). These values were several orders of magnitude greater than the recommended value. The risk outcomes showed that children were more susceptible to cancer risk from PTEs exposure. Furthermore, Cr presented the highest values of CR for adults and children through ingestion and dermal routes.
3.2.3. Sensitivity Analysis
The sensitivity analysis was performed to identify the key variables contributing significantly to cancer and non-cancer risk (Figure 6 and Figure S2). Concerning probabilistic carcinogenic and non-carcinogenic risk estimation by exposure to surface water, the sensitivity analysis showed that exposure duration (ED) presented the strongest positive effect on the risk outcome for both receptors. However, the exposure time (ET) and exposure frequency (EF) also played an important role in the risk results.
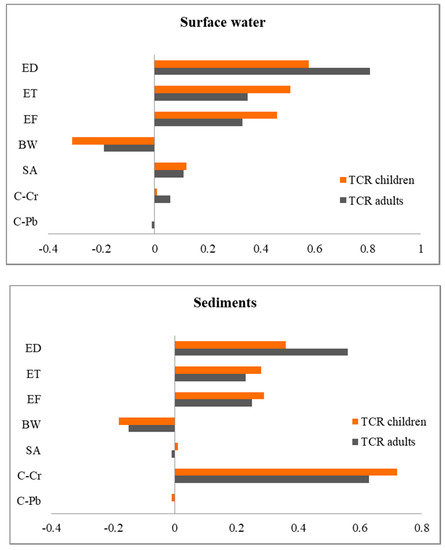
Figure 6.
Sensitivity analysis results from identifying the relative contribution of input variables on TCR for both receptors exposed to surface waters and sediments.
Regarding exposure to polluted sediments, the sensitivity analysis results showed that the concentration of PTEs and the exposure duration (ED) were the main influential variables to HI and TCR for both receptors. On the other hand, exposure time (ET) and exposure frequency (EF) had less influence on the risk outcomes.
4. Discussion
4.1. Potential Impacts of PTEs on Human Health
Rivers are severely polluted with PTEs in the study site. The high levels of PTEs are possibly generated from urban pollution, fish farming, gold mining, and municipal landfill areas without waste treatment [37]. The concentration of PTEs in surface waters from the study area were in the following order: Ni > Pb > Cr > Cu > Zn > Hg > Cd > Al, with the highest values detected close to the mining operations. On the other hand, for sediments, the concentrations of PTEs varied widely among the sampling locations following the decreasing order: Al > Zn > Cu > Cr > Ni > Pb > Cd > Hg (Figure S3). The risk outcomes showed that the sampling locations near mining areas presented the highest values of HI and TCR for exposure to surface water. Furthermore, landfills were the second pollution source related to TCR for children. For sediments, all sampling locations were above the safe exposure limit (TCR > 1 × 10−5) for the inhabitants exposed to polluted rivers. The main sources of risk were fish farming, mining, and landfills, mainly for children (Figure 7).
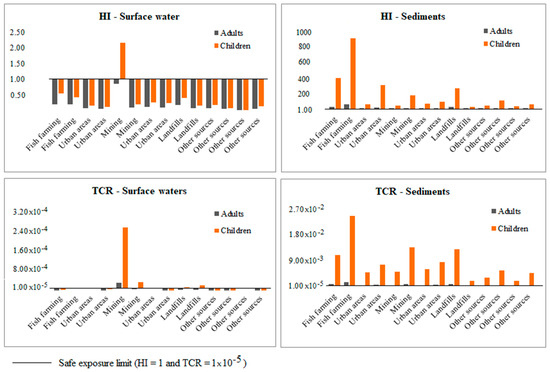
Figure 7.
Results of HI and TCR, and their relationship with the possible sources of contamination reported in the sector.
The presence of PTEs in surface waters and sediment samples showed serious human health implications for both children and adult receptors. Similar findings were found by Castilhos et al. [6] and de Souza et al. [56] in gold mining operations in the Brazilian Amazon, who identified mining pollution as a potential hazard for people who live near the mining areas. In addition, Barraza et al. [35] and Maurice et al. [34] reported HQ and CR values above the recommended thresholds due to exposure to polluted zones impacted by oil activities in the Ecuadorian Amazon. In this line, further investigations are needed to identify all potential sources of hazard for the inhabitants of the Ecuadorian Amazon.
Deterministic and Probabilistic Quantification
The overall HI and TCR values by both deterministic and probabilistic methods showed that the non-carcinogenic and carcinogenic risks were above the safe exposure limit, although the probabilistic risk outcomes were higher than the deterministic ones. For surface waters, the HI estimated by the deterministic method was 1.85 and 4.83 for adults and children, respectively. In contrast, the probabilistic HI ranged from 2.89 to 1.77 × 104 for adults and 34.91 to 10.27 × 104 for children. Regarding sediments, while the deterministic HI was 1.99 × 102 for adults and 2.66 × 103 for children, the probabilistic HI ranged from 0.1 to 62.78 × 105 for adults and 0.8 to 15.45 × 105 for children. Concerning the cancer risk, the deterministic TCR associated with water exposure was 4.31 × 10−5 and 3.42 × 10−4 for adults and children, respectively, but the probabilistic TCR ranged from 3.24 × 10−7 to 3.47 × 10−3 for adults and 1.02 × 10−6 to 4.61 × 10−4 for children. For sediments, the deterministic TCR was 5.67 × 10−3 for adults and 1.06 × 10−3 for children, but the probabilistic TCR ranged from 4.00 × 10−8 to 4.17 × 10−2 for adults and 9.30 × 10−7 to 4.09 × 10−2 for children. As expected, results showed that children are generally more exposed to PTEs than adults. Furthermore, the main contributor to the aggregate risk and total carcinogenic risk in residents was the accidental ingestion of sediments.
The advantage of probabilistic methods over deterministic ones is that the latter provides probabilistic predictions and a better understanding of the risk levels to which the receptors are exposed. The risk distributions offer information on the percentage of the population with cancer or non-cancer risks above the level of acceptability [45]. Furthermore, the sensitivity analysis performed from the probabilistic results allows for the evaluation of the significance of the input variables in the risk outcomes. In this study, the risk estimate was most sensitive to the concentration of PTEs and exposure duration (ED).
4.2. Environmental Management and Public Policy
The risk assessment is a useful instrument to give quantitative meaning to problems of environmental exposure to pollutants, and most importantly for prioritizing corrective actions [58]. In the study area, the probability of an individual developing cancer over a lifetime as a result of exposure to PTEs was higher than the acceptable levels. In this line, strategic policies on reducing exposure are needed to avoid the detrimental health effects of the residents. In addition, future studies to assess the risk of the vulnerable populations, including children and pregnant women, should be carried out to identify the occurrence of PTE-associated diseases through different exposure routes. The risk related to the ingestion of local crops and fish must be monitored, which would raise the aggregate risk figures, since some PTEs can enter the human body through the food chain [44,45].
The studies on environmental pollution and human health risk in the Amazon have focused on Hg contamination related to gold mining operations, deforestation, and damming of rivers [59]. However, the presence of PTEs such as Cd, Cr, and Pb and the human health risk through multiple exposure pathways have not been documented accordingly [56]. Many studies have reported excessive concentrations of PTEs in Ecuadorian Amazon rivers [29,31,32,54,55]. Therefore, it is very likely that these high levels of PTEs are related to the appearance of serious health problems in local populations.
The results suggest that mining activities in the area represent a potential hazard for the population since PTEs occur in gold processing areas, mainly in the informal mining sector. It is also common in many rural areas of developing countries, which rely on unskilled workers to mine and process gold. Furthermore, fish farming and landfills contributed to the HI and TCR through exposure to polluted sediments. Therefore, this study sheds light on the need for continuous environmental monitoring to identify the origin of PTEs in Amazon rivers and their effect on the health of the inhabitants. This information can support public strategies to control the quality and use of local rivers. Environmental and public health supervisory institutions should monitor the impact of anthropogenic activities in the area and adopt effective measures to decrease the risk to human health from exposure to pollutants.
5. Conclusions
This study provides preliminary information on the health risk of PTEs for adults and children, both of which are river users in the Ecuadorian Amazon. The risk outcomes by both deterministic and probabilistic methods showed that exposure in local rivers is unsafe for human health. The highest risk for adults and children was related to exposure to polluted sediments through incidental ingestion and dermal contact routes. Therefore, it is advisable to assess the bioavailable concentrations of PTEs in sediments. The risk associated with exposure to contaminated waters also was extremely high for adults and children receptors.
The sensitivity analysis identified the concentration of PTEs and exposure duration, the two most important variables for health risk calculation. While the non-cancer risk was associated with Al, Cd, Cr, Hg, and Pb exposure, Cr was the main contributor to the overall cancer risk, representing a major concern. Considering that the elements are presented as different species, with different toxicity and bioavailability, it is necessary to evaluate chemical speciation for a more robust risk assessment.
The human health risk outcomes of this study need to be further investigated. Although the exposure values taken from the literature provide valid information, population-specific parameters should be determined locally to obtain site-specific risk outcomes. Epidemiological studies are the most consistent mechanisms for public health control and intervention. However, risk assessment is a suitable instrument to estimate health harms derived from exposure to pollutants. Adequate regulatory strategies and continuous environmental monitoring could reduce the pollution in Amazon Rivers and, therefore, reduce human health risks.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4441/13/5/613/s1. Figure S1: Point risk map of HI and TCR for both age groups exposed to polluted sediments, Figure S2: Sensitivity analysis result to identify the relative contribution of input variables on HI for both receptors, Figure S3: Spatial concentration of PTEs in surface waters and sediments, Table S1: Reference doses (RfD) and slope factors (SF) for elements evaluated in the risk assessment.
Author Contributions
Conceptualization, S.J.-O.; data curation, K.E.S. and B.S.; formal analysis, S.J.-O. and K.E.S.; investigation, I.G.-G. and B.S.; methodology, S.J.-O. and M.O.; supervision, M.-J.G.-M., M.O. and D.B.; validation, K.E.S.; writing—original draft, S.J.-O. and I.G.-G.; writing—review & editing, M.-J.G.-M., M.O. and D.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pan, L.; Fang, G.; Wang, Y.; Wang, L.; Su, B.; Li, D.; Xiang, B. Potentially Toxic Element Pollution Levels and Risk Assessment of Soils and Sediments in the Upstream River, Miyun Reservoir, China. Int. J. Environ. Res. Public Health 2018, 15, 2364. [Google Scholar] [CrossRef]
- Rehman, I.U.; Ishaq, M.; Ali, L.; Muhammad, S.; Din, I.U.; Yaseen, M.; Ullah, H. Potentially toxic elements’ occurrence and risk assessment through water and soil of Chitral urban environment, Pakistan: A case study. Environ. Geochem. Health 2020, 42, 4355–4368. [Google Scholar] [CrossRef]
- Saha, N.; Rahman, M.S.; Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W. Industrial metal pollution in water and probabilistic assessment of human health risk. J. Environ. Manag. 2017, 185, 70–78. [Google Scholar] [CrossRef]
- Guzmán-Martínez, F.; Arranz-González, J.C.; Ortega, M.F.; García-Martínez, M.J.; Rodríguez-Gómez, V. A new ranking scale for assessing leaching potential pollution from abandoned mining wastes based on the Mexican official leaching test. J. Environ. Manag. 2020, 273, 111139. [Google Scholar] [CrossRef] [PubMed]
- WHO. Water Quality Drinking Water, 4th ed.; World Health Organization: Geneva, Switzerland, 2011.
- Castilhos, Z.; Rodrigues-Filho, S.; Cesar, R.; Rodrigues, A.P.; Villas-Bôas, R.; De Jesus, I.; Lima, M.; Faial, K.; Miranda, A.; Brabo, E.; et al. Human exposure and risk assessment associated with mercury contamination in artisanal gold mining areas in the Brazilian Amazon. Environ. Sci. Pollut. Res. 2015, 22, 11255–11264. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.-P.; Chen, J.-S.; Chien, Y.-C.; Chen, C.-F. Spatial analysis of the risk to human health from exposure to arsenic contaminated groundwater: A kriging approach. Sci. Total. Environ. 2018, 627, 1048–1057. [Google Scholar] [CrossRef]
- Xu, Z.; Lu, Q.; Xu, X.; Feng, X.; Liang, L.; Liu, L.; Li, C.; Chen, Z.; Qiu, G. Multi-pathway mercury health risk assessment, categorization and prioritization in an abandoned mercury mining area: A pilot study for implementation of the Minamata Convention. Chemosphere 2020, 260, 127582. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, R.; Fan, L.; Chen, T.; Bai, Y.; Yu, Q.; Liu, Y. Assessment of multiple exposure to chemical elements and health risks among residents near Huodehong lead-zinc mining area in Yunnan, Southwest China. Chemosphere 2017, 174, 613–627. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Wang, X.; Wang, R.; Liu, G. Health risk assessment of potentially harmful elements in subsidence water bodies using a Monte Carlo approach: An example from the Huainan coal mining area, China. Ecotoxicol. Environ. Saf. 2019, 171, 737–745. [Google Scholar] [CrossRef]
- IARC. Monographs on the evaluation of carcinogenic risks to humans. In International Agency for Research on Cancer; World Health Organization: Lyon, France, 1987. [Google Scholar]
- Leikin, J.B.; Paloucek, F.P. Poisoning and Toxicology Handbook, 4th ed.; Taylor & Francis Group: Boca Raton, FL, USA, 2007; ISBN 9780429195648. [Google Scholar] [CrossRef]
- Adimalla, N.; Chen, J.; Qian, H. Spatial characteristics of heavy metal contamination and potential human health risk assessment of urban soils: A case study from an urban region of South India. Ecotoxicol. Environ. Saf. 2020, 194, 110406. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Liu, J.; Wang, Q.; Hong, H.; Zhao, W.; Chen, S.; Yan, C.; Lu, H. Geochemical and probabilistic human health risk of chromium in mangrove sediments: A case study in Fujian, China. Chemosphere 2019, 233, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Gitter, A.; Mena, K.D.; Wagner, K.L.; Boellstorff, D.E.; Borel, K.E.; Gregory, L.F.; Gentry, T.J.; Karthikeyan, R. Human Health Risks Associated with Recreational Waters: Preliminary Approach of Integrating Quantitative Microbial Risk Assessment with Microbial Source Tracking. Water 2020, 12, 327. [Google Scholar] [CrossRef]
- Huang, Y.; Zuo, R.; Li, J.; Wu, J.; Zhai, Y.; Teng, Y. The Spatial and Temporal Variability of Groundwater Vulnerability and Human Health Risk in the Limin District, Harbin, China. Water 2018, 10, 686. [Google Scholar] [CrossRef]
- Dooyema, C.A.; Neri, A.; Lo, Y.-C.; Durant, J.; Dargan, P.I.; Swarthout, T.; Biya, O.; Gidado, S.O.; Haladu, S.; Sani-Gwarzo, N.; et al. Outbreak of Fatal Childhood Lead Poisoning Related to Artisanal Gold Mining in Northwestern Nigeria, 2010. Environ. Health Perspect. 2012, 120, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Chen, J.; Zhang, J.; Zhang, H.; Qiao, L.; Men, Y. Heavy metals in rice and garden vegetables and their potential health risks to inhabitants in the vicinity of an industrial zone in Jiangsu, China. J. Environ. Sci. 2010, 22, 1792–1799. [Google Scholar] [CrossRef]
- USEPA. Guidelines for Carcinogen Risk Assessment; Environmental Protection Agency: Washington, DC, USA, 2005.
- ACHHRA. Environmental Health Risk Assessment. Guidelines for assessing Human Health Risk from Environmental Hazards; Australian Centre for Human Health Risk Assessment: Melbourne, Australia, 2017. [Google Scholar]
- USEPA. Risk Assessment Guidance for Superfund Volume I: Human Health Evaluation Manual (Part E, Supplemental Guidance for Dermal Risk Assessment); Environmental Protection Agency: Washington, DC, USA, 2004.
- Singh, D.D.; Thind, P.S.; Sharma, M.; Sahoo, S.; John, S. Environmentally Sensitive Elements in Groundwater of an Industrial Town in India: Spatial Distribution and Human Health Risk. Water 2019, 11, 2350. [Google Scholar] [CrossRef]
- Castresana, G.P.; Roldán, E.C.; Suastegui, W.A.G.; Perales, J.L.M.; Montalvo, A.C.; Silva, A.H. Evaluation of Health Risks due to Heavy Metals in a Rural Population Exposed to Atoyac River Pollution in Puebla, Mexico. Water 2019, 11, 277. [Google Scholar] [CrossRef]
- Rajasekhar, B.; Nambi, I.M.; Govindarajan, S.K. Human health risk assessment for exposure to BTEXN in an urban aquifer using deterministic and probabilistic methods: A case study of Chennai city, India. Environ. Pollut. 2020, 265, 114814. [Google Scholar] [CrossRef]
- Barrio-Parra, F.; Izquierdo-Díaz, M.; Dominguez-Castillo, A.; Medina, R.; De Miguel, E. Human-health probabilistic risk assessment: The role of exposure factors in an urban garden scenario. Landsc. Urban Plan. 2019, 185, 191–199. [Google Scholar] [CrossRef]
- USEPA. Risk Assessment Guidance for Superfund: Volume III-Part A, Process for Conducting Probabilistic Risk Assessment; Environmental Protection Agency: Washington, DC, USA, 2001.
- Harris, M.J.; Stinson, J.; Landis, W.G. A Bayesian Approach to Integrated Ecological and Human Health Risk Assessment for the South River, Virginia Mercury-Contaminated Site. Risk Anal. 2017, 37, 1341–1357. [Google Scholar] [CrossRef]
- Webb, J.; Mainville, N.; Mergler, D.; Lucotte, M.; Betancourt, O.; Davidson, R.; Cueva, E.; Quizhpe, E. Mercury in Fish-eating Communities of the Andean Amazon, Napo River Valley, Ecuador. EcoHealth 2004, 1, SU59–SU71. [Google Scholar] [CrossRef]
- López-Blanco, C.; Collahuazo, L.; Torres, S.; Chinchay, L.; Ayala, D.; Benítez, P. Mercury Pollution in Soils from the Yacuambi River (Ecuadorian Amazon) as a Result of Gold Placer Mining. Bull. Environ. Contam. Toxicol. 2015, 95, 311–316. [Google Scholar] [CrossRef]
- Mainville, N.; Webb, J.; Lucotte, M.; Davidson, R.; Betancourt, O.; Cueva, E.; Mergler, D. Decrease of soil fertility and release of mercury following deforestation in the Andean Amazon, Napo River Valley, Ecuador. Sci. Total. Environ. 2006, 368, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Mora, A.; Jumbo-Flores, D.; González-Merizalde, M.; Bermeo-Flores, S.A.; Alvarez-Figueroa, P.; Mahlknecht, J.; Hernández-Antonio, A. Heavy Metal Enrichment Factors in Fluvial Sediments of an Amazonian Basin Impacted by Gold Mining. Bull. Environ. Contam. Toxicol. 2019, 102, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Requelme, M.R.; Ramos, J.; Angélica, R.; Brabo, E. Assessment of Hg-contamination in soils and stream sediments in the mineral district of Nambija, Ecuadorian Amazon (example of an impacted area affected by artisanal gold mining). Appl. Geochem. 2003, 18, 371–381. [Google Scholar] [CrossRef]
- Vargas, G.C.; Au, W.W.; Izzotti, A. Public health issues from crude-oil production in the Ecuadorian Amazon territories. Sci. Total. Environ. 2020, 719, 134647. [Google Scholar] [CrossRef] [PubMed]
- Maurice, L.; López, F.; Becerra, S.; Jamhoury, H.; Le Menach, K.; Dévier, M.-H.; Budzinski, H.; Prunier, J.; Juteau-Martineau, G.; Ochoa-Herrera, V.; et al. Drinking water quality in areas impacted by oil activities in Ecuador: Associated health risks and social perception of human exposure. Sci. Total. Environ. 2019, 690, 1203–1217. [Google Scholar] [CrossRef] [PubMed]
- Barraza, F.; Maurice, L.; Uzu, G.; Becerra, S.; López, F.; Ochoa-Herrera, V.; Ruales, J.; Schreck, E. Distribution, contents and health risk assessment of metal(loid)s in small-scale farms in the Ecuadorian Amazon: An insight into impacts of oil activities. Sci. Total. Environ. 2018, 106–120. [Google Scholar] [CrossRef] [PubMed]
- Laraque, A.; Bernal, C.; Bourrel, L.; Darrozes, J.; Christophoul, F.; Armijos, E.; Fraizy, P.; Pombosa, R.; Guyot, J.L. Sediment budget of the Napo River, Amazon basin, Ecuador and Peru. Hydrol. Process. 2009, 23, 3509–3524. [Google Scholar] [CrossRef]
- Capparelli, M.V.; Moulatlet, G.M.; Abessa, D.M.D.S.; Lucas-Solis, O.; Rosero, B.; Galarza, E.; Tuba, D.; Carpintero, N.; Ochoa-Herrera, V.; Cipriani-Avila, I. An integrative approach to identify the impacts of multiple metal contamination sources on the Eastern Andean foothills of the Ecuadorian Amazonia. Sci. Total. Environ. 2020, 709, 136088. [Google Scholar] [CrossRef]
- R Core Team: A language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020.
- USEPA. Exposure Factors Handbook: 2011 Edition; Environmental Protection Agency: Washington, DC, USA, 2011.
- Risk Assessment Information System (RAIS), Environmental Protection Agency. Available online: https://rais.ornl.gov/ (accessed on 12 October 2020).
- USEPA. Toxicological Review of Hexavalent Chromium; Environmental Protection Agency: Washington, DC, USA, 1998.
- EFSA. Scientific Opinion on the Risks to Public Health Related to the Presence of Chromium in Food and Drinking Water; European Food Safety Authority: Parma, Italy, 2014. [Google Scholar]
- Alfonso, M.; Ferreira, L.; Durán, R. El Mercurio Como Contaminante Ambiental y Agente Neurotóxico; Universidade de Vigo: Vigo, España, 2010; ISBN 978-84-8158-500-1. [Google Scholar]
- EFSA. Scientific Opinion on the risk for Public Health Related to the Presence of Mercury and Methylmercury in Food; European Food Safety Authority: Parma, Italy, 2012. [Google Scholar]
- Jiménez-Oyola, S.; García-Martínez, M.-J.; Ortega, M.F.; Bolonio, D.; Rodríguez, C.; Esbrí, J.-M.; Llamas, J.F.; Higueras, P.; Oyola, S.J. Multi-pathway human exposure risk assessment using Bayesian modeling at the historically largest mercury mining district. Ecotoxicol. Environ. Saf. 2020, 201, 110833. [Google Scholar] [CrossRef] [PubMed]
- Kaur, L.; Rishi, M.S.; Siddiqui, A.U. Deterministic and probabilistic health risk assessment techniques to evaluate non-carcinogenic human health risk (NHHR) due to fluoride and nitrate in groundwater of Panipat, Haryana, India. Environ. Pollut. 2020, 259, 113711. [Google Scholar] [CrossRef] [PubMed]
- Tong, R.; Cheng, M.; Zhang, L.; Liu, M.; Yang, X.; Li, X.; Yin, W. The construction dust-induced occupational health risk using Monte-Carlo simulation. J. Clean. Prod. 2018, 184, 598–608. [Google Scholar] [CrossRef]
- Low, K.H.; Zain, S.M.; Abas, M.R.; Salleh, K.M.; Teo, Y.Y. Distribution and health risk assessment of trace metals in freshwater tilapia from three different aquaculture sites in Jelebu Region (Malaysia). Food Chem. 2015, 177, 390–396. [Google Scholar] [CrossRef]
- Israeli, M.; Nelson, C.B. Distribution and Expected Time of Residence for U.S. Households. Risk Anal. 1992, 12, 65–72. [Google Scholar] [CrossRef]
- Spence, L.; Walden, T. RISC4 User’s Manual: Cambridge, UK. 2001. Available online: https://www.groundwatersoftware.com/risc.htm (accessed on 24 February 2021).
- Anderson, E.; Browne, N.; Dulestky, S.; Raming, J.; Warn, T. Development an Statistical Distributions or Ranges of Standard Factos Used in Exposure Assessments, EPA/600/8-85/010; Environmental Protection Agency: Washington, DC, USA, 1985. [Google Scholar]
- Carr, C. American Industrial Health Council: Exposure Factors Sourcebook. Regul. Toxicol. Pharmacol. 1994, 20, 212. [Google Scholar] [CrossRef]
- Goldblum, D.K.; Rak, A.; Ponnapalli, M.D.; Clayton, C.J. The Fort Totten mercury pollution risk assessment: A case history. J. Hazard. Mater. 2006, 136, 406–417. [Google Scholar] [CrossRef]
- Appleton, J.D.; Williams, T.M.; Orbea, H.; Carrasco, M. Fluvial Contamination Associated with Artisanal Gold Mining in the Ponce Enríquez, Portovelo-Zaruma and Nambija Areas, Ecuador. Water Air Soil Pollut. 2001, 131, 19–39. [Google Scholar] [CrossRef]
- González-Merizalde, M.V.; Menezes-Filho, J.A.; Cruz-Erazo, C.T.; Bermeo-Flores, S.A.; Sánchez-Castillo, M.O.; Hernández-Bonilla, D.; Mora, A. Manganese and Mercury Levels in Water, Sediments, and Children Living Near Gold-Mining Areas of the Nangaritza River Basin, Ecuadorian Amazon. Arch. Environ. Contam. Toxicol. 2016, 71, 171–182. [Google Scholar] [CrossRef]
- De Souza, E.S.; Texeira, R.A.; Da Costa, H.S.C.; Oliveira, F.J.; Melo, L.C.A.; Faial, K.D.C.F.; Fernandes, A.R. Assessment of risk to human health from simultaneous exposure to multiple contaminants in an artisanal gold mine in Serra Pelada, Pará, Brazil. Sci. Total. Environ. 2017, 576, 683–695. [Google Scholar] [CrossRef]
- Bonotto, D.M.; Wijesiri, B.; Vergotti, M.; Da Silveira, E.G.; Goonetilleke, A. Assessing mercury pollution in Amazon River tributaries using a Bayesian Network approach. Ecotoxicol. Environ. Saf. 2018, 166, 354–358. [Google Scholar] [CrossRef]
- De Miguel, E.; Clavijo, D.; Ortega, M.F.; Gómez, A. Probabilistic meta-analysis of risk from the exposure to Hg in artisanal gold mining communities in Colombia. Chemosphere 2014, 108, 183–189. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gochfeld, M. Cases of mercury exposure, bioavailability, and absorption. Ecotoxicol. Environ. Saf. 2003, 56, 174–179. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).







