Adsorption of Malachite Green Dye onto Mesoporous Natural Inorganic Clays: Their Equilibrium Isotherm and Kinetics Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Pretreatment of NICs Samples
2.3. Characterization of NICs
2.4. Batch Adsorption Studies
3. Result and Discussion
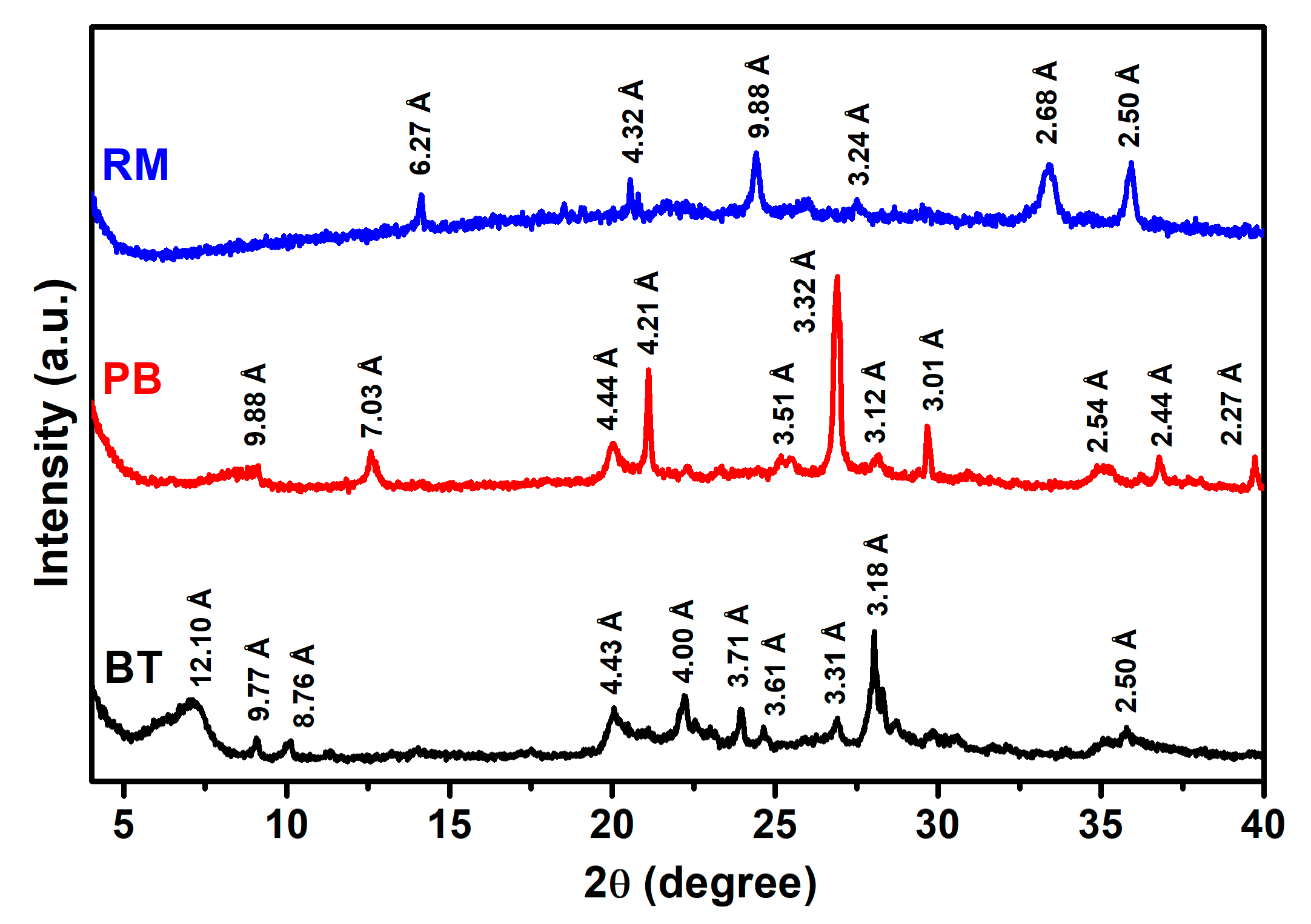
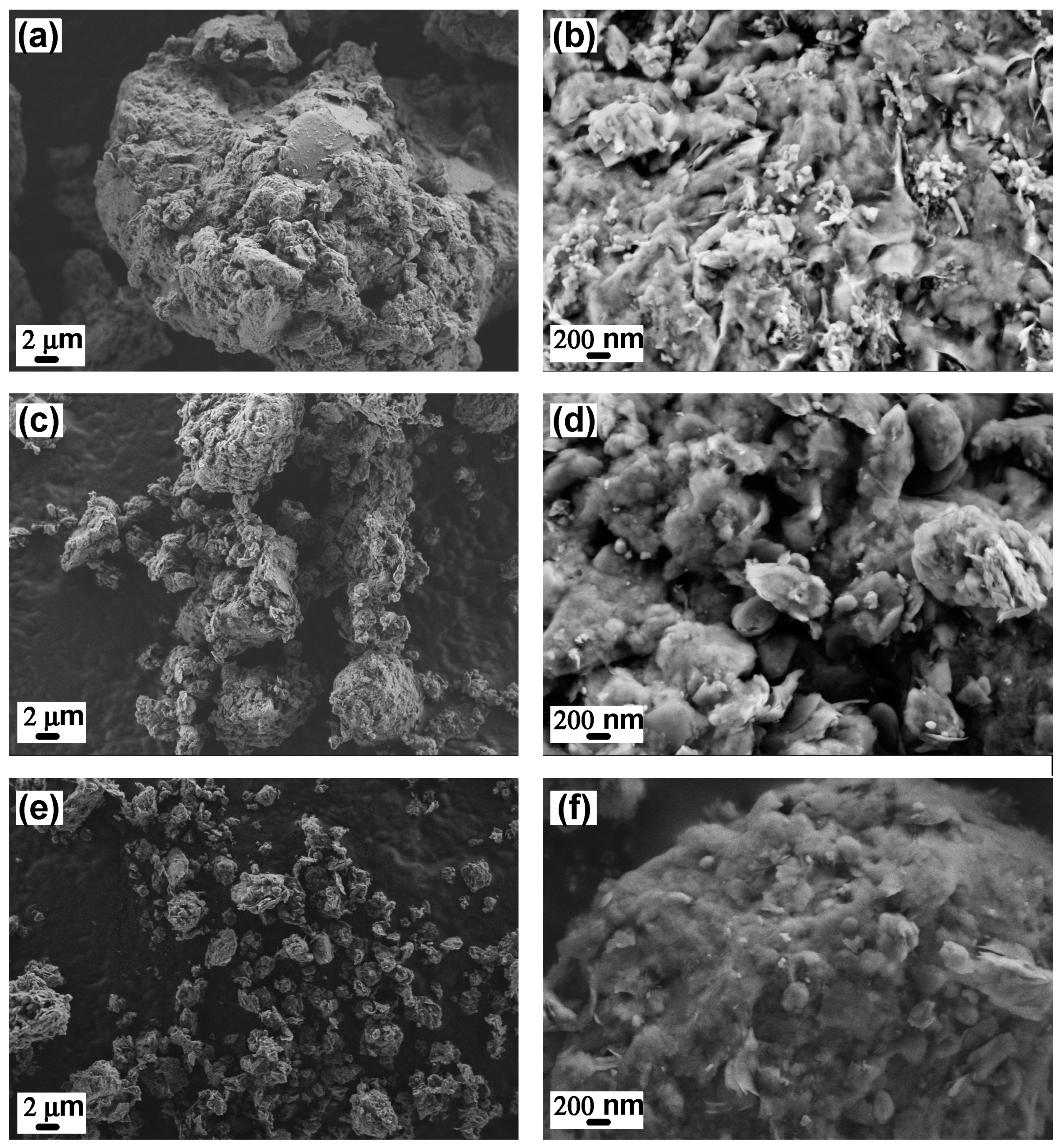
3.1. Characterization of NICs
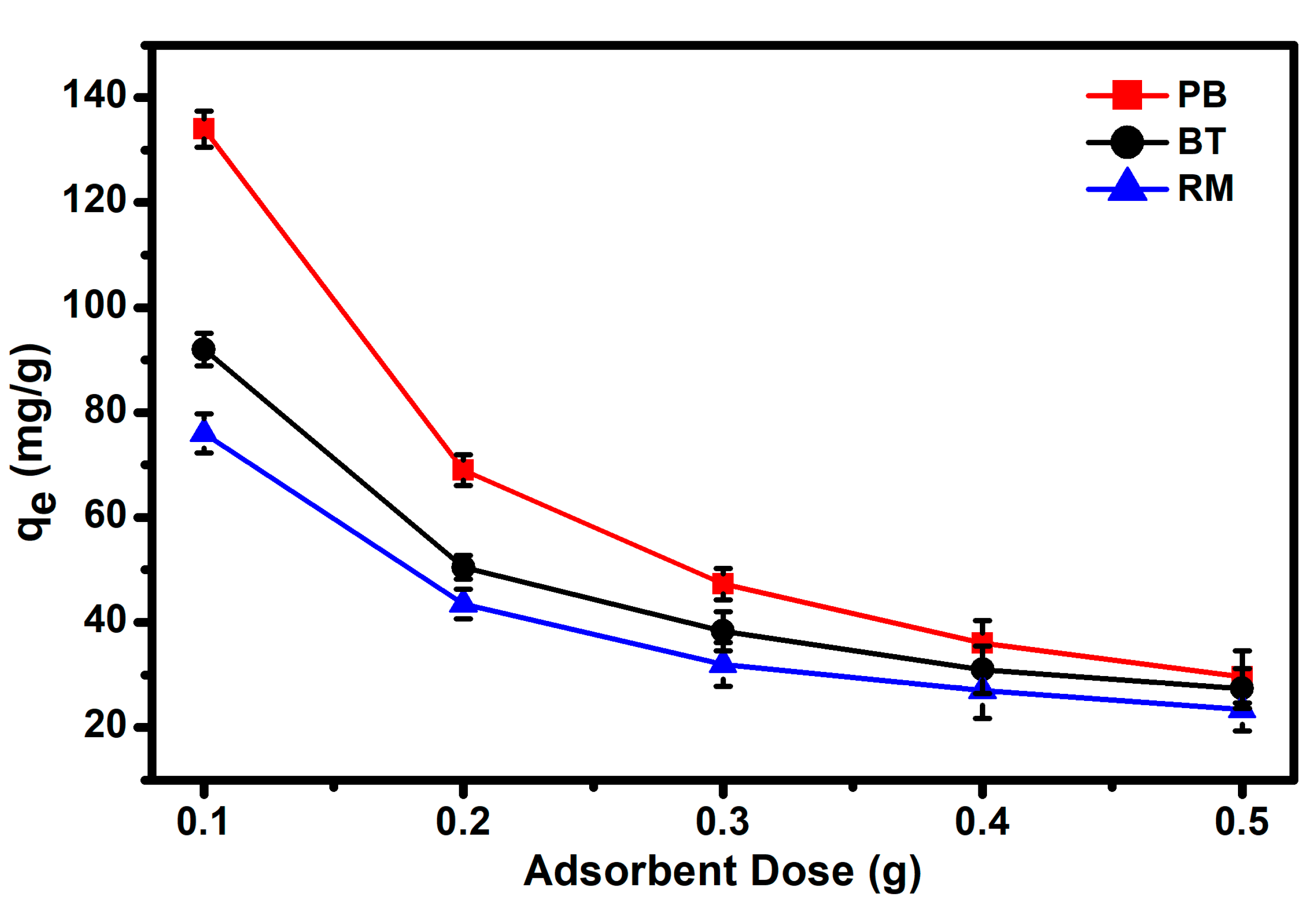
3.2. Adsorption Studies of MG Dye
3.3. Adsorption Kinetics
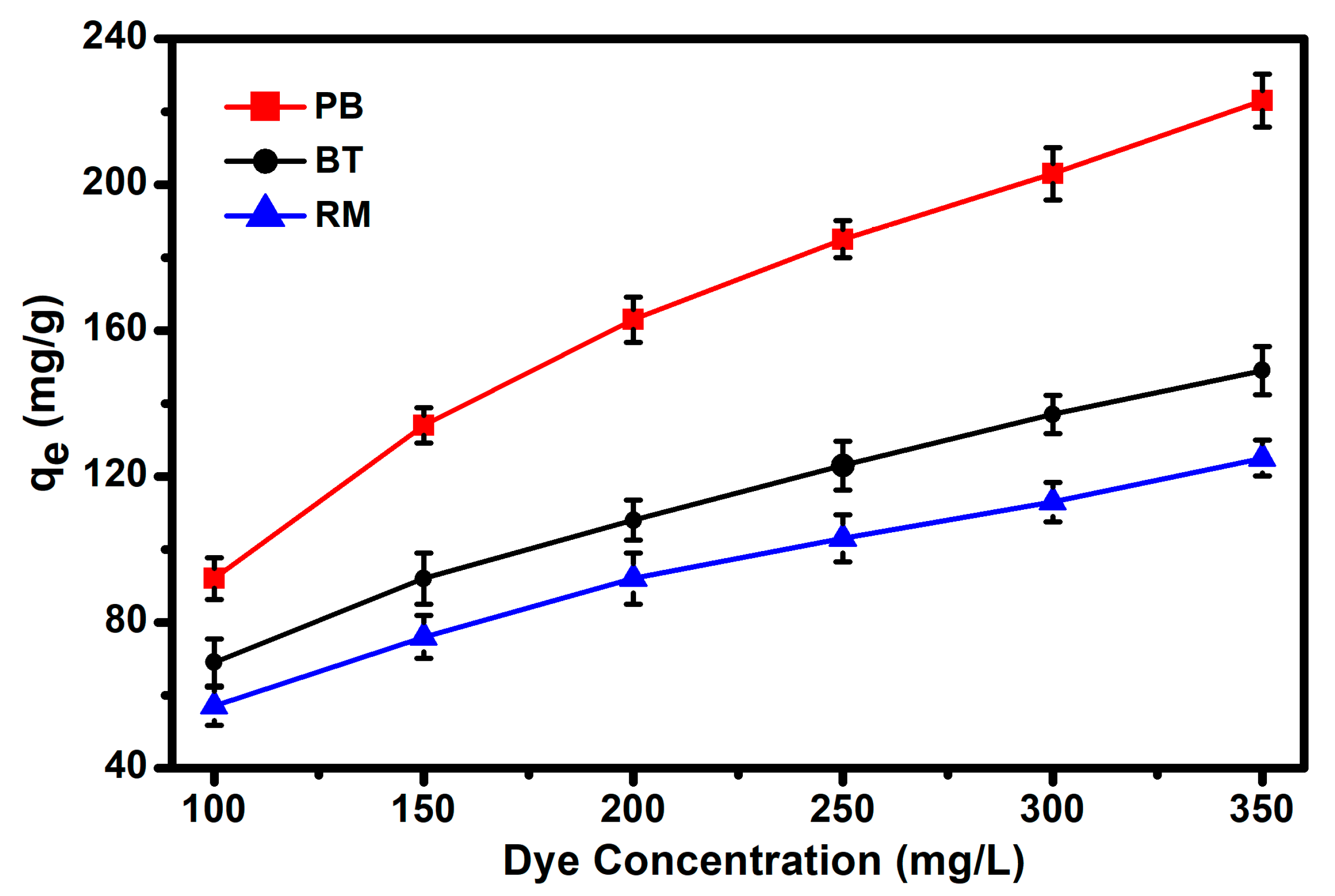
3.4. Adsorption Isotherm
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gamoudi, S.; Srasra, E. Adsorption of organic dyes by HDPy+-modified clay: Effect of molecular structure on the adsorption. J. Mol. Struct. 2019, 1193, 522–531. [Google Scholar] [CrossRef]
- Garg, D.; Kumar, S.; Sharma, K.; Majumder, C. Application of waste peanut shells to form activated carbon and its utilization for the removal of Acid Yellow 36 from wastewater. Groundw. Sustain. Dev. 2019, 8, 512–519. [Google Scholar] [CrossRef]
- Rashid, A.; Khan, S.; Ayub, M.; Sardar, T.; Jehan, S.; Zahir, S.; Khan, M.S.; Muhammad, J.; Khan, R.; Ali, A. Mapping human health risk from exposure to potential toxic metal contamination in groundwater of Lower Dir, Pakistan: Application of multivariate and geographical information system. Chemosphere 2019, 225, 785–795. [Google Scholar] [CrossRef]
- Amarasooriya, A.; Kawakami, T. Removal of fluoride, hardness and alkalinity from groundwater by electrolysis. Groundw. Sustain. Dev. 2019, 9, 100231. [Google Scholar] [CrossRef]
- Adebayo, T.B.; Abegunrin, T.P.; Awe, G.O.; Are, K.S.; Guo, H.; Onofua, O.E.; Adegbola, G.A.; Ojediran, J.O. Geospatial mapping and suitability classification of groundwater quality for agriculture and domestic uses in a Precambrian basement complex. Groundw. Sustain. Dev. 2021, 12, 100497. [Google Scholar] [CrossRef]
- Ahmed, M.; Mashkoor, F.; Nasar, A. Development, characterization, and utilization of magnetized orange peel waste as a novel adsorbent for the confiscation of crystal violet dye from aqueous solution. Groundw. Sustain. Dev. 2020, 10, 100322. [Google Scholar] [CrossRef]
- Priya, K.; Aswin, K.; Indu, M.; Adarsh, S. Assessment of hydrogeochemical processes in the aquifers of Coimbatore city, India with special reference to nickel contamination. Groundw. Sustain. Dev. 2020, 11, 100393. [Google Scholar] [CrossRef]
- Bolisetty, S.; Peydayesh, M.; Mezzenga, R. Sustainable technologies for water purification from heavy metals: Review and analysis. Chem. Soc. Rev. 2019, 48, 463–487. [Google Scholar] [CrossRef] [PubMed]
- Pillai, P.; Dharaskar, S.; Shah, M.; Sultania, R. Determination of fluoride removal using silica nano adsorbent modified by rice husk from water. Groundw. Sustain. Dev. 2020, 11, 100423. [Google Scholar] [CrossRef]
- Hou, M.-F.; Ma, C.-X.; Zhang, W.-D.; Tang, X.-Y.; Fan, Y.-N.; Wan, H.-F. Removal of rhodamine B using iron-pillared bentonite. J. Hazard. Mater. 2011, 186, 1118–1123. [Google Scholar] [CrossRef]
- Chu, H.; Liu, X.; Liu, B.; Zhu, G.; Lei, W.; Du, H.; Liu, J.; Li, J.; Li, C.; Sun, C. Hexagonal 2H-MoSe 2 broad spectrum active photocatalyst for Cr (VI) reduction. Sci. Rep. 2016, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Saeed, M.; Munir, M.; Nafees, M.; Shah, S.S.A.; Ullah, H.; Waseem, A. Synthesis, characterization and applications of silylation based grafted bentonites for the removal of Sudan dyes: Isothermal, kinetic and thermodynamic studies. Microporous Mesoporous Mater. 2020, 291, 109697. [Google Scholar] [CrossRef]
- Sarma, G.K.; Gupta, S.S.; Bhattacharyya, K.G. RETRACTED: Adsorption of Crystal Violet on Raw and Acid-Treated Montmorillonite, K10, in Aqueous Suspension; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Hou, H.; Zhou, R.; Wu, P.; Wu, L. Removal of Congo red dye from aqueous solution with hydroxyapatite/chitosan composite. Chem. Eng. J. 2012, 211, 336–342. [Google Scholar] [CrossRef]
- Tsai, W.; Chang, C.; Lin, M.; Chien, S.; Sun, H.; Hsieh, M. Adsorption of acid dye onto activated carbons prepared from agricultural waste bagasse by ZnCl2 activation. Chemosphere 2001, 45, 51–58. [Google Scholar] [CrossRef]
- Raghu, M.; Kumar, K.Y.; Prashanth, M.; Prasanna, B.; Vinuth, R.; Kumar, C.P. Adsorption and antimicrobial studies of chemically bonded magnetic graphene oxide-Fe3O4 nanocomposite for water purification. J. Water Process Eng. 2017, 17, 22–31. [Google Scholar] [CrossRef]
- Crini, G.; Peindy, H.N.; Gimbert, F.; Robert, C. Removal of CI Basic Green 4 (Malachite Green) from aqueous solutions by adsorption using cyclodextrin-based adsorbent: Kinetic and equilibrium studies. Sep. Purif. Technol. 2007, 53, 97–110. [Google Scholar] [CrossRef]
- Tsai, W.-T.; Chen, H.-R. Removal of malachite green from aqueous solution using low-cost chlorella-based biomass. J. Hazard. Mater. 2010, 175, 844–849. [Google Scholar] [CrossRef] [PubMed]
- Saha, P.; Chowdhury, S.; Gupta, S.; Kumar, I.; Kumar, R. Assessment on the removal of malachite green using tamarind fruit shell as biosorbent. Clean Soil Air Water 2010, 38, 437–445. [Google Scholar] [CrossRef]
- Srivastava, S.; Sinha, R.; Roy, D. Toxicological effects of malachite green. Aquat. Toxicol. 2004, 66, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.; Saha, P. Sea shell powder as a new adsorbent to remove Basic Green 4 (Malachite Green) from aqueous solutions: Equilibrium, kinetic and thermodynamic studies. Chem. Eng. J. 2010, 164, 168–177. [Google Scholar] [CrossRef]
- Nethaji, S.; Sivasamy, A.; Thennarasu, G.; Saravanan, S. Adsorption of Malachite Green dye onto activated carbon derived from Borassus aethiopum flower biomass. J. Hazard. Mater. 2010, 181, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Song, Y.; Zhou, L. Facile synthesis of polyamidoamine dendrimer gel with multiple amine groups as a super adsorbent for highly efficient and selective removal of anionic dyes. J. Colloid Interface Sci. 2019, 546, 351–360. [Google Scholar] [CrossRef]
- Kheirabadi, M.; Samadi, M.; Asadian, E.; Zhou, Y.; Dong, C.; Zhang, J.; Moshfegh, A.Z. Well-designed Ag/ZnO/3D graphene structure for dye removal: Adsorption, photocatalysis and physical separation capabilities. J. Colloid Interface Sci. 2019, 537, 66–78. [Google Scholar] [CrossRef]
- Yuan, Y.-J.; Chen, D.; Yu, Z.-T.; Zou, Z.-G. Cadmium sulfide-based nanomaterials for photocatalytic hydrogen production. J. Mater. Chem. A 2018, 6, 11606–11630. [Google Scholar] [CrossRef]
- Fu, J.; Yu, J.; Jiang, C.; Cheng, B. g-C3N4-Based heterostructured photocatalysts. Adv. Energy Mater. 2018, 8, 1701503. [Google Scholar] [CrossRef]
- SMoghaddam, S.; Moghaddam, M.A.; Arami, M. Coagulation/flocculation process for dye removal using sludge from water treatment plant: Optimization through response surface methodology. J. Hazard. Mater. 2010, 175, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhao, Y.; Moutinho, J.; Shao, J.; Zydney, A.L.; He, Y. Recovery of small dye molecules from aqueous solutions using charged ultrafiltration membranes. J. Hazard. Mater. 2015, 284, 58–64. [Google Scholar] [CrossRef]
- Ikhlaq, A.; Brown, D.R.; Kasprzyk-Hordern, B. Catalytic ozonation for the removal of organic contaminants in water on alumina. Appl. Catal. B Environ. 2015, 165, 408–418. [Google Scholar] [CrossRef]
- Jayanthi, S.; Eswar, N.K.; Singh, S.A.; Chatterjee, K.; Madras, G.; Sood, A. Macroporous three-dimensional graphene oxide foams for dye adsorption and antibacterial applications. RSC Adv. 2016, 6, 1231–1242. [Google Scholar] [CrossRef]
- Bentahar, S.; Dbik, A.; el Khomri, M.; el Messaoudi, N.; Lacherai, A. Adsorption of methylene blue, crystal violet and congo red from binary and ternary systems with natural clay: Kinetic, isotherm, and thermodynamic. J. Environ. Chem. Eng. 2017, 5, 5921–5932. [Google Scholar] [CrossRef]
- Basaleh, A.A.; Al-Malack, M.H.; Saleh, T.A. Methylene Blue removal using polyamide-vermiculite nanocomposites: Kinetics, equilibrium and thermodynamic study. J. Environ. Chem. Eng. 2019, 7, 103107. [Google Scholar] [CrossRef]
- Alkan, M.; Doğan, M.; Turhan, Y.; Demirbaş, Ö.; Turan, P. Adsorption kinetics and mechanism of maxilon blue 5G dye on sepiolite from aqueous solutions. Chem. Eng. J. 2008, 139, 213–223. [Google Scholar] [CrossRef]
- Chakraborty, S.; Chowdhury, S.; Saha, P.D. Adsorption of crystal violet from aqueous solution onto sugarcane bagasse: Central composite design for optimization of process variables. J. Water Reuse Desalin. 2012, 2, 55–65. [Google Scholar] [CrossRef]
- Mittal, A.; Mittal, J.; Malviya, A.; Kaur, D.; Gupta, V. Adsorption of hazardous dye crystal violet from wastewater by waste materials. J. Colloid Interface Sci. 2010, 343, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhao, J.; Zhong, A.; Jin, Y. Removal capacity and adsorption mechanism of heat-treated palygorskite clay for methylene blue. Chem. Eng. J. 2011, 174, 143–150. [Google Scholar] [CrossRef]
- De Queiroga, L.N.F.; Franca, D.B.; Rodrigues, F.; Santos, I.M.; Fonseca, M.G.; Jaber, M. Functionalized bentonites for dye adsorption: Depollution and production of new pigments. J. Environ. Chem. Eng. 2019, 7, 103333. [Google Scholar] [CrossRef]
- Vimonses, V.; Lei, S.; Jin, B.; Chow, C.W.; Saint, C. Kinetic study and equilibrium isotherm analysis of Congo Red adsorption by clay materials. Chem. Eng. J. 2009, 148, 354–364. [Google Scholar] [CrossRef]
- Olusegun, S.J.; Lima, L.F.d.; Mohallem, N.D.S. Enhancement of adsorption capacity of clay through spray drying and surface modification process for wastewater treatment. Chem. Eng. J. 2018, 334, 1719–1728. [Google Scholar] [CrossRef]
- Munir, M.; Nazar, M.F.; Zafar, M.N.; Zubair, M.; Ashfaq, M.; Hosseini-Bandegharaei, A.; Khan, S.U.-D.; Ahmad, A. Effective Adsorptive Removal of Methylene Blue from Water by Didodecyldimethylammonium Bromide-Modified Brown Clay. ACS Omega 2020, 5, 16711–16721. [Google Scholar] [CrossRef]
- Brito, D.F.; Filho, E.C.d.; Fonseca, M.G.; Jaber, M. Organophilic bentonites obtained by microwave heating as adsorbents for anionic dyes. J. Environ. Chem. Eng. 2018, 6, 7080–7090. [Google Scholar] [CrossRef]
- Javed, S.H.; Zahir, A.; Khan, A.; Afzal, S.; Mansha, M. Adsorption of Mordant Red 73 dye on acid activated bentonite: Kinetics and thermodynamic study. J. Mol. Liq. 2018, 254, 398–405. [Google Scholar] [CrossRef]
- Elmoubarki, R.; Mahjoubi, F.; Tounsadi, H.; Moustadraf, J.; Abdennouri, M.; Zouhri, A.; el Albani, A.; Barka, N. Adsorption of textile dyes on raw and decanted Moroccan clays: Kinetics, equilibrium and thermodynamics. Water Resour. Ind. 2015, 9, 16–29. [Google Scholar] [CrossRef]
- Chaari, I.; Fakhfakh, E.; Medhioub, M.; Jamoussi, F. Comparative study on adsorption of cationic and anionic dyes by smectite rich natural clays. J. Mol. Struct. 2019, 1179, 672–677. [Google Scholar] [CrossRef]
- Agatzini-Leonardou, S.; Oustadakis, P.; Tsakiridis, P.; Markopoulos, C. Titanium leaching from red mud by diluted sulfuric acid at atmospheric pressure. J. Hazard. Mater. 2008, 157, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Makhoukhi, B.; Djab, M.; Didi, M.A. Adsorption of Telon dyes onto bis-imidazolium modified bentonite in aqueous solutions. J. Environ. Chem. Eng. 2015, 3, 1384–1392. [Google Scholar] [CrossRef]
- Madejová, J. FTIR techniques in clay mineral studies. Vib. Spectrosc. 2003, 31, 1–10. [Google Scholar] [CrossRef]
- Toor, M.; Jin, B.; Dai, S.; Vimonses, V. Activating natural bentonite as a cost-effective adsorbent for removal of Congo-red in wastewater. J. Ind. Eng. Chem. 2015, 21, 653–661. [Google Scholar] [CrossRef]
- Jawad, A.H.; Abdulhameed, A.S. Mesoporous Iraqi red kaolin clay as an efficient adsorbent for methylene blue dye: Adsorption kinetic, isotherm and mechanism study. Surf. Interfaces 2020, 18, 100422. [Google Scholar] [CrossRef]
- Hassanien, M.M.; Abou-El-Sherbini, K.S.; Al-Muaikel, N.S. Immobilization of methylene blue onto bentonite and its application in the extraction of mercury (II). J. Hazard. Mater. 2010, 178, 94–100. [Google Scholar] [CrossRef]
- Ahmadi, A.; Foroutan, R.; Esmaeili, H.; Tamjidi, S. The role of bentonite clay and bentonite clay@ MnFe2O4 composite and their physico-chemical properties on the removal of Cr (III) and Cr (VI) from aqueous media. Environ. Sci. Pollut. Res. 2020, 27, 1–14. [Google Scholar] [CrossRef]
- el Ouardi, M.; Laabd, M.; Oualid, H.A.; Brahmi, Y.; Abaamrane, A.; Elouahli, A.; Addi, A.A.; Laknifli, A. Efficient removal of p-nitrophenol from water using montmorillonite clay: Insights into the adsorption mechanism, process optimization, and regeneration. Environ. Sci. Pollut. Res. 2019, 26, 19615–19631. [Google Scholar] [CrossRef] [PubMed]
- Marrakchi, F.; Khanday, W.; Asif, M.; Hameed, B. Cross-linked chitosan/sepiolite composite for the adsorption of methylene blue and reactive orange 16. Int. J. Biol. Macromol. 2016, 93, 1231–1239. [Google Scholar] [CrossRef]
- Bentahar, S.; Dbik, A.; el Khomri, M.; el Messaoudi, N.; Lacherai, A. Removal of a cationic dye from aqueous solution by natural clay. Groundw. Sustain. Dev. 2018, 6, 255–262. [Google Scholar] [CrossRef]
- Ullah, H.; Nafees, M.; Iqbal, F.; Awan, S.; Shah, A.; Waseem, A. Adsorption Kinetics of Malachite green and Methylene blue from aqueous solutions using surfactant-modified Organoclays. Acta Chim. Slov. 2017, 64, 449–460. [Google Scholar] [CrossRef] [PubMed]
- Doğan, M.; Özdemir, Y.; Alkan, M. Adsorption kinetics and mechanism of cationic methyl violet and methylene blue dyes onto sepiolite. Dye. Pigment. 2007, 75, 701–713. [Google Scholar] [CrossRef]
- Munagapati, V.S.; Yarramuthi, V.; Nadavala, S.K.; Alla, S.R.; Abburi, K. Biosorption of Cu (II), Cd (II) and Pb (II) by Acacia leucocephala bark powder: Kinetics, equilibrium and thermodynamics. Chem. Eng. J. 2010, 157, 357–365. [Google Scholar] [CrossRef]
- Fil, B.A. Isotherm, kinetic, and thermodynamic studies on the adsorption behavior of malachite green dye onto montmorillonite clay. Part. Sci. Technol. 2016, 34, 118–126. [Google Scholar] [CrossRef]
- Ghanizadeh, G.; Asgari, G. Adsorption kinetics and isotherm of methylene blue and its removal from aqueous solution using bone charcoal. React. Kinet. Mech. Catal. 2011, 102, 127–142. [Google Scholar] [CrossRef]
- Rehman, M.S.U.; Munir, M.; Ashfaq, M.; Rashid, N.; Nazar, M.F.; Danish, M.; Han, J.-I. Adsorption of Brilliant Green dye from aqueous solution onto red clay. Chem. Eng. J. 2013, 228, 54–62. [Google Scholar] [CrossRef]
- Karim, A.B.; Mounir, B.; Hachkar, M.; Bakasse, M.; Yaacoubi, A. Removal of Basic Red 46 dye from aqueous solution by adsorption onto Moroccan clay. J. Hazard. Mater. 2009, 168, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Bhatti, H.N.; Zaman, Q.; Kausar, A.; Noreen, S.; Iqbal, M. Efficient remediation of Zr (IV) using citrus peel waste biomass: Kinetic, equilibrium and thermodynamic studies. Ecol. Eng. 2016, 95, 216–228. [Google Scholar] [CrossRef]
- Ullah, R.; Iftikhar, F.J.; Ajmal, M.; Shah, A.; Akhter, M.S.; Ullah, H.; Waseem, A. Modified clays as an efficient adsorbent for brilliant green, ethyl violet and allura red dyes: Kinetic and thermodynamic Studies. Pol. J. Environ. Stud. 2020, 29, 3831–3839. [Google Scholar] [CrossRef]
- Tehrani-Bagha, A.; Nikkar, H.; Mahmoodi, N.; Markazi, M.; Menger, F. The sorption of cationic dyes onto kaolin: Kinetic, isotherm and thermodynamic studies. Desalination 2011, 266, 274–280. [Google Scholar] [CrossRef]
- Saha, P.; Chowdhury, S.; Gupta, S.; Kumar, I. Insight into adsorption equilibrium, kinetics and thermodynamics of Malachite Green onto clayey soil of Indian origin. Chem. Eng. J. 2010, 165, 874–882. [Google Scholar] [CrossRef]
- Tian, L.; Zhang, J.; Shi, H.; Li, N.; Ping, Q. Adsorption of malachite green by diatomite: Equilibrium isotherms and kinetic studies. J. Dispers. Sci. Technol. 2016, 37, 1059–1066. [Google Scholar] [CrossRef]
- Hameed, B.; El-Khaiary, M. Malachite green adsorption by rattan sawdust: Isotherm, kinetic and mechanism modeling. J. Hazard. Mater. 2008, 159, 574–579. [Google Scholar] [CrossRef]
- Sartape, A.S.; Mandhare, A.M.; Jadhav, V.V.; Raut, P.D.; Anuse, M.A.; Kolekar, S.S. Removal of malachite green dye from aqueous solution with adsorption technique using Limonia acidissima (wood apple) shell as low cost adsorbent. Arab. J. Chem. 2017, 10, S3229–S3238. [Google Scholar] [CrossRef]
- Dahri, M.K.; Kooh, M.R.R.; Lim, L.B. Water remediation using low cost adsorbent walnut shell for removal of malachite green: Equilibrium, kinetics, thermodynamic and regeneration studies. J. Environ. Chem. Eng. 2014, 2, 1434–1444. [Google Scholar] [CrossRef]
- Chowdhury, S.; Das, P. Mechanistic, kinetic, and thermodynamic evaluation of adsorption of hazardous malachite green onto conch shell powder. Sep. Sci. Technol. 2011, 46, 1966–1976. [Google Scholar] [CrossRef]









| Parameter | Chemical Composition (%) | ||
|---|---|---|---|
| PB Clay | BT Clay | RM Clay | |
| Al2O3 | 56.3 | 60.7 | 18.7 |
| SiO2 | 18 | 16.4 | 15.3 |
| Fe2O3 | 10.5 | 5.54 | 44.34 |
| CaO | 4.41 | 4.68 | 1.36 |
| K2O | 3.51 | 1.1 | 0.38 |
| MgO | 3.1 | 3.4 | 0.47 |
| Na2O | 1.6 | 6.8 | 12 |
| TiO2 | 1.21 | 0.63 | 6.27 |
| Parameter | Unit | PB Clay | BT Clay | RM Clay |
|---|---|---|---|---|
| Surface Area | m2/g | 115.99 | 38.306 | 16.796 |
| Pore Volume | cm3/g | 0.1527 | 0.0711 | 0.0656 |
| Pore Size | nm | 9.6055 | 19.168 | 25.834 |
| Adsorbent | Pseudo-First Order | Pseudo-Second Order | ||||||
|---|---|---|---|---|---|---|---|---|
| Dye | q(exp) (mg/g) | qe (calc) (mg/g) | K1 (min−1) | R2 | qe (calc) (mg/g) | K2 (mg/g) | R2 | |
| PB clay | MG | 134 | 105.22 | 0.01645 | 0.935 | 147.06 | 0.007 | 0.975 |
| BT clay | MG | 92 | 89.74 | 0.01641 | 0.928 | 113.63 | 0.009 | 0.953 |
| RM clay | MG | 76 | 67.70 | 0.01460 | 0.996 | 95.24 | 0.010 | 0.992 |
| Adsorbent | Langmuir | Freundlich | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Dye | q(exp) (mg/g) | qm (mg/g) | KL (L/mg) | R2 | RL | nF | KF (mg/g) | R2 | |
| PB clay | MG | 223 | 243.90 | 0.064 | 0.994 | 0.135 | 3.373 | 53.83 | 0.968 |
| BT clay | MG | 149 | 188.68 | 0.016 | 0.990 | 0.382 | 2.462 | 17.30 | 0.998 |
| RM clay | MG | 125 | 172.41 | 0.011 | 0.993 | 0.481 | 2.153 | 10.15 | 0.995 |
| Adsorbent | Isotherm | qm (mg/g) | References |
|---|---|---|---|
| Kaolin | Langmuir | 52 | [64] |
| Clayey soil | Langmuir | 78.57 | [65] |
| Diatomite | Langmuir | 23.64 | [66] |
| Rattan sawdust | Langmuir | 62.71 | [67] |
| Wood apple shell | Langmuir | 34.56 | [68] |
| Walnut shell | Langmuir | 90.8 | [69] |
| Conch shell powder | Langmuir | 92.25 | [70] |
| Sea shell powder | Langmuir | 42.33 | [21] |
| PB clay | Langmuir | 223 | This study |
| BT clay | Langmuir | 149 | This study |
| RM clay | Langmuir | 125 | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ullah, S.; Ur Rahman, A.; Ullah, F.; Rashid, A.; Arshad, T.; Viglašová, E.; Galamboš, M.; Mahmoodi, N.M.; Ullah, H. Adsorption of Malachite Green Dye onto Mesoporous Natural Inorganic Clays: Their Equilibrium Isotherm and Kinetics Studies. Water 2021, 13, 965. https://doi.org/10.3390/w13070965
Ullah S, Ur Rahman A, Ullah F, Rashid A, Arshad T, Viglašová E, Galamboš M, Mahmoodi NM, Ullah H. Adsorption of Malachite Green Dye onto Mesoporous Natural Inorganic Clays: Their Equilibrium Isotherm and Kinetics Studies. Water. 2021; 13(7):965. https://doi.org/10.3390/w13070965
Chicago/Turabian StyleUllah, Sami, Altaf Ur Rahman, Fida Ullah, Abdur Rashid, Tausif Arshad, Eva Viglašová, Michal Galamboš, Niyaz Mohammad Mahmoodi, and Haseeb Ullah. 2021. "Adsorption of Malachite Green Dye onto Mesoporous Natural Inorganic Clays: Their Equilibrium Isotherm and Kinetics Studies" Water 13, no. 7: 965. https://doi.org/10.3390/w13070965
APA StyleUllah, S., Ur Rahman, A., Ullah, F., Rashid, A., Arshad, T., Viglašová, E., Galamboš, M., Mahmoodi, N. M., & Ullah, H. (2021). Adsorption of Malachite Green Dye onto Mesoporous Natural Inorganic Clays: Their Equilibrium Isotherm and Kinetics Studies. Water, 13(7), 965. https://doi.org/10.3390/w13070965









