Removal of Cationic Organic Dye from Aqueous Solution by Chemical and Pyrolysis Activated Ulva lactuca
Abstract
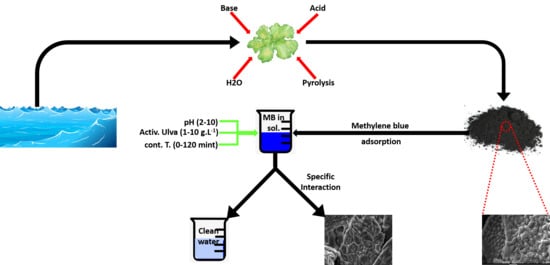
:1. Introduction
2. Materials and Methods
2.1. Adsorbate
2.2. Adsorbents
2.3. Surface Modification of Algal Biomass
2.4. Characterization of the Modified and Nonmodified Algae
2.4.1. Determination of the Specific Surface Area
2.4.2. FTIR Analysis
2.4.3. Morphological Characteristics of the U. lactuca Surface
2.5. Study of the Biosorption Process
2.6. Kinetic Study of Biosorption
2.7. Biosorption Isotherm
3. Results and Discussion
3.1. Specific Surface Area of Algal Biomass
3.2. Functional Group Alterations Before and After Biosorption
3.3. SEM Analysis
3.4. Contact Time
3.5. Effect of Adsorbent Dose
3.6. Effect of pH on the Biosorption Process
3.7. Biosorption Dynamics Kinetics
3.7.1. Model of the Pseudo-First-Order Kinetics (Lagergren Model)
3.7.2. Model of the Pseudo-Second-Order Kinetics
3.7.3. Intraparticle Diffusion Model
3.8. Elovich’s Model
3.9. Adsorption Isotherm
| Adsorbents | Adsorption Capacity (mg.g−1) | References |
|---|---|---|
| Ulva lactuca (UL-OH) | 625.0 | Present study |
| Poly(methacrylic acid) modified biomass of baker’s yeast | 869.6 | [84] |
| Poly(amic acid) modified biomass of baker’s yeast | 680.3 | [85] |
| Caulerpa lentillifera | 417.0 | [86] |
| Alga Sargassum muticum seaweed | 279.2 | [9] |
| Enteromorpha spp. | 274.0 | [87] |
| Activated sludge biomass | 256.4 | [88] |
| Dead macrofungi (Fomes fomentarius) | 232.7 | [89] |
| Dead macrofungi (Phellinus igniarius) | 204.4 | [89] |
| Hydrilla verticillata | 198.0 | [90] |
| Moss | 185.0 | [5] |
| Algae Gelidium | 171.0 | [91] |
| Duckweed (Spirodela polyrrhiza) (at pH 9) | 144.9 | [92] |
| Water hyacinth root | 128.9 | [5] |
| Duckweed (Spirodela polyrrhiza) (at pH 7) | 119.0 | [92] |
| Algal waste | 104.0 | [91] |
| Composite material | 74.0 | [91] |
| Unmodified biomass of baker’s yeast | 51.5 | [84,85] |
| Green alga Ulva lactuca | 40.2 | [27] |
| Brown alga Cystoseira barbatula Kutzing | 38.6 | [93] |
| Dead Streptomyces rimosus | 34.3 | [94] |
| Dead fungus Aspergillus niger | 18.5 | [95] |
| Posidonia oceanica (L.) fibres | 5.6 | [87] |
| Caulerpa racemosa var. cylindracea | 5.2 | [96] |
| Living biomass | 1.2 | [95] |
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kannan, N.; Sundaram, M.M. Kinetics and mechanism of removal of methylene blue by adsorption on various carbons—A comparative study. Dye. Pigment. 2001, 51, 25–40. [Google Scholar] [CrossRef]
- Sarma, G.K.; SenGupta, S.; Bhattacharyya, K.G. Methylene Blue Adsorption on Natural and Modified Clays. Sep. Sci. Technol. 2011, 46, 1602–1614. [Google Scholar] [CrossRef]
- Hao, O.J.K.; Kim, H.; Chaing, P.C. Decolorization of wastewater. Critical Reviews. Environ. Sci. Technol. 2000, 30, 449–505. [Google Scholar] [CrossRef]
- Gong, R.; Ding, Y.; Li, M.; Yang, C.; Liu, H.; Sun, Y. Utilization of powdered peanut hull as biosorbent for removal of anionic dyes from aqueous solution. Dye. Pigment. 2005, 64, 187–192. [Google Scholar] [CrossRef]
- Low, K.S.; Lee, C.K.; Tan, K.K. Biosorption of basic dyes by water hyacinth roots. Bioresour. Technol. 1995, 52, 79–83. [Google Scholar] [CrossRef]
- Lee, C.K.; Low, K.S.; Chung, L.C. Removal of Some Organic Dyes by Hexane-Extracted Spent Bleaching Earth. J. Chem. Technol. Biotechnol. 1997, 69, 93–99. [Google Scholar] [CrossRef]
- Banat, F.; Al-Asheh, S.; Al-Makhadmeh, L. Evaluation of the use of raw and activated date pits as potential adsorbents for dye containing waters. Process. Biochem. 2003, 39, 193–202. [Google Scholar] [CrossRef]
- Ab Rahman, I.; Saad, B. Utilization of Guava Seeds as a Source of Activated Carbon for Removal of Methylene Blue from Aqueous Solution. Malays. J. Chem. 2003, 5, 008–014. [Google Scholar]
- Rubin, E.; Rodriguez, P.; Herrero, R.; Cremades, J.; Barbara, I.; Vicente, M.E.S.D. Removal of Methylene Blue from aqueous solutions using as biosorbentSargassum muticum: An invasive macroalga in Europe. J. Chem. Technol. Biotechnol. 2005, 80, 291–298. [Google Scholar] [CrossRef] [Green Version]
- Rajeshwarisivaraj; Subburam, V. Activated parthenium carbon as an adsorbent for the removal of dyes and heavy metal ions from aqueous solution. Bioresour. Technol. 2002, 85, 205–206. [Google Scholar] [CrossRef]
- Fu, Y.; Viraraghavan, T. Fungal decolorization of dye wastewaters: A review. Bioresour. Technol. 2001, 79, 251–262. [Google Scholar] [CrossRef]
- Rashid, N.; Rehman, M.S.U.; Han, J.-I. Recycling and reuse of spent microalgal biomass for sustainable biofuels. Biochem. Eng. J. 2013, 75, 101–107. [Google Scholar] [CrossRef]
- Ponnusamy, S.K.; Pavithra, J.; Suriya, S.; Ramesh, M.; Kumar, K. Sargassum wightii, a marine alga is the source for the production of algal oil, bio-oil, and application in the dye wastewater treatment. Desalin. Water Treat. 2014, 55, 1–17. [Google Scholar]
- Abbas, M.N.; Al-Hermizy, S.M.M.; Abudi, Z.N.; Ibrahim, T.A. Phenol Biosorption from Polluted Aqueous Solutions by Ulva Lactuca Alga Using Batch Mode Unit. J. Ecol. Eng. 2019, 20, 225–235. [Google Scholar] [CrossRef]
- Alsufyani, T.; Engelen, A.H.; Diekmann, O.E.; Kuegler, S.; Wichard, T. Prevalence and mechanism of polyunsaturated aldehydes production in the green tide forming macroalgal genus Ulva (Ulvales, Chlorophyta). Chem. Phys. Lipids 2014, 183, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, W.M.; Hassan, A.F.; Azab, Y.A. Biosorption of toxic heavy metals from aqueous solution by Ulva lactuca activated carbon. Egypt. J. Basic Appl. Sci. 2016, 3, 241–249. [Google Scholar] [CrossRef] [Green Version]
- Wong, M.H.; Kwok, T.T.; Ho, K.C. Heavy metals inUlva lactuca collected within Tolo Harbour, an almost landlocked sea. Aquat. Ecol. 1982, 16, 223–230. [Google Scholar] [CrossRef]
- Mourad, F.A.; El-Azim, H.A. Use of green alga Ulva lactuca (L.) as an indicator to heavy metal pollution at intertidal waters in Suez Gulf, Aqaba Gulf and Suez Canal, Egypt. Egypt. J. Aquat. Biol. Fish. 2019, 23, 437–449. [Google Scholar] [CrossRef] [Green Version]
- Salima, A.; Benaouda, B.; Noureddine, B.; Duclaux, L. Application of Ulva lactuca and Systoceira stricta algae-based activated carbons to hazardous cationic dyes removal from industrial effluents. Water Res. 2013, 47, 3375–3388. [Google Scholar] [CrossRef] [PubMed]
- Wong, Y.; Szeto, Y.; Cheung, W.; McKay, G. Adsorption of acid dyes on chitosan—Equilibrium isotherm analyses. Process. Biochem. 2004, 39, 695–704. [Google Scholar] [CrossRef]
- Tan, I.; Ahmad, A.; Hameed, B. Adsorption of basic dye on high-surface-area activated carbon prepared from coconut husk: Equilibrium, kinetic and thermodynamic studies. J. Hazard. Mater. 2008, 154, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Nazem, M.A.; Zare, M.H.; Shirazian, S. Preparation and optimization of activated nano-carbon production using physical activation by water steam from agricultural wastes. RSC Adv. 2020, 10, 1463–1475. [Google Scholar] [CrossRef] [Green Version]
- Trabucco, A.; Marquez, F. Structure of the Glomerular Tuft. J. Urol. 1952, 67, 235–255. [Google Scholar] [CrossRef]
- Nahil, M.A.; Williams, P.T. Pore characteristics of activated carbons from the phosphoric acid chemical activation of cotton stalks. Biomass- Bioenergy 2012, 37, 142–149. [Google Scholar] [CrossRef]
- van Oss, C.J. A review of: “Active Carbon.” R.C. Bansal, J.B. Donnet and F. Stoeckli; Marcel Dekker, New York, 1988. pp. 482, $135. J. Dispers. Sci. Technol. 1990, 11, 323. [Google Scholar] [CrossRef]
- El Nemr, A.; Shoaib, A.G.M.; El Sikaily, A.; Mohamed, A.E.-D.A.; Hassan, A.F. Evaluation of Cationic Methylene Blue Dye Removal by High Surface Area Mesoporous Activated Carbon Derived from Ulva lactuca. Environ. Process. 2021, 8, 311–332. [Google Scholar] [CrossRef]
- El Sikaily, A.; Khaled, A.; El Nemr, A.; Abdelwahab, O. Removal of Methylene Blue from aqueous solution by marine green algaUlva lactuca. Chem. Ecol. 2006, 22, 149–157. [Google Scholar] [CrossRef]
- Pratiwi, D.; Prasetyo, D.J.; Poeloengasih, C.D. Adsorption of Methylene Blue dye using Marine algae Ulva lactuca. IOP Conf. Ser. Earth Environ. Sci. 2019, 251, 012012. [Google Scholar] [CrossRef] [Green Version]
- Zeb, J.; Sultan, M.; Tahir, H. Removal of basic dye methylene blue by using bioabsorbents Ulva lactuca and Sargassum. Afr. J. Biotechnol. 2008, 7, 2649–2655. [Google Scholar]
- Makeswari, M.; Santhi, T.; Ezhilarasi, M.R. Adsorption of methylene blue dye by citric acid modified leaves of Ricinus communis from aqueous solutions. J. Chem. Pharm. Res. 2016, 8, 452–462. [Google Scholar]
- Cazetta, A.L.; Vargas, A.M.M.; Nogami, E.M.; Kunita, M.H.; Guilherme, M.R.; Martins, A.C.; Silva, T.L.; Moraes, J.C.G.; Almeida, V.C. NaOH-activated carbon of high surface area produced from coconut shell: Kinetics and equilibrium studies from the methylene blue adsorption. Chem. Eng. J. 2011, 174, 117–125. [Google Scholar] [CrossRef]
- Sultan, H.; Ahmed, N.; Mubashir, M.; Danish, S. Chemical production of acidified activated carbon and its influences on soil fertility comparative to thermo-pyrolyzed biochar. Sci. Rep. 2020, 10, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Tseng, R.-L. Mesopore control of high surface area NaOH-activated carbon. J. Colloid Interface Sci. 2006, 303, 494–502. [Google Scholar] [CrossRef]
- Wahlström, N.; Edlund, U.; Pavia, H.; Toth, G.; Jaworski, A.; Pell, A.J.; Choong, F.X.; Shirani, H.; Nilsson, K.P.R.; Richter-Dahlfors, A. Cellulose from the green macroalgae Ulva lactuca: Isolation, characterization, optotracing, and production of cellulose nanofibrils. Cellulose 2020, 27, 3707–3725. [Google Scholar] [CrossRef] [Green Version]
- Mayeko, A.K.K.; Vesituluta, P.N.; Di Phanzu, J.N.; Muanda, D.M.W.; Bakambo, G.E.; Lopaka, B.I.; Mulangala, J.M. Adsorption de la quinine bichlorhydrate sur un charbon actif peu coûteux à base de la Bagasse de canne à sucre imprégnée de l’acide phosphorique. Int. J. Biol. Chem. Sci. 2012, 6. [Google Scholar] [CrossRef]
- Adamson, W. Physical Chemistry of Surfaces, 4th ed.; John Wiley & Sons: Hoboken, NJ, USA, 1982; p. 373. [Google Scholar]
- El-Jamal, M.M.; Ncibi, M.C. Biosorption of methylene blue by chaetophora elegans algae: Kinetics, equilibrium and thermodynamic studies. Acta Chim. Slov. 2012, 59, 24–31. [Google Scholar] [PubMed]
- Lagergren, S. Zur theorie der sogenannten adsorption geloster stoffe. Kungliga Svenska Vetenskapsakademiens. Handlingar 1898, 24, 1–39. [Google Scholar]
- Ho, Y.S. Adsorption of Heavy Metals from Waste Streams by Peat; University of Birmingham: Birmingham, UK, 1995. [Google Scholar]
- Weber, W.J.; Morris, J.C. Kinetics of adsorption carbon from solutions. J. Sanit. Eng. Div. 1963, 89, 31–60. [Google Scholar] [CrossRef]
- Chien, S.H.; Clayton, W.R. Application of Elovich Equation to the Kinetics of Phosphate Release and Sorption in Soils. Soil Sci. Soc. Am. J. 1980, 44, 265–268. [Google Scholar] [CrossRef]
- Hamdaoui, O. Batch study of liquid-phase adsorption of methylene blue using cedar sawdust and crushed brick. J. Hazard. Mater. 2006, 135, 264–273. [Google Scholar] [CrossRef]
- Weber, T.W.; Chakravorti, R.K. Pore and solid diffusion models for fixed-bed adsorbers. AIChE J. 1974, 20, 228–238. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J.; Zheng, Y.; Wang, A. Adsorption of methylene blue by kapok fiber treated by sodium chlorite optimized with response surface methodology. Chem. Eng. J. 2012, 184, 248–255. [Google Scholar] [CrossRef]
- El-Sikaily, A.; El Nemr, A.; Khaled, A.; Abdelwehab, O. Removal of toxic chromium from wastewater using green alga Ulva lactuca and its activated carbon. J. Hazard. Mater. 2007, 148, 216–228. [Google Scholar] [CrossRef]
- Tchuifon, D.R.T. Biosorption of amaranth red in aqueous solution onto treated and untreated lignocellulosic materials (pineapple peelings and coconut shells). J. Mater. Environ. Sci. 2017, 8, 4199–4212. [Google Scholar]
- Liang, S.; Guo, X.; Feng, N.; Tian, Q. Isotherms, kinetics and thermodynamic studies of adsorption of Cu2+ from aqueous solutions by Mg2+/K+ type orange peel adsorbents. J. Hazard. Mater. 2010, 174, 756–762. [Google Scholar] [CrossRef]
- M’Sakni, N.H.; Majdoub, H.; Roudesli, S.; Picton, L.; Le Cerf, D.; Rihouey, C.; Morvan, C. Composition, structure and solution properties of polysaccharides extracted from leaves of Mesembryanthenum crystallinum. Eur. Polym. J. 2006, 42, 786–795. [Google Scholar] [CrossRef]
- Al-Qodah, Z.; Al-Shannag, M.; Amro, A.; Assirey, E.; Bob, M.; Bani-Melhem, K.; Alkasrawi, M. Impact of surface modification of green algal biomass by phosphorylation on the removal of copper(II) ions from water. Turk. J. Chem. 2017, 41, 190–208. [Google Scholar] [CrossRef]
- Bartošová, A.; Blinová, L.; Gerulová, K. Characterisation Of Polysacharides And Lipids From Selected Green Algae Species By FTIR-ATR Spectroscopy. Res. Pap. Fac. Mater. Sci. Technol. Slovak Univ. Technol. 2015, 23, 97–102. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Fan, S.; Li, Y. Removal Behavior of Methylene Blue from Aqueous Solution by Tea Waste: Kinetics, Isotherms and Mechanism. Int. J. Environ. Res. Public Heal. 2018, 15, 1321. [Google Scholar] [CrossRef] [Green Version]
- Xia, Y.; Yao, Q.; Zhang, W.; Zhang, Y.; Zhao, M. Comparative adsorption of methylene blue by magnetic baker’s yeast and EDTAD-modified magnetic baker’s yeast: Equilibrium and kinetic study. Arab. J. Chem. 2019, 12, 2448–2456. [Google Scholar] [CrossRef] [Green Version]
- Rahmawati, F.; Ridassepri, A.F.; Chairunnisa; Wijayanta, A.T.; Nakabayashi, K.; Miyawaki, J.; Miyazaki, T. Carbon from Bagasse Activated with Water Vapor and Its Adsorption Performance for Methylene Blue. Appl. Sci. 2021, 11, 678. [Google Scholar] [CrossRef]
- Ijagbemi, C.O.; Chun, J.I.; Han, D.H.; Cho, H.Y.; O, S.J.; Kim, D.S. Methylene Blue adsorption from aqueous solution by activated carbon: Effect of acidic and alkaline solution treatments. J. Environ. Sci. Heal Part A 2010, 45, 958–967. [Google Scholar] [CrossRef] [PubMed]
- Jawad, A.H.; Ngoh, Y.S.; Radzun, K.A. Utilization of watermelon (Citrullus lanatus) rinds as a natural low-cost biosorbent for adsorption of methylene blue: Kinetic, equilibrium and thermodynamic studies. J. Taibah Univ. Sci. 2018, 12, 371–381. [Google Scholar] [CrossRef] [Green Version]
- Jawad, A.H.; AbdulHameed, A.S.; Mastuli, M.S. Acid-factionalized biomass material for methylene blue dye removal: A comprehensive adsorption and mechanism study. J. Taibah Univ. Sci. 2020, 14, 305–313. [Google Scholar] [CrossRef] [Green Version]
- Annadurai, G.; Juang, R.-S.; Lee, D.-J. Use of cellulose-based wastes for adsorption of dyes from aqueous solutions. J. Hazard. Mater. 2002, 92, 263–274. [Google Scholar] [CrossRef]
- Seghier, A.; Hadjel, H.; Benderdouche, N. Sorption of Methylene Blue Dye from Aqueous Solution Using an Agricultural Waste. Trends Green Chem. 2017, 3. [Google Scholar] [CrossRef]
- Deokar, R. Enteromorpha Intestinalis/: Low Cost Biosorbents for Biosorption Methylene Blue. Int. J. Recent Sci. Res. 2016, 7, 9291–9297. [Google Scholar]
- Trabelsi, L.; M’Sakni, N.H.; Ouada, H.B.; Bacha, H.; Roudesli, S. Partial characterization of extracellular polysaccharides produced by cyanobacterium Arthrospira platensis. Biotechnol. Bioprocess Eng. 2009, 14, 27–31. [Google Scholar] [CrossRef]
- Ovchinnikov, O.; Evtukhova, A.V.; Kondratenko, T.; Smirnov, M.S.; Khokhlov, V.; Erina, O. Manifestation of intermo-lecular interactions in FTIR spectra of methylene blue molecules. Vib. Spectrosc. 2016, 86, 181–189. [Google Scholar] [CrossRef]
- Abou, O.; Abdellaoui, H.Y.; Laabd, M.; El Ouardi, M.; Brahmi, Y.; Iazza, M.; Abou Oualid, J. Eco-Efficient Green Seaweed Codium decorticatum Biosorbent for Textile Dyes: Characterization, Mechanism, Recyclability, and RSM Optimization. ACS Omega 2020, 5, 22192–22207. [Google Scholar] [CrossRef]
- Deokar, R. Biosorption of Methylene Blue and Malachite Green From Binary Solution onto Ulva lactuca. Int. Jour. Microbiol. Appl. Sci. 2014, 3, 295–304. [Google Scholar]
- Karim, A.; Mounir, B.; Hachkar, M.; Bakasse, M.; Yaacoubi, A. Élimination du colorant basique « Bleu de Méthylène » en solution aqueuse par l’argile de Safi. Rev. Sci. Eau 2010, 23, 375. [Google Scholar]
- Gupta, V.; Mittal, A.; Gajbe, V. Adsorption and desorption studies of a water soluble dye, Quinoline Yellow, using waste materials. J. Colloid Interface Sci. 2005, 284, 89–98. [Google Scholar] [CrossRef]
- Tsai, W.-T.; Hsu, H.-C.; Su, T.-Y.; Lin, K.-Y.; Lin, C.-M.; Dai, T.-H. The adsorption of cationic dye from aqueous solution onto acid-activated andesite. J. Hazard. Mater. 2007, 147, 1056–1062. [Google Scholar] [CrossRef] [PubMed]
- Bennani Karim, A.; Mounir, B.; Hachkar, M.; Bakasse, M.; Rais, Z.; Yaacoubi, A. Dynamic adsorption of BR46 dye and raw textile effluent on Moroccan clay to solve the drought problem. J. Water Sci. Environ. Technol. 2017, 2, 2508–9250. [Google Scholar]
- Namasivayam, C.; Muniasamy, N.; Gayatri, K.; Rani, M.; Ranganathan, K. Removal of dyes from aqueous solutions by cellulosic waste orange peel. Bioresour. Technol. 1996, 57, 37–43. [Google Scholar] [CrossRef]
- Uddin, M.T.; Rahman, M.A.; Rukanuzzaman, M.; Islam, M.A. A potential low cost adsorbent for the removal of cationic dyes from aqueous solutions. Appl. Water Sci. 2017, 7, 2831–2842. [Google Scholar] [CrossRef]
- Bilal, M.; Rasheed, T.; Sosa-Hernández, J.E.; Raza, A.; Nabeel, F.; Iqbal, H.M.N. Biosorption: An Interplay between Marine Algae and Potentially Toxic Elements—A Review. Mar. Drugs 2018, 16, 65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doğan, M.; Alkan, M. Removal of methyl violet from aqueous solution by perlite. J. Colloid Interface Sci. 2003, 267, 32–41. [Google Scholar] [CrossRef]
- Alzaydien, A.S. Adsorption of Methylene Blue from Aqueous Solution onto a Low-Cost Natural Jordanian Tripoli. Am. J. Environ. Sci. 2009, 5, 197–208. [Google Scholar] [CrossRef] [Green Version]
- Low, K.S.; Chow, S.W. Chrome Sludge as an Adsorbent for Colour Removal AU—Lee, C. K. Environ. Technol. 1996, 17, 1023–1028. [Google Scholar]
- Acemioglu, B. Batch kinetic study of sorption of methylene blue by perlite. Chem. Eng. J. 2005, 106, 73–81. [Google Scholar] [CrossRef]
- Panday, K.K.; Prasad, G.; Singh, V.N. Use of wollastonite for the treatment of Cu(II) rich effluents. Water Air Soil Pollut. 1986, 27, 287–296. [Google Scholar] [CrossRef]
- Juang, R.-S.; Chen, M.-L. Application of the Elovich Equation to the Kinetics of Metal Sorption with Solvent-Impregnated Resins. Ind. Eng. Chem. Res. 1997, 36, 813–820. [Google Scholar] [CrossRef]
- Chowdhury, S.; Saha, P.D. Biosorption of methylene blue from aqueous solutions by a waste biomaterial: Hen feathers. Appl. Water Sci. 2012, 2, 209–219. [Google Scholar] [CrossRef] [Green Version]
- Maurya, R.; Ghosh, T.; Paliwal, C.; Shrivastav, A.; Chokshi, K.; Pancha, I.; Ghosh, A.; Mishra, S. Biosorption of Methylene Blue by De-Oiled Algal Biomass: Equilibrium, Kinetics and Artificial Neural Network Modelling. PLoS ONE 2014, 9, e109545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hameed, B.; El-Khaiary, M. Removal of basic dye from aqueous medium using a novel agricultural waste material: Pumpkin seed hull. J. Hazard. Mater. 2008, 155, 601–609. [Google Scholar] [CrossRef]
- Chaleshtori, A.A.; Meghadddam, F.M.; Sadeghi, M.M.; Rahimi, R.R.; Hemati, S.; Ahmadi, A.A. Removal of acid red 18 (Azo-dye) from aqueous solution by adsorption onto activated charcoal prepared from almond shell. J. Environ. Sci. Manag. 2017, 20, 9–16. [Google Scholar]
- Kamga, F.T. Modeling adsorption mechanism of paraquat onto Ayous (Triplochiton scleroxylon) wood sawdust. Appl. Water Sci. 2018, 9, 1. [Google Scholar] [CrossRef] [Green Version]
- Pathania, D.; Sharma, S.; Singh, P. Removal of methylene blue by adsorption onto activated carbon developed from Ficus carica bast. Arab. J. Chem. 2017, 10, S1445–S1451. [Google Scholar] [CrossRef] [Green Version]
- Rafatullah, M.; Sulaiman, O.; Hashim, R.; Ahmad, A. Adsorption of methylene blue on low-cost adsorbents: A review. J. Hazard. Mater. 2010, 177, 70–80. [Google Scholar] [CrossRef]
- Yu, J.-X.; Li, B.-H.; Sun, X.-M.; Yuan, J.; Chi, R.-A. Polymer modified biomass of baker’s yeast for enhancement adsorption of methylene blue, rhodamine B and basic magenta. J. Hazard. Mater. 2009, 168, 1147–1154. [Google Scholar] [CrossRef]
- Yu, J.-X.; Li, B.-H.; Sun, X.-M.; Yuan, J.; Chi, R.-A. Poly(Amic Acid)-Modified Biomass of Baker’s Yeast for Enhancement Adsorption of Methylene Blue and Basic Magenta. Appl. Biochem. Biotechnol. 2009, 160, 1394–1406. [Google Scholar] [CrossRef]
- Marungrueng, K.; Pavasant, P. High performance biosorbent (Caulerpa lentillifera) for basic dye removal. Bioresour. Technol. 2007, 98, 1567–1572. [Google Scholar] [CrossRef]
- Ncibi, M.C.; Ben Hamissa, A.; Fathallah, A.; Kortas, M.; Baklouti, T.; Mahjoub, B.; Seffen, M. Biosorptive uptake of methylene blue using Mediterranean green alga Enteromorpha spp. J. Hazard. Mater. 2009, 170, 1050–1055. [Google Scholar] [CrossRef]
- Gulnaz, O.; Kaya, A.; Matyar, F.; Arikan, B. Sorption of basic dyes from aqueous solution by activated sludge. J. Hazard. Mater. 2004, 108, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Maurya, N.S.; Mittal, A.K.; Cornel, P.; Rother, E. Biosorption of dyes using dead macro fungi: Effect of dye structure, ionic strength and pH. Bioresour. Technol. 2006, 97, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Low, K.; Lee, C.; Heng, L. Sorption of basic dyes byHydrilla verticillata. Environ. Technol. 1994, 15, 115–124. [Google Scholar] [CrossRef]
- Vilar, V.J.P.; Botelho, C.M.S.; Boaventura, R.A.R. Methylene blue adsorption by algal biomass based materials: Biosorbents characterization and process behaviour. J. Hazard. Mater. 2007, 147, 120–132. [Google Scholar] [CrossRef]
- Waranusantigul, P.; Pokethitiyook, P.; Kruatrachue, M.; Upatham, E. Kinetics of basic dye (methylene blue) biosorption by giant duckweed (Spirodela polyrrhiza). Environ. Pollut. 2003, 125, 385–392. [Google Scholar] [CrossRef]
- Caparkaya, D.; Cavas, L. Biosorption of Methylene Blue by a Brown Alga Cystoseira barbatula Kutzing. Acta Chim. Slov. 2008, 55, 547–553. [Google Scholar]
- Nacèra, Y.; Aicha, B. Equilibrium and kinetic modelling of methylene blue biosorption by pretreated dead streptomyces rimosus: Effect of temperature. Chem. Eng. J. 2006, 119, 121–125. [Google Scholar] [CrossRef]
- Fu, Y.; Viraraghavan, T. Removal of a Dye from an Aqueous Solution by the Fungus Aspergillus niger. Water Qual. Res. J. 2000, 35, 95–112. [Google Scholar] [CrossRef]
- Cengiz, S.; Cavas, L. Removal of methylene blue by invasive marine seaweed: Caulerpa racemosa var. cylindracea. Bioresour. Technol. 2008, 99, 2357–2363. [Google Scholar] [CrossRef] [PubMed]
- Vakili, M.; Rafatullah, M.; Yuan, J.; Zwain, H.M.; Mojiri, A.; Gholami, Z.; Gholami, F.; Wang, W.; Giwa, A.S.; Yu, Y.; et al. Nickel ion removal from aqueous solutions through the adsorption process: A review. Rev. Chem. Eng. 2019. [Google Scholar] [CrossRef]

















| UL-T | UL-OH | UL-WIS | UL-H | UL-NA | |
|---|---|---|---|---|---|
| 249 | 545 | 437 | 290 | 235 | Qm (mol g−1) |
| 263 | 575 | 460 | 305 | 247 | SMB (m2 g−1) |
| l Band Assignment from the Literature | Main Peak (cm−1) from Adsorbent (U. lactuca) | Main Peak (cm−1) from Adsorbate (MB) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| UL-NA | UL-WIS | UL-OH | UL-H | UL-T | MB | |||||||
| Bef. | Aft. | Bef. | Aft. | Bef. | Aft. | Bef. | Aft. | Bef. | Aft. | Functional Groups of MB | Λ cm−1 of FT-IR Spectrum | |
| Water v(O‒H) stretching protein v(N‒H) stretching | 3307 | 3389 3324 | 3350 | 3332 3360 | 3350 | 3381 3317 | 3434 | 3399 | 3352 | - | ‒NH/‒OH stretching vibration absorbance | 3410 |
| Lipid-carbohydrate mainly vas (CH2) and vs. (CH2) stretching | 2958 2895 | 2948 - | 2940 2895 | 2934 2891 | 2952 2895 | 2938 2230 | 2938 2895 | 2958 2240 | 2962 2895 | 2954 - | symmetrical stretching C‒H of ‒CH2 peak | 2928 |
| Protein amide I band mainly v(C=O) stretching | 1638 | 1638 | 1638 | 1644 | 1640 | 1626 | 1697 1595 | 1709 1620 | 1614 | 1609 | Vibrational bands of the =N + (CH3)2 | (1640–1650) |
| Protein amide II band mainly δ(NH) bending and v(C‒N) stretching | 1579 | 1589 | 1571 | 1573 | - | 1589 | 1565 | 1560 | - | - | stretching band of C‒O, C‒N from the amide II | 1572 |
| Protein δas (CH2) and δas (CH3) bending of methyl, Lipid δas (CH2) bending of methyl | 1430 | 1434 | 1428 | 1428 | 1436 | 1436 | 1414 | 1462 | 1426 | 1434 | symmetrical stretching peak of carboxyl (‒COOH) | 1442 |
| Protein δs(CH2) and δs(CH3) bending of methyl Carboxylic Acid vs. (C‒O) of COO‒ groups of carboxylates Lipid δs(N(CH3)3) bending of methyl | 1369 | 1342 | 1358 | 1363 | 1368 | 1383 | 1389 | 1342 | - | 1395 | - | |
| Nucleic acid (other phosphate containing compounds) vas(>P=O) stretching of phosphodiesters | 1236 | 1294 1244 | 1291 | 1285 | 1248 | 1248 1300 | - | 1197 | - | 1244 | - | |
| Carbohydrate v(C‒O‒C) of Polysaccharides | 1151 | - | 1159 | - | 1159 | - | 1163 | - | 1154 | - | bending band of N‒H and C‒N from the amide III band | 1140 |
| Sulfate groups as S = O, C−O−S and v(C‒S‒C) of sulphated polysaccharides | 1048 1050 | 1030 1046 | 1032 | 1032 | 1030 | 1026 | 1026 995 | 1026 | 1067 | 1040 | Vibrational bands of C-S-C | (615–625, 1095) |
| 848 | 895 | 893 | 901 | 850 | 942 889 | 801 | 926 810 | 863 | 801 | bending band of N‒H and C‒N from the amide III band | 854 | |
| UL-NA | UL-WIS | UL-OH | UL-H | UL-T | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | Before | After | Before | After | |
| C | 46.53 | 47.35 | 25.84 | 57.69 | 48.35 | 51.61 | 64.35 | 71.79 | 71.28 | 71.65 |
| O | 46.95 | 48.89 | 3.65 | 41.13 | 48.81 | 47.15 | 34.68 | 26.38 | 28.11 | 26.42 |
| Na | 0.75 | - | - | - | 0.79 | - | 0.41 | - | - | - |
| Mg | 2.36 | 1.90 | 0.02 | 0.55 | 0.82 | 0.56 | - | 0.34 | 0.33 | 0.36 |
| S | 1.77 | 1.44 | - | 0.25 | 1.00 | 0.47 | 0.28 | 0.37 | 0.14 | 0.28 |
| Cl | 0.06 | - | - | - | - | - | - | - | - | - |
| K | 0.28 | - | - | - | - | - | - | - | - | - |
| Ca | 0.43 | 0,42 | 0.02 | 0.37 | 0.23 | 0.21 | 0.27 | 0.57 | 0.15 | 1.04 |
| Contact Time (min) | MB mg L−1 | Removal% | Capacity Adsorption mg g−1 | Algal Dose g L−1 | pH | Treatment | References | |
|---|---|---|---|---|---|---|---|---|
| 20 mg L−1 | 6 g L−1 | 20 mg L−1 | ||||||
| 75 | 16 | 78.44 | 75.00 | 263.2 | 5 | 8 | UL-NA | Current study |
| 70.28 | 90.34 | 303 | UL-WIS | |||||
| 87.61 | 95.35 | 625 | UL-OH | |||||
| 5.00 | 95.38 | 2 | UL-H | |||||
| 8.04 | 95.37 | 2.9 | UL-T | |||||
| 45 | 25 | 75 | 40.2 | 1.25 | 10 | SW ꝉ +TW ꝉ +ddW ꝉ + dried (100 °C) | [27] | |
| 60 | 100 | 65.68 | 200 | 2 | 7 | SW ꝉ +TW ꝉ +dried (RT ꝉ) | [63] | |
| 110 | 25 | 91.92 | NA | 1.25 | 8 | SW ꝉ +TW ꝉ +ddW ꝉ + dried (100 °C) | [28] | |
| 180 | 200 | 96.59 | 344.83 | 1.5 | 11.16 | TW ꝉ +dW ꝉ + dried (105 °C) + ZnCl2 + 110 °C + 700 °C | [26] | |
| UL-NA. | UL-T | UL-H | UL-OH | UL-WIS | ||
|---|---|---|---|---|---|---|
| 0.0276 | 0.0069 | 0.0023 | 0.0276 | 0.0115 | K1 (min−1) | Model of pseudo-first |
| 648.63 | 22.59 | 234.42 | 220.29 | 441.57 | qe* (mg·g−1) | |
| 860.81 | 342.9 | 426.58 | 1990.7 | 2600.73 | qe* (the.) * (mg·g−1) | |
| 0.974 | 0.762 | 0.937 | 0.806 | 0.969 | R2 | |
| 1000 | 333.3 | 62.5 | 1000 | 1000 | K2 (g·mg−1 min−1) | Model of pseudo-second |
| 33.34 × 10−6 | 20.55 × 10−6 | 755.16 × 10−6 | 500.00 × 10−6 | 111.11 × 10−6 | qe* (mg·g−1) | |
| 24.46 × 10−6 | 27.51 | 942.59 × 10−6 | 596.09 × 10−6 | 59.06 × 10−6 | qe* (the.) (mg·g−1) | |
| 0.986 | 0.592 | 0.940 | 0.999 | 0.998 | R2 | |
| 546.37 | −847 × 10−6 | 16.46 | 4316 106 | 1350 | α | Elovich |
| 0.0049 | −0.0736 | 0.1069 | 0.0293 | 0.0113 | β | model |
| 0.979 | 0.094 | 0.867 | 0.951 | 0.978 | R2 | |
| 143.05 | 19.31 | 16.23 | 18.99 | 46.82 | Kint1 | Intraparticle diffusion model |
| (mg·g−1 min1/2) | ||||||
| −215.8 | −33.1 | −34.7 | 651.7 | 287.7 | C (cm2.s−1) | |
| 0.978 | 0.760 | 0.978 | 0.761 | 0.917 | R2 | |
| 23.46 | 3.52 | 4.73 | 9.20 | 14.18 | Kint2 | |
| (mg·g−1·min1/2) | ||||||
| 472.3 | 51.8 | 1.9 | 702 | 505.3 | D* (cm2·s−1) | |
| 0.793 | 0.188 | 0.892 | 0.998 | 0.999 | R2 | |
| Freundlich Constants | Langmuir Constants | |||||||
|---|---|---|---|---|---|---|---|---|
| R2 | Kf | n | 1/n | R2 | RL | KL | qmax (mg/g) | |
| (L/mg) | ||||||||
| 0.9762 | 1847 | 1.54 | 0.65 | 0.9836 | 0.12 | 0.363 | 303.0 | UL-WIS |
| 0.9819 | 899 | 5.55 | 0.18 | 0.9985 | 0.40 | 0.083 | 625.0 | UL-OH |
| 0.9943 | ID | 0.05 | 20.04 | 0.9575 | 0.47 | 0.062 | 2.0 | UL-H |
| 0.9478 | ID | 0.05 | 19.22 | 0.9096 | 0.47 | 0.064 | 2.9 | UL-T |
| 0.9403 | 1665 | 1.56 | 0.64 | 0.9789 | 0.13 | 0.369 | 263.0 | UL-NA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
M’sakni, N.H.; Alsufyani, T. Removal of Cationic Organic Dye from Aqueous Solution by Chemical and Pyrolysis Activated Ulva lactuca. Water 2021, 13, 1154. https://doi.org/10.3390/w13091154
M’sakni NH, Alsufyani T. Removal of Cationic Organic Dye from Aqueous Solution by Chemical and Pyrolysis Activated Ulva lactuca. Water. 2021; 13(9):1154. https://doi.org/10.3390/w13091154
Chicago/Turabian StyleM’sakni, Nour Houda, and Taghreed Alsufyani. 2021. "Removal of Cationic Organic Dye from Aqueous Solution by Chemical and Pyrolysis Activated Ulva lactuca" Water 13, no. 9: 1154. https://doi.org/10.3390/w13091154
APA StyleM’sakni, N. H., & Alsufyani, T. (2021). Removal of Cationic Organic Dye from Aqueous Solution by Chemical and Pyrolysis Activated Ulva lactuca. Water, 13(9), 1154. https://doi.org/10.3390/w13091154