A Comprehensive Review on Membrane Fouling: Mathematical Modelling, Prediction, Diagnosis, and Mitigation
Abstract
1. Introduction
- Feed chemistry and composition, i.e., pH, ionic strength, and foulant concentration.
- Concentration polarization (CP): CP can be broadly described as the deposition of rejected solutes on the membrane’s surface, creating a region near the membrane with spatially varying concentrations known as the polarized layer. This added resistance causes an increase in the osmotic pressure across the membrane, which decreases the driving force of the process (transmembrane pressure (TMP)), the permeate flux and the observed solute rejection, all of which increase the possibility of membrane fouling [5,7].
- Membrane properties include membrane material type, porosity, hydrophobicity, surface charges, membrane morphology, and molecular weight cut-off (MWCO).
- Process operating conditions such as temperature, pressure, aeration, permeate flux, and several other hydrodynamic conditions.
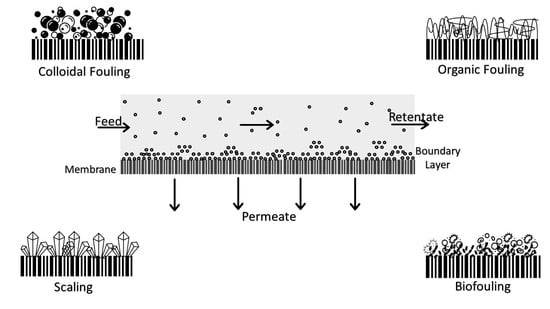
2. Membrane Foulants
2.1. Particulate Fouling
2.2. Organic Fouling
2.3. Inorganic Fouling
2.4. Biofouling
- Flux decline: as with the case of particulate fouling discussed earlier, the biofilm formation increases the resistance and reduces the permeate flux.
- Membrane biodeterioration: damage to the membrane’s structure due to acidic byproducts resulting from the microorganism’s biological activity.
- Deteriorated salt retention: inhibition of conventional transport mechanisms and increased CP effects across the membrane, due to the accumulation of dissolved salts and ions on the surface.
- Increased differential pressure: this is due to the increased resistance caused by biofilm formation.
- Higher energy requirements: the high-pressure requirements and the decline in permeate flux result in increased energy consumption.
- Frequent chemical cleaning: the cleaning process disrupts the membrane plant operation and shortens membrane life.
3. Fouling Prediction
- Pilot plant evaluation of the system’s performance;
- The use of fouling indices; and
- The use of predictive models.
3.1. Pilot Plant Evaluation of System Performance
3.2. Membrane Fouling Indices
3.2.1. Silt Density Index (SDI)
3.2.2. Modified Fouling Index (MFI)
3.2.3. Langelier Saturation Index (LSI) and Stiff and Davis Saturation Index (S&DSI)
3.2.4. Total and Dissolved Organic Carbon (TOC and DOC)
3.3. Predictive Models
4. Membrane Integrity and Fouling Diagnosis
- A thorough assessment of the malfunctioning membrane’s conductivity profiles followed by an evaluation of the extent of deviation from expected performance;
- Examination of the malfunctioning membrane’s peripheral matrix and identifying any defective components (i.e., interconnectors, end-seals, spacers, O-rings).
- External inspection: the different components that constitute the RO membrane are visually examined to diagnose the damaged zones. The core tubes, fiberglass castings, and anti-telescoping devices (ATDs) are carefully checked for potential impairments, including any obvious accumulation of foulants, crystals, scales, and biofilms [62,65,66];
- Mechanical integrity tests: several direct and indirect techniques have been developed to evaluate membrane integrity. Direct methods mainly utilize pressure-driven approaches to specify any grooves or channels in the sheets of the membrane, whereas indirect methods assess the overall integrity of the membrane’s structure [67];
- Dye test: dye testing is used to test damage to the surface of some membrane materials. Commonly used dyes include Congo Red, Methyl blue, and Rhodamine B. A fairly intense color is seen on damaged surfaces, particularly where the damage permits the access of a rather large dye molecule to the exposed surface on the porous supporting layer. If the membrane is intact, a uniformly colored stain would be observed [62,65];
- Cell test: a cell test is carried out to evaluate the performance of the malfunctioning membrane by comparing it against a new one, namely through a comparison of the differences in salt rejection and flux rates. The test is conducted by extracting an autopsied element from the defective membrane, followed by soaking it in deionized (DI) water to clean it from fouling residue and buildup, then inspecting its performance and comparing it against the standard performance of new membrane elements [62,66,68];
- Thorough analysis of the foulants: characterization techniques like scanning electron microscopy (SEM), energy dispersive X-ray (EDaX), Fujiwara oxidation testing, thermogravimetric analysis, and biological reactivity testing are commonly used to depict the membrane’s surface conditions and topography, and distinguish the different types of accumulated foulants [62,65].
5. Fouling Mitigation
5.1. Feedwater Pretreatment
5.2. Operational Conditions Optimization
5.3. Membrane Monitoring and Cleaning
- Sponge ball cleaning: this method is only applicable for tubular modules, and involves scrubbing foulants from the membrane’s surface using a sponge ball made out of polyurethane or another material [105]. The sponge ball cleaning regiment is usually utilized when the membrane is used to treat heavily polluted feedwaters like wastewater and industrial process water [105,106,107].
- Alternative flushing: this method is mostly applicable for the removal of colloidal particles from the membrane’s surface. It entails applying alternative rounds of high-pressure cross-flow water from the permeate side to the feed side and vice versa, which creates turbulence and causes the adsorbed foulants to release from the membrane [107]. It is important to optimize the forward and backward flush times to avoid compromising the membrane’s recovery efficiency, yet ensure complete cleaning cycles of the membrane modules [107,108].
- Backwashing: this method is commonly used in industries as it can retain the membrane’s flux before fouling to a very good extent. It cleans the membrane’s clogged pores by creating a negative pressure gradient across the membrane such that the applied hydraulic pressure on the permeate side exceeds the operating pressure of the module [106]. The flush creates turbulence across the membrane’s surface, loosening the foulants from the surface and out of the pores. Nonetheless, backwashing is not suitable for cleaning irreversible fouling which is characterized by clogging due to the treatment of highly concentrated colloidal solutions [106,109].
- Air flushing: this method, commonly referred to as air sparging, is more suitable for cleaning tubular and flat sheet membranes than fiber and spiral wound modules [107]. It follows the normal flushing procedure except that air is supplied to create bubbles which further augment the produced turbulence, thus enhancing the dislodgement of deposits from the membrane. It can be applied during the filtration process or scheduled periodically, as it is a rapid cleaning method that does not require chemicals and can be easily integrated into an existing membrane system. However, the effectiveness of this cleaning method is limited and the pumping requirements can be costly [110].
- Chemical cleaning: this method involves the use of chemical reagents that react with the foulants and reduce their affinity to the membrane surface, making it easier to remove these deposits. The choice of chemical agents is very important because they should not damage the membrane structure or compromise its integrity in any way. The most commonly used chemicals include acids, bases, surfactants, and chelating agents [5,69]. Low pH cleaners are mostly used to remove colloidal particulates and inorganic scales, while organic foulants and microorganisms are best removed using high pH agents [111]. Hacıfazlıoğlu et al. [112] investigated the effect of chemical cleaning on fouling control in a mini pilot-scale NF and RO system installed at a wastewater treatment plant in the ITOB Industrial Organized Zone located in Izmir/Turkey. The dual-step chemical cleaning (acid cleaning followed by alkaline cleaning) process proposed by the researchers was applied to NF and RO membranes employed for the desalination of MBR effluent discharged from a wastewater treatment plant. The study results showed that the cleaning efficiency increased with increasing cleaning contact time with chemicals.
5.4. Surface Modification and Novel Membrane Materials
5.4.1. Physical Surface Modification
5.4.2. Chemical Surface Modification
- Hydrophilization treatment: in this method, an antifouling lining is hydrophilized onto the membrane’s surface through chemical reactions with protic acids (i.e., hydrofluoric, hydrochloric, sulfuric, and nitric acids), ethanol, or 2-propanol [10,132,133]. Miyamoto et al. [134] investigated the blending of polyvinylpyrrolidone (PVP) into a polysulfone (PSf) membrane. PSf membranes are hydrophobic and are susceptible to fouling by NOM. Therefore, a hydrophilization treatment is conducted using non-solvent-induced phase separation, in which PVP is added to the PSf membrane.
- Radical grafting: in this method, free radicals are produced and then reacted with the membrane’s monomers to graft its surface. Wei et al. [135] conducted a radical grafting study in which they used 3-allyl-5,5-dimethylhydantoin (ADMH) as the grafting monomer. The modified membrane was tested for biofouling resistance in a microbial-cell suspension, where it showed augmented microbial adsorption rejection and enhanced flux rates compared to the unmodified membrane. Another study carried out by Isawi et al. [136] investigated grafting RO membranes with ZnO nanoparticles (NPs). The ZnO-NPs-grafted membrane achieved improved salt, dissolved bivalent ions (i.e., Ca2+ and SO42−), and monovalent ions (Cl− and Na+) rejection percentages of 97%, 99%, and 98%, respectively.
- Chemical coupling: this method entails reacting the active free carboxylic acid and primary amine groups on the surface of the PA-RO with chemical reagents to induce antifouling behaviors [132,137]. Hu et al. [138] covalently attached PVA to the surface of PA-TFC RO membranes. The covalent attachment of PVA resulted in improved surface hydrophilicity, enhanced salt rejection ability, and a slightly increased surface roughness. Additionally, the modification improved the membrane antifouling characteristics to a variety of foulants, including BSA and dodecyltrimethyl ammonium bromide.
- Plasma treatment: this method is further classified into plasma polymerization and plasma-induced polymerization. In the former, plasma is used to induce the accumulation of a layer of polymers onto the surface of TFC and PA-RO membranes. While, in the latter, plasma initiates the activation of oxides and/or hydroxides on the membrane’s surface, which are then involved in other polymerization methods [132,139]. A study by Safarpour et al. [140] used interfacial polymerization to synthesize TFC-RO membranes and modified them by adding dimethyl sulfoxide and glycerol. The modified membranes were characterized using SEM, FTIR, and contact angle measurements. It was reported that using dimethyl sulfoxide and glycerol as additives increased the permeate flux, surface roughness, and hydrophilicity of the membranes while maintaining the same salt rejection performance. Jahangiri et al. [141] applied the dielectric barrier discharge (DBD) plasma method to enhance the antifouling characteristics of a PA-TFC-RO membrane. The novel membrane was characterized using SEM, attenuated total reflectance FITR (ATR-FITR), and contact angle measurements, which revealed changes to the membrane’s surface morphology. ATR-FITR images showed hydrogen bonding on the surface of the modified membrane, and the contact angle measurement showed that the hydrophilicity increased, leading to a boost in surface roughness. The modified membranes had improved performance in terms of salt rejection, permeate flux, and BSA filtration. Another study by Hirsch et al. [142] modified the surface of TFC-RO membranes by combining three methods, namely, plasma activation, plasma bromination, and surface-initiated atom transfer radical polymerization (si-ATRP). Although the synthesized membranes suffered from unstable coating adhesion, the highest biofilm reduction reached was 85.4%, which is a significant enhancement.
5.4.3. Novel Membrane Materials
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prihasto, N.; Liu, Q.F.; Kim, S.H. Pre-treatment strategies for seawater desalination by reverse osmosis system. Desalination 2009, 249, 308–316. [Google Scholar] [CrossRef]
- Henthorne, L.; Boysen, B. State-of-the-art of reverse osmosis desalination pretreatment. Desalination 2015, 356, 129–139. [Google Scholar] [CrossRef]
- Al-Juboori, R.A.; Yusaf, T. Biofouling in RO system: Mechanisms, monitoring and controlling. Desalination 2012, 302, 1–23. [Google Scholar] [CrossRef]
- Ochando-Pulido, J.M.; Stoller, M.; Di Palma, L.; Martínez-Ferez, A. On the optimization of a flocculation process as fouling inhibiting pretreatment on an ultrafiltration membrane during olive mill effluents treatment. Desalination 2016, 393, 151–158. [Google Scholar] [CrossRef]
- Jiang, S.; Li, Y.; Ladewig, B.P. A review of reverse osmosis membrane fouling and control strategies. Sci. Total Environ. 2017, 595, 567–583. [Google Scholar] [CrossRef]
- Guo, W.; Ngo, H.H.; Li, J. A mini-review on membrane fouling. Bioresour. Technol. 2012, 122, 27–34. [Google Scholar] [CrossRef]
- Field, R. Fundamentals of fouling. In Membranes for Water Treatment; Peinemann, K.-V., Pereira Nunes, S., Eds.; Wiley-VCH: Weinheim, Germany, 2010; Volume 4, pp. 1–23. [Google Scholar]
- Colloid|Definition of Colloid by Merriam-Webster. Available online: https://www.merriam-webster.com/dictionary/colloid (accessed on 29 January 2020).
- Yiantsios, S.G.; Sioutopoulos, D.; Karabelas, A.J. Colloidal fouling of RO membranes: An overview of key issues and efforts to develop improved prediction techniques. Desalination 2005, 183, 257–272. [Google Scholar] [CrossRef]
- Qasim, M.; Badrelzaman, M.; Darwish, N.N.; Darwish, N.A.; Hilal, N. Reverse osmosis desalination: A state-of-the-art review. Desalination 2019, 459, 59–104. [Google Scholar] [CrossRef]
- Tang, C.Y.; Chong, T.H.; Fane, A.G. Colloidal interactions and fouling of NF and RO membranes: A review. Adv. Colloid Interface Sci. 2011, 164, 126–143. [Google Scholar] [CrossRef]
- Kalafatakis, S.; Zarebska, A.; Lange, L.; Hélix-Nielsen, C.; Skiadas, I.V.; Gavala, H.N. Biofouling mitigation approaches during water recovery from fermented broth via forward osmosis. Membranes 2020, 10, 307. [Google Scholar] [CrossRef]
- Maddah, H.; Chogle, A. Biofouling in reverse osmosis: Phenomena, monitoring, controlling and remediation. Appl. Water Sci. 2017, 7, 2637–2651. [Google Scholar] [CrossRef]
- Donlan, R.M. Biofilms: Microbial life on surfaces. Emerg. Infect. Dis. 2002, 8, 881–890. [Google Scholar] [CrossRef]
- Chai-Hoon, C.K.O.O.; Mohammad, A.; Suja, F.; Zainal, M. Use and Development of Fouling Index in Predicting Membrane Fouling. Sep. Purif. Rev. 2013, 42. [Google Scholar] [CrossRef]
- Sim, L.N.; Chong, T.H.; Taheri, A.H.; Sim, S.T.V.; Lai, L.; Krantz, W.B.; Fane, A.G. A review of fouling indices and monitoring techniques for reverse osmosis. Desalination 2018, 434, 169–188. [Google Scholar] [CrossRef]
- Rudolph, G.; Virtanen, T.; Ferrando, M.; Güell, C.; Lipnizki, F.; Kallioinen, M. A review of in situ real-time monitoring techniques for membrane fouling in the biotechnology, biorefinery and food sectors. J. Membr. Sci. 2019, 588, 117221. [Google Scholar] [CrossRef]
- Ruigómez, I.; Vera, L.; González, E.; Rodríguez-Sevilla, J. Pilot plant study of a new rotating hollow fibre membrane module for improved performance of an anaerobic submerged MBR. J. Membr. Sci. 2016, 514, 105–113. [Google Scholar] [CrossRef]
- Melián-Martel, N.; Sadhwani Alonso, J.J.; Ruiz-García, A. Combined silica and sodium alginate fouling of spiral-wound reverse osmosis membranes for seawater desalination. Desalination 2018, 439, 25–30. [Google Scholar] [CrossRef]
- Sioutopoulos, D.; Karabelas, A.; Mappas, V. Membrane fouling due to protein—Polysaccharide mixtures in dead-end ultrafiltration; the effect of permeation flux on fouling resistance. Membranes 2019, 9, 21. [Google Scholar] [CrossRef]
- Hernández, M.; Melián-Martel, N.; Ruiz-García, A.; Río-Gamero, B. Del Fouling characterization during initial stage of cross-flow ultrafiltration. J. Water Process Eng. 2020, 38, 101611. [Google Scholar] [CrossRef]
- Neubrand, W.; Vogler, S.; Ernst, M.; Jekel, M. Lab and pilot scale investigations on membrane fouling during the ultrafiltration of surface water. Desalination 2010, 250, 968–972. [Google Scholar] [CrossRef]
- Kim, L.H.; Nava-Ocampo, M.; van Loosdrecht, M.C.M.; Kruithof, J.C.; Vrouwenvelder, J.S. The membrane fouling simulator: Development, application, and early-warning of biofouling in RO treatment. Desalin. Water Treat. 2018, 126, 1–23. [Google Scholar] [CrossRef]
- Vrouwenvelder, J.S.; van Paassen, J.A.M.; Wessels, L.P.; van Dam, A.F.; Bakker, S.M. The Membrane Fouling Simulator: A practical tool for fouling prediction and control. J. Membr. Sci. 2006, 281, 316–324. [Google Scholar] [CrossRef]
- Massons-Gassol, G.; Gilabert-Oriol, G.; Johnson, J.; Arrowood, T. Comparing Biofouling Development in Membrane Fouling Simulators and Spiral-Wound Reverse Osmosis Elements Using River Water and Municipal Wastewater. Ind. Eng. Chem. Res. 2017, 56, 11628–11633. [Google Scholar] [CrossRef]
- B.V. Convergence Industry. Membrane Fouling Simulator. Available online: https://www.con-vergence.com/product/membrane-fouling-simulator/ (accessed on 27 April 2021).
- Bagheri, M.; Akbari, A.; Mirbagheri, S.A. Advanced control of membrane fouling in filtration systems using artificial intelligence and machine learning techniques: A critical review. Process Saf. Environ. Prot. 2019, 123, 229–252. [Google Scholar] [CrossRef]
- Aidan, A.; Abdel-Jabbar, N.; Ibrahim, T.H.; Nenov, V.; Mjalli, F. Neural network modeling and optimization of scheduling backwash for membrane bioreactor. Clean Technol. Environ. Policy 2008, 10, 389–395. [Google Scholar] [CrossRef]
- Curcio, S.; Calabrò, V.; Iorio, G. Reduction and control of flux decline in cross-flow membrane processes modeled by artificial neural networks. J. Membr. Sci. 2006, 286, 125–132. [Google Scholar] [CrossRef]
- Li, C.; Wang, X. Application of MBR Membrane Flux Prediction Based on Elman Neural Network. DEStech Trans. Eng. Technol. Res. 2017. [Google Scholar] [CrossRef]
- Hedayati Moghaddam, A.; Hazrati, H.; Sargolzaei, J.; Shayegan, J. Assessing and simulation of membrane technology for modifying starchy wastewater treatment. Appl. Water Sci. 2017, 7, 2753–2765. [Google Scholar] [CrossRef]
- Ruiz-García, A.; Melián-Martel, N.; Nuez, I. Short review on predicting fouling in RO desalination. Membranes 2017, 7, 62. [Google Scholar] [CrossRef]
- ASTM D4189—07(2014) Standard Test Method for Silt Density Index (SDI) of Water. Available online: https://www.astm.org/Standards/D4189.htm (accessed on 21 June 2020).
- Alhadidi, A.; Kemperman, A.J.B.; Schippers, J.C.; Blankert, B.; Wessling, M.; van der Meer, W.G.J. SDI normalization and alternatives. Desalination 2011, 279, 390–403. [Google Scholar] [CrossRef]
- Alhadidi, A.M.M.; Blankert, B.; Kemperman, A.; Schurer, R.; Schippers, J.; Wessling, M. Limitations, improvements and alternatives of the silt density index. Desalin. Water Treat. 2012, 51, 1–10. [Google Scholar] [CrossRef]
- Schippers, J.C.; Verdouw, J. The modified fouling index, a method of determining the fouling characteristics of water. Desalination 1980, 32, 137–148. [Google Scholar] [CrossRef]
- Boerlage, S.F.E.; Kennedy, M.D.; Bonne, P.A.C.; Galjaard, G.; Schippers, J.C. Prediction of flux decline in membrane systems due to particulate fouling. Desalination 1997, 113, 231–233. [Google Scholar] [CrossRef]
- Boerlage, S.F.E.; Kennedy, M.D.; Dickson, M.R.; El-Hodali, D.E.Y.; Schippers, J.C. The modified fouling index using ultrafiltration membranes (MFI-UF): Characterisation, filtration mechanisms and proposed reference membrane. J. Membr. Sci. 2002, 197, 1–21. [Google Scholar] [CrossRef]
- Cai, B.J.; Baudin, I.; Ng, H.Y. A modified fouling index (MFI40) and fouling predicting approach for ultrafiltration of secondary effluents. J. Water Reuse Desalin. 2019, 9, 67–82. [Google Scholar] [CrossRef]
- Mirzabeygi, M.; Naji, M.; Yousefi, N.; Shams, M.; Biglari, H.; Mahvi, A.H. Evaluation of corrosion and scaling tendency indices in water distribution system: A case study of Torbat Heydariye, Iran. Desalin. Water Treat. 2016, 57, 25918–25926. [Google Scholar] [CrossRef]
- Leitz, F.; Guerra, K. Water Chemistry Analysis for Water Conveyance, Storage, and Desalination Projects Manuals and Standards Program; US Department of the Interior, Bureau of Reclamation: Denver, CO, USA, 2013.
- Jepsen, K.L.; Bram, M.V.; Pedersen, S.; Yang, Z. Membrane fouling for produced water treatment: A review study from a process control perspective. Water 2018, 10, 847. [Google Scholar] [CrossRef]
- Wilf, M.; Klinko, K. Performance of commercial seawater membranes. Desalination 1994, 96, 465–478. [Google Scholar] [CrossRef]
- Chen, K.L.; Song, L.; Ong, S.L.; Ng, W.J. The development of membrane fouling in full-scale RO processes. J. Membr. Sci. 2004, 232, 63–72. [Google Scholar] [CrossRef]
- Duclos-Orsello, C.; Li, W.; Ho, C.C. A three mechanism model to describe fouling of microfiltration membranes. J. Membr. Sci. 2006, 280, 856–866. [Google Scholar] [CrossRef]
- Mondal, S.; De, S. A fouling model for steady state crossflow membrane filtration considering sequential intermediate pore blocking and cake formation. Sep. Purif. Technol. 2010, 75, 222–228. [Google Scholar] [CrossRef]
- Chang, E.E.; Yang, S.Y.; Huang, C.P.; Liang, C.H.; Chiang, P.C. Assessing the fouling mechanisms of high-pressure nanofiltration membrane using the modified Hermia model and the resistance-in-series model. Sep. Purif. Technol. 2011, 79, 329–336. [Google Scholar] [CrossRef]
- Virtanen, T.; Reinikainen, S.P.; Lahti, J.; Mänttäri, M.; Kallioinen, M. Visual tool for real-time monitoring of membrane fouling via Raman spectroscopy and process model based on principal component analysis. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef]
- Jolliffe, I.T.; Cadima, J. Principal component analysis: A review and recent developments. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2016, 374, 20150202. [Google Scholar] [CrossRef] [PubMed]
- Koonani, H.; Amirinejad, M. Combined three mechanisms models for membrane fouling during microfiltration. J. Membr. Sci. Res. 2019, 5, 274–282. [Google Scholar]
- Ruiz-García, A.; Nuez, I. Long-term performance decline in a brackish water reverse osmosis desalination plant. Predictive model for the water permeability coefficient. Desalination 2016, 397, 101–107. [Google Scholar] [CrossRef]
- Corbatón-Báguena, M.J.; Vincent-Vela, M.C.; Gozálvez-Zafrilla, J.M.; Álvarez-Blanco, S.; Lora-García, J.; Catalán-Martínez, D. Comparison between artificial neural networks and Hermia’s models to assess ultrafiltration performance. Sep. Purif. Technol. 2016, 170, 434–444. [Google Scholar] [CrossRef]
- Barello, M.; Manca, D.; Patel, R.; Mujtaba, I.M. Neural network based correlation for estimating water permeability constant in RO desalination process under fouling. Desalination 2014, 345, 101–111. [Google Scholar] [CrossRef]
- Park, S.; Baek, S.S.; Pyo, J.C.; Pachepsky, Y.; Park, J.; Cho, K.H. Deep neural networks for modeling fouling growth and flux decline during NF/RO membrane filtration. J. Membr. Sci. 2019, 587. [Google Scholar] [CrossRef]
- Lim, S.J.; Kim, Y.M.; Park, H.; Ki, S.; Jeong, K.; Seo, J.; Chae, S.H.; Kim, J.H. Enhancing accuracy of membrane fouling prediction using hybrid machine learning models. Desalin. Water Treat. 2019, 146, 22–28. [Google Scholar] [CrossRef]
- Han, H.G.; Zhang, H.J.; Liu, Z.; Qiao, J.F. Data-driven decision-making for wastewater treatment process. Control Eng. Pract. 2020, 96, 104305. [Google Scholar] [CrossRef]
- Koo, C.H.; Mohammad, A.W.; Suja’, F.; Meor Talib, M.Z. Review of the effect of selected physicochemical factors on membrane fouling propensity based on fouling indices. Desalination 2012, 287, 167–177. [Google Scholar] [CrossRef]
- Sim, L.N.; Ye, Y.; Chen, V.; Fane, A.G. Crossflow Sampler Modified Fouling Index Ultrafiltration (CFS-MFIUF)-An alternative Fouling Index. J. Membr. Sci. 2010, 360, 174–184. [Google Scholar] [CrossRef]
- Langelier-Explanation. Available online: https://www.lenntech.com/ro/index/langelier-explanation.htm (accessed on 3 February 2020).
- Yu, Y.; Lee, S.; Hong, K.; Hong, S. Evaluation of membrane fouling potential by multiple membrane array system (MMAS): Measurements and applications. J. Membr. Sci. 2010, 362, 279–288. [Google Scholar] [CrossRef]
- Ait Messaoudene, N.; Naceur, M.W. The Modified Fouling Index Revisited: Proposal of a Novel Dimensionless Fouling Index for Membranes—IJERT. Int. J. Eng. Res. Technol. 2014, 3, 193–198. [Google Scholar]
- Voutchkov, N. Diagnostics of Membrane Fouling and Scaling. In Pretreatment for Reverse Osmosis Desalination; Elsevier: Amsterdam, The Netherlands, 2017; pp. 43–64. [Google Scholar]
- Vrouwenvelder, J.S.; van der Kooij, D. Diagnosis of fouling problems of NF and RO membrane installations by a quick scan. Desalination 2003, 153, 121–124. [Google Scholar] [CrossRef]
- Vrouwenvelder, J.S.; Kappelhof, J.W.N.M.; Heijman, S.G.J.; Schippers, J.C.; van der Kooij, D. Tools for fouling diagnosis of NF and RO membranes and assessment of the fouling potential of feed water. Desalination 2003, 157, 361–365. [Google Scholar] [CrossRef]
- Leitz, F. Membrane Element Autopsy Manual. Security 1996, 157, 361–365. [Google Scholar]
- Membrane Autopsy & Analysis—MF, UF, NF & RO Membrane Inspection. Available online: https://www.pwtchemicals.com/services/membrane-autopsy/ (accessed on 12 February 2020).
- Ostarcevic, E.R.; Jacangelo, J.; Gray, S.R.; Cran, M.J. Current and emerging techniques for high-pressure membrane integrity testing. Membranes 2018, 8, 60. [Google Scholar] [CrossRef]
- Schipolowski, T.; Jezowska, A.; Wozny, G. Reliability of membrane test cell measurements. Desalination 2006, 189, 71–80. [Google Scholar] [CrossRef]
- Goh, P.S.; Lau, W.J.; Othman, M.H.D.; Ismail, A.F. Membrane fouling in desalination and its mitigation strategies. Desalination 2018, 425, 130–155. [Google Scholar] [CrossRef]
- Voutchkov, N. Membrane Foulants and Saline Water Pretreatment. In Pretreatment for Reverse Osmosis Desalination; Elsevier: Amsterdam, The Netherlands, 2017; pp. 11–41. [Google Scholar]
- Jamaly, S.; Darwish, N.N.; Ahmed, I.; Hasan, S.W. A short review on reverse osmosis pretreatment technologies. Desalination 2014, 354, 30–38. [Google Scholar] [CrossRef]
- Kavitha, J.; Rajalakshmi, M.; Phani, A.R.; Padaki, M. Pretreatment processes for seawater reverse osmosis desalination systems—A review. J. Water Process Eng. 2019, 32, 100926. [Google Scholar] [CrossRef]
- Lee, W.J.; Ng, Z.C.; Hubadillah, S.K.; Goh, P.S.; Lau, W.J.; Othman, M.H.D.; Ismail, A.F.; Hilal, N. Fouling mitigation in forward osmosis and membrane distillation for desalination. Desalination 2020, 480, 114338. [Google Scholar] [CrossRef]
- Ebrahim, S.; Bou-Hamed, S.; Abdel-Jawad, M.; Burney, N. Microfiltration system as a pretreatment for RO units: Technical and economic assessment. Desalination 1997, 109, 165–175. [Google Scholar] [CrossRef]
- Lee, J.; Oh, B.S.; Kim, S.; Kim, S.J.; Hong, S.K.; Kim, I.S. Fate of Bacillus sp. and Pseudomonas sp. isolated from seawater during chlorination and microfiltration as pretreatments of a desalination plant. J. Membr. Sci. 2010, 349, 208–216. [Google Scholar] [CrossRef]
- Jeong, S.; Choi, Y.J.; Nguyen, T.V.; Vigneswaran, S.; Hwang, T.M. Submerged membrane hybrid systems as pretreatment in seawater reverse osmosis (SWRO): Optimisation and fouling mechanism determination. J. Membr. Sci. 2012, 411–412, 173–181. [Google Scholar] [CrossRef]
- Hakami, M.W.; Alkhudhiri, A.; Al-Batty, S.; Zacharof, M.P.; Maddy, J.; Hilal, N. Ceramic microfiltration membranes in wastewater treatment: Filtration behavior, fouling and prevention. Membranes 2020, 10, 248. [Google Scholar] [CrossRef] [PubMed]
- Chua, K.T.; Hawlader, M.N.A.; Malek, A. Pretreatment of seawater: Results of pilot trials in Singapore. Desalination 2003, 159, 225–243. [Google Scholar] [CrossRef]
- Gu, H.; Rahardianto, A.; Gao, L.X.; Caro, X.P.; Giralt, J.; Rallo, R.; Christofides, P.D.; Cohen, Y. Fouling indicators for field monitoring the effectiveness of operational strategies of ultrafiltration as pretreatment for seawater desalination. Desalination 2018, 431, 86–99. [Google Scholar] [CrossRef]
- Monnot, M.; Laborie, S.; Cabassud, C. Granular activated carbon filtration plus ultrafiltration as a pretreatment to seawater desalination lines: Impact on water quality and UF fouling. Desalination 2016, 383, 1–11. [Google Scholar] [CrossRef]
- Anis, S.F.; Hashaikeh, R.; Hilal, N. Reverse osmosis pretreatment technologies and future trends: A comprehensive review. Desalination 2019, 452, 159–195. [Google Scholar] [CrossRef]
- Talaeipour, M.; Nouri, J.; Hassani, A.H.; Mahvi, A.H. An investigation of desalination by nanofiltration, reverse osmosis and integrated (hybrid NF/RO) membranes employed in brackish water treatment. J. Environ. Health Sci. Eng. 2017, 15. [Google Scholar] [CrossRef]
- Hassan, A.M.; Farooque, A.M.; Jamaluddin, A.T.M.; Al-Amoudi, A.S.; Al-Sofi, M.A.; Al-Rubaian, A.F.; Kither, N.M.; Al-Tisan, I.A.R.; Rowaili, A. Demonstration plant based on the new NF-SWRO process. Desalination 2000, 131, 157–171. [Google Scholar] [CrossRef]
- Al-Amoudi, A.S.; Farooque, A.M. Performance restoration and autopsy of NF membranes used in seawater pretreatment. Desalination 2005, 178, 261–271. [Google Scholar] [CrossRef]
- Al-Hajouri, A.A.; Al-Amoudi, A.S.; Farooque, A.M. Long term experience in the operation of nanofiltration pretreatment unit for seawater desalination at SWCC SWRO plant. Desalin. Water Treat. 2013, 51, 1861–1873. [Google Scholar] [CrossRef]
- Shirazi, S.; Lin, C.J.; Chen, D. Inorganic fouling of pressure-driven membrane processes—A critical review. Desalination 2010, 250, 236–248. [Google Scholar] [CrossRef]
- Ashfaq, M.Y.; Al-Ghouti, M.A.; Da’na, D.A.; Qiblawey, H.; Zouari, N. Investigating the effect of temperature on calcium sulfate scaling of reverse osmosis membranes using FTIR, SEM-EDX and multivariate analysis. Sci. Total Environ. 2020, 703, 134726. [Google Scholar] [CrossRef]
- Farhat, N.M.; Vrouwenvelder, J.S.; Van Loosdrecht, M.C.M.; Bucs, S.S.; Staal, M. Effect of water temperature on biofouling development in reverse osmosis membrane systems. Water Res. 2016, 103, 149–159. [Google Scholar] [CrossRef]
- Saeki, D.; Karkhanechi, H.; Matsuura, H.; Matsuyama, H. Effect of operating conditions on biofouling in reverse osmosis membrane processes: Bacterial adhesion, biofilm formation, and permeate flux decrease. Desalination 2016, 378, 74–79. [Google Scholar] [CrossRef]
- Dupont. Troubleshooting: Membrane Element Evaluation: Vacuum Decay Test Tech Manual; DuPont de Nemours Inc.: Wilmington, DE, USA, 2019. [Google Scholar]
- Niewersch, C.; Rieth, C.; Hailemariam, L.; Oriol, G.G.; Warczok, J. Reverse osmosis membrane element integrity evaluation using imperfection model. Desalination 2020, 476, 114175. [Google Scholar] [CrossRef]
- Chapman, M.; Linton, W.K. Methods for Monitoring the Integrity of Reverse Osmosis and Nanofiltration Membrane Systems; Department of Interior, Bureau of Reclamation: Denver, CO, USA, 2000.
- Standard Practice for Integrity Testing of Water Filtration Membrane Systems; ASTM International: West Conshohocken, PA, USA, 2003.
- Kruithof, J.C.; Kamp, P.C.; Folmer, H.C.; Nederlof, M.M.; Van Hoof, S.C.J.M. Development of a membrane integrity monitoring strategy for the UF/RO Heemskerk drinking water treatment plant. Water Sci. Technol. Water Supply 2001, 1, 261–271. [Google Scholar] [CrossRef]
- Reclamation. Desalination and Water Purification Research and Development Program Evaluation of Conventional and Membrane Pretreatment for Seawater Reverse Osmosis; Department of Interior, Bureau of Reclamation: Denver, CO, USA, 2005.
- Crozes, G.F.; Sethi, S.; Mi, B.; Curl, J.; Marias, B. Improving membrane integrity monitoring indirect methods to reduce plant downtime and increase microbial removal credit. Desalination 2002, 149, 493–497. [Google Scholar] [CrossRef]
- Adham, S.; Gagliardo, P.; Smith, D.; Ross, D.; Gramith, K.; Trussell, R. Monitoring the integrity of reverse osmosis membranes. Desalination 1998, 119, 143–150. [Google Scholar] [CrossRef]
- Pype, M.L.; Patureau, D.; Wery, N.; Poussade, Y.; Gernjak, W. Monitoring reverse osmosis performance: Conductivity versus fluorescence excitation-emission matrix (EEM). J. Membr. Sci. 2013, 428, 205–211. [Google Scholar] [CrossRef]
- Huang, X.; Min, J.H.; Lu, W.; Jaktar, K.; Yu, C.; Jiang, S.C. Evaluation of methods for reverse osmosis membrane integrity monitoring for wastewater reuse. J. Water Process Eng. 2015, 7, 161–168. [Google Scholar] [CrossRef]
- Fritzmann, C.; Löwenberg, J.; Wintgens, T.; Melin, T. State-of-the-art of reverse osmosis desalination. Desalination 2007, 216, 1–76. [Google Scholar] [CrossRef]
- Vedavyasan, C.V. Pretreatment trends—An overview. Desalination 2007, 203, 296–299. [Google Scholar] [CrossRef]
- Li, W.; Liu, X.; Wang, Y.-N.; Chong, T.H.; Tang, C.Y.; Fane, A.G. Analyzing the Evolution of Membrane Fouling via a Novel Method Based on 3D Optical Coherence Tomography Imaging. Environ. Sci. Technol. 2016, 50, 6930–6939. [Google Scholar] [CrossRef] [PubMed]
- Arnal, J.M.; García-Fayos, B.; Sancho, M. Membrane Cleaning. In Expanding Issues in Desalination Edited; Ning, R.Y., Ed.; Intech Open: Rijeka, Croatia, 2011; ISBN 9789533076249. [Google Scholar]
- Lin, J.C.T.; Lee, D.J.; Huang, C. Membrane fouling mitigation: Membrane cleaning. Sep. Sci. Technol. 2010, 45, 858–872. [Google Scholar] [CrossRef]
- Ebrahim, S. Cleaning and regeneration of membranes in desalination and wastewater applications: State-of-the-art. Desalination 1994, 96, 225–238. [Google Scholar] [CrossRef]
- Obotey Ezugbe, E.; Rathilal, S. Membrane Technologies in Wastewater Treatment: A Review. Membranes 2020, 10, 89. [Google Scholar] [CrossRef] [PubMed]
- Arnal, J.M.; García-Fayos, B.; Sancho, M. Chapter 3: Membrane cleaning. Expand. Issues Desalin. 2011, 63–84. [Google Scholar]
- Membrane Cleaning Technologies. Available online: https://www.lenntech.com/membrane-cleaning.htm (accessed on 7 July 2020).
- Zhao, Y.J.; Wu, K.F.; Wang, Z.J.; Zhao, L.; Li, S.S. Fouling and cleaning of membrane—A literature review. J. Environ. Sci. Engl. 2000, 12, 241–251. [Google Scholar]
- Nath, K. Membrane Separation Processes; PHI Learning Private Limited: Delhi, India, 2017; ISBN 8120352912. [Google Scholar]
- Andes, K.; Bartels, C.R.; Liu, E.; Sheehy Presenter, N.; Manager-Hydranautics, D. Methods for Enhanced Cleaning of Fouled Ro Elements; The International Desalination Association World Congress on Desalination and Water Reuse: Tianjin, China, 2013. [Google Scholar]
- Hacıfazlıoğlu, M.C.; Parlar, İ.; Pek, T.; Kabay, N. Evaluation of chemical cleaning to control fouling on nanofiltration and reverse osmosis membranes after desalination of MBR effluent. Desalination 2019, 466, 44–51. [Google Scholar] [CrossRef]
- Garcia-Fayos, B.; Arnal, J.M.; Gimenez, A.; Álvarez, S.; Sancho, M. Study of ultrasonically enhanced chemical cleaning of SWRO membranes at pilot plant scale. Desalin. Water Treat. 2017, 88, 1–7. [Google Scholar] [CrossRef]
- Thanu, D.P.R.; Zhao, M.; Han, Z.; Keswani, M. Fundamentals and Applications of Sonic Technology. In Developments in Surface Contamination and Cleaning: Applications of Cleaning Techniques; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–48. [Google Scholar]
- Qasim, M.; Darwish, N.N.; Mhiyo, S.; Darwish, N.A.; Hilal, N. The use of ultrasound to mitigate membrane fouling in desalination and water treatment. Desalination 2018, 443, 143–164. [Google Scholar] [CrossRef]
- Kyllönen, H.M.; Pirkonen, P.; Nyström, M. Membrane filtration enhanced by ultrasound: A review. Desalination 2005, 181, 319–335. [Google Scholar] [CrossRef]
- Alventosa-Delara, E.; Barredo-Damas, S.; Alcaina-Miranda, M.I.; Iborra-Clar, M.I. Study and optimization of the ultrasound-enhanced cleaning of an ultrafiltration ceramic membrane through a combined experimental-statistical approach. Ultrason. Sonochem. 2014, 21, 1222–1234. [Google Scholar] [CrossRef]
- Ahmad, A.L.; Che Lah, N.F.; Ismail, S.; Ooi, B.S. Membrane antifouling methods and alternatives: Ultrasound approach. Sep. Purif. Rev. 2012, 41, 318–346. [Google Scholar] [CrossRef]
- Shahkaramipour, N.; Tran, T.N.; Ramanan, S.; Lin, H. Membranes with surface-enhanced antifouling properties for water purification. Membranes 2017, 7, 13. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, Y.; He, M.; Su, Y.; Zhao, X.; Elimelech, M.; Jiang, Z. Antifouling membranes for sustainable water purification: Strategies and mechanisms. Chem. Soc. Rev. 2016, 45, 5888–5924. [Google Scholar] [CrossRef]
- Saqib, J.; Aljundi, I.H. Membrane fouling and modification using surface treatment and layer-by-layer assembly of polyelectrolytes: State-of-the-art review. J. Water Process Eng. 2016, 11, 68–87. [Google Scholar] [CrossRef]
- Son, M.; Yang, W.; Bucs, S.S.; Nava-Ocampo, M.F.; Vrouwenvelder, J.S.; Logan, B.E. Polyelectrolyte-Based Sacrificial Protective Layer for Fouling Control in Reverse Osmosis Desalination. Environ. Sci. Technol. Lett. 2018, 5, 584–590. [Google Scholar] [CrossRef]
- Li, Q.; Chen, G.Q.; Liu, L.; Kentish, S.E. Spray assisted layer-by-layer assembled one-bilayer polyelectrolyte reverse osmosis membranes. J. Membr. Sci. 2018, 564, 501–507. [Google Scholar] [CrossRef]
- Halakoo, E.; Feng, X. Layer-by-layer assembly of polyethyleneimine/graphene oxide membranes for desalination of high-salinity water via pervaporation. Sep. Purif. Technol. 2020, 234, 116077. [Google Scholar] [CrossRef]
- Zhang, Y.; Wan, Y.; Pan, G.; Shi, H.; Yan, H.; Xu, J.; Guo, M.; Wang, Z.; Liu, Y. Surface modification of polyamide reverse osmosis membrane with sulfonated polyvinyl alcohol for antifouling. Appl. Surf. Sci. 2017, 419, 177–187. [Google Scholar] [CrossRef]
- Falath, W.; Sabir, A.; Jacob, K.I. Novel reverse osmosis membranes composed of modified PVA/Gum Arabic conjugates: Biofouling mitigation and chlorine resistance enhancement. Carbohydr. Polym. 2017, 155, 28–39. [Google Scholar] [CrossRef]
- Anis, S.F.; Hashaikeh, R.; Hilal, N. Flux and salt rejection enhancement of polyvinyl(alcohol) reverse osmosis membranes using nano-zeolite. Desalination 2019, 470, 114104. [Google Scholar] [CrossRef]
- Shao, F.; Dong, L.; Dong, H.; Zhang, Q.; Zhao, M.; Yu, L.; Pang, B.; Chen, Y. Graphene oxide modified polyamide reverse osmosis membranes with enhanced chlorine resistance. J. Membr. Sci. 2017, 525, 9–17. [Google Scholar] [CrossRef]
- Nurkhamidah, S.; Devi, B.C.; Febriansyah, B.A.; Ramadhani, A.; Nyamiati, R.D.; Rahmawati, Y.; Chafidz, A. Characteristics of Cellulose Acetate/Polyethylene Glycol membrane with the addition of Graphene Oxide by using surface coating method. IOP Conf. Ser. Mater. Sci. Eng. 2020, 732, 12002. [Google Scholar]
- Gholami, S.; Rezvani, A.; Vatanpour, V.; Cortina, J.L. Improving the chlorine resistance property of polyamide TFC RO membrane by polyethylene glycol diacrylate (PEGDA) coating. Desalination 2018, 443, 245–255. [Google Scholar] [CrossRef]
- Zhang, Y.; Wan, Y.; Guo, M.; Pan, G.; Shi, H.; Yao, X.; Liu, Y. Surface modification on thin-film composite reverse osmosis membrane by cation complexation for antifouling. J. Polym. Res. 2019, 26, 1–12. [Google Scholar] [CrossRef]
- Kang, G.D.; Cao, Y.M. Development of antifouling reverse osmosis membranes for water treatment: A review. Water Res. 2012, 46, 584–600. [Google Scholar] [PubMed]
- Kulkarni, A.; Mukherjee, D.; Gill, W.N. Flux enhancement by hydrophilization of thin film composite reverse osmosis membranes. J. Membr. Sci. 1996, 114, 39–50. [Google Scholar] [CrossRef]
- Miyamoto, K.; Ikeshima, D.; Furutani, T.; Xiao, H.; Yonezu, A.; Chen, X. On the surface hydrophilization of a blended polysulfone membrane: Atomic force microscopy measurement and molecular dynamics simulation. Surf. Topogr. Metrol. Prop. 2019, 7, 35003. [Google Scholar] [CrossRef]
- Wei, X.; Wang, Z.; Zhang, Z.; Wang, J.; Wang, S. Surface modification of commercial aromatic polyamide reverse osmosis membranes by graft polymerization of 3-allyl-5,5-dimethylhydantoin. J. Membr. Sci. 2010, 351, 222–233. [Google Scholar] [CrossRef]
- Isawi, H.; El-Sayed, M.H.; Feng, X.; Shawky, H.; Abdel Mottaleb, M.S. Surface nanostructuring of thin film composite membranes via grafting polymerization and incorporation of ZnO nanoparticles. Appl. Surf. Sci. 2016, 385, 268–281. [Google Scholar] [CrossRef]
- Asadollahi, M.; Bastani, D.; Musavi, S.A. Enhancement of surface properties and performance of reverse osmosis membranes after surface modification: A review. Desalination 2017, 420, 330–383. [Google Scholar] [CrossRef]
- Hu, Y.; Lu, K.; Yan, F.; Shi, Y.; Yu, P.; Yu, S.; Li, S.; Gao, C. Enhancing the performance of aromatic polyamide reverse osmosis membrane by surface modification via covalent attachment of polyvinyl alcohol (PVA). J. Membr. Sci. 2016, 501, 209–219. [Google Scholar]
- Ankoliya, D.; Mehta, B.; Raval, H. Advances in surface modification techniques of reverse osmosis membrane over the years. Sep. Sci. Technol. 2019, 54, 293–310. [Google Scholar] [CrossRef]
- Safarpour, M.; Vatanpour, V.; Khataee, A.; Zarrabi, H.; Gholami, P.; Yekavalangi, M.E. High flux and fouling resistant reverse osmosis membrane modified with plasma treated natural zeolite. Desalination 2017, 411, 89–100. [Google Scholar] [CrossRef]
- Jahangiri, F.; Asadollahi, M.; Mousavi, S.A.; Farhadi, F. Improvement of performance of polyamide reverse osmosis membranes using dielectric barrier discharge plasma treatment as a novel surface modification method. Polym. Eng. Sci. 2019, 59, E468–E475. [Google Scholar] [CrossRef]
- Hirsch, U.; Ruehl, M.; Teuscher, N.; Heilmann, A. Antifouling coatings via plasma polymerization and atom transfer radical polymerization on thin film composite membranes for reverse osmosis. Appl. Surf. Sci. 2018, 436, 207–216. [Google Scholar] [CrossRef]
- Choi, J.; Choi, Y.; Ju, J.; Park, Y.; Lee, S. Inorganic fouling mitigation using the baffle system in direct contact membrane distillation (DCMD). Desalination 2020, 496, 114714. [Google Scholar] [CrossRef]
- Saleem, H.; Zaidi, S.J. Nanoparticles in reverse osmosis membranes for desalination: A state of the art review. Desalination 2020, 475, 114171. [Google Scholar] [CrossRef]
- Farahbaksh, J.; Delnavaz, M.; Vatanpour, V. Investigation of raw and oxidized multiwalled carbon nanotubes in fabrication of reverse osmosis polyamide membranes for improvement in desalination and antifouling properties. Desalination 2017, 410, 1–9. [Google Scholar] [CrossRef]
- Rajakumaran, R.; Boddu, V.; Kumar, M.; Shalaby, M.S.; Abdallah, H.; Chetty, R. Effect of ZnO morphology on GO-ZnO modified polyamide reverse osmosis membranes for desalination. Desalination 2019, 467, 245–256. [Google Scholar] [CrossRef]
- Fathizadeh, M.; Tien, H.N.; Khivantsev, K.; Song, Z.; Zhou, F.; Yu, M. Polyamide/nitrogen-doped graphene oxide quantum dots (N-GOQD) thin film nanocomposite reverse osmosis membranes for high flux desalination. Desalination 2019, 451, 125–132. [Google Scholar] [CrossRef]
- Ragunath, S.; Roy, S.; Mitra, S. Selective hydrophilization of the permeate surface to enhance flux in membrane distillation. Sep. Purif. Technol. 2016, 170, 427–433. [Google Scholar] [CrossRef]
- Tong, T.; Zhao, S.; Boo, C.; Hashmi, S.M.; Elimelech, M. Relating Silica Scaling in Reverse Osmosis to Membrane Surface Properties. Environ. Sci. Technol. 2017, 51, 4396–4406. [Google Scholar] [CrossRef]
- Lin, S.; Huang, H.; Zeng, Y.; Zhang, L.; Hou, L. Facile surface modification by aldehydes to enhance chlorine resistance of polyamide thin film composite membranes. J. Membr. Sci. 2016, 518, 40–49. [Google Scholar] [CrossRef]
- Vatanpour, V.; Zoqi, N. Surface modification of commercial seawater reverse osmosis membranes by grafting of hydrophilic monomer blended with carboxylated multiwalled carbon nanotubes. Appl. Surf. Sci. 2017, 396, 1478–1489. [Google Scholar] [CrossRef]
- Li, K.; Lee, B.; Kim, Y. High performance reverse osmosis membrane with carbon nanotube support layer. J. Membr. Sci. 2019, 592, 117358. [Google Scholar]
- Hu, X.; Sun, J.; Peng, R.; Tang, Q.; Luo, Y.; Yu, P. Novel thin-film composite reverse osmosis membrane with superior water flux using parallel magnetic field induced magnetic multi-walled carbon nanotubes. J. Clean. Prod. 2020, 242, 118423. [Google Scholar] [CrossRef]
- Ahmed, F.E.; Hashaikeh, R.; Hilal, N. Fouling control in reverse osmosis membranes through modification with conductive carbon nanostructures. Desalination 2019, 470, 114118. [Google Scholar]
- Ma, R.; Ji, Y.L.; Weng, X.D.; An, Q.F.; Gao, C.J. High-flux and fouling-resistant reverse osmosis membrane prepared with incorporating zwitterionic amine monomers via interfacial polymerization. Desalination 2016, 381, 100–110. [Google Scholar] [CrossRef]
- Ma, R.; Ji, Y.L.; Guo, Y.S.; Mi, Y.F.; An, Q.F.; Gao, C.J. Fabrication of antifouling reverse osmosis membranes by incorporating zwitterionic colloids nanoparticles for brackish water desalination. Desalination 2017, 416, 35–44. [Google Scholar]
- Yang, Z.; Saeki, D.; Matsuyama, H. Zwitterionic polymer modification of polyamide reverse-osmosis membranes via surface amination and atom transfer radical polymerization for anti-biofouling. J. Membr. Sci. 2018, 550, 332–339. [Google Scholar]
- Li, S.L.; Wu, P.; Wang, J.; Wang, J.; Hu, Y. Fabrication of high performance polyamide reverse osmosis membrane from monomer 4-morpholino-m-phenylenediamine and tailoring with zwitterions. Desalination 2020, 473, 114169. [Google Scholar] [CrossRef]
- Mahdavi, H.; Rahimi, A. Zwitterion functionalized graphene oxide/polyamide thin film nanocomposite membrane: Towards improved anti-fouling performance for reverse osmosis. Desalination 2018, 433, 94–107. [Google Scholar] [CrossRef]
- Khorshidi, B.; Biswas, I.; Ghosh, T.; Thundat, T.; Sadrzadeh, M. Robust fabrication of thin film polyamide-TiO2 nanocomposite membranes with enhanced thermal stability and anti-biofouling propensity. Sci. Rep. 2018, 8, 1–10. [Google Scholar]
- Lee, T.H.; Oh, J.Y.; Hong, S.P.; Lee, J.M.; Roh, S.M.; Kim, S.H.; Park, H.B. ZIF-8 particle size effects on reverse osmosis performance of polyamide thin-film nanocomposite membranes: Importance of particle deposition. J. Membr. Sci. 2019, 570–571, 23–33. [Google Scholar] [CrossRef]
- Armendariz Ontiveros, M.; Quintero, Y.; Llanquilef, A.; Morel, M.; Argentel Martínez, L.; García García, A.; Garcia, A. Anti-Biofouling and Desalination Properties of Thin Film Composite Reverse Osmosis Membranes Modified with Copper and Iron Nanoparticles. Materials 2019, 12, 2081. [Google Scholar] [CrossRef] [PubMed]
- Mamah, S.C.; Goh, P.S.; Ismail, A.F.; Suzaimi, N.D.; Ahmad, N.A.; Lee, W.J. Flux enhancement in reverse osmosis membranes induced by synergistic effect of incorporated palygorskite/chitin hybrid nanomaterial. J. Environ. Chem. Eng. 2021, 9, 105432. [Google Scholar] [CrossRef]
- Ashfaq, M.Y.; Al-Ghouti, M.A.; Zouari, N. Functionalization of reverse osmosis membrane with graphene oxide to reduce both membrane scaling and biofouling. Carbon N. Y. 2020, 166, 374–387. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, X.; Di Yuan, Y.; Yuan, H.; Li, N.; Ying, Y.; Peh, S.B.; Wang, Y.; Cheng, Y.; Cai, Y.; et al. Thin-Film Nanocomposite Membranes Containing Water-Stable Zirconium Metal-Organic Cages for Desalination. ACS Mater. Lett. 2021, 3, 268–274. [Google Scholar]
- Zhao, Q.; Zhao, D.L.; Chung, T.S. Thin-film nanocomposite membranes incorporated with defective ZIF-8 nanoparticles for brackish water and seawater desalination. J. Membr. Sci. 2021, 625, 119158. [Google Scholar] [CrossRef]
- Wenten, I.G. Khoiruddin Reverse osmosis applications: Prospect and challenges. Desalination 2016, 391, 112–125. [Google Scholar] [CrossRef]
| Microorganism | Examples |
|---|---|
| Bacteria | Mycobacterium, Flavobacterium, Pseudomonas, Corynebacterium, Bacillus, Arthrobacte, Acinetobacter, Cytophaga, Moraxella, Micrococcus, Serratia, Lactobacillus, Aeromonas |
| Fungi | Penicillium, Trichoderma, Mucor, Fusarium, Aspergillus |
| LSI Value | Interpretation |
|---|---|
| <0 | Undersaturation of CaCO3 ions in the feedwater; scaling will not form. |
| 0 | Neutral feedwater, the index is inconclusive. |
| >0 | Supersaturation of CaCO3 ions in the feedwater; scaling is highly probable. |
| LSI Value | Interpretation |
|---|---|
| 2 | Non-corrosive scaling |
| 0.5 | Slight corrosive scaling |
| 0 | Neutral, probable pitting corrosion occurrence |
| −0.5 | Primitive corrosion with no scaling |
| −2 | Serious corrosion |
| Index | Membrane | Foulant | Filtration and Operating Mode | Test | Equation | Advantages | Limitations | Refs. |
|---|---|---|---|---|---|---|---|---|
| SDI | MF flat sheet 0.45 µm | Particulate matter | Dead end and constant pressure | t vs. V |
|
| [16,32,57] | |
| SDI+ | MF flat sheet 0.45 µm | Particulate matter | Dead end and constant pressure | t vs. V | - | 1. Considers variation in temperature, pressure, and membrane resistance. |
| [16,32,35,57] |
| SDIv | MF flat sheet 0.45 µm | Particulate matter | Dead end and constant pressure | t vs. V |
| Inaccurate results if the foulants size is smaller than 45 µm. | [16,32,35,57] | |
| MFI | MF flat sheet 0.45 µm | Particulate matter | Dead end and constant pressure | t/V vs. V |
| Inaccurate results if the foulants size is smaller than 45 µm. | [16,32,57] | |
| MFI-UFconst. flux | UF, flat sheet, 10-200 kDa | Colloids | Dead end and constant flux | Δp vs. t or Δt/ΔV vs. V | 1. Most industrial-scale RO filtration processes are constant flow processes. |
| [16,32,57] | |
| NF-MFI | NF | Organic matter | Dead end and constant pressure | t/(V/A) vs. V/A | 1. Able to filter out the organic matter. |
| [16,32,57] | |
| CFS-MFI | MF, UF, and NF | Particulate matter | Crossflow, dead-end, and constant pressure | t/V vs. V |
| Operating under constant pressure. | [16,32,57,58] | |
| CFI | MF and NF | All types of foulants | Constant pressure | t/V vs. V |
|
| [16,32,57] | |
| LSI | All membranes | CaCO3 | - | - |
| Does not quantify how much scale or calcium carbonate would precipitate at equilibrium conditions. | [16,32,57,59] | |
| S&DSI | All membranes | CaCO3 | - | - |
| Inconsiderate of the precipitation reaction kinetics thus fails to consider the induction time required for precipitate formation. | [16,32,57,59] | |
| SI | All membranes | CaCO3, CaSO4, BaSO4 and SiO2 | - | - |
| Ksp value is very sensitive to changes in operative parameters or to the presence of contaminants/impurities. | [16] | |
| MMAS | MF, UF, and NF | Particulate, colloids and organic matter | Dead end and constant pressure | t/V vs. V | - |
|
| [32,60] |
| DFI | MF flat sheet 0.45 µm | Particulate matter | Dead end and constant pressure | t/V vs. V |
| more accuracy and reliability tests are needed to validate the test | [32,61] |
| Model | Characteristics | Equation |
|---|---|---|
| 1 | Standard blocking, intermediate pore blockage, and cake formation | |
| 2 | Standard blocking, complete pore blockage, and cake formation (using the Hagen–Poiseuille law) | |
| 3 | Standard blocking, intermediate pore blockage, and cake formation (using the Hagen–Poiseuille law) | |
| 4 | Zero-order standard blocking, complete pore blockage, cake formation | |
| 5 | Zero-order standard blocking, intermediate pore blockage, and cake formation |
| Monitoring Technique | Membrane | Mode | Description | Advantages | Limitations | Refs. |
|---|---|---|---|---|---|---|
| Pressure decay test | MF, UF, and NF | Offline | The pressure test uses a low-pressure air supply that is applied to the permeate side of the membrane. If the membrane’s integrity is compromised or suffers from leak spots; air would pass through. The air transfer would occur only if the applied pressure exceeds the defected site’s bubble point pressure. | 1. Suitable for a wide array of membranes; from RO to MF, and a vast range of configurations; including hollow fibers, tubular and flat sheets. |
| [90,92,93] |
| Vacuum decay test | NF and RO | Offline | The membrane element is placed into a clean water bath for several hours, then drained. The permeate tube is plugged then a vacuum is applied to measure vacuum decay rates. If the decay rate exceeds 10kPa/min, then the membrane’s integrity is compromised. | 1. Suitable for a wide array of membranes; from RO to MF, and a vast range of configurations; including hollow fibers, tubular and flat sheets. | 1. Applicable to separate elements in a system, not to the full system. | [67,90,92] |
| Particles tracking | MF and UF | Online | The concentration of a specific particle is tracked in both the feed and the permeate. | 1. Simple and rapid solution for membrane fouling analysis. |
| [92,94] |
| Turbidity monitoring | MF and UF | Online | Turbidity levels are measured in both the feed and the permeate. | 1. Simple and rapid solution for membrane fouling analysis. |
| [95,96] |
| TOC monitoring | NF and RO | Online | TOC concentrations are measured in both the feed and the permeate. | 1. Suitable for a wide array of membranes; from RO to NF, and a vast range of configurations; including hollow fibers, tubular and flat sheets. Applicable to full-scale setups. | Expensive equipment. | [92,93,97] |
| Sulfate tracking | NF and RO | Offline | Sulfate concentrations are measured in both the feed and the permeate. | 1. Suitable for a wide array of membranes; from RO to NF, and a vast range of configurations; including hollow fibers, tubular and flat sheets. Applicable to full-scale setups. | Expensive equipment. | [67] |
| Conductivity monitoring | NF and RO | Online | The conductivity is monitored in both the feed and the permeate. | Can assess the performance of critical control points. | Time-consuming. | [97,98] |
| Marker-based test (challenge test or seeding method) | MF, UF, NF, and RO | Offline | The feed is supplemented by microorganisms which are then tracked to the permeate. | 1. Assesses viruses’ removal efficiency New markers, like fluorescence-tagged nanoparticles, can provide high-resolution results. | 1. Requires seeding. Expensive. | [67,91] |
| Pulse integrity test | NF and RO | Online | The pulse of a highly rejected particle, i.e., magnesium sulfate is measured and monitored. |
| 1. Unsuitable for NF membranes as the elements themselves have substantial conductivity which can alter the accuracy of the test’s results. | [67,91] |
| Fluorescence excitation-emission matrix spectroscopy | NF and RO | Online | The concentration of microspheres in both the feed and the permeate is measured by fluorescence spectroscopy. | Up to 4 log10 removal reported.Results can be cross-checked with conductivity-based tests. | 1. Expensive due to the cost of particles. | [91,98] |
| Flow cytometry | NF and Ro | Offline | An optical analysis approach to quantify and characterize cells in a liquid matrix. | 1. Highly sensitive, thus accurate and can detect a wide range of membrane integrity problems. |
| [91,99] |
| Conventional Pretreatment Method | Advantages | Disadvantages |
|---|---|---|
| Coagulation/flocculation |
|
|
| Chlorination |
|
|
| Media filtration |
|
|
| Acidification |
|
|
| Ozonation | 1. Does not affect the integrity of the feed in terms of odor or taste. |
|
| DAF | 1. Cost-effective. | 1. Scraper-related issues. |
| Scale inhibitors | 1. Inhibits crystallization-induced scale formation. | 1. Overdosing of scale inhibitors can cause detrimental damage to RO membranes. |
| UV |
| 1. Can lead to biofilm formation. |
| Aspect of Comparison | Conventional Pretreatment Methods | Membrane Pretreatment Methods |
|---|---|---|
| Capital cost | lower than non-conventional membrane-based methods | Higher than conventional methods but new developments are causing costs to decline. |
| Carbon footprint | High | Low |
| Energy requirements | Low | High |
| Chemical costs | High | Low |
| Quality of permeate | SDI < 4 for 90% of the time inconsistent quality indicators. Turbidity: <1.0 NTU. | SDI < 2.5 for 100% of the time. Constant quality indicators. Turbidity < 0.1 NTU. |
| Method | Modifier | Test Conditions | Permeate Flux (Lm−2 h−1) | Salt Rejection (%) | Ref. |
|---|---|---|---|---|---|
| Surface coating | PDDA and PSS | 2000 ppm NaCl solution at 4.1 MPa | 15.5 | 99 | [122] |
| LbL surface coating | Pluronic F127 amphiphilic triblock copolymer | 2 g/L NaCl solution at 4 MPa | 30 | 94 | [123] |
| LbL surface coating | PEI and GO | 200 g/L NaCl at 65 °C | 8.4 kg m−2 h−1 | 99.9 | [124] |
| Surface coating | SPVA | 2000 ppm NaCl solution at 1.55 MPa | 42.6 | 99.18 | [125] |
| Surface coating | Pluronic F127 and Gum arabic | 2000 mg/L NaCl solution at 55.2 bar | - | 98 | [126] |
| Slip casting | Nano zeolite-Y | 25,000 mg/L NaCl solution at 25 bar | 5.1 | 99.52 | [127] |
| Spin coating | GO | 1 mg/mL NaCl solution at 1.5 MPa and 25 °C | - | 95.3 | [128] |
| Surface coating | GO | - | 1356 | 37 | [129] |
| Surface coating | PEGDA | 2000 ppm NaCl solution at 20 bar and 25 °C | - | 99 | [130] |
| Cation complexation | PEG | 2000 ppm NaCl solution at 1.55 MPa | 40.8 | 99.04 | [131] |
| Hydrophilization treatment | PVP | - | - | - | [134] |
| Hydrophilization treatment | Chromic acid | 60 °C | 61 | - | [148] |
| Free radical grafting | ADMH | 2000 ppm NaCl solution at 1.5 MPa and 25 °C | 184.5 | 95.8 | [135] |
| Free radical grafting | ZnO NPs | 2000 mg/l NaCl solution at 15 bar and 25 °C | 35 | 97 | [136] |
| Radical grafting | ADMH | 35mM NaCl solution at 27.6 bar and 22 °C | - | 99.1 | [149] |
| Chemical coupling | PVA | 500 mg/L NaCl solution at 5 bar and 25 °C | 27 | 98.46 | [138] |
| Chemical coupling | Aldehydes | 2000 ppm NaCl solution at 1.6MPa and 25 °C | 37.5 | 98.6 | [150] |
| Glow discharge plasma treatment | Clinoptilolite | 16,000 ppm NaCl solution at 1.5 MPa and 25 °C | - | 97.12 | [140] |
| Dielectric barrier discharge plasma treatment | - | - | - | - | [141] |
| Plasma polymerization and si-ATRP | HEMA, MPC, and SBMA | - | 6042 | 99 | [142] |
| Modifier | Test Conditions | Permeate flux (Lm−2 h−1) | Salt rejection (%) | Ref. |
|---|---|---|---|---|
| Carboxylated CNT | 2000 mg/L NaCl and 500 mg/L BSA solutions at 15 bar and 25 °C | - | 94 | [151] |
| CNT | 2000 mg/L NaCl solution at 15 bar and 25 °C | 25.9 | 96 | [145] |
| CNT | 2000 mg/L NaCl solution at 15.5 bar | 128.6 | 98.3 | [152] |
| Magnetic multi-walled CNT | 2 g/L NaCl, 2 g/L Na2SO4, 2 g/L MgSO4 solutions at 1 MPa and 25 °C | 11.39 (NaCl), 10.84 (Na2SO4) and 11.10 (MgSO4) | 97.04 (NaCl), 96 (Na2SO4) and 95.31 (MgSO4) | [153] |
| CNT | 25,000 ppm NaCl solution at 24 bar | 1.78 | 99.91 | [154] |
| Zwitterionic diamine monomer N-aminoethyl piperazine | 2000 ppm NaCl solution at 1.5 MPa and 25 °C | 54.5 | 98.3 | [155] |
| Zwitterionic colloid nanoparticles | 2000 ppm NaCl solution at 1.5 MPa and 25 °C | 37.3 | 96.5 | [156] |
| Zwitterionic polymer | 0.85 wt% NaCl solution at 1.5 MPa and 30 °C | 50.48 | 96.9 | [157] |
| Zwitterionic monomer | 2000 ppm NaCl solution at 15 bar and 25 °C | 24.75 | 98.5 | [158] |
| Zwitterionic GO | 1000 ppm NaCl solution at 12 bar | 17.52 | 94.8 | [159] |
| TiO2 NPs | 2000 ppm NaCl solution at 1.52 MPa and 65 °C | 24.3 | 97 | [160] |
| GO-ZnO | 2000 mg/L NaCl solution at 20 bar and 25 °C | 31.42 | 96.3 | [146] |
| N-GOQD | 2000 ppm NaCl solution at 15 bar | 24.9 | 93 | [147] |
| ZIF-8 | 2 g/L NaCl solution at 15.5 bar and 25 °C | 51.925 | 98.5 | [161] |
| Fe NPs and Cu NPs | 1000mg/L NaCl solution at 300 psi and 25 °C | 8.4 (Fe NPs) and 3 (Cu NPs) | 92.96 (Fe NPs) and 74.36 (Cu NPs) | [162] |
| palygorskite-chitin (PAL-CH) hybrid nanomaterial | 2000 ppm NaCl solution at 15 bar | 2.4 | 93, 95, 98.5, and 96.6 for PA/PSF-PAL-CH1, PA/PSF-PAL-CH2, PA/PSF-PAL-CH3 and PA/PSF-PAL-CH4 | [163] |
| GO | 800 mg/L CaCl2 and Na2SO4 at 25℃ and 20 bar | - | 98 | [164] |
| zirconium metal–organic cages | 2000 ppm NaCl at 25 °C and 15.5 bar | 22.79 | 94.7 | [165] |
| dZIF-8 | 2000 ppm NaCl solution at 20 bar (brackish water) and 32,000 ppm NaCl at 50 bar (seawater) | 52.2 (brackish water), 38 (seawater) | 98.6 (brackish water), 98.8 (seawater) | [166] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
AlSawaftah, N.; Abuwatfa, W.; Darwish, N.; Husseini, G. A Comprehensive Review on Membrane Fouling: Mathematical Modelling, Prediction, Diagnosis, and Mitigation. Water 2021, 13, 1327. https://doi.org/10.3390/w13091327
AlSawaftah N, Abuwatfa W, Darwish N, Husseini G. A Comprehensive Review on Membrane Fouling: Mathematical Modelling, Prediction, Diagnosis, and Mitigation. Water. 2021; 13(9):1327. https://doi.org/10.3390/w13091327
Chicago/Turabian StyleAlSawaftah, Nour, Waad Abuwatfa, Naif Darwish, and Ghaleb Husseini. 2021. "A Comprehensive Review on Membrane Fouling: Mathematical Modelling, Prediction, Diagnosis, and Mitigation" Water 13, no. 9: 1327. https://doi.org/10.3390/w13091327
APA StyleAlSawaftah, N., Abuwatfa, W., Darwish, N., & Husseini, G. (2021). A Comprehensive Review on Membrane Fouling: Mathematical Modelling, Prediction, Diagnosis, and Mitigation. Water, 13(9), 1327. https://doi.org/10.3390/w13091327