Establishing Baseline Assessment Levels for Monitoring Coastal Heavy Metals Using Foraminiferal Shells: A Case Study from the Southeastern Mediterranean
Abstract
:1. Introduction
2. Materials and Methods
2.1. Field Sampling
2.2. Sample Preparation
2.3. Geochemical Analyses
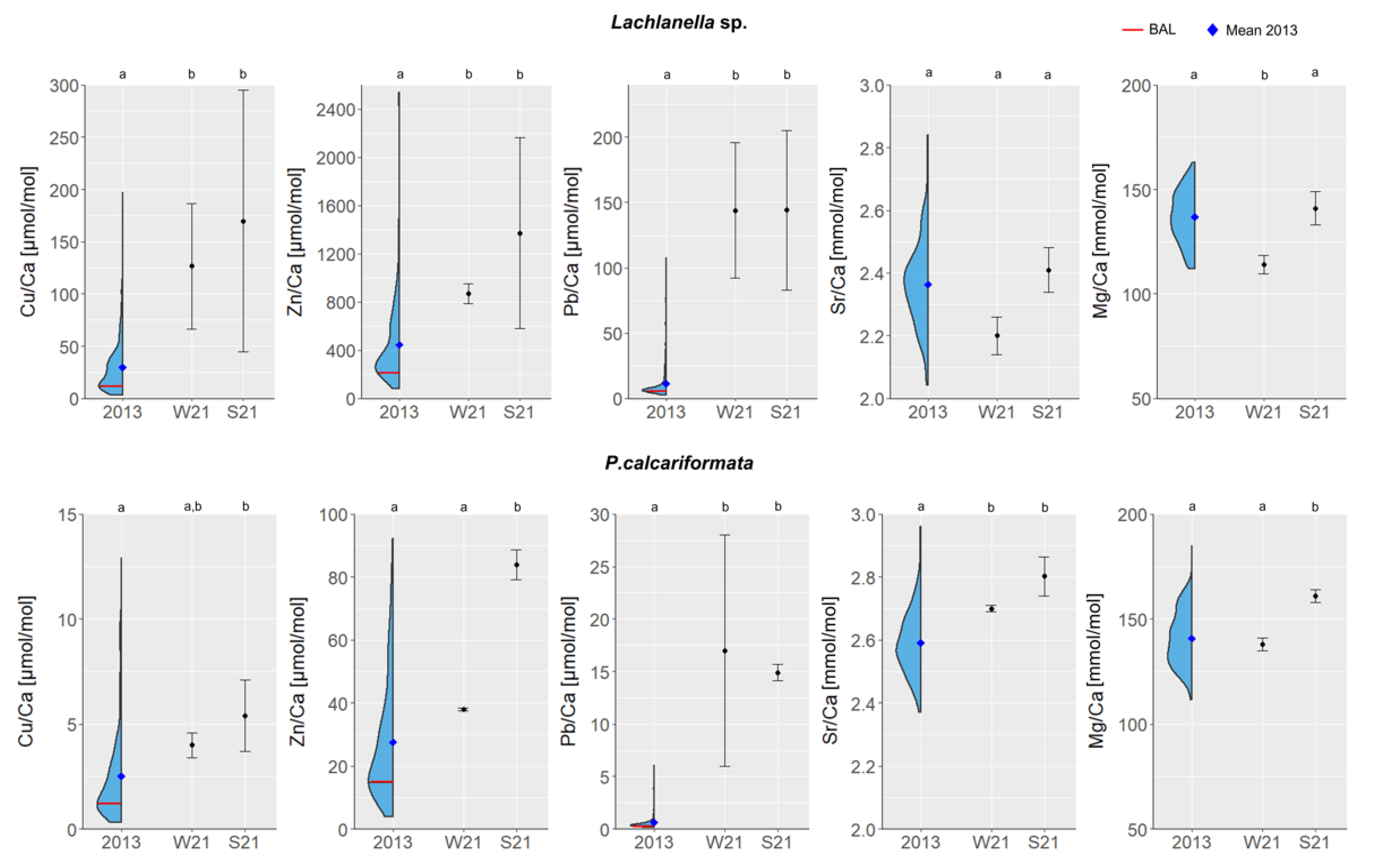
3. Results
Metals/Ca Records of Lachlanella sp. and P. calcariformata
4. Discussion
4.1. Calculation of Baseline Assessment Levels (BALs)
- The fifth lower percentile of the metal/Ca LA-ICPMS dataset of each species, as a cut off value of the lower distribution tail of each metal, representing the relative best (clean) water quality at the time of sampling, considering the analytical/treatment potential error. The cut off values were chosen based on the principle adopted for assessing the pivot values for heavy metals in sediments, which is the concentration of elements in a non-polluted sand fraction [44]. The pivot values are supposed to be the lowest possible concentrations of normalized heavy metals, and the assessment of the pivot values is performed by comparing them to the ranked heavy metal concentrations of all available data [45,46]. The pivot values are supposed to be close to the lowest tail of ranked concentrations, which explains why we chose the fifth lower percentile in this study.
- Addition of natural metal/Ca variability in seawater using the variability of Sr/Ca in each species as a non-anthropogenic proxy. Strontium is a very abundant alkaline earth metal whose concentration in seawater is of an overwhelmingly natural origin [47], and is one of the most common elements substituted for calcium in calcitic shells (e.g., [9]). Thus, the observed variability in Sr/Ca was used here as a tracer of the natural variation recorded in the shells related to seawater composition. The ±2 standard deviation (in RSD%) of the observed variation in Sr/Ca was determined as 12% for Lachlanella and 8% for P. calcariformata, which were used as an indication for the natural metal/Ca variations, and thus added to the defined level in 1 above.
- Addition of biological natural variability (variations within a population, and within individual specimens) was assessed based on laboratory culturing experiments per species. These variations for each metal/Ca were assessed based on the laboratory culturing of the two species from unpublished data associated with the study of Titelboim et al. (2017) [31] (see Supplementary Excel File S2) that were exposed to similar conditions (temperature, salinity, light and seawater HM composition), and analyzed by LA-ICPMS. The relative standard deviation (RSD%) for each metal/Ca was used to evaluate the natural biological variability between and within specimens (i.e., biological noise [48]).
4.2. Application of the BAL and Implications for Future Monitoring Efforts
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zuykov, M.; Pelletier, E.; Harper, D.A.T. Bivalve mollusks in metal pollution studies: From bioaccumulation to biomonitoring. Chemosphere 2013, 93, 201–208. [Google Scholar] [CrossRef]
- Jan, A.T.; Azam, M.; Siddiqui, K.; Ali, A.; Choi, I.; Haq, Q.M.R. Heavy Metals and Human Health: Mechanistic Insight into Toxicity and Counter Defense System of Antioxidants. Int. J. Mol. Sci. 2015, 16, 29592–29630. [Google Scholar] [CrossRef] [Green Version]
- Batley, G.E. Electroanalytical techniques for the determination of heavy metals in seawater. Mar. Chem. 1983, 12, 107–117. [Google Scholar] [CrossRef]
- Phillips, D.J.H. The use of biological indicator organisms to monitor trace metal pollution in marine and estuarine environments-a review. Environ. Pollut. 1977, 13, 281–317. [Google Scholar] [CrossRef]
- Edge, K.J.; Dafforn, K.A.; Simpson, S.L.; Roach, A.C.; Johnston, E.L. A biomarker of contaminant exposure is effective in large scale assessment of ten estuaries. Chemosphere 2014, 100, 16–26. [Google Scholar] [CrossRef]
- Goldberg, E.D.; Bertine, K.K. Beyond the Mussel Watch—new directions for monitoring marine pollution. Sci. Total Environ. 2000, 247, 165–174. [Google Scholar] [CrossRef]
- Steinhardt, J.; Butler, P.G.; Carroll, M.L.; Hartley, J. The Application of Long-Lived Bivalve Sclerochronology in Environmental Baseline Monitoring. Front. Mar. Sci. 2016, 3, 176. [Google Scholar] [CrossRef] [Green Version]
- Lingard, S.M.; Evans, R.D.; Bourgoin, B.P. Method for the Estimation of Organic-Bound and Crystal-Bound Metal Concentrations in Bivalve Shells. Bull. Environ. Contam. Toxicol 1992, 48, 179–184. [Google Scholar] [CrossRef]
- Sen Gupta, B.K.; Lea, D.W. Trace elements in foraminiferal calcite. In Modern Foraminifera; Springer: Berlin/Heidelberg, Germany, 1999; pp. 259–277. [Google Scholar]
- Katz, M.E.; Cramer, B.S.; Franzese, A.; Hönisch, B.; Miller, K.G.; Rosenthal, Y.; Wright, J.D. Traditional and emerging geochemical proxies in foraminifera. J. Foraminifer. Res. 2010, 40, 165–192. [Google Scholar] [CrossRef]
- Alve, E. Benthic forameniferal responses to esturine pollution: A review. J. Foraminifer. Res. 1995, 25, 190–203. [Google Scholar] [CrossRef]
- Nigam, R.; Saraswat, R.; Panchang, R. Application of foraminifers in ecotoxicology: Retrospect, perspect and prospect. Environ. Int. 2006, 32, 273–283. [Google Scholar] [CrossRef]
- Alve, E.; Korsun, S.; Schönfeld, J.; Dijkstra, N.; Golikova, E.; Hess, S.; Husum, K.; Panieri, G. Foram-AMBI: A sensitivity index based on benthic foraminiferal faunas from North-East Atlantic and Arctic fjords, continental shelves and slopes. Mar. Micropaleontol. 2016, 122, 1–12. [Google Scholar] [CrossRef]
- Schönfeld, J.; Alve, E.; Geslin, E.; Jorissen, F.J.; Korsun, S.; Spezzaferri, S.; Abramovich, S.; Almogi-Labin, A.; du Chatelet, E.A.; Barras, C.; et al. The FOBIMO (FOraminiferal BIo-MOnitoring) initiative-Towards a standardised protocol for soft-bottom benthic foraminiferal monitoring studies. Mar. Micropaleontol. 2012, 94–95, 1–13. [Google Scholar] [CrossRef]
- Bouchet, V.; Alve, E.; Rygg, B.; Telford, R.J. Benthic foraminifera provide a promising tool for ecological quality assessment of marine waters. Ecol. Indic. 2012, 23, 66–75. [Google Scholar] [CrossRef]
- de Nooijer, L.J.; Reichart, G.-J.; Dueñas-Bohórquez, A.; Wolthers, M.; Ernst, S.R.; Mason, P.R.D.; Van Der Zwaan, G.J. Copper incorporation in foraminiferal calcite: Results from culturing experiments. Biogeosciences 2007, 4, 493–504. [Google Scholar] [CrossRef] [Green Version]
- Frontalini, F.; Buosi, C.; Da Pelo, S.; Coccioni, R.; Cherchi, A.; Bucci, C. Benthic foraminifera as bio-indicators of trace element pollution in the heavily contaminated Santa Gilla lagoon (Cagliari, Italy). Mar. Pollut. Bull. 2009, 58, 858–877. [Google Scholar] [CrossRef]
- Munsel, D.; Kramar, U.; Dissard, D.; Nehrke, G.; Berner, Z.; Bijma, J.; Reichart, G.-J.; Neumann, T. Incorporation of ‘hydrothermal’ elements in foraminiferal calcite: Results from culturing experiments. Results Cult. Exp. 2010, 10, 2339. [Google Scholar] [CrossRef] [Green Version]
- Nardelli, M.P.; Malferrari, D.; Ferretti, A.; Bartolini, A.; Sabbatini, A.; Negri, A. Zinc incorporation in the miliolid foraminifer Pseudotriloculina rotunda under laboratory conditions. Mar. Micropaleontol. 2016, 126, 42–49. [Google Scholar] [CrossRef]
- Smith, C.W.; Fehrenbacher, J.S.; Goldstein, S.T. Incorporation of heavy metals in experimentally grown foraminifera from Sapelo Island, Georgia and Little Duck Key, Florida, USA. Mar. Micropaleontol. 2020, 156, 101854. [Google Scholar] [CrossRef]
- Titelboim, D.; Sadekov, A.; Hyams-Kaphzan, O.; Almogi-Labin, A.; Herut, B.; Kucera, M.; Abramovich, S. Foraminiferal single chamber analyses of heavy metals as a tool for monitoring permanent and short term anthropogenic footprints. Mar. Pollut. Bull. 2018, 128, 65–71. [Google Scholar] [CrossRef]
- Youssef, M. Heavy metals contamination and distribution of benthic foraminifera from the Red Sea coastal area, Jeddah, Saudi Arabia. Oceanologia 2015, 57, 236–250. [Google Scholar] [CrossRef] [Green Version]
- Titelboim, D.; Sadekov, A.; Blumenfeld, M.; Almogi-Labin, A.; Herut, B.; Halicz, L.; Benaltabet, T.; Torfstein, A.; Kucera, M.; Abramovich, S. Monitoring of heavy metals in seawater using single chamber foraminiferal sclerochronology. Ecol. Indic. 2021, 120, 106931. [Google Scholar] [CrossRef]
- Oron, S.; Sadekov, A.; Katz, T.; Goodman-Tchernov, B. Benthic foraminifera geochemistry as a monitoring tool for heavy metal and phosphorus pollution—A post fish-farm removal case study. Mar. Pollut. Bull. 2021, 168, 112443. [Google Scholar] [CrossRef]
- Schmidt, S.; Hathorne, E.C.; Schönfeld, J.; Garbe, D. Heavy metal uptake of near-shore benthic foraminifera during multi-metal culturing experiments. Biogeosciences 2022, 19, 629–664. [Google Scholar] [CrossRef]
- Boyle, E.A. Cadmium, zinc, copper, and barium in foraminifera tests. Earth Planet. Sci. Lett. 1981, 53, 11–35. [Google Scholar] [CrossRef]
- Elderfield, H.; Bertram, C.J.; Erez, J. A biomineralization model for the incorporation of trace elements into foraminiferal calcium carbonate. Earth Planet. Sci. Lett. 1996, 142, 409–423. [Google Scholar] [CrossRef]
- Erez, J. The Source of Ions for Biomineralization in Foraminifera and Their Implications for Paleoceanographic Proxies. Rev. Mineral. Geochem. 2003, 54, 115–149. [Google Scholar] [CrossRef] [Green Version]
- Havach, S.M.; Chandler, G.T.; Wilson-Finelli, A.; Shaw, T.J. Experimental determination of trace element partition coefficients in cultured benthic foraminifera. Geochim. Cosmochim. Acta 2001, 65, 1277–1283. [Google Scholar] [CrossRef]
- Barras, C.; Mouret, A.; Nardelli, M.P.; Metzger, E.; Petersen, J.; La, C.; Filipsson, H.L.; Jorissen, F. Experimental calibration of manganese incorporation in foraminiferal calcite. Geochim. Cosmochim. Acta 2018, 237, 49–64. [Google Scholar] [CrossRef]
- Titelboim, D.; Sadekov, A.; Almogi-Labin, A.; Herut, B.; Kucera, M.; Schmidt, C.; Hyams-Kaphzan, O.; Abramovich, S. Geochemical signatures of benthic foraminiferal shells from a heat-polluted shallow marine environment provide field evidence for growth and calcification under extreme warmth. Glob. Change Biol. 2017, 23, 4346–4353. [Google Scholar] [CrossRef]
- Titelboim, D.; Almogi-Labin, A.; Herut, B.; Kucera, M.; Schmidt, C.; Hyams-Kaphzan, O.; Ovadia, O.; Abramovich, S. Selective responses of benthic foraminifera to thermal pollution. Mar. Pollut. Bull. 2016, 105, 324–336. [Google Scholar] [CrossRef]
- Schmidt, C.; Morard, R.; Almogi-Labin, A.; Weinmann, A.E.; Titelboim, D.; Abramovich, S.; Kucera, M. Recent Invasion of the Symbiont-Bearing Foraminifera Pararotalia into the Eastern Mediterranean Facilitated by the Ongoing Warming Trend. PLoS ONE 2015, 10, e0132917. [Google Scholar] [CrossRef]
- Schmidt, C.; Titelboim, D.; Brandt, J.; Herut, B.; Abramovich, S.; Almogi-Labin, A.; Kucera, M. Extremely heat tolerant photo-symbiosis in a shallow marine benthic foraminifera. Sci. Rep. 2016, 6, 30930. [Google Scholar] [CrossRef]
- Manda, S.; Titelboim, D.; Ashckenazi-Polivoda, S.; Almogi-Labin, A.; Herut, B.; Abramovich, S. Epiphytic benthic foraminiferal preferences for macroalgal habitats: Implications for coastal warming. Mar. Environ. Res. 2020, 161, 105084. [Google Scholar] [CrossRef]
- Fehrenbacher, J.S.; Spero, H.J.; Russell, A.D.; Vetter, L.; Eggins, S. Optimizing LA-ICP-MS analytical procedures for elemental depth profiling of foraminifera shells. Chem. Geol. 2015, 407–408, 2–9. [Google Scholar] [CrossRef] [Green Version]
- Benaltabet, T.; Gutner-Hoch, E.; Torfstein, A. Heavy Metal, Rare Earth Element and Pb Isotope Dynamics in Mussels During a Depuration Experiment in the Gulf of Aqaba, Northern Red Sea. Front. Mar. Sci. 2021, 8, 2001. [Google Scholar] [CrossRef]
- Biller, D.V.; Bruland, K.W. Analysis of Mn, Fe, Co, Ni, Cu, Zn, Cd, and Pb in seawater using the Nobias-chelate PA1 resin and magnetic sector inductively coupled plasma mass spectrometry (ICP-MS). Mar. Chem. 2012, 130–131, 12–20. [Google Scholar] [CrossRef]
- Yoshiki, S.; Shouhei Urushihara, S.N.; Tomoharu Minami, K.N. Multielemental Determination of GEOTRACES Key Trace Metals in Seawater by ICPMS after Preconcentration Using an Ethylenediaminetriacetic Acid Chelating Resin. Anal. Chem. 2008, 80, 6267–6273. [Google Scholar]
- Middag, R.; Rolison, J.M.; George, E.; Gerringa, L.J.A.; Rijkenberg, M.J.A.; Stirling, C.H. Basin scale distributions of dissolved manganese, nickel, zinc and cadmium in the Mediterranean Sea. Mar. Chem. 2022, 238, 104063. [Google Scholar] [CrossRef]
- Schumacker, R.; Tomek, S. Understanding Statistics Using R; Springer: New York, NY, USA, 2013. [Google Scholar]
- Van Dijk, I.; de Nooijer, L.J.; Reichart, G.-J. Trends in element incorporation in hyaline and porcelaneous foraminifera as a function of pCO2. Biogeosciences 2017, 14, 497–510. [Google Scholar] [CrossRef] [Green Version]
- Sagar, N.; Sadekov, A.; Scott, P.; Jenner, T.; Vadiveloo, A.; Moheimani, N.R.; McCulloch, M. Geochemistry of large benthic foraminifera Amphisorus hemprichii as a high-resolution proxy for lead pollution in coastal environments. Mar. Pollut. Bull. 2021, 162, 111918. [Google Scholar] [CrossRef] [PubMed]
- Kersten, M.; Smedes, F. Normalization procedures for sediment contaminants in spatial and temporal trend monitoring. J. Environ. Monit. 2002, 4, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Assessment of data collected under the Co-ordinated Environmental Monitoring Programme. In Proceedings of the OSPAR Commission, London, UK, 4 May 2005; p. 115.
- Herut, B.; Sandler, A. Normalization methods for pollutants in marine sediments: Review and recommendations for the Mediterranean. IOLR Rep. H 2006, 18, 23. [Google Scholar]
- Lebrato, M.; Garbe-, D.; Müller, M.N.; Blanco-Ameijeiras, S.; Feely, R.A.; Lorenzoni, L.; Molinero, J.C.; Bremer, K.; Jones, D.O.B.; Iglesias-Rodriguez, D.; et al. Global variability in seawater Mg:Ca and Sr:Ca ratios in the modern ocean. Proc. Natl. Acad. Sci. USA 2020, 117, 22281–22292. [Google Scholar] [CrossRef] [PubMed]
- De Nooijer, L.J.; Hathorne, E.C.; Reichart, G.J.; Langer, G.; Bijma, J. Variability in calcitic Mg/Ca and Sr/Ca ratios in clones of the benthic foraminifer Ammonia tepida. Mar. Micropaleontol. 2014, 107, 32–43. [Google Scholar] [CrossRef] [Green Version]

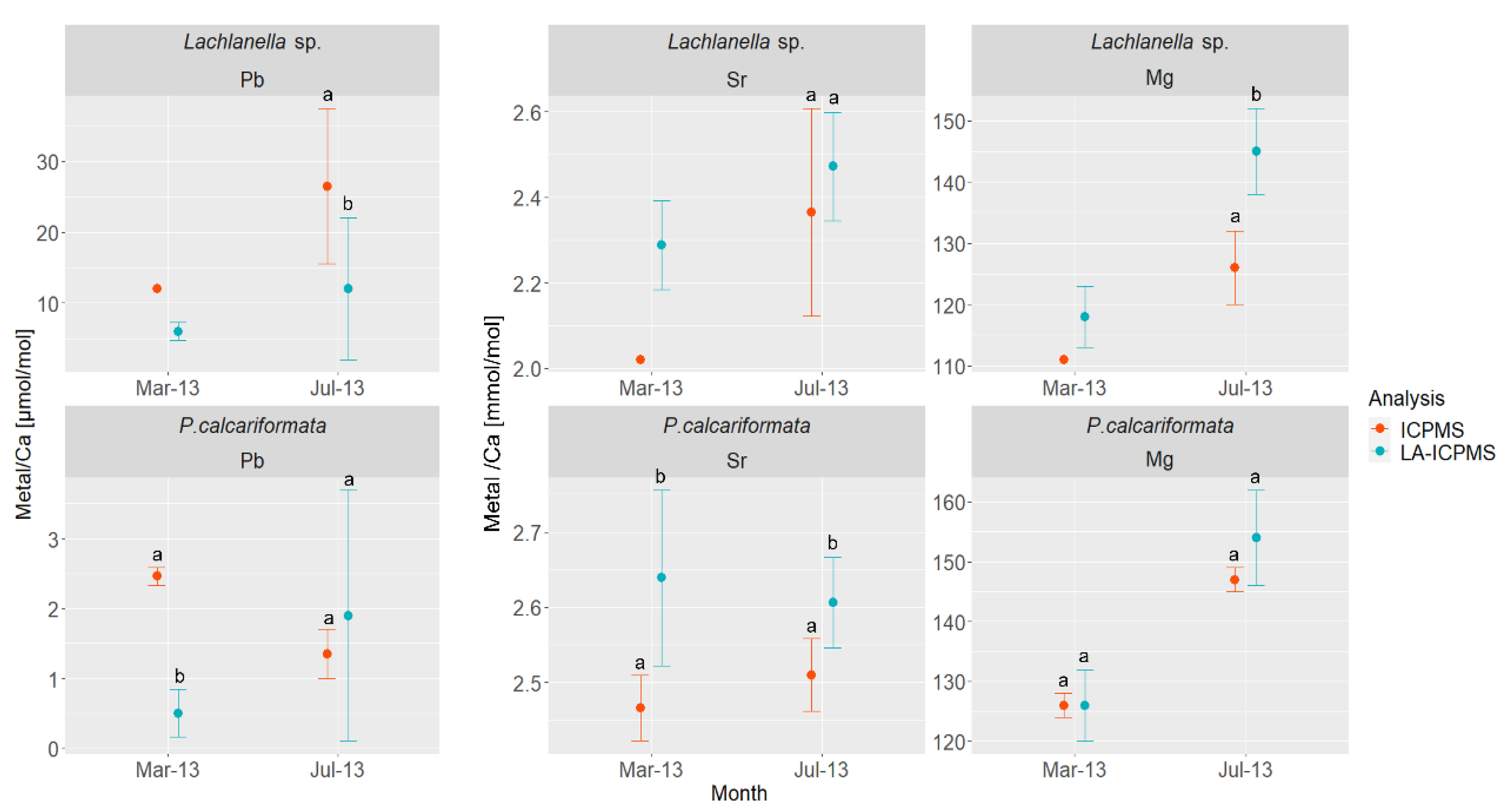

| Analytical Method | Date | n | N | Cu/Ca [μmol/mol] | DCu | Zn/Ca [μmol/mol] | DZn | Pb/Ca [μmol/mol] | DPb × 103 | Sr/Ca [mmol/mol] | Mg/Ca [mmol/mol] |
|---|---|---|---|---|---|---|---|---|---|---|---|
| ICPMS | March 2021 | 4 | 20 | 130 ± 60 | 39 | 870 ± 90 | 286 | 145 ± 50 | 5.1 | 2.22 ± 0.06 | 110 ± 4 |
| August 2021 | 6 | 20 | 170 ± 130 | 29 | 1370 ± 800 | 285 | 144 ± 60 | 2.9 | 2.41 ± 0.07 | 140 ± 8 | |
| LA-ICPMS | March 2013–January 2014 | 246 | 30 ± 25 | 450 ± 310 | 11 ± 13 | 2.36 ± 0.13 | 140 ± 10 | ||||
| March 2013 | 40 | 20 ± 20 | 220 ± 50 | 6 ± 1 | 2.29 ± 0.10 | 120 ± 5 | |||||
| July 2013 | 40 | 30 ± 20 | 600 ± 300 | 12 ± 10 | 2.47 ± 0.13 | 145 ± 7 | |||||
| ICPMS | March 2013 | 1 | 20 | N/A | N/A | 12 | 2.02 | 111 | |||
| July 2013 | 5 | 20 | N/A | N/A | 30 ± 10 | 2.34 ± 0.22 | 130 ± 6 |
| Analytical Method | Date | n | N | Cu/Ca [μmol/mol] | DCu | Zn/Ca [μmol/mol] | DZn | Pb/Ca [μmol/mol] | DPb × 103 | Sr/Ca [mmol/mol] | Mg/Ca [mmol/mol] |
|---|---|---|---|---|---|---|---|---|---|---|---|
| ICPMS | March 2021 | 4 | 100 | 4 ± 1 | 18 | 40 ± 1 | 169 | 17 ± 10 | 2.9 | 2.70 ± 0.01 | 138 ± 3 |
| August 2021 | 6 | 50 | 5 ± 2 | 21 | 84 ± 5 | 170 | 15 ± 1 | 1.9 | 2.80 ± 0.06 | 161 ± 3 | |
| LA-ICPMS | March 2013–January 2014 | 167 | 3 ± 2 | 31 ± 24 | 0.6 ± 0.8 | 2.59 ± 0.10 | 141 ± 13 | ||||
| March 2013 | 25 | 4 ± 3 | 19 ± 1 | 0.5 ± 0.3 | 2.64 ± 0.12 | 126 ± 6 | |||||
| July 2013 | 67 | 4 ± 3 | 15 ± 12 | 1.9 ± 1.8 | 2.61 ± 0.06 | 154 ± 8 | |||||
| ICPMS | March 2013 | 2 | 50 | N/A | N/A | 2.5 ± 0.1 | 2.47 ± 0.04 | 126 ± 2 | |||
| July 2013 | 4 | 50 | N/A | N/A | 1.4 ± 0.4 | 2.51 ± 0.05 | 147 ± 2 |
| Species | Rotaliid/Miliolid | Cu/Ca [μmol/mol] | Zn/Ca [μmol/mol] | Pb/Ca [μmol/mol] | Sr/Ca [mmol/mol] | Mg/Ca [mmol/mol] | Reference |
|---|---|---|---|---|---|---|---|
| Pseudotriloculina rotunda | M | 1122 ± 30 | [19] | ||||
| Amphistegina lobifera | R | 21 ± 7 | 0.76 ± 0.49 | [23] | |||
| Amphistegina lessonii | R | 31 ± 27 | 0.53 ± 0.25 | ||||
| Operculina ammonoides | R | 0.5 ± 0.5 | 13 ± 9 | 2.52 ± 0.17 | 158 ± 11 | [24] | |
| Archaias angulatus | M | 88 ± 5 | 2.20 ± 0.01 | 138 ± 1 | [42] | ||
| Sorites marginalis | M | 74 ± 11 | 2.00 ± 0.01 | 144 ± 1 | |||
| Laevipeneroplis bradyi | M | 74 ± 6 | 2.20 ± 0.01 | 136 ± 1 | |||
| Peneroplis pertusus | M | 53 ± 11 | 2.10 ± 0.07 | 126 ± 2 | |||
| Heterostegina antillarum | R | 36 ± 15 | 2.70 ± 0.02 | 141.3 ± 0.3 | |||
| Planorbulina acervalis | R | 32 ± 7 | 3.10 ± 0.02 | 140 ± 1 | |||
| Amphisorus hemprichii | M | 2.4 ± 0.2 | [43] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoober, L.; Titelboim, D.; Abramovich, S.; Herut, B.; Teutsch, N.; Benaltabet, T.; Torfstein, A. Establishing Baseline Assessment Levels for Monitoring Coastal Heavy Metals Using Foraminiferal Shells: A Case Study from the Southeastern Mediterranean. Water 2022, 14, 1532. https://doi.org/10.3390/w14101532
Hoober L, Titelboim D, Abramovich S, Herut B, Teutsch N, Benaltabet T, Torfstein A. Establishing Baseline Assessment Levels for Monitoring Coastal Heavy Metals Using Foraminiferal Shells: A Case Study from the Southeastern Mediterranean. Water. 2022; 14(10):1532. https://doi.org/10.3390/w14101532
Chicago/Turabian StyleHoober, Lin, Danna Titelboim, Sigal Abramovich, Barak Herut, Nadya Teutsch, Tal Benaltabet, and Adi Torfstein. 2022. "Establishing Baseline Assessment Levels for Monitoring Coastal Heavy Metals Using Foraminiferal Shells: A Case Study from the Southeastern Mediterranean" Water 14, no. 10: 1532. https://doi.org/10.3390/w14101532






