Visible Light Photocatalyst and Antibacterial Activity of BFO (Bismuth Ferrite) Nanoparticles from Honey
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Section
2.3. Characterisation
2.4. Antibacterial Activity
2.5. Photocatalytic Activity
3. Results and Discussions
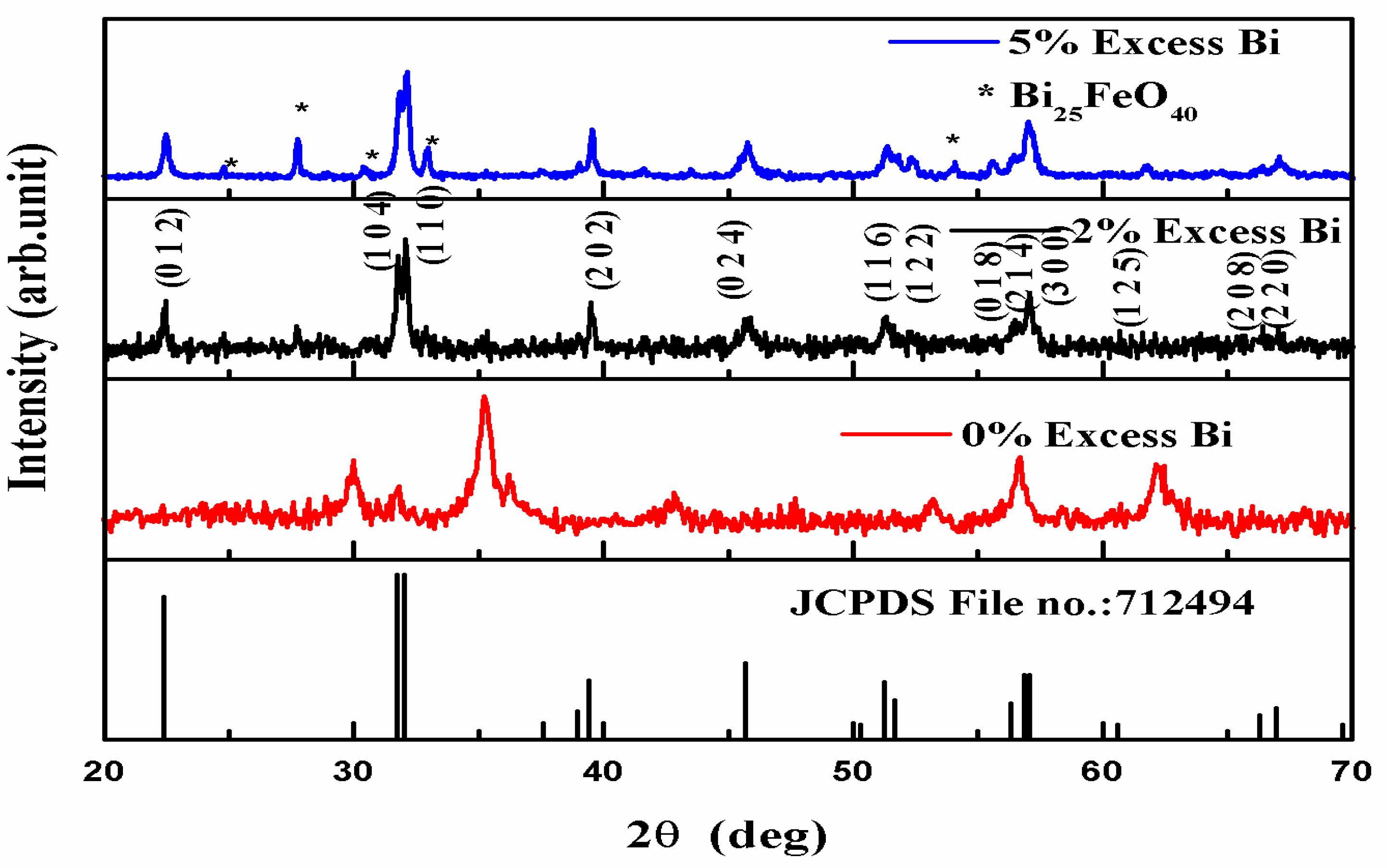
3.1. XRD Analysis
3.2. FTIR Analysis
3.3. UV-DRS Analysis
3.4. PL Analysis
3.5. FESEM Analysis
3.6. XPS Analysis
3.7. Antibacterial Activity
3.8. Photocatalytic Dye Degradation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rouhani, Z.; Karimi-Sabet, J.; Mehdipourghazi, M.; Hadi, A.; Dastbaz, A. Response surface optimization of hydrothermal synthesis of Bismuth ferrite nanoparticles under supercritical water conditions: Application for photocatalytic degradation of Tetracycline. Environ. Nanotechnol. Monit. Manag. 2019, 11, 100198. [Google Scholar] [CrossRef]
- Zeljković, S.; Ivas, T.; Maruyama, H.; Nino, J.C. Structural, magnetic and optical properties of BiFeO3 synthesized by the solvent-deficient method. Ceram. Int. 2019, 45, 19793–19798. [Google Scholar] [CrossRef]
- Altaf, S.; Ali, K.; Khan, H.M.; Sardar, K.; Kamran, K.; Raza, M.A. Low Temperature Synthesis and Characterization of Bismuth Ferrite (Bi2Fe4O9) Nanoparticles by Using Hydrothermal Method. Dig. J. Nanomater. Biostructures 2019, 14, 727–733. [Google Scholar]
- Gautam, A.; Singh, K.; Sen, K.; Kotnala, R.; Singh, M. Crystal structure and magnetic property of Nd doped BiFeO3 nanocrytallites. Mater. Lett. 2011, 65, 591–594. [Google Scholar] [CrossRef]
- Priyadharsini, P.; Pradeep, A.; Sathyamoorthy, B.; Chandrasekaran, G. Enhanced multiferroic properties in La and Ce co-doped BiFeO3 nanoparticles. J. Phys. Chem. Solids 2014, 75, 797–802. [Google Scholar] [CrossRef]
- Muneeswaran, M.; Giridharan, N.V. Effect of Dy-substitution on the structural, vibrational, and multiferroic properties of BiFeO3 nanoparticles. J. Appl. Phys. 2014, 115, 214109. [Google Scholar] [CrossRef]
- Ishikawa, K.L. Nonlinear optical response of graphene in time domain. Phys. Rev. B 2010, 82, 201402. [Google Scholar] [CrossRef] [Green Version]
- Arora, M.; Sati, P.C.; Chauhan, S.; Singh, H.; Yadav, K.L.; Chhoker, S.; Kumar, M. Structural, magnetic and optical properties of Bi1–xDyxFeO3 nanoparticles synthesized by sol–gel method. Mater. Lett. 2013, 96, 71–73. [Google Scholar] [CrossRef]
- Wang, L.; Han, Y.; Jia, G.; Zhang, C.; Liu, Y.; Liu, L.; Yin, K. Facile preparation, characterization and photocatalytic properties of multifunctional BiFeO3 nanocrystals. J. Nanosci. Nanotechnol. 2011, 11, 5207–5209. [Google Scholar] [CrossRef]
- Feng, Y.-N.; Wang, H.-C.; Luo, Y.-D.; Shen, Y.; Lin, Y.-H. Ferromagnetic and photocatalytic behaviors observed in Cadoped BiFeO3 nanofibres. J. Appl. Phys. 2013, 113, 146101. [Google Scholar] [CrossRef]
- Chakrabarti, K.; Sarkar, B.; Ashok, V.D.; Das, K.; Chaudhuri, S.S.; Mitra, A.; De, S.K. Exchange bias effect in BiFeO3-NiO nanocomposite. J. Appl. Phys. 2014, 115, 013906. [Google Scholar] [CrossRef]
- Hasan, M.; Islam, M.F.; Mahbub, R.; Hossain, M.S.; Hakim, M.A. A soft chemical route to the synthesis of BiFeO3 nanoparticles with enhanced magnetization. Mater. Res. Bull. 2016, 73, 179–186. [Google Scholar] [CrossRef]
- Li, S.; Nechache, R.; Davalos, I.A.V.; Goupil, G.; Nikolova, L.; Nicklaus, M.; Laverdiere, J.; Ruediger, A.; Rosei, F. Ultrafast Microwave Hydrothermal Synthesis of BiFeO3 Nanoplates. J. Am. Ceram. Soc. 2013, 96, 3155–3162. [Google Scholar] [CrossRef]
- Fang, L.; Liu, J.; Ju, S.; Zheng, F.; Dong, W.; Shen, M. Experimental and theoretical evidence of enhanced ferromagnetism in sonochemical synthesized BiFeO3 nanoparticles. Appl. Phys. Lett. 2010, 97, 242501. [Google Scholar] [CrossRef]
- Khan, U.S.; Adeela, N.; Javed, K.; Riaz, S.; Ali, H.H.; Iqbal, M.; Han, X.F.; Naseem, S. Influence of cobalt doping on structural and magnetic properties of BiFeO3 nanoparticles. J. Nanoparticle Res. 2015, 17, 429. [Google Scholar] [CrossRef]
- Sharma, S.; Bhattacharya, A. Drinking water contamination and treatment techniques. Appl. Water Sci. 2017, 7, 1043–1067. [Google Scholar] [CrossRef] [Green Version]
- Naushad, M.; Sharma, G.; Alothman, Z.A. Photodegradation of toxic dye using Gum Arabic-crosslinked-poly (acrylamide)/Ni (OH) 2/FeOOH nanocomposites hydrogel. J. Clean. Prod. 2019, 241, 118263. [Google Scholar] [CrossRef]
- Ali, I.; Alharbi, O.M.L.; Alothman, Z.A.; Al-Mohaimeed, A.M.; Alwarthan, A. Modeling of fenuron pesticide adsorption on CNTs for mechanistic insight and removal in water. Environ. Res. 2019, 170, 389–397. [Google Scholar] [CrossRef]
- Wabaidur, S.M.; Khan, M.A.; Siddiqui, M.R.; Otero, M.; Jeon, B.H.; Alothman, Z.A.; Hakami, A.A.H. Oxygenated functionalities enriched MWCNTs decorated with silica coated spinel ferrite—A nanocomposite for potentially rapid and efficient de-colorization of aquatic environment. J. Mol. Liq. 2020, 317, 113916. [Google Scholar] [CrossRef]
- Alothman, Z.A. A Review: Fundamental Aspects of Silicate Mesoporous Materials. Materials 2012, 5, 2874–2902. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.A.; Alqadami, A.A.; Wabaidur, S.M.; Siddiqui, M.R.; Jeon, B.H.; Alshareef, S.A.; Alothman, Z.A.; Hamedelniel, A.E. Oil industry waste based non-magnetic and magnetic hydrochar to sequester potentially toxic post-transition metal ions from water. J. Hazard. Mater. 2020, 400, 123247. [Google Scholar] [CrossRef] [PubMed]
- Satar, N.S.A.; Adnan, R.; Lee, H.L.; Hall, S.R.; Kobayashi, T.; Kassim, M.H.M.; Kaus, N.H.M. Facile green synthesis of ytrium-doped BiFeO3 with highly efficient photocatalytic degradation towards methylene blue. Ceram. Int. 2019, 45, 15964–15973. [Google Scholar] [CrossRef]
- Abushad, M.; Khan, W.; Naseem, S.; Husain, S.; Nadeem, M.; Ansari, A. Influence of Mn doping on microstructure, optical, dielectric and magnetic properties of BiFeO3 nanoceramics synthesized via sol–gel method. Ceram. Int. 2019, 45, 7437–7445. [Google Scholar] [CrossRef]
- Wang, Z.B.; Aldalbahi, A.; Ahamad, T.; Alshehri, S.M.; Feng, P.X. Preparation of BiFeO3 and its photoelectric performance as photoanode of DSSC. Ceram. Int. 2021, 47, 27565–27570. [Google Scholar] [CrossRef]
- Aliyu, A.; Srivastava, C. Correlation between growth texture, crystallite size, lattice strain and corrosion behavior of cop-per-carbon nanotube composite coatings. Surf. Coat. Technol. 2021, 405, 126596. [Google Scholar] [CrossRef]
- Ranjithkumar, B.; Ramalingam, H.; Srinivas, C.; Magesh, G.; Rahale, C.S.; El-Metwaly, N.M.; Shekar, B.C. Natural fuels (Honey and Cow urine) assisted combustion synthesis of zinc oxide nanoparticles for antimicrobial activities. Ceram. Int. 2021, 47, 14475–14481. [Google Scholar] [CrossRef]
- Ichangi, A.; Lê, K.; Queraltó, A.; Grosch, M.; Weissing, R.; Ünlü, F.; Chijioke, A.K.; Verma, A.; Fischer, T.; Surmenev, R.; et al. Electrospun BiFeO3 Nanofibers for Vibrational Energy Harvesting Application. Adv. Eng. Mater. 2021. [Google Scholar] [CrossRef]
- Kossar, S.; Amiruddin, R.; Rasool, A. Study on thickness-dependence characteristics of bismuth ferrite (BFO) for ultraviolet (UV) photodetector application. Micro Nano Syst. Lett. 2021, 9, 1–10. [Google Scholar] [CrossRef]
- Sharmila, M.; Jothi Mani, R.; Kader, A.; Ahmad, A.; Eldesoky, G.E.; Yahya, A.E.; Bahajjaj, A.A.A. Photocatalytic and Biological Activity of ZnO Nanoparticles Using Honey. Coatings 2021, 11, 1046. [Google Scholar] [CrossRef]
- Chen, X.; Liu, B.; Li, X.; An, T.T.; Zhou, Y.; Li, G.; Wu-Smart, J.; Alvarez, S.; Naldrett, M.J.; Eudy, J.; et al. Identification of anti-inflammatory vesicle-like nanoparticles in honey. J. Extracell. Vesicles 2021, 10, e12069. [Google Scholar] [CrossRef]
- Vishwakarma, A.K.; Tripathi, P.; Srivastava, A.; Sinha, A.; Srivastava, O. Band gap engineering of Gd and Co doped BiFeO3 and their application in hydrogen production through photoelectrochemical route. Int. J. Hydrogen Energy 2017, 42, 22677–22686. [Google Scholar] [CrossRef]
- Bharathkumar, S.; Sakar, M.; Vinod, K.R.; Balakumar, S. Versatility of electrospinning in the fabrication of fibrous mat and mesh nanostructures of bismuth ferrite (BiFeO3) and their magnetic and photocatalytic activities. Phys. Chem. Chem. Phys. 2015, 17, 17745–17754. [Google Scholar] [CrossRef] [PubMed]
- Aghdam, T.R.; Mehrizadeh, H.; Salari, D.; Tseng, H.H.; Niaei, A.; Amini, A. Photocatalytic removal of NOx over immobilized BiFeO3 nanoparticles and effect of operational parameters. Korean J. Chem. Eng. 2018, 35, 994–999. [Google Scholar] [CrossRef]
- Pattnaik, S.P.; Behera, A.; Martha, S.; Acharya, R.; Parida, K. Synthesis, photoelectrochemical properties and solar light-induced photocatalytic activity of bismuth ferrite nanoparticles. J. Nanoparticle Res. 2018, 20, 10. [Google Scholar] [CrossRef]
- Prashanthi, K.; Thakur, G.; Thundat, T. Surface enhanced strong visible photoluminescence from one-dimensional multiferroic BiFeO3 nanostructures. Surf. Sci. 2012, 606, L83–L86. [Google Scholar] [CrossRef]
- Mishra, D.K.; Qi, X. Energy levels and photoluminescence properties of nickel-doped bismuth ferrite. J. Alloys Compd. 2010, 504, 27–31. [Google Scholar] [CrossRef]
- Prashanthi, K.; Gupta, M.; Tsui, Y.Y.; Thundat, T. Effect of annealing atmosphere on microstructural and photoluminescence characteristics of multiferroic BiFeO3 thin films prepared by pulsed laser deposition technique. Appl. Phys. A 2013, 110, 903–907. [Google Scholar] [CrossRef]
- Miriyala, N.; Prashanthi, K.; Thundat, T. Oxygen vacancy dominant strong visible photoluminescence from BiFeO3 nanotubes. Phys. Status Solidi (RRL)—Rapid Res. Lett. 2013, 7, 668–671. [Google Scholar] [CrossRef]
- Li, J.; Huang, X.; Zhao, X.; Chen, L.J.; Yan, X.P. pH-Responsive Torpedo-Like Persistent Luminescence Nanoparticles for Autofluorescence-Free Biosensing and High-Level Information Encryption. Angew. Chem. 2021, 133, 2428–2435. [Google Scholar] [CrossRef]
- Ramazanov, S.; Sobola, D.; Ţălu, Ş.; Orudzev, F.; Arman, A.; Kaspar, P.; Dallaev, R.; Ramazanov, G. Multiferroic behavior of the func-tionalized surface of a flexible substrate by deposition of Bi2O3 and Fe2O3. Microsc. Res. Tech. 2022, 85, 1300–1310. [Google Scholar] [CrossRef]
- Tian, C.; Yao, Q.; Tong, Z.; Zhou, H.; Rao, G.; Deng, J.; Wang, Z.; Wang, J. Effects of Sm-doping on microstructure, magnetic and microwave absorption properties of BiFeO3. J. Rare Earths 2021, 39, 835–843. [Google Scholar] [CrossRef]
- Ahamad, T.; Aldalbahi, A.; Alshehri, S.M.; Alotaibi, S.; Alzahly, S.; Wang, Z.B.; Feng, P.X. Enhanced photovoltaic performance of dye-sensitized solar cells based Ag2O doped BiFeO3 hetrostructures. Sol. Energy 2021, 220, 758–765. [Google Scholar] [CrossRef]
- Ramadan, W.; Feldhoff, A.; Bahnemann, D. Assessing the photocatalytic oxygen evolution reaction of BiFeO3 loaded with IrO2 nanoparticles as cocatalyst. Sol. Energy Mater. Sol. Cells 2021, 232, 111349. [Google Scholar] [CrossRef]
- Amrillah, T.; Hermawan, A.; Yin, S.; Juang, J.Y. Formation and physical properties of the self-assembled BFO–CFO vertically aligned nanocomposite on a CFO-buffered two-dimensional flexible mica substrate. RSC Adv. 2021, 11, 15539–15545. [Google Scholar] [CrossRef]
- Gajendiran, J.; Raj, S.G.; Kumar, G.R.; Gnanam, S.; Ramya, J.R.; Senthil, V.P. Photoluminescence Properties and Antibacterial Activity of BiFeO3 and BiFeO3-CoFe2O4 Composite. J. Electron. Mater. 2022, 51, 8–16. [Google Scholar] [CrossRef]
- Rameshkumar, C.; Gayathri, R.; Subalakshmi, R. Synthesis and characterization of undopped bismuth ferrite oxide nanoparticles for the application of cancer treatment. Mater. Today Proc. 2021, 43, 3662–3665. [Google Scholar] [CrossRef]
- Chouker, M.A.; Abdallah, H.; Zeiz, A.; El-Dakdouki, M.H. Host-quest inclusion complex of quinoxaline-1, 4-dioxide derivative with 2-hydroxypropyl-β-cyclodextrin: Preparation, characterization, and antibacterial activity. J. Mol. Struct. 2021, 1235, 130273. [Google Scholar] [CrossRef]
- Ahmed, H.M.; Roy, A.; Wahab, M.; Ahmed, M.; Othman-Qadir, G.; Elesawy, B.H.; Khandaker, M.U.; Islam, M.N.; Emran, T.B. Applications of nanomaterials in agri food and pharmaceutical industry. J. Nanomater. 2021, 2021, 1472096. [Google Scholar] [CrossRef]
- Nabavi, S.; Behzad, S. Herbal Drugs and Natural Products in the light of Nanotechnology and Nanomedicine for Developing Drug Formulations. Mini-Rev. Med. Chem. 2021, 21, 302–313. [Google Scholar] [CrossRef]
- Roy, A.; Ananda Murthy, H.C.; Ahmed, H.M.; Islam, M.N.; Prasad, R. Phytogenic Synthesis of Metal/Metal Oxide Nanoparticles for Degradation of Dyes. J. Renew. Mater. 2022, 10, 1911. [Google Scholar] [CrossRef]
- Shimi, A.K.; Ahmed, H.M.; Wahab, M.; Katheria, S.; Wabaidur, S.M.; Eldesoky, G.E.; Islam, M.A.; Rane, K.P. Synthesis and Ap-plications of Green Synthesized TiO2 Nanoparticles for Photocatalytic Dye Degradation and Antibacterial Activity. J. Nanomater. 2022, 2022, 7060388. [Google Scholar] [CrossRef]
- Pan, Z.; Liu, M.; Zhang, G.; Zhuzhang, H.; Wang, X. Molecular Triazine–Heptazine Junctions Promoting Exciton Dissociation for Overall Water Splitting with Visible Light. J. Phys. Chem. C 2021, 125, 9818–9826. [Google Scholar] [CrossRef]
- Pan, Z.; Zhao, M.; Zhuzhang, H.; Zhang, G.; Anpo, M.; Wang, X. Gradient Zn-Doped Poly Heptazine Imides Integrated with a van der Waals Homojunction Boosting Visible Light-Driven Water Oxidation Activities. ACS Catal. 2021, 11, 13463–13471. [Google Scholar] [CrossRef]
- Siddique, M.; Khan, N.M.; Saeed, M. Photocatalytic Activity of Bismuth Ferrite Nanoparticles Synthesized via Sol-Gel Route. Z. Für Phys. Chem. 2019, 233, 595–607. [Google Scholar] [CrossRef]
- Puhan, A.; Bhushan, B.; Satpathy, S.; Meena, S.S.; Nayak, A.; Rout, D. Facile single phase synthesis of Sr, Co co-doped BiFeO3 nanoparticles for boosting photocatalytic and magnetic properties. Appl. Surf. Sci. 2019, 493, 593–604. [Google Scholar] [CrossRef]
- Ponraj, C.; Vinitha, G.; Daniel, J. Photocatalytic degradation of acid red-85 dye by nickel substituted bismuth ferrite nanoparticles. Mater. Res. Express 2019, 6, 084006. [Google Scholar] [CrossRef]
- Jaffari, Z.; Lam, S.-M.; Sin, J.-C.; Zeng, H. Boosting visible light photocatalytic and antibacterial performance by decoration of silver on magnetic spindle-like bismuth ferrite. Mater. Sci. Semicond. Process. 2019, 101, 103–115. [Google Scholar] [CrossRef]
- Irfan, S.; Zhuanghao, Z.; Li, F.; Chen, Y.-X.; Liang, G.-X.; Luo, J.-T.; Ping, F. Critical review: Bismuth ferrite as an emerging visible light active nanostructured photocatalyst. J. Mater. Res. Technol. 2019, 8, 6375–6389. [Google Scholar] [CrossRef]
- Dumitru, R.; Ianculescu, A.; Păcurariu, C.; Lupa, L.; Pop, A.; Vasile, B.S.; Surdu, V.-A.; Manea, F. BiFeO3-synthesis, characterization and its photocatalytic activity towards doxorubicin degradation from water. Ceram. Int. 2019, 45, 2789–2802. [Google Scholar] [CrossRef]
- Duan, Q.; Kong, F.; Han, X.; Jiang, Y.; Liu, T.; Chang, Y.; Zhou, L.; Qin, G.; Zhang, X. Synthesis and characterization of morphology-controllable BiFeO3 particles with efficient photocatalytic activity. Mater. Res. Bull. 2019, 112, 104–108. [Google Scholar] [CrossRef]








| Parameters | Different Precursors of BiFeO3 Nanoparticles Samples | Standard JCPDS File No.: 71-2494 | |
|---|---|---|---|
| 2%Excess | 5%Excess | ||
| a = b (Å) | 5.5651 | 5.5696 | 5.587 |
| c (Å) | 13.8760 | 13.8714 | 13.867 |
| V (Å)3 | 372.1664 | 372.6507 | 374.94 |
| Sample Name | Crystallite Size (nm) | Microstrain (m−4) | Dislocation Density (m−2) |
|---|---|---|---|
| 0% Excess | 19.2 | 7.04 × 10−5 | 2.71 × 1017 |
| 2% Excess | 10.51 | 1.66 × 10−3 | 9.05 × 1017 |
| 5% Excess | 26.84 | 7.04 × 10−4 | 1.39 × 1017 |
| S. No. | Nanoparticles | Dye | Source | Degradation % | Time (Min) | XRD (nm) | Reference |
|---|---|---|---|---|---|---|---|
| 1. | BFO | RB | Visible | 94 | 180 | 14.38 | [54] |
| 2. | BFO | RhB | Visible | 62.3 | 60 | 50.63 | [55] |
| 3. | BFO | AR-85 | Visible | 60 | 60 | 57 | [56] |
| 4. | Ag-BFO | SY | Visible | 84.4 | 240 | - | [57] |
| 5. | Ag-BFO | MB | Visible | 91.2 | 240 | - | [58] |
| 6. | Se-BFO | CR | Visible | 95 | 120 | - | [58] |
| 7. | BFO | DOX | UV | 79 | 160 | 17.6 | [59] |
| 8. | BFO | MB | Visible | 60 | 120 | - | [60] |
| 9. | BFO | MB | Sunlight | 87 | 120 | 14 | [22] |
| 10. | BFO | MB | Sunlight | 96.6 | 180 | 14 | [22] |
| 11. | Y-BFO | MB | Sunlight | 92.6 | 120 | 14 | [22] |
| 12. | Y-BFO | MB | Sunlight | 97.6 | 180 | 14 | [22] |
| 13. | BFO | TC | UV | 92 | 180 | 29.46 | [1] |
| 14. | BFO | TC | Visible | 99 | 180 | 29.46 | [1] |
| 15. | BFO | MB | Visible | 66 | 120 | 19.2 | Current work |
| 16. | BFO | MB | Visible | 81 | 120 | 10.51 | Current work |
| 17. | BFO | MB | Visible | 68 | 120 | 26.8 | Current work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharmila, M.; Mani, R.J.; Parvathiraja, C.; Kader, S.M.A.; Siddiqui, M.R.; Wabaidur, S.M.; Islam, M.A.; Lai, W.-C. Visible Light Photocatalyst and Antibacterial Activity of BFO (Bismuth Ferrite) Nanoparticles from Honey. Water 2022, 14, 1545. https://doi.org/10.3390/w14101545
Sharmila M, Mani RJ, Parvathiraja C, Kader SMA, Siddiqui MR, Wabaidur SM, Islam MA, Lai W-C. Visible Light Photocatalyst and Antibacterial Activity of BFO (Bismuth Ferrite) Nanoparticles from Honey. Water. 2022; 14(10):1545. https://doi.org/10.3390/w14101545
Chicago/Turabian StyleSharmila, M., R. Jothi Mani, C. Parvathiraja, S. M. Abdul Kader, Masoom Raza Siddiqui, Saikh Mohammad Wabaidur, Md Ataul Islam, and Wen-Cheng Lai. 2022. "Visible Light Photocatalyst and Antibacterial Activity of BFO (Bismuth Ferrite) Nanoparticles from Honey" Water 14, no. 10: 1545. https://doi.org/10.3390/w14101545
APA StyleSharmila, M., Mani, R. J., Parvathiraja, C., Kader, S. M. A., Siddiqui, M. R., Wabaidur, S. M., Islam, M. A., & Lai, W.-C. (2022). Visible Light Photocatalyst and Antibacterial Activity of BFO (Bismuth Ferrite) Nanoparticles from Honey. Water, 14(10), 1545. https://doi.org/10.3390/w14101545








