Food Sources for Benthic Grazers in Trophic Networks of Macrophyte Habitats in a Transitional Baltic Ecosystem
Abstract
1. Introduction
2. Materials and Methods
2.1. Characteristics of the Sampling Sites
2.2. Sample Collection
2.3. Sample Preparation and Analysis
- -
- Three food sources (macrophytes, their epiphytes, and sedimentary organic matter and seston < 150 µm (small)) to estimate the diet of four species of gastropods at both sites and habitats (Table S1);
- -
- Six food sources (epiphytes on C. contraria, epiphytes on S. pectinata, sedimentary organic matter, seston < 150 µm (small), epiphytes of C. glomerata and seston > 150 µm (large), macrophytes) to estimate the diet of amphipods at the northern site;
- -
- Six food sources (C. contraria, P. perfoliatus, epiphytes on different macrophyte species, seston > 150 µm (large), sedimentary organic matter and seston < 150 µm (small)) to estimate the diet of amphipods at the southern site.
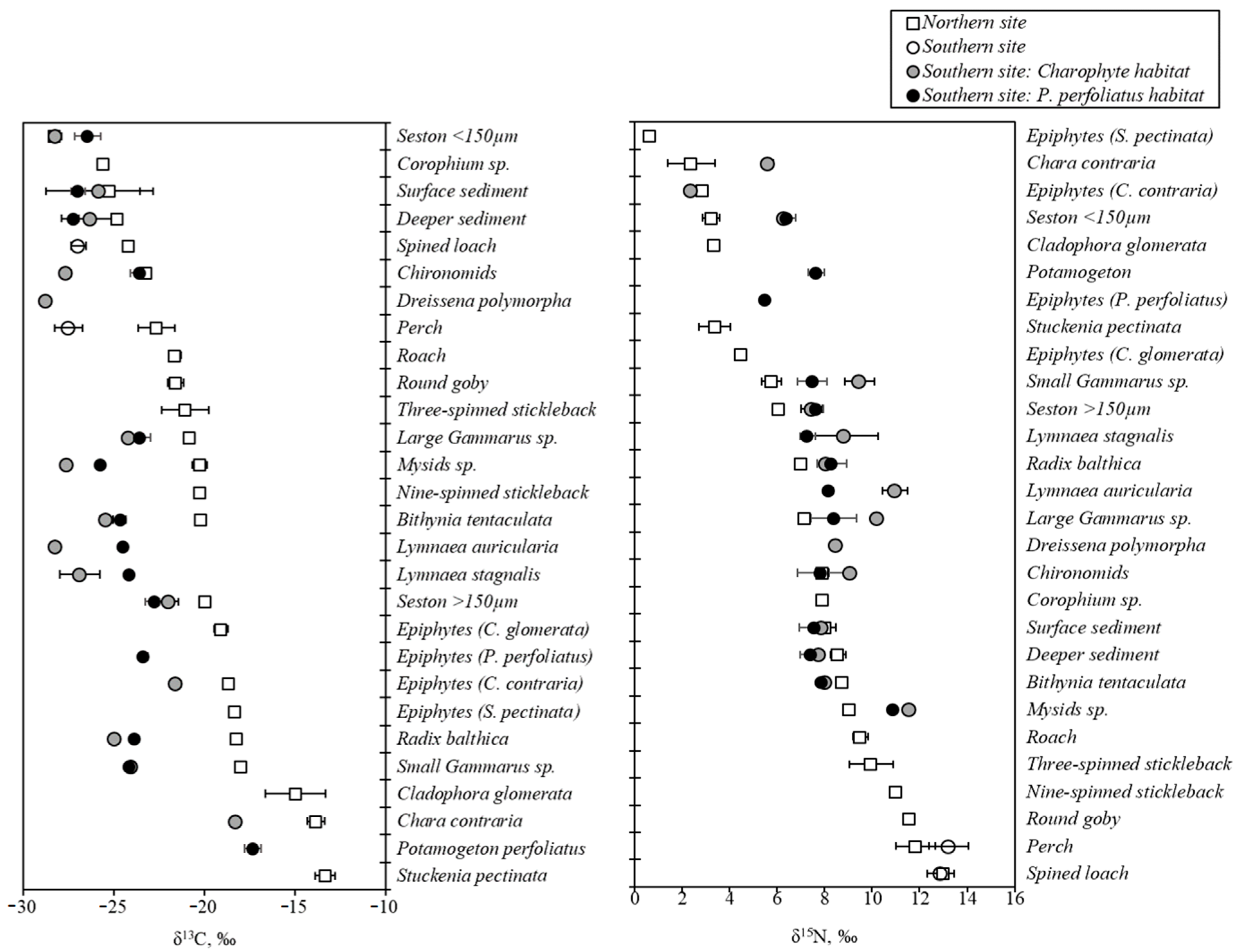
3. Results
3.1. Trophic Networks in Macrophyte Habitats
3.2. Macrophytes and Epiphytes: Differences among Taxa, Habitats and Sites
3.3. Seston and Sediment: Comparisons among Size Fractions/Layers and Sites
3.4. Consumer Tissues Reflecting Isotopic Signals of Food Sources
3.5. Food Sources for Gastropods and Amphipods
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gopal, B. Should ‘Wetlands’ Cover All Aquatic Ecosystems and Do Macrophytes Make a Difference to Their Ecosystem Services? Folia Geobot. 2016, 51, 209–226. [Google Scholar] [CrossRef]
- Savage, C.; Thrush, S.F.; Lohrer, A.M.; Hewitt, J.E. Ecosystem Services Transcend Boundaries: Estuaries Provide Resource Subsidies and Influence Functional Diversity in Coastal Benthic Communities. PLoS ONE 2012, 7, e42708. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.J.; Broadley, A.; Adame, M.F.; Branch, T.A.; Turschwell, M.P.; Connolly, R.M. The Assessment of Fishery Status Depends on Fish Habitats. Fish 2019, 20, 1–14. [Google Scholar] [CrossRef]
- Macreadie, P.; Jarvis, J.; Trevathan-Tackett, S.M.; Bellgrove, A. Seagrasses and Macroalgae: Importance, Vulnerability and Impacts Identifying Victorian Coastal Areas with High Ecosystem Rehabilitation Potential View Project The Role of Z. Marina Seed Physiology on Viability and Resilience View Project. In Climate Change Impacts on Fisheries and Aquaculture: A Global Analysis; Delve Publishing: Burlington, ON, Canada, 2017; pp. 729–770. [Google Scholar]
- Horn, S.; Coll, M.; Asmus, H.; Dolch, T. Food Web Models Reveal Potential Ecosystem Effects of Seagrass Recovery in the Northern Wadden Sea. Restor. Ecol. 2020, 29, e13328. [Google Scholar] [CrossRef]
- Mulderij, G.; Mau, B.; van Donk, E.; Gross, E.M. Allelopathic Activity of Stratiotes Aloides on Phytoplankton—towards Identification of Allelopathic Substances. In Shallow Lakes in a Changing World; Springer: Berlin/Heidelberg, Germany, 2007; pp. 89–100. [Google Scholar] [CrossRef][Green Version]
- Krause-Jensen, D.; Sagert, S.; Schubert, H.; Boström, C. Empirical Relationships Linking Distribution and Abundance of Marine Vegetation to Eutrophication. Ecol. Indic. 2008, 8, 515–529. [Google Scholar] [CrossRef]
- Hidding, B.; Bakker, E.S.; Keuper, F.; de Boer, T.; de Vries, P.P.; Nolet, B.A. Differences in Tolerance of Pondweeds and Charophytes to Vertebrate Herbivores in a Shallow Baltic Estuary. Aquat. Bot. 2010, 93, 123–128. [Google Scholar] [CrossRef]
- Lesutienė, J. Food Web of the Curonian Lagoon: Organic Matter Sources and Feeding of Mysids. Ph.D. Thesis, Klaipeda University, Klaipeda, Lithuania, 2009. [Google Scholar]
- Kahma, T.I.; Karlson, A.M.L.; Sun, X.; Mörth, C.M.; Humborg, C.; Norkko, A.; Rodil, I.F. Macroalgae Fuels Coastal Soft-Sediment Macrofauna: A Triple-Isotope Approach across Spatial Scales. Mar. Environ. Res. 2020, 162, 105163. [Google Scholar] [CrossRef]
- Haines, E.B.; Montague, C.L. Food Sources of Estuarine Invertebrates Analyzed Using 13C/12C Ratios. Ecology 1979, 60, 48–56. [Google Scholar] [CrossRef]
- Kotta, J.; Torn, K.; Martin, G.; Orav-Kotta, H.; Paalme, T. Seasonal Variation in Invertebrate Grazing on Chara Connivens and C. Tomentosa in Kõiguste Bay, NE Baltic Sea. Helgol. Mar. Res. 2004, 58, 71–76. [Google Scholar] [CrossRef]
- Vizzini, S.; Sarà, G.; Michener, R.H.; Mazzola, A. The Role and Contribution of the Seagrass posidonia Oceanica (L.) Delile Organic Matter for Secondary Consumers as Revealed by Carbon and Nitrogen Stable Isotope Analysis. Acta Oecol. 2002, 23, 277–285. [Google Scholar] [CrossRef]
- Puche, E.; Rojo, C.; Ramos-Jiliberto, R.; Rodrigo, M.A.; Puche, E.; Rojo, C.; Rodrigo, M.A. Structure and Vulnerability of the Multi-Interaction Network in Macrophyte-Dominated Lakes. OIKOS 2020, 129, 35–48. [Google Scholar] [CrossRef]
- Papastergiadou, E.; Kagalou, I.; Stefanidis, K.; Retalis, A.; Leonardos, I. Effects of Anthropogenic Influences on the Trophic State, Land Uses and Aquatic Vegetation in a Shallow Mediterranean Lake: Implications for Restoration. Water Resour. Manag. 2009, 24, 415–435. [Google Scholar] [CrossRef]
- Peterson, B.J.; Fry, B. Stable Isotopes in Ecosystem Studies. Ann. Rev. Ecol. Syst. 1987, 18, 293–320. [Google Scholar] [CrossRef]
- Post, D.M. Using stable isotopes to estimate trophic position: Models, methods, and assumptions. Ecology 2002, 83, 703–718. [Google Scholar] [CrossRef]
- France, R.L. Carbon-13 Enrichment in Benthic Compared to Planktonic Algae: Foodweb Implications. Mar. Ecol. Prog. Ser. 1995, 124, 307–312. [Google Scholar] [CrossRef]
- Fry, B.; Sherr, E.B. Δ13C Measurements as Indicators of Carbon Flow in Marine and Freshwater Ecosystems. In Stable Isotopes in Ecological Research; Springer: New York, NY, USA, 1989; pp. 196–229. [Google Scholar] [CrossRef]
- Gasiūnaitė, Z.R.; Daunys, D.; Olenin, S.; Razinkovas, A. The Curonian Lagoon. In Ecology of Baltic Coastal Waters; Springer: Berlin/Heidelberg, Germany, 2008; pp. 197–215. [Google Scholar] [CrossRef]
- Razinkovas-Baziukas, A.; Barisevičiūtė, R.; Baziukė, D.; Pilkaitytė, R.; Ruginis, T. The Distribution of Carbon Stable Isotopes as an Indicator of Temporal and Spatial Dynamic and Distribution of Autochtonic and Allochtonic Organic Matter in a Shallow Estuarine Lagoon. Comput. Sci. Tech. 2015, 3, 413–432. [Google Scholar] [CrossRef][Green Version]
- Nelson, W.G. Development of an Epiphyte Indicator of Nutrient Enrichment: A Critical Evaluation of Observational and Experimental Studies. Ecol. Indic. 2017, 79, 207–227. [Google Scholar] [CrossRef] [PubMed]
- Politi, T.; Zilius, M.; Bartoli, M.; Bučas, M. Amphipods’ Grazing and Excretion Loop Facilitates Chara Contraria Persistence in a Eutrophic Lagoon. Aquat. Bot. 2021, 171, 103378. [Google Scholar] [CrossRef]
- Orth, R.J.; van Montfrans, J. Epiphyte-Seagrass Relationships with an Emphasis on the Role of Micrograzing: A Review. Aquat. Bot. 1984, 18, 43–69. [Google Scholar] [CrossRef]
- Vybernaite-Lubiene, I.; Zilius, M.; Saltyte-Vaisiauske, L.; Bartoli, M. Recent Trends (2012–2016) of N, Si, and P Export from the Nemunas River Watershed: Loads, Unbalanced Stoichiometry, and Threats for Downstream Aquatic Ecosystems. Water 2018, 10, 1178. [Google Scholar] [CrossRef]
- Mežine, J.; Ferrarin, C.; Vaičiute, D.; Idzelyte, R.; Zemlys, P.; Umgiesser, G. Sediment Transport Mechanisms in a Lagoon with High River Discharge and Sediment Loading. Water 2019, 11, 1970. [Google Scholar] [CrossRef]
- Voss, M.; Asmala, E.; Bartl, I.; Carstensen, J.; Conley, D.J.; Dippner, J.W.; Humborg, C.; Lukkari, K.; Petkuviene, J.; Reader, H.; et al. Origin and Fate of Dissolved Organic Matter in Four Shallow Baltic Sea Estuaries. Biogeochemistry 2021, 154, 385–403. [Google Scholar] [CrossRef]
- Morkune, R.; Petkuviene, J.; Bružas, M.; Morkunas, J.; Bartoli, M. Monthly Abundance Patterns and the Potential Role of Waterbirds as Phosphorus Sources to a Hypertrophic Baltic Lagoon. Water 2020, 12, 1392. [Google Scholar] [CrossRef]
- Zemlys, P.; Ferrarin, C.; Umgiesser, G.; Gulbinskas, S.; Bellafiore, D. Investigation of Saline Water Intrusions into the Curonian Lagoon (Lithuania) and Two-Layer Flow in the Klaipeda Strait Using Finite Element Hydrodynamic Model. Ocean Sci. 2013, 9, 573–584. [Google Scholar] [CrossRef]
- Kataržytė, M.; Vaičiūtė, D.; Bučas, M.; Gyraitė, G.; Petkuvienė, J. Microorganisms Associated with Charophytes under Different Salinity Conditions. Oceanologia 2017, 59, 177–186. [Google Scholar] [CrossRef]
- He, D.; Ren, L.; Wu, Q. Epiphytic Bacterial Communities on Two Common Submerged Macrophytes in Taihu Lake: Diversity and Host-Specificity. Chin. J. Oceanol. Limnol. 2012, 30, 237–247. [Google Scholar] [CrossRef]
- Marcelina, Z.; Adam, S.; Pierre, R. Spatial and Temporal Variability of Organic Matter Sources and Food Web Structure across Benthic Habitats in a Low Diversity System (Southern Baltic Sea). J. Sea Res. 2018, 141, 47–60. [Google Scholar] [CrossRef]
- Pidgeon, B.; Buckle, D.; Humphrey, C.; Boyden, J.; Luxon, R. Monitoring of Impacts of Ranger Uranium Mine on Fish Communities in Shallow Lowland Billabongs in 2002. 2003. Available online: https://www.awe.gov.au/sites/default/files/documents/ir443.pdf (accessed on 1 February 2022).
- Signa, G.; Mazzola, A.; Kairo, J.; Vizzini, S. Small-Scale Variability in Geomorphological Settings Influences Mangrove-Derived Organic Matter Export in a Tropical Bay. Biogeosciences 2017, 14, 617–629. [Google Scholar] [CrossRef]
- Beaudoin, C.P.; Prepas, E.E.; Tonn, W.M.; Wassenaar, L.I.; Kotak, B.G. A Stable Carbon and Nitrogen Isotope Study of Lake Food Webs in Canada’s Boreal Plain. Freshw. Biol. 2001, 46, 465–477. [Google Scholar] [CrossRef]
- R Core Development Team. R: A Language and Environment for Statistical Computing. 2013. Available online: http://www.R-project.org/ (accessed on 5 February 2021).
- Elith, J.; Leathwick, J.R.; Hastie, T. A working guide to boosted regression trees. J. Anim. Ecol. 2008, 77, 802–813. [Google Scholar] [CrossRef]
- Hijmans, R.J.; Phillips, S.; Leathwick, J.; Elith, J. Dismo: Species Distribution Modeling. R Package Version 1.3-5. 2021. Available online: https://cran.r-project.org/package=dismo (accessed on 1 February 2022).
- Parnell, A.C.; Inger, R.; Bearhop, S.; Jackson, A.L. Source Partitioning Using Stable Isotopes: Coping with Too Much Variation. PLoS ONE 2010, 5, e9672. [Google Scholar] [CrossRef] [PubMed]
- DeNiro, M.J.; Epstein, S. Influence of Diet on the Distribution of Carbon Isotopes in Animals. Geochim. Cosmochim. Acta 1978, 42, 495–506. [Google Scholar] [CrossRef]
- Fry, B. Stable Isotope Ecology; Springer: New York, NY, USA, 2006. [Google Scholar]
- Olenin, S. Benthic Zonation of the Eastern Gotland Basin, Baltic Sea. Neth. J. Aquat. Ecol. 1997, 30, 265–282. [Google Scholar] [CrossRef]
- Froese, R.; Pauly, D. (Eds.) FishBase 2000: Concepts Designs and Data Sources—Google Knygos; ICLARM: Los Banos, Laguna, 2000. [Google Scholar]
- Rolff, C.; Elmgren, R. Use of Riverine Organic Matter in Plankton Food Webs of the Baltic Sea. Mar. Ecol. Prog. Ser. 2000, 197, 81–101. [Google Scholar] [CrossRef]
- Morkune, R. Food Web of the Lithuanian Baltic Sea Coastal Zone: Structure and Organic Matter Flows. Ph.D. Thesis, Klaipeda University, Klaipėda, Lithuania, 2017. [Google Scholar]
- Megens, L.; van der Plicht, J.; de Leeuw, J.W.; Smedes, F. Stable Carbon and Radiocarbon Isotope Compositions of Particle Size Fractions to Determine Origins of Sedimentary Organic Matter in an Estuary. Org. Geochem. 2002, 33, 945–952. [Google Scholar] [CrossRef][Green Version]
- Haines, E.B. The Origins of Detritus in Georgia Salt Marsh Estuaries. Oikos 1977, 29, 254. [Google Scholar] [CrossRef]
- Bartoli, M.; Nizzoli, D.; Zilius, M.; Bresciani, M.; Pusceddu, A.; Bianchelli, S.; Sundbäck, K.; Razinkovas-Baziukas, A.; Viaroli, P. Denitrification, Nitrogen Uptake, and Organic Matter Quality Undergo Different Seasonality in Sandy and Muddy Sediments of a Turbid Estuary. Front. Microbiol. 2021, 11, 3524. [Google Scholar] [CrossRef]
- Chappuis, E.; Seriñá, V.; Martí, E.; Ballesteros, E.; Gacia, E. Decrypting Stable-Isotope (Δ13C and Δ15N) Variability in Aquatic Plants. Freshw. Biol. 2017, 62, 1807–1818. [Google Scholar] [CrossRef]
- Sebastian-Gonzalez, E.; Navarro, J.; Sánchez-Zapata, J.A.; Botella, F.; Delgado Huertas, A. Water Quality and Avian Inputs as Sources of Isotopic Variability in Aquatic Macrophytes and Macroinvertebrates. J. Limnol. 2011, 71, 191–199. [Google Scholar] [CrossRef]
- Matuszak, A.; Voigt, C.C.; Storch, I.; Bauer, H.G.; Quillfeldt, P. Depth-Specific and Spatiotemporal Variation of Δ13C and Δ15N in Charophytes of Lake Constance: Implications for Food Web Studies. Rapid Commun. Mass Spectrom. 2011, 25, 2089–2094. [Google Scholar] [CrossRef]
- Karosiene, J.; Paskauskas, R. Spatial Variability of Epiphyton Communities Structure in a Temperate Estuarine Lagoon. Estuar. Coast. Shelf. Sci. 2012, 114, 100–104. [Google Scholar] [CrossRef]
- Bengtsson, M.M.; Bühler, A.; Brauer, A.; Dahlke, S.; Schubert, H.; Blindow, I. Eelgrass Leaf Surface Microbiomes Are Locally Variable and Highly Correlated with Epibiotic Eukaryotes. Front. Microbiol. 2017, 8, 1312. [Google Scholar] [CrossRef] [PubMed]
- Whalen, M.A.; Duffy, J.E.; Grace, J.B. Temporal Shifts in Top-down vs. Bottom-up Control of Epiphytic Algae in a Seagrass Ecosystem. Ecology 2013, 94, 510–520. [Google Scholar] [CrossRef] [PubMed]
- Ardón, M.; Morse, J.L.; Colman, B.P.; Bernhardt, E.S. Drought-Induced Saltwater Incursion Leads to Increased Wetland Nitrogen Export. Glob. Chang. Biol. 2013, 19, 2976–2985. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.A.; Lauer, N.T.; Hackney, C.T. Soil Phosphorus Dynamics and Saltwater Intrusion in a Florida Estuary. Wetlands 2014, 34, 535–544. [Google Scholar] [CrossRef]
- Puche, E.; Rojo, C.; Segura, M.; Rodrigo, M.A. Macrophyte Meadows Mediate the Response of the Sediment Microbial Community to Ultraviolet Radiation. Hydrobiologia 2021, 848, 4569–4583. [Google Scholar] [CrossRef]
- Brendelberger, H.; Jurgens, S. Suspension feeding in Bithynia tentaculata (Prosobranchia, Bithyniidae), as affected by body size, food and temperature. Oecologia 1993, 94, 36–42. [Google Scholar] [CrossRef]
- Zettler Michael, L.; Darius, D. Long-term macrozoobenthos changes in a shallow boreal lagoon: Comparison of a recent biodiversity inventory with historical data. Limnologica 2007, 37, 170–185. [Google Scholar] [CrossRef]
- Trebitz, A.; Shepard, G.; Brady, V.; Schmude, K. The non-native faucet snail (Bithynia tentaculata) makes the leap to Lake Superior. J. Great Lakes Res. 2015, 41, 1197–1200. [Google Scholar] [CrossRef]
- Berezina, N.A.; Maximov, A.A. Abundance and food preferences of amphipods (Crustacea: Amphipoda) in the Eastern Gulf of Finland, Baltic Sea. Journal of Siberian Federal University. Biology 2016, 9, 409. [Google Scholar]
- Berthold, M.; Porsche, C.; Hofmann, A.; Nowak, P. Combined effects of salinity and temperature on grazing activity of invasive Gammarus tigrinus on charophytes. Repr. Serv. Biol. 2021, 1–44. [Google Scholar] [CrossRef]
- Repečka, R. The Species Composition of the Ichthyofauna in the Lithuanian Economic Zone of the Baltic Sea and the Curonian Lagoon and Its Changes in Recent Years. Acta Zool. Litu. 2012, 13, 149–157. [Google Scholar] [CrossRef]
- Ložys, L. The Growth of Pikeperch (Sander lucioperca L.) and Perch (Perca fluviatilis L.) under Different Water Temperature and Salinity Conditions in the Curonian Lagoon and Lithuanian Coastal Waters of the Baltic Sea. In Proceedings of the Biology of the Baltic Sea; Springer: Dordrecht, The Netherlands, 2004; pp. 105–113. [Google Scholar]





| Taxon/Group | Feeding Strategy | Northern Site | Southern Site | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mixed Habitat of Chara contraria, Cladophora glomerata and Stuckenia pectinata | Separate Habitats of Chara contraria/Potamogeton perfoliatus/not Assigned to Any Habitat | ||||||||
| n | δ13C, ‰ | δ15N, ‰, | TL | n | δ13C, ‰ | δ15N, ‰, | TL | ||
| Algae | |||||||||
| Chara contraria | Prod. | 3 | −13.82 ± 0.49 | 2.39 ± 0.99 | 3/-/- | −18.28 ± 0.19 | 5.62 ± 0.22 | ||
| Cladophora glomerata | Prod. | 2 | −14.96 ± 1.65 | 3.37 ± 0.16 | 0 | ||||
| Plants | |||||||||
| Potamogeton perfoliatus | Prod. | 0 | -/3/- | −17.32 ± 0.46 | 7.65 ± 0.34 | ||||
| Stuckenia pectinata | Prod. | 3 | −13.32 ± 0.55 | 3.37 ± 0.66 | 0 | ||||
| Epiphytes | |||||||||
| Epiphytes on C. contraria | Prod. | 3 | −18.65 ± 0.27 | 2.88 ± 0.14 | 3/-/- | −21.57 ± 0.28 | 2.35 ± 0.11 | ||
| Epiphytes on C. glomerata | Prod. | 2 | −19.09 ± 0.38 | 4.49 ± 0.06 | 0 | ||||
| Epiphytes on P. perfoliatus | Prod. | 0 | -/3/- | −23.35 ± 0.22 | 5.51 ± 0.02 | ||||
| Epiphytes on S. pectinata | Prod. | 2 | −18.30 ± 0.01 | 0.64 ± 0.16 | 0 | ||||
| Seston fractions | |||||||||
| Seston < 150 µm | Mix | 3 | −28.28 ± 0.07 | 3.22 ± 0.35 | 3/3/- | −28.24 ± 0.33/−26.44 ± 0.73 | 6.27 ± 0.03/6.42 ± 0.35 | ||
| Seston > 150 µm | Mix | 2 | −19.96 ± 0.10 | 6.08 ± 0.05 | 2/3/- | −21.98 ± 0.52/−22.75 ± 0.53 | 7.46 ± 0.45/7.64 ± 0.33 | ||
| Sediment layers | |||||||||
| 0.5–2 cm | Mix | 3 | −24.8 ± 0.27 | 8.57 ± 0.32 | 6/6/- | −26.32 ± 1.59/−27.21 ± 0.27 | 7.76 ± 0.23/7.41 ± 0.45 | ||
| 0–0.5 cm | Mix | 3 | −25.25 ± 1.69 | 8.01 ± 0.48 | 6/6/- | −25.8 ± 2.96/−26.98 ± 0.40 | 7.86 ± 0.37/7.58 ± 0.67 | ||
| Mollusca | |||||||||
| Bithynia tentaculata | Surf. dep. | 3 | −20.21 ± 0.03 | 8.75 ± 0.13 | 2.3 | 3/4/- | −25.42 ± 0.37/−24.61 ± 0.26 | 8.04 ± 0.18/7.89 ± 0.14 | 2.0 |
| Dreissena polymorpha | Susp. filt. | 0 | 3/-/- | −28.79 ± 0.08 | 8.48 ± 0.11 | 2.1 | |||
| Lymnaea auricularia | Surf. dep. | 0 | 3/2/- | −28.23 ± 0.25/−24.47 ± 0.09 | 10.97 ± 0.52/8.16 ± 0.22 | 2.5 | |||
| Lymnaea stagnalis | Surf. dep. | 0 | 2/3/- | −26.88 ± 1.09/−24.16 ± 0.07 | 8.82 ± 1.43/7.29 ± 0.31 | 1.9 | |||
| Radix balthica | Surf. dep. | 3 | −18.21 ± 0.19 | 6.99 ± 0.26 | 1.7 | 3/4/- | −24.95 ± 0.17/−23.83 ± 0.21 | 8.08 ± 0.06/8.29 ± 0.62 | 2.0 |
| Worms | |||||||||
| Chironomids | Subs. dep. | 3 | −23.25 ± 0.15 | 7.9 ± 0.28 | 2.0 | 3/5/- | −27.68 ± 0.33/−23.56 ± 0.51 | 9.1 ± 0.14/7.85 ± 1.00 | 2.1 |
| Crustaceans | |||||||||
| Corophium sp. | Susp. filt., Surf. dep. | 3 | −25.57 ± 0.11 | 7.9 ± 0.24 | 2.0 | 0 | |||
| Gammarus sp. (large) | Omniv. | 4 | −20.8 ± 0.01 | 7.17 ± 0.07 | 1.8 | 3/3/- | −24.2 ± 0.08/−23.59 ± 0.61 | 10.2 ± 0.12/8.39 ± 0.97 | 2.4 |
| Gammarus sp. (small) | Omniv. | 4 | −18.0 ± 0.21 | 5.75 ± 0.42 | 1.4 | 2/3/- | −24.04 ± 0.01/−24.14 ± 0.11 | 9.47 ± 0.62/7.48 ± 0.64 | 2.1 |
| Mysidae | Susp. filt., Surf. dep., Omniv. | 3 | −20.25 ± 0.40 | 9.03 ± 0.09 | 2.3 | 3/3/- | −27.62 ± 0.25/−25.73 ± 0.05 | 11.57 ± 0.23/10.9 ± 0.08 | 2.9 |
| Fish | |||||||||
| Nine-spined stickleback (Pungitius pungitius) | Zoobenth., Zooplank. | 4 | −20.22 ± 0.29 | 10.98 ± 0.08 | 2.9 | 0 | |||
| European perch (Perca fluviatilis) | Zoobenth., Nect., Pred. | 3 | −22.65 ± 1.01 | 11.83 ± 0.84 | 3.2 | -/2/1 | /−27.95 ± 0.05/−26.63 | /13.56 ± 0.80/12.53 | 3.5 |
| Roach (Rutilus rutilus) | Herbiv., Zoobenth., Zooplank. | 4 | −21.61 ± 0.34 | 9.51 ± 0.32 | 2.5 | 0 | |||
| Round goby (Neogobius melanostomus) | Zoobenth. | 4 | −21.59 ± 0.44 | 11.56 ± 0.15 | 3.1 | 0 | |||
| Spined loach (Cobitis taenia) | Zoobenth., Zooplank., Herb. | 3 | −24.18 ± 0.23 | 13 ± 0.09 | 3.5 | 1/2/- | −26.65/−27.13 ± 0.40 | 13.48/12.60 ± 0.29 | 3.4 |
| Three-spined stickleback (Gasterosteus aculeatus) | Zoobenth., Zooplank. | 4 | −21.05 ± 1.31 | 9.95 ± 0.93 | 2.6 | 0 | |||
| Consumer Taxa | δ13C | δ15N | ||||
|---|---|---|---|---|---|---|
| t-Test | p | Site Comparison | t-Test | p | Site Comparison | |
| Fish | ||||||
| Cobitis taenia | t = 10.50, df = 3.23 | *** | N > S | t = 0.32, df = 2.08 | ns | N = S |
| Perca fluviatilis | t = 6.66, df = 3.73 | ** | N > S | t = −2.03, df = 3.99 | ns | N = S |
| Mollusca | ||||||
| Bithynia tentaculata | t = 24.15, df = 6.09 | *** | N > S | t = 8.09, df = 4.78 | *** | N > S |
| Radix balthica | t = 23.43, df = 7.77 | *** | N > S | t = −5.31, df = 6.88 | ** | N < S |
| Crustaceans | ||||||
| Gammarus sp. (small) | U-test | * | N > S | t = −4.33, df = 5.10 | ** | N < S |
| Gammarus sp. (large) | U-test | ** | N > S | U-test, W = 24 | ** | N < S |
| Mysids | U-test | * | N > S | U-test, W = 18 | * | N < S |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morkūnė, R.; Bučas, M.; Kataržytė, M.; Politi, T.; Vaičiūtė, D.; Vizzini, S.; Martin, G. Food Sources for Benthic Grazers in Trophic Networks of Macrophyte Habitats in a Transitional Baltic Ecosystem. Water 2022, 14, 1565. https://doi.org/10.3390/w14101565
Morkūnė R, Bučas M, Kataržytė M, Politi T, Vaičiūtė D, Vizzini S, Martin G. Food Sources for Benthic Grazers in Trophic Networks of Macrophyte Habitats in a Transitional Baltic Ecosystem. Water. 2022; 14(10):1565. https://doi.org/10.3390/w14101565
Chicago/Turabian StyleMorkūnė, Rasa, Martynas Bučas, Marija Kataržytė, Tobia Politi, Diana Vaičiūtė, Salvatrice Vizzini, and Georg Martin. 2022. "Food Sources for Benthic Grazers in Trophic Networks of Macrophyte Habitats in a Transitional Baltic Ecosystem" Water 14, no. 10: 1565. https://doi.org/10.3390/w14101565
APA StyleMorkūnė, R., Bučas, M., Kataržytė, M., Politi, T., Vaičiūtė, D., Vizzini, S., & Martin, G. (2022). Food Sources for Benthic Grazers in Trophic Networks of Macrophyte Habitats in a Transitional Baltic Ecosystem. Water, 14(10), 1565. https://doi.org/10.3390/w14101565






