Performance Evaluation of a Hybrid Enhanced Membrane Bioreactor (eMBR) System Treating Synthetic Textile Effluent
Abstract
:1. Introduction
2. Materials and Methods
2.1. Composition of Synthetic Textile Wastewater
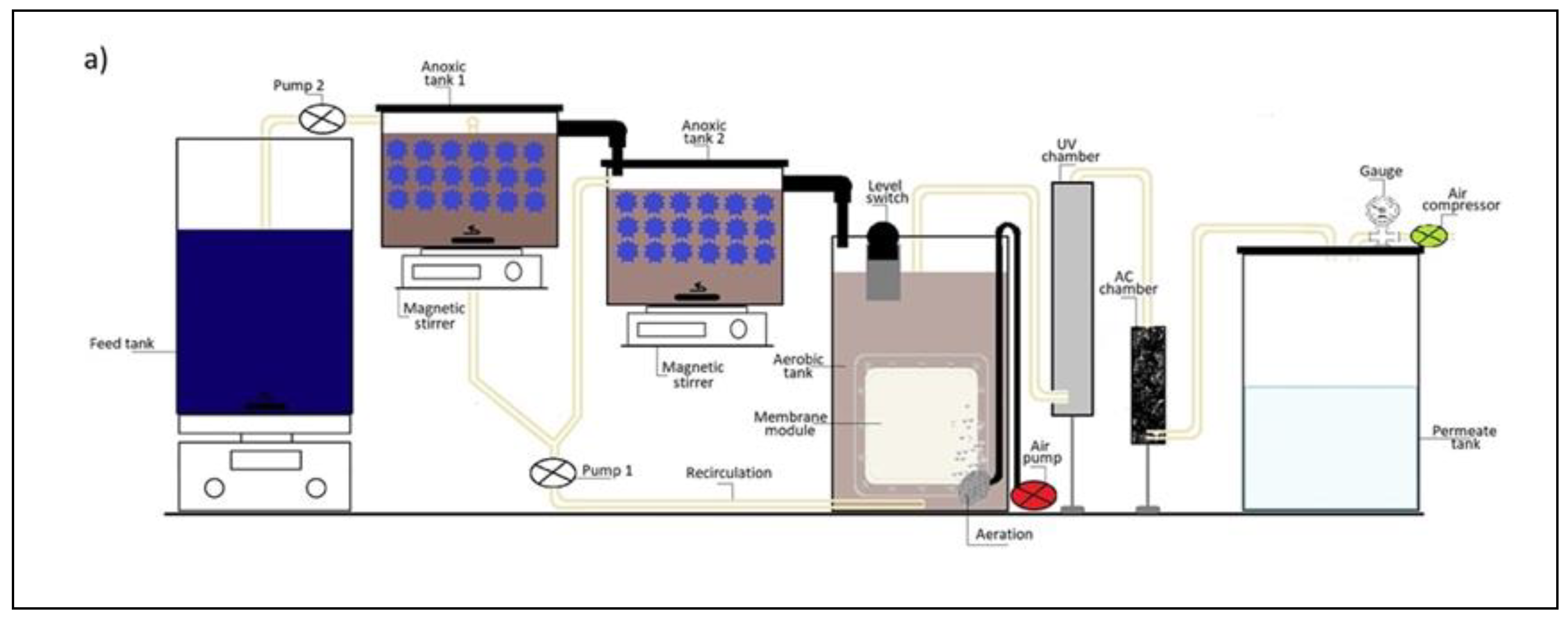
2.2. Experimental Setup of Hybrid eMBR System
2.3. Analytical Methodologies
2.4. Operational Parameters of Enhanced Membrane Bioreactor
3. Results and Discussion
3.1. Operational Phases of the eMBR
3.2. Monitoring of the Hybrid eMBR System Parameters
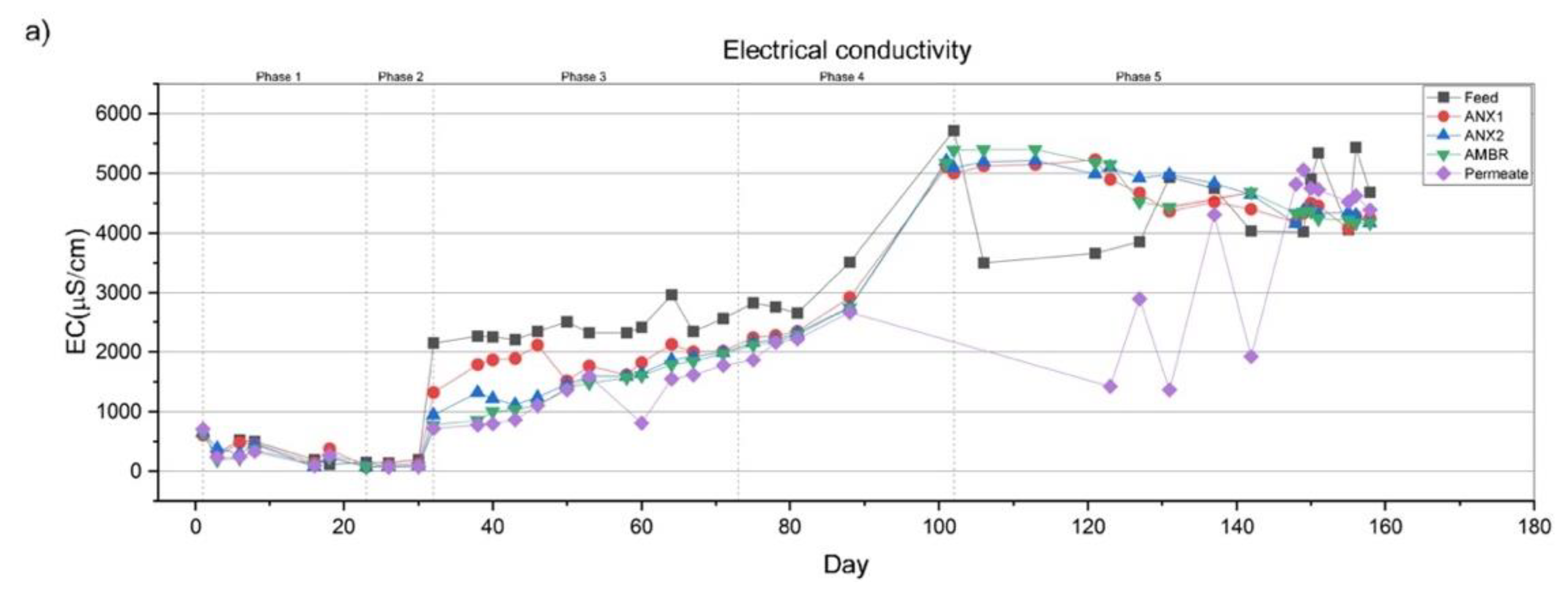
3.2.1. Electrical Conductivity and Total Dissolved Solids
3.2.2. Temperature Variation
3.2.3. Dissolved Oxygen (DO) and Oxidation Reduction Potential (ORP)
3.2.4. Hydrogen Potential (pH)
- Nutrient requirements (C:N:P ratio): Minimum ratios required for optimal microbial growth in the aerobic and anaerobic processes are 100:5:1 (aerobic processes) and 330:5:1 (anaerobic processes) [39]. In this study, during phases 3, 4, and 5, the ratio of nutrients was 3.66:TN:1, 3.16:2.15:1, and 3.25:2.49:1, respectively.
3.2.5. Temporal Variation of Flux and Transmembrane Pressure (TMP)
3.2.6. Mixed Liquor Suspended Solids (MLSS) and Mixed Liquor Volatile Suspended Solids (MLVSS)
3.3. Treated Water Quality
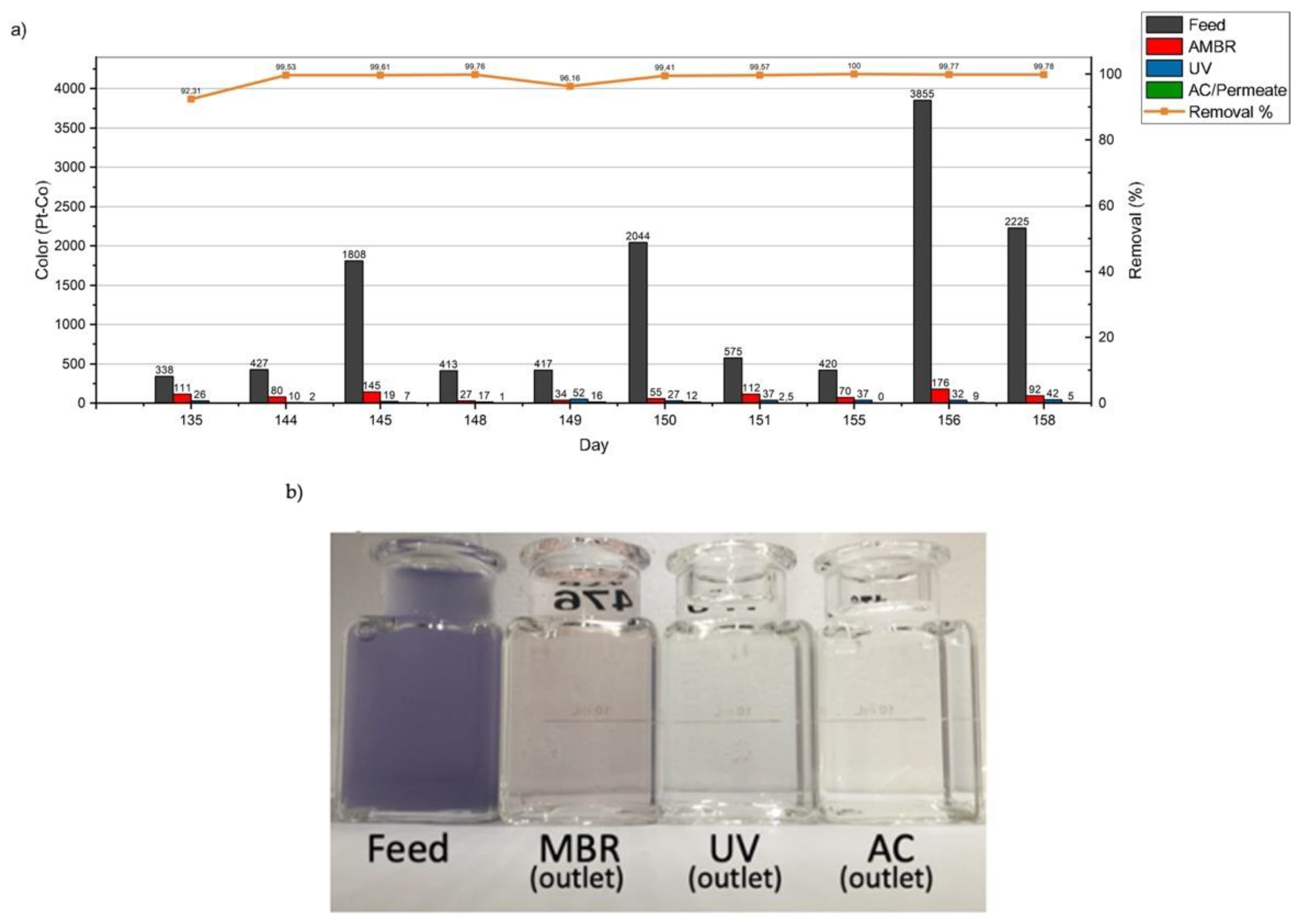
3.3.1. Color
3.3.2. Turbidity
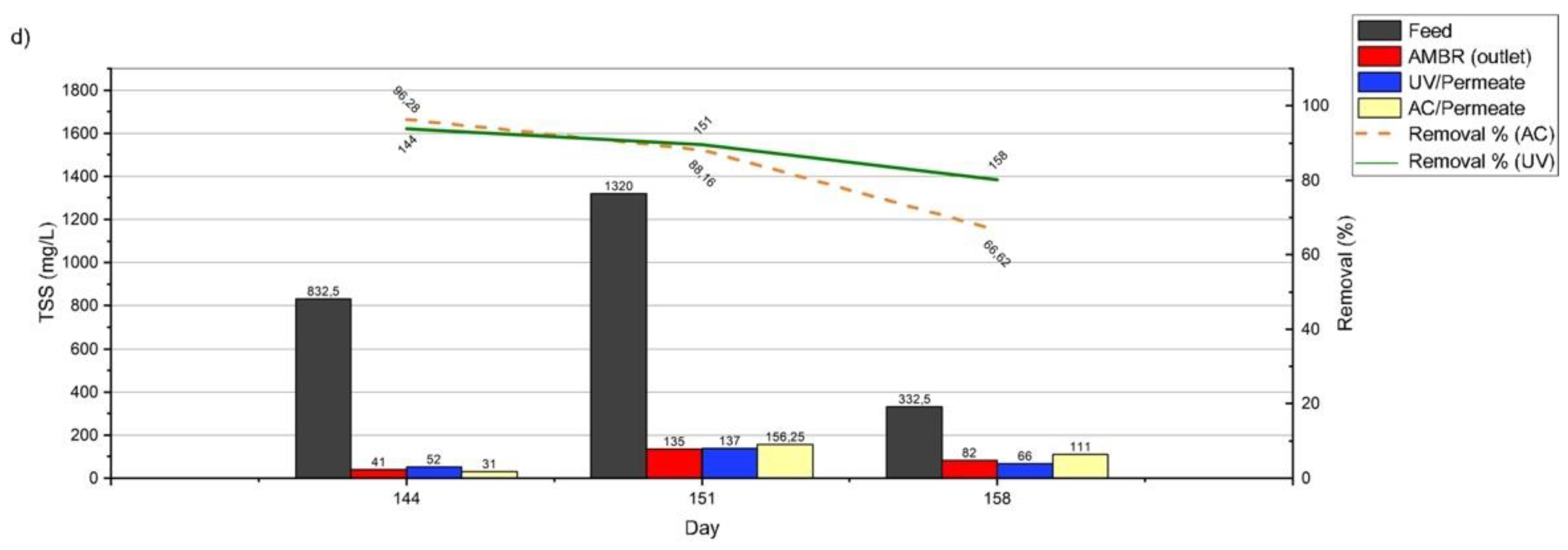
3.3.3. Chemical Oxygen Demand (COD), Total Organic Carbon (TOC), and Total suspended Solids (TSS)
| MBR Configuration | Types of Wastewater | Removal Efficiencies | Membrane Specification | References |
|---|---|---|---|---|
| Membrane bioreactor (dsMBR) incorporating two ultrafiltration (UF) side-stream membrane modules | Dyehouse wastewater of a textile company | COD: 75% Turbidity: 94% Color: 28.6% TSS: 70.6–100% TOC: 2.5–91.5% | Norit X-flow Airlift™ UF-membrane/109 tubular UF-membranes with a diameter of 5 mm and surface area of 0.046 m2 | [54] |
| Submerged membrane bioreactor integrated with ozonation and photocatalysis | Real textile wastewater | COD: 93% Turbidity: 99.9% Color: 94% TSS: 99.9% TOC: 80% | PVDF hollow fiber membrane with 0.6 m2 surface area and pore size of 0.1 μm | [38] |
| Sequential anaerobic–aerobic (MBSBBR and SBR) | Synthetic textile dyeing wastewater containing three commercial reactive azo dyes was considered. | COD: 77.1% ± 7.9% Color: 79.9% ± 1.5% | Flat-sheet microfiltration chlorinated polyethylene membrane (KUBOTA, Japan) with 0.4 μm pore size and surface area of 0.1 m2 | [59] |
| Submerged membrane bioreactor (SMBR) | Synthetic textile dye wastewater | COD: 90% Color: 20–70% | Membrane: UF, membrane material: PES, pore size: 0.050 μm, dimensions: 25 × 25 cm | [60] |
| Sequential anaerobic moving bed bioreactor and aerobic membrane bioreactor (AnMBBR and AeMBR). | Real textile wastewater from Dye Textile Industry | COD: 54.8–64.42% Color: 20–70% | Flat-sheet membranes, polyethersulfone (PES), total surface area and pore size of 0.0196 m2 and 0.45 μm | [61] |
| Aerobic membrane bioreactor (MBR) | Synthetic textile Reactive Dye wastewater of cotton and cellulose fibers | COD: 76–94% Turbidity: 100% Color: 66–98% | External tubular crossflow microfiltration (MF), alumina/alumina, 30 cm, 0.2 μm, 7.53 × 10−3 m2, internal/external diameter 8/10 mm | [62] |
| Aerobic membrane bioreactor (MBR-NF) | Real textile wastewater from Dye Textile Industry | Turbidity: 62–72% Color: 93–94% | Flat-sheet UF membrane | [63] |
| Bioaugmented membrane bioreactor (MBR) with a GAC-packed zone | Synthetic textile dye wastewater | Color: 70–100% TOC: 96% | A 4.5 cm compact bundle (packing density=56%) of microporous (0.4 mm) hydrophilic-treated, polyethylene hollow fibers, obtained from Mitsubishi Rayon, Japan | [64] |
| Combined moving bed biofilm reactors and a membrane filtration system (MBBRs, anaerobic–aerobic in series, and MF) | Azo dye Reactive Brilliant Red X-3B-containing synthetic wastewater | COD: 85% Color: 90% TSS: 94% | Hollow fiber PVDF, pore size of 0.02 μm, housing of 1880 mm-long, and a diameter of 220 mm | [50] |
| Aerobic reactor | Decolorization of Reactive Red 11 and 152 azo dyes | Color: 100% | - | [51] |
| Anaerobic–aerobic, one and two-stage processes for the biological treatment | Synthetic wastewaters containing Reactive Black 5 (RB5) | COD: 81–90% Color: 73–92% TOC: partial mineralization of the RB5 | - | [52] |
3.3.4. Scanning Electron Microscopy (SEM) and Energy Dispersive Spectroscopy (EDS) for Membrane Analysis
3.3.5. Summary and Evaluation of the Results Achieved during the Study
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Radhakrishnan, S. Sustainable consumption and production patterns in fashion. In The UN Sustainable Development Goals for the Textile and Fashion Industry; Gardetti, M.A., Muthu, S.S., Eds.; Textile Science and Clothing Technology; Springer: Singapore, 2020. [Google Scholar]
- Rather, L.J.; Bhat, A.K.; Mohammad, F.; Jameel, S.; Ganie, S.A.; Dar, O.A. Advances in the Sustainable Technologies for Water Conservation in Textile Industries. In Water in Textiles and Fashion; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Bilińska, L.; Gmurek, M.; Ledakowicz, S. Comparison between Industrial and Simulated Textile Wastewater Treatment by AOPs—Biodegradability, Toxicity and Cost Assessment. Chem. Eng. J. 2016, 306, 550–559. [Google Scholar] [CrossRef] [Green Version]
- Sarayu, K.; Sandhya, S. Current Technologies for Biological Treatment of Textile Wastewater-A Review. Appl. Biochem. Biotechnol. 2012, 167, 645–661. [Google Scholar] [CrossRef] [PubMed]
- Kant, R. Textile Dyeing Industry an Environmental Hazard. Nat. Sci. 2012, 04, 22–26. [Google Scholar] [CrossRef] [Green Version]
- GilPavas, E.; Dobrosz-Gómez, I.; Gómez-García, M.Á. Optimization and Toxicity Assessment of a Combined Electrocoagulation, H2O2/Fe2+/UV and Activated Carbon Adsorption for Textile Wastewater Treatment. Sci. Total Environ. 2019, 651, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Lellis, B.; Fávaro-Polonio, C.Z.; Pamphile, J.A.; Polonio, J.C. Effects of Textile Dyes on Health and the Environment and Bioremediation Potential of Living Organisms. Biotechnol. Res. Innov. 2019, 3, 275–290. [Google Scholar] [CrossRef]
- Takahashi, N.; Kumagai, T.; Shimizu, M.; Suzuki, T.; Ohtsuki, T. Removal of Dissolved Organic Carbon and Color from Dyeing Wastewater by Pre-Ozonation and Subsequent Biological Treatment Using Test-Scale Plant. Ozone Sci. Eng. 2007, 29, 139–145. [Google Scholar] [CrossRef]
- Sivaram, N.M.; Barik, D.; Gopal, P.M. Toxic Waste from Textile Industries. In Energy from Toxic Organic Waste for Heat and Power Generation; Barik, D., Ed.; Woodhead Publishing: Sawston, UK, 2018; pp. 43–54. [Google Scholar]
- Yaqub, M.; Lee, W. Zero-Liquid Discharge (ZLD) Technology for Resource Recovery from Wastewater: A Review. Sci. Total Environ. 2019, 681, 551–563. [Google Scholar] [CrossRef]
- Paździor, K.; Wrębiak, J.; Klepacz-Smółka, A.; Gmurek, M.; Bilińska, L.; Kos, L.; Sójka-Ledakowicz, J.; Ledakowicz, S. Influence of Ozonation and Biodegradation on Toxicity of Industrial Textile Wastewater. J. Environ. Manag. 2017, 195, 166–173. [Google Scholar] [CrossRef]
- Naim, M.M.; El Abd, Y.M. Removal and Recovery of Dyestuffs from Dyeing Wastewaters. Sep. Purif. Methods 2002, 31, 171–228. [Google Scholar] [CrossRef]
- Shoukat, R.; Khan, S.J.; Jamal, Y. Hybrid Anaerobic-Aerobic Biological Treatment for Real Textile Wastewater. J. Water Process Eng. 2019, 29, 100804. [Google Scholar] [CrossRef]
- Verma, A.K.; Dash, R.R.; Bhunia, P. A Review on Chemical Coagulation/Flocculation Technologies for Removal of Colour from Textile Wastewaters. J. Environ. Manag. 2012, 93, 154–168. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghouti, M.A.; Khraisheh, M.A.M.; Allen, S.J.; Ahmad, M.N. The Removal of Dyes from Textile Wastewater: A Study of the Physical Characteristics and Adsorption Mechanisms of Diatomaceous Earth. J. Environ. Manag. 2003, 69, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Marcucci, M.; Ciardelli, G.; Matteucci, A.; Ranieri, L.; Russo, M. Experimental Campaigns on Textile Wastewater for Reuse by Means of Different Membrane Processes. Desalination 2002, 149, 137–143. [Google Scholar] [CrossRef]
- Soares, P.A.; Batalha, M.; Souza, S.M.A.G.U.; Boaventura, R.A.R.; Vilar, V.J.P. Enhancement of a Solar Photo-Fenton Reaction with Ferric-Organic Ligands for the Treatment of Acrylic-Textile Dyeing Wastewater. J. Environ. Manag. 2015, 152, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, S.; Titus, A.; Gnanamani, A.; Mandal, A.B.; Sekaran, G. Treatment of Textile Wastewater by Homogeneous and Heterogeneous Fenton Oxidation Processes. Desalination 2011, 281, 438–445. [Google Scholar] [CrossRef]
- Kang, S.-F.; Liao, C.-H.; Po, S.-T. Decolorization of Textile Wastewater by Photo-Fenton Oxidation Technology. Chemosphere 2000, 41, 1287–1294. [Google Scholar] [CrossRef]
- Martínez-Huitle, C.A.; dos Santos, E.V.; de Araújo, D.M.; Panizza, M. Applicability of Diamond Electrode/Anode to the Electrochemical Treatment of a Real Textile Effluent. J. Electroanal. Chem. 2012, 674, 103–107. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Chen, H.; Yuan, R.; Wang, F.; Ma, F.; Zhou, B. Intensified Degradation of Textile Wastewater Using a Novel Treatment of Hydrodynamic Cavitation with the Combination of Ozone. J. Environ. Chem. Eng. 2020, 8, 103959. [Google Scholar] [CrossRef]
- Davis, M.L. Water and Wastewater Engineering, Professional Edition: Design Principles and Practice, 1st ed.; McGraw-Hill: New York, NY, USA, 2010; ISBN 0-07-171384-0. [Google Scholar]
- Fonseca Couto, C.; Lange, L.C.; Santos Amaral, M.C. A Critical Review on Membrane Separation Processes Applied to Remove Pharmaceutically Active Compounds from Water and Wastewater. J. Water Process Eng. 2018, 26, 156–175. [Google Scholar] [CrossRef]
- Chollom, M.N.; Rathilal, S.; Pillay, V.L.; Alfa, D. The Applicability of Nanofiltration for the Treatment and Reuse of Textile Reactive Dye Effluent. Water SA 2015, 41, 398–405. [Google Scholar] [CrossRef] [Green Version]
- Lafi, R.; Gzara, L.; Lajimi, R.H.; Hafiane, A. Treatment of Textile Wastewater by a Hybrid Ultrafiltration/Electrodialysis Process. Chem. Eng. Process.—Process Intensif. 2018, 132, 105–113. [Google Scholar] [CrossRef]
- Jalilnejad, E.; Sadeghpour, P.; Ghasemzadeh, K. Advances in Membrane Bioreactor Technology; Elsevier Inc.: Amsterdam, The Netherlands, 2019; ISBN 978-0-12816-8-226. [Google Scholar]
- Xiao, K.; Xu, Y.; Liang, S.; Lei, T.; Sun, J.; Wen, X.; Zhang, H.; Chen, C.; Huang, X. Engineering Application of Membrane Bioreactor for Wastewater Treatment in China: Current State and Future Prospect. Front. Environ. Sci. Eng. 2014, 8, 805–819. [Google Scholar] [CrossRef]
- Meng, F.; Zhang, S.; Oh, Y.; Zhou, Z.; Shin, H.S.; Chae, S.R. Fouling in Membrane Bioreactors: An Updated Review. Water Res. 2017, 114, 151–180. [Google Scholar] [CrossRef] [PubMed]
- Krzeminski, P.; Leverette, L.; Malamis, S.; Katsou, E. Membrane Bioreactors—A Review on Recent Developments in Energy Reduction, Fouling Control, Novel Configurations, LCA and Market Prospects. J. Membr. Sci. 2017, 527, 207–227. [Google Scholar] [CrossRef] [Green Version]
- Liao, Y.; Bokhary, A.; Maleki, E.; Liao, B. A Review of Membrane Fouling and Its Control in Algal-Related Membrane Processes. Bioresour. Technol. 2018, 264, 343–358. [Google Scholar] [CrossRef]
- Jegatheesan, V.; Pramanik, B.K.; Chen, J.; Navaratna, D.; Chang, C.Y.; Shu, L. Treatment of Textile Wastewater with Membrane Bioreactor: A Critical Review. Bioresour. Technol. 2016, 204, 202–212. [Google Scholar] [CrossRef]
- Moazzem, S.; Ravishankar, H.; Fan, L.; Roddick, F.; Jegatheesan, V. Application of Enhanced Membrane Bioreactor (EMBR) for the Reuse of Carwash Wastewater. J. Environ. Manag. 2020, 254, 109780. [Google Scholar] [CrossRef]
- Baird, R.B.; Eaton, A.D.; Rice, E.W. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association: Washington, DC, USA, 2017. [Google Scholar]
- Metropolitan Water District of Southern California, U.S. Department of the Interior. Salinity Management Study, Final Report; Technical Appendix: Salinity and Total Dissolved Solids; Bookman-Edmonston Engineering, Inc.: Olympia, WA, USA, 1999.
- Speight, J.G. The Properties of Water. In Natural Water Remediation; Elsevier: Amsterdam, The Netherlands, 2020; ISBN 978-0-12803-8-109. [Google Scholar]
- He, H.; Chen, Y.; Li, X.; Cheng, Y.; Yang, C.; Zeng, G. Influence of Salinity on Microorganisms in Activated Sludge Processes: A Review. Int. Biodeterior. Biodegrad. 2017, 119, 520–527. [Google Scholar] [CrossRef]
- Hong, J.; Li, W.; Lin, B.; Zhan, M.; Liu, C.; Chen, B.Y. Deciphering the Effect of Salinity on the Performance of Submerged Membrane Bioreactor for Aquaculture of Bacterial Community. Desalination 2013, 316, 23–30. [Google Scholar] [CrossRef]
- Sathya, U.; Keerthi; Nithya, M.; Balasubramanian, N. Evaluation of Advanced Oxidation Processes (AOPs) Integrated Membrane Bioreactor (MBR) for the Real Textile Wastewater Treatment. J. Environ. Manag. 2019, 246, 768–775. [Google Scholar] [CrossRef]
- Seneviratne, M. Wastewater Treatment Technologies ZDHC-Roadmap to Zero Programme. Ministry of Environment and Forest, “Guide for Assessment of Effluent Treatment Plant”; Amsterdam, The Netherlands. 2008, p. 119. Available online: https://uploads-ssl.webflow.com/5c4065f2d6b53e08a1b03de7/5db6f50d7a90f4e4a47725cf_Wastewater_Treatment_Technologies_for_the_Textile_Industry-FINAL.pdf (accessed on 17 May 2022).
- Patel, H.; Vashi, R.T. Characterization and Treatment of Textile Wastewater; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Gerardi, M.H. ORP Management in Wastewater as an Indicator of Process Efficiency. YSI Yellow Springs 2008. Available online: http://www.ysi.com/media/pdfs/A567-ORP-Management-in-Wastewater-as-an-Indicator-of-Process-Efficiency.pdf (accessed on 27 February 2017).
- Metcalf & Eddy, Inc.; Tchobanoglous, G.; Stensel, H.D.; Tsuchihashi, R.; Burton, F.L.; Abu-Orf, M.; Bowden, G.; Pfrang, W. Wastewater Engineering Treatment and Resource, 5th ed.; McGraw-Hill: New York, NY, USA, 2014; ISBN 978-0-07340-1-188. [Google Scholar]
- Till, B.A.; Weathers, L.J.; Alvarez, P.J.J. Fe(O)-Supported Autotrophic Denitrification. Environ. Sci. Technol. 1998, 32, 634–639. [Google Scholar] [CrossRef]
- Ghafari, S.; Hasan, M.; Aroua, M.K. Effect of Carbon Dioxide and Bicarbonate as Inorganic Carbon Sources on Growth and Adaptation of Autohydrogenotrophic Denitrifying Bacteria. J. Hazard. Mater. 2009, 162, 1507–1513. [Google Scholar] [CrossRef] [PubMed]
- Palmer, M.; Hatley, H. The Role of Surfactants in Wastewater Treatment: Impact, Removal and Future Techniques: A Critical Review. Water Res. 2018, 147, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Grady, C.P.L., Jr.; Love, N.G.; Filipe, C.D.M.; Daigger, G.T. Biological Wastewater Treatment; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Metcalf & Eddy, Inc.; Tchobanoglous, G.; Stensel, H.D.; Burton, F.L. Wastewater Engineering: Treatment and Reuse, 4th ed.; McGraw-Hill: New York, NY, USA, 2003; Volume 4, ISBN 0071122508. [Google Scholar]
- Uygur, A.; Kargi, F. Salt Inhibition on Biological Nutrient Removal from Saline Wastewater in a Sequencing Batch Reactor. Enzyme Microbial Technol. 2004, 34, 313–318. [Google Scholar] [CrossRef]
- Seneviratne, M. Wastewater Treatment Technologies, 1st ed.; ZDHC: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Dong, B.; Chen, H.; Yang, Y.; He, Q.; Dai, X. Treatment of Printing and Dyeing Wastewater Using MBBR Followed by Membrane Separation Process. Desalination Water Treat. 2014, 52, 4562–4567. [Google Scholar] [CrossRef]
- Kodam, K.M.; Kodam, K.M.; Gawai, K.R. Decolorisation of Reactive Red 11 and 152 Azo Dyes under Aerobic Conditions; CSIR: New Delhi, India, 2006; Volume 5. [Google Scholar]
- Bonakdarpour, B.; Vyrides, I.; Stuckey, D.C. Comparison of the Performance of One Stage and Two Stage Sequential Anaerobic-Aerobic Biological Processes for the Treatment of Reactive-Azo-Dye-Containing Synthetic Wastewaters. Int. Biodeterior. Biodegrad. 2011, 65, 591–599. [Google Scholar] [CrossRef]
- Sari Erkan, H.; Çağlak, A.; Soysaloglu, A.; Takatas, B.; Onkal Engin, G. Performance Evaluation of Conventional Membrane Bioreactor and Moving Bed Membrane Bioreactor for Synthetic Textile Wastewater Treatment. J. Water Process Eng. 2020, 38, 101631. [Google Scholar] [CrossRef]
- de Jager, D.; Sheldon, M.S.; Edwards, W. Colour Removal from Textile Wastewater Using a Pilot-Scale Dual-Stage MBR and Subsequent RO System. Sep. Purif. Technol. 2014, 135, 135–144. [Google Scholar] [CrossRef]
- Yao, M.; Nan, J.; Chen, T. Effect of Particle Size Distribution on Turbidity under Various Water Quality Levels during Flocculation Processes. Desalination 2014, 354, 116–124. [Google Scholar] [CrossRef]
- Sun, F.; Sun, B.; Hu, J.; He, Y.; Wu, W. Organics and Nitrogen Removal from Textile Auxiliaries Wastewater with A2O-MBR in a Pilot-Scale. J. Hazard. Mater. 2015, 286, 416–424. [Google Scholar] [CrossRef] [PubMed]
- Moazzem, S. Application of Ceramic Ultrafiltration/Reverse Osmosis Membranes and Enhanced Membrane Bioreactor for the Reuse of Car Wash Wastewater. Master’s Thesis, RMIT University, Melbourne, VIC, Australia, 2017. [Google Scholar]
- Hashempour, Y.; Nasseri, M.; Mohseni-Bandpei, A.; Motesaddi, S.; Eslamizadeh, M. Assessing vulnerability to climate change for total organic carbon in a system of drinking water supply. Sustain. Cities Soc. 2020, 53, 101904. [Google Scholar] [CrossRef]
- Azimi, B.; Abdollahzadeh-Sharghi, E.; Bonakdarpour, B. Anaerobic-Aerobic Processes for the Treatment of Textile Dyeing Wastewater Containing Three Commercial Reactive Azo Dyes: Effect of Number of Stages and Bioreactor Type. Chin. J. Chem. Eng. 2021, 39, 228–239. [Google Scholar] [CrossRef]
- Deowan, S.A.; Galiano, F.; Hoinkis, J.; Figoli, A.; Drioli, E. Submerged Membrane Bioreactor (SMBR) for Treatment of Textile Dye Wastewatertowards Developing Novel MBR Process. APCBEE Procedia 2013, 5, 259–264. [Google Scholar] [CrossRef] [Green Version]
- Kozak, M.; Cırık, K.; Dolaz, M.; Başak, S. Evaluation of Textile Wastewater Treatment in Sequential Anaerobic Moving Bed Bioreactor—Aerobic Membrane Bioreactor. Process Biochem. 2021, 105, 62–71. [Google Scholar] [CrossRef]
- Khouni, I.; Louhichi, G.; Ghrabi, A. Assessing the Performances of an Aerobic Membrane Bioreactor for Textile Wastewater Treatment: Influence of Dye Mass Loading Rate and Biomass Concentration. Process Saf. Environ. Prot. 2020, 135, 364–382. [Google Scholar] [CrossRef]
- Li, K.; Liu, Q.; Fang, F.; Wu, X.; Xin, J.; Sun, S.; Wei, Y.; Ruan, R.; Chen, P.; Wang, Y.; et al. Influence of Nanofiltration Concentrate Recirculation on Performance and Economic Feasibility of a Pilot-Scale Membrane Bioreactor-Nanofiltration Hybrid Process for Textile Wastewater Treatment with High Water Recovery. J. Clean. Prod. 2020, 261, 121067. [Google Scholar] [CrossRef]
- Hai, F.I.; Yamamoto, K.; Nakajima, F.; Fukushi, K. Bioaugmented Membrane Bioreactor (MBR) with a GAC-Packed Zone for High Rate Textile Wastewater Treatment. Water Res. 2011, 45, 2199–2206. [Google Scholar] [CrossRef] [Green Version]
- Musa, M.A.; Idrus, S.; Man, H.C.; Daud, N.N.N. Wastewater Treatment and Biogas Recovery Using Anaerobic Membrane Bioreactors (AnMBRs): Strategies and Achievements. Energies 2018, 11, 1675. [Google Scholar] [CrossRef] [Green Version]
- Tan, X.; Acquah, I.; Liu, H.; Li, W.; Tan, S. A Critical Review on Saline Wastewater Treatment by Membrane Bioreactor (MBR) from a Microbial Perspective. Chemosphere 2019, 220, 1150–1162. [Google Scholar] [CrossRef]







| Substance | Concentration (mg/L) |
|---|---|
| Glucose | 3000 |
| Ammonium chloride | 1146 |
| Monopotassium phosphate | 143.10 |
| Calcium chloride | 29.19 |
| Magnesium sulfate heptahydrate | 9.73 |
| Iron (III) chloride | 1.00 |
| Sodium bicarbonate | 500 |
| Cobalt (II) chloride | 0.1 |
| Zinc chloride | 0.1 |
| Reactive Black 05 (RB05) | 7.5 |
| Reactive Blue 222 (RB222) | 7.5 |
| Analysis | Equipment/Standard | Location of Sample Collection |
|---|---|---|
| Chemical oxygen demand (COD) | DR5000 Spectrophotometer (HACH Company, Loveland, CO, USA) + HACH test kits/DR5000 Spectrophotometer—Procedures from the Manual | Outlet of all tanks/UV chamber/GAC column |
| Total phosphorus (TP) | ||
| Total nitrogen (TN) | ||
| Ammonia | ||
| Nitrate | Thermo Scientific Dionex Aquion Ion Chromatography System (Thermo Scientific, Waltham, MA, United States) | Outlet of all tanks/UV chamber/GAC column |
| Nitrite | ||
| Color | DR5000 Spectrophotometer (HACH Company, Loveland, CO, USA)/Method 8025—True Color | Feed: at the inlet MBR/UV/GAC: at the outlet |
| Dissolved oxygen (DO) | HQ40D Portable Multi + Probes LDO101; CDC401; MTC101; PHC201/HQ40D + Probes Manuals (HACH Company, Loveland, CO, USA) | Inlet of feed/ANX/MBR/permeate |
| Electrical conductivity (EC) | ||
| Oxide reduction potential (ORP) | ||
| pH | ||
| Temperature (T) | ||
| Heavy Metals | Atomic Absorption spectroscopy—VARIAN AA240 FS (Varian -previous, now it is part of Agilent-, Palo Alto, CA, USA) | Outlet of all tanks/UV chamber/GAC column |
| Mixed liquor suspended solids (MLSS) | [33]—2540 D. Total Suspended Solids Dried at 103–105 °C | Inlet of ANX/MBR |
| Mixed liquor volatile suspended solids (MLVSS) | [30,32]—2540 D. Total Suspended Solids Dried at 103–105 °C | Inlet of ANX/MBR |
| Total Organic Carbon (TOC) | TOC-L Shimadzu Analyzer (Shimadzu, Kyoto, Japan) | Outlet of all tanks/UV chamber/GAC column |
| Total Suspended Solids (TSS) | [30]—2540 D. Total Suspended Solids Dried at 103–105 °C | Feed: at the inlet MBR/UV/AC: at the outlet |
| Turbidity | Hach (2100N) turbidimeter/ [30]—2130 TURBITY (HACH Company, Loveland, CO, USA) | Feed: at the inlet MBR/UV/AC: at the outlet |
| SEM analysis of membrane | Scanning Electron Microscopy—Quanta 200 (FEI, Hillsboro, OR, USA) | New and used membrane |
| SEM analysis of activated carbon | Scanning Electron Microscopy—Quanta 200 (FEI, Hillsboro, OR, USA) | New and used AC |
| Parameters | GSWW 100% | GSWW 75% + TSWW 25% | GSWW 50% + TSWW 50% | GSWW 25% + TSWW 75% | TSWW 100% |
|---|---|---|---|---|---|
| Stage 1 | Stage 2 | Stage 3 | |||
| Phase 1 | Phase 2 | Phase 3 | Phase 4 | Phase 5 | |
| Period of operation (day) (number of days) | 1–22 (23) | 23–31 (7) | 32–72 (40) | 73–101 (28) | 102–158 (56) |
| Average of system flow rate (L/day) | - | 1.06 | 0.56 | 0.87 | 1.06 |
| Flux (L/m2h) | - | 2.25 | 1.19 | 1.85 | 2.26 |
| Hydraulic retention time, HRT (day) | - | 19.85 | 37.41 | 24.18 | 19.12 |
| Return activated sludge, RAS (L/day) | - | - | 3.05 | 2.98 | 1.76 |
| Influent C:N:P ratio | - | 1681:N:P | 3.66:N:1 | 3.16:2.15:1 | 3.25:2.49:1 |
| Parameter | Unit | Phase 1 | Phase 2 | Phase 3 | Phase 4 | Phase 5 |
|---|---|---|---|---|---|---|
| DO | mg/L | 2.74 | 2.76 | 0.53 | 2.74 | 2.98 |
| EC | µS/cm | 329.7 | 169.2 | 2388 | 3490 | 4383 |
| ORP | mV | 62.7 | 186.5 | 48.3 | −71.4 | −139.9 |
| pH | - | 6.67 | 5.47 | 5.05 | 6.36 | 7.63 |
| T | °C | 23.7 | 33.3 | 42.1 | 37 | 30.6 |
| TSS | mg/L | 757.5 | - | 398.3 | 631 | 828.3 |
| TDS | mg/L | 311 | 106 | 1650 | 3121 | 4393 |
| COD | mg/L | - | 1681 | 333 | 313 | 296 |
| NH3-N | mg/L | - | 61 | 155 | 215 | 167 |
| TN | mg/L | - | - | - | 275 | 227 |
| TP | mg/L | - | - | 97 | 99.4 | 92.3 |
| TOC | mg/L | - | - | 625 | 2747 | 1108 |
| True Color | Pt-Co | - | - | - | - | 1252 |
| Turbidity | NTU | - | 232 | 243 | 70 | 132 |
| Zinc | mg/L | - | - | 0.045 | 0.006 | 0.92 |
| Iron | mg/L | - | - | 0.13 | - | 0.55 |
| Tank | Phase 1 | Phase 2 | Phase 3 | Phase 4 | Phase 5 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| MLSS | MVLSS | MLSS | MVLSS | MLSS | MVLSS | MLSS | MVLSS | MLSS | MVLSS | |
| ANX1 | 610 | - | - | - | 1143 | - | 2257 | 1633 | 1801 | 1389 |
| ANX2 | 775 | - | - | - | 833 | - | 1750 | 1173 | 2153 | 1386 |
| AMBR | 963 | - | - | - | 473 | - | 1847 | 1287 | 1394 | 1026 |
| Element | wt.% | |
|---|---|---|
| New Membrane | Membrane Used to Filter TSWW | |
| N | - | 17.36 |
| O | 4.17 | 46.50 |
| F | 94.48 | 1.24 |
| Na | - | 3.32 |
| Mg | - | 0.29 |
| Al | - | 1.46 |
| Si | - | 3.30 |
| P | - | 8.36 |
| S | 0.40 | 3.59 |
| Cl | - | 2.26 |
| K | - | 6.44 |
| Ca | - | 1.61 |
| Fe | - | 1.05 |
| Cu | 0.95 | 0.58 |
| Zn | - | 0.31 |
| Ir * | - | 2.34 |
| Total: | 100.00 | 100.00 |
| Parameter | Unit | Permeate in Phase 5 | Evaluation |
|---|---|---|---|
| DO | mg/L | 7.04 | S |
| EC | µS/cm | 3731 | NS |
| ORP | mV | 67 | S |
| pH | - | 7.98 | S |
| T | °C | 21.1 | S |
| TSS | mg/L | 0 | 100.0% |
| TDS | mg/L | 2388 | 0% |
| COD | mg/L | 54 | 81.8% |
| NH3-N | mg/L | 10 | 94.0% |
| TN | mg/L | 61 | 73.1% |
| TP | mg/L | 264 | 42% * |
| TOC | mg/L | 85 | 92.3% |
| True Color | Pt-Co | 5 | 99.6% |
| Turbidity | NTU | 0.96 | 99.3% |
| Zn | mg/L | 0.002 | 99.8% |
| Parameter | Unit | Permeate Phase 5 | OEKO-TEX®® | Levi Strauss | NIKE | Adidas | C&A | China | India | Brazil | USA | Reuse |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pH | - | 7.98 | 6–8 | 6–9 | 6–9 | 6–9 | 6–9 | 6–9 | 5.5–9 | 5–9 | 5.5–11 | 6.5–8.5 |
| T | °C | 21.1 | 25–40 | 37 | N/A | N/A | 37 | N/A | variation >5 °C | <40 | 60 | N/A |
| TSS | mg/L | 0 | 15–100 | 30 | 30 | 50 | 30 | 20–100 | 100 | 0 | N/A | 30 |
| TDS | mg/L | 2388 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| COD | mg/L | 54 | 30–200 | N/A | 200 | 125 | 200 | 60–200 | N/A | N/A | N/A | 60 |
| NH3-N | mg/L | 10 | 2.5–10 | N/A | N/A | N/A | N/A | 8–20 | N/A | 20 | N/A | 10 |
| TN | mg/L | 61 | N/A | N/A | N/A | 10 | N/A | 15–30 | N/A | N/A | N/A | none |
| TP | mg/L | 264 | 0.5–5 | N/A | N/A | 2 | N/A | 0.5–1.5 | N/A | N/A | N/A | 1 |
| TOC | mg/L | 85 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| True Color | Pt-Co | 5 | N/A | N/A | 150 | N/A | 150 | 30–80 | 400 | N/A | N/A | 30 |
| Turbidity | NTU | 0.96 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | 5 |
| Zn | mg/L | 0.002 | 0.3–5 | 1 | N/A | 1 | 1 | 2–5 | 5 | 5 | 25 | N/A |
| Fe | mg/L | 0.87 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | 15 | N/A | none |
| % of Compliance * | 71 | 100 | 100 | 67 | 100 | 75 | 100 | 100 | 100 | 67 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, K.R.M.d.; Bergamasco, R.; Jegatheesan, V. Performance Evaluation of a Hybrid Enhanced Membrane Bioreactor (eMBR) System Treating Synthetic Textile Effluent. Water 2022, 14, 1708. https://doi.org/10.3390/w14111708
Santos KRMd, Bergamasco R, Jegatheesan V. Performance Evaluation of a Hybrid Enhanced Membrane Bioreactor (eMBR) System Treating Synthetic Textile Effluent. Water. 2022; 14(11):1708. https://doi.org/10.3390/w14111708
Chicago/Turabian StyleSantos, Kryssian Romeiro Manoel dos, Rosangela Bergamasco, and Veeriah Jegatheesan. 2022. "Performance Evaluation of a Hybrid Enhanced Membrane Bioreactor (eMBR) System Treating Synthetic Textile Effluent" Water 14, no. 11: 1708. https://doi.org/10.3390/w14111708







