Removal of METH through Tertiary or Advanced Treatment in a WWTP
Abstract
:1. Introduction
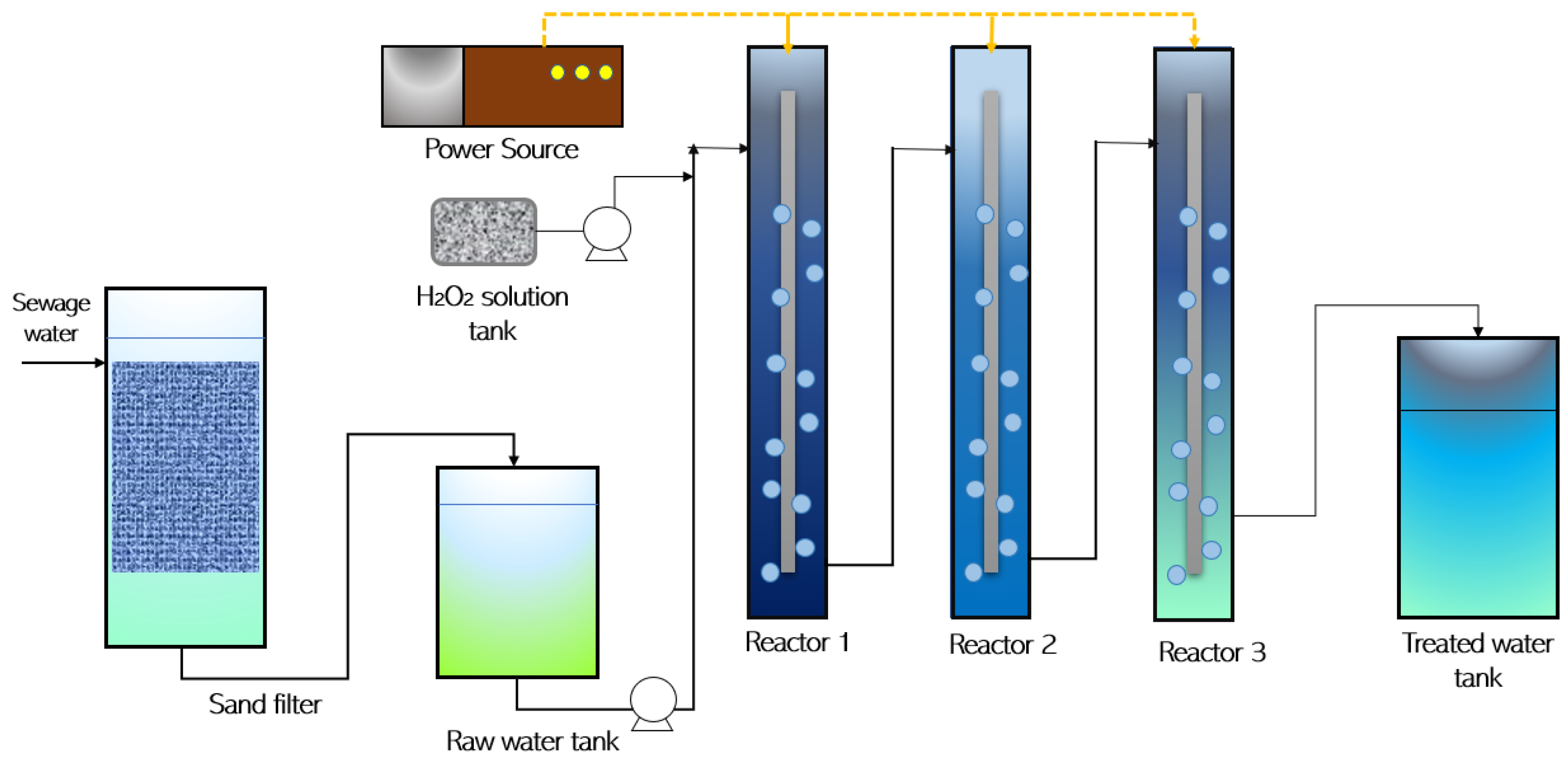
2. Materials and Methods
3. Results
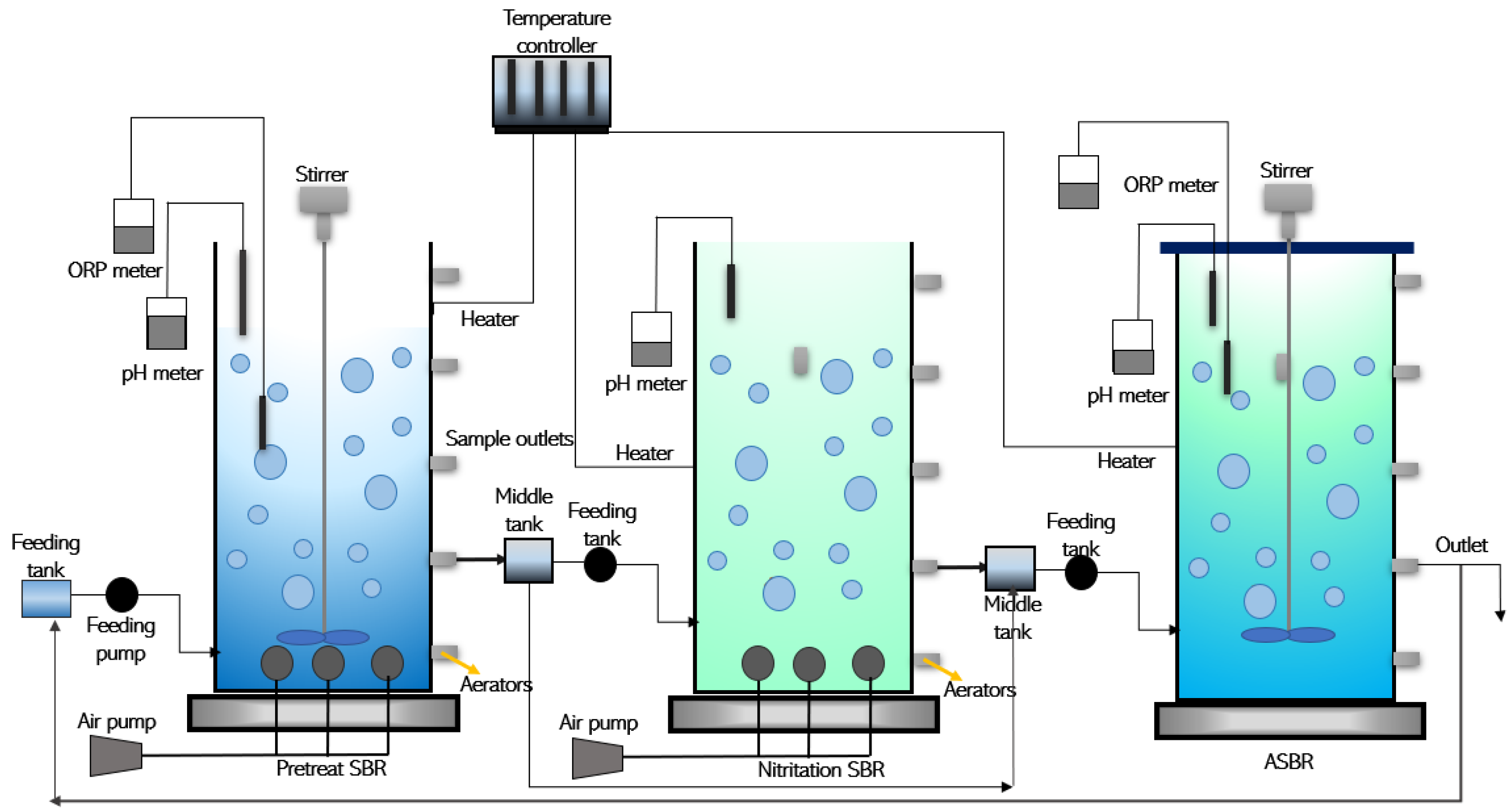
3.1. Sequential Batch Reactor (SBR) Activated Sludge Process
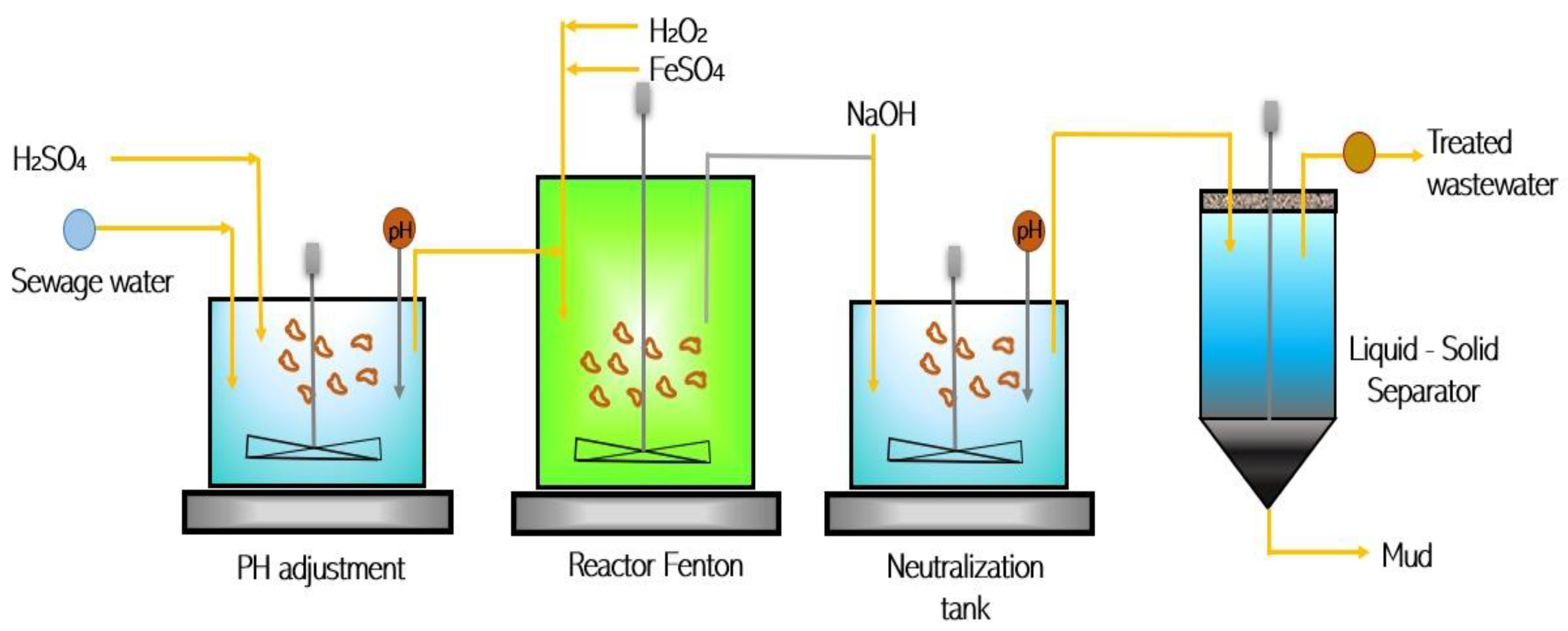
3.2. Modified Fenton Reaction (FR)
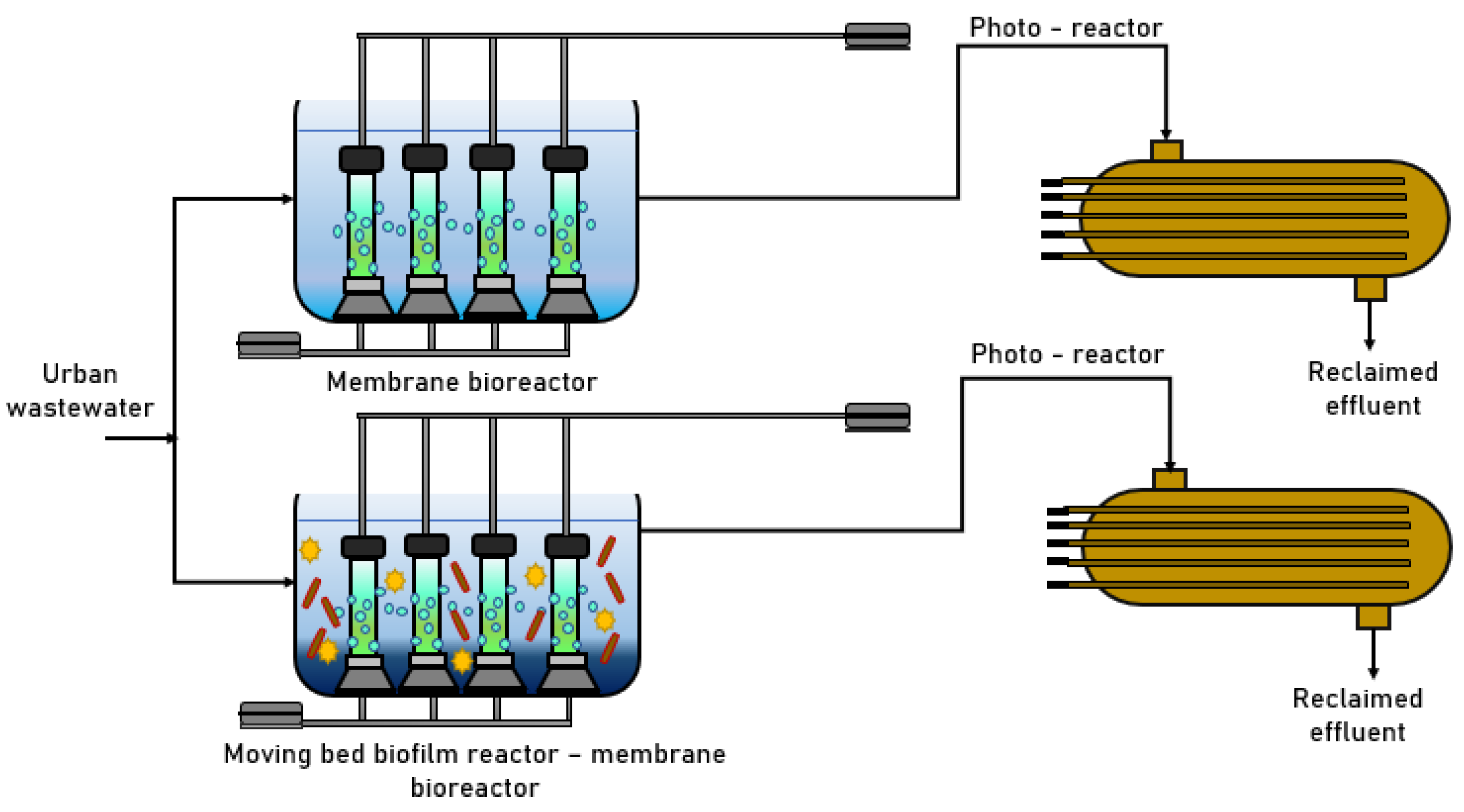
3.3. Mixed-Flow Bioreactor
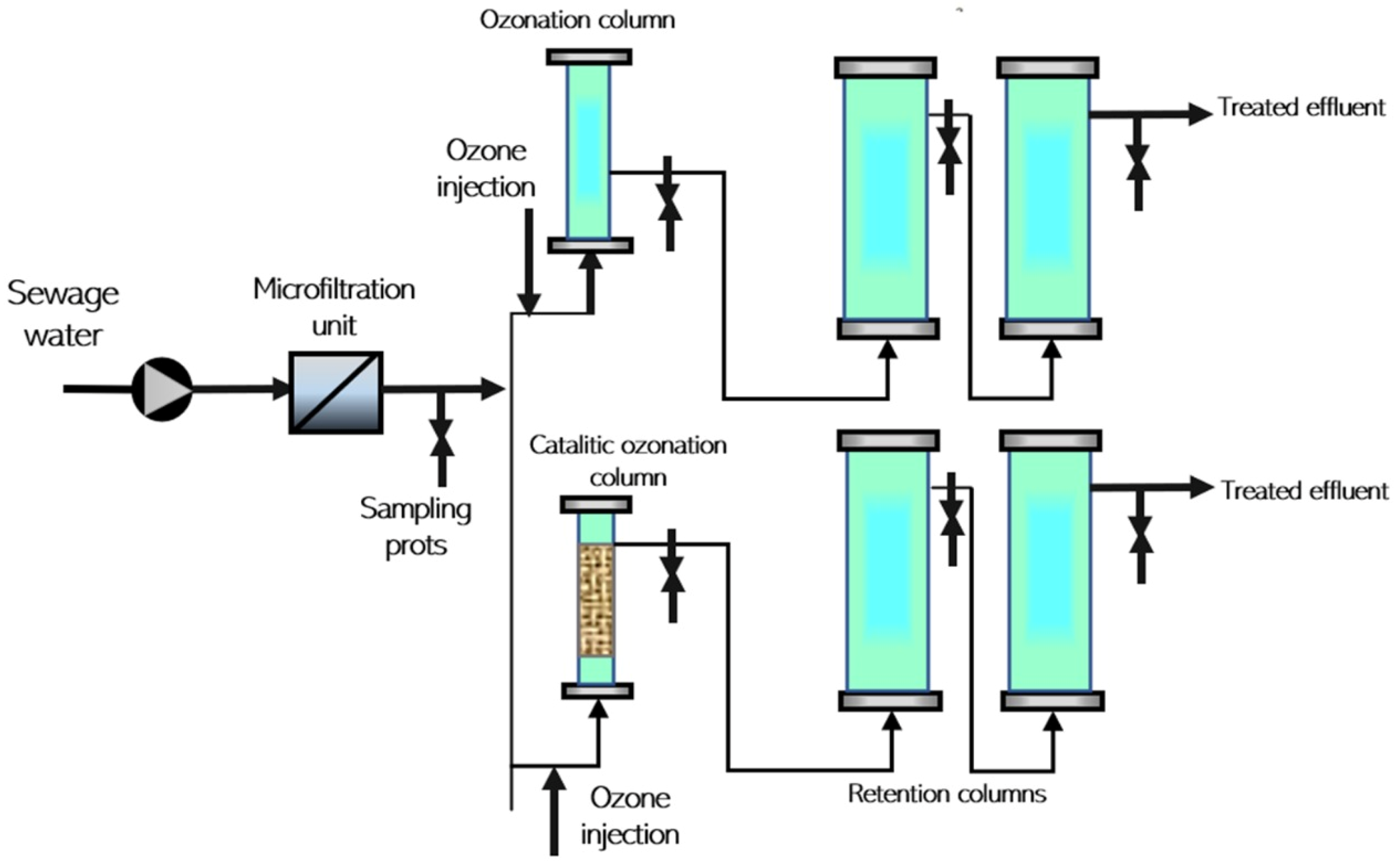
3.4. Ozonation
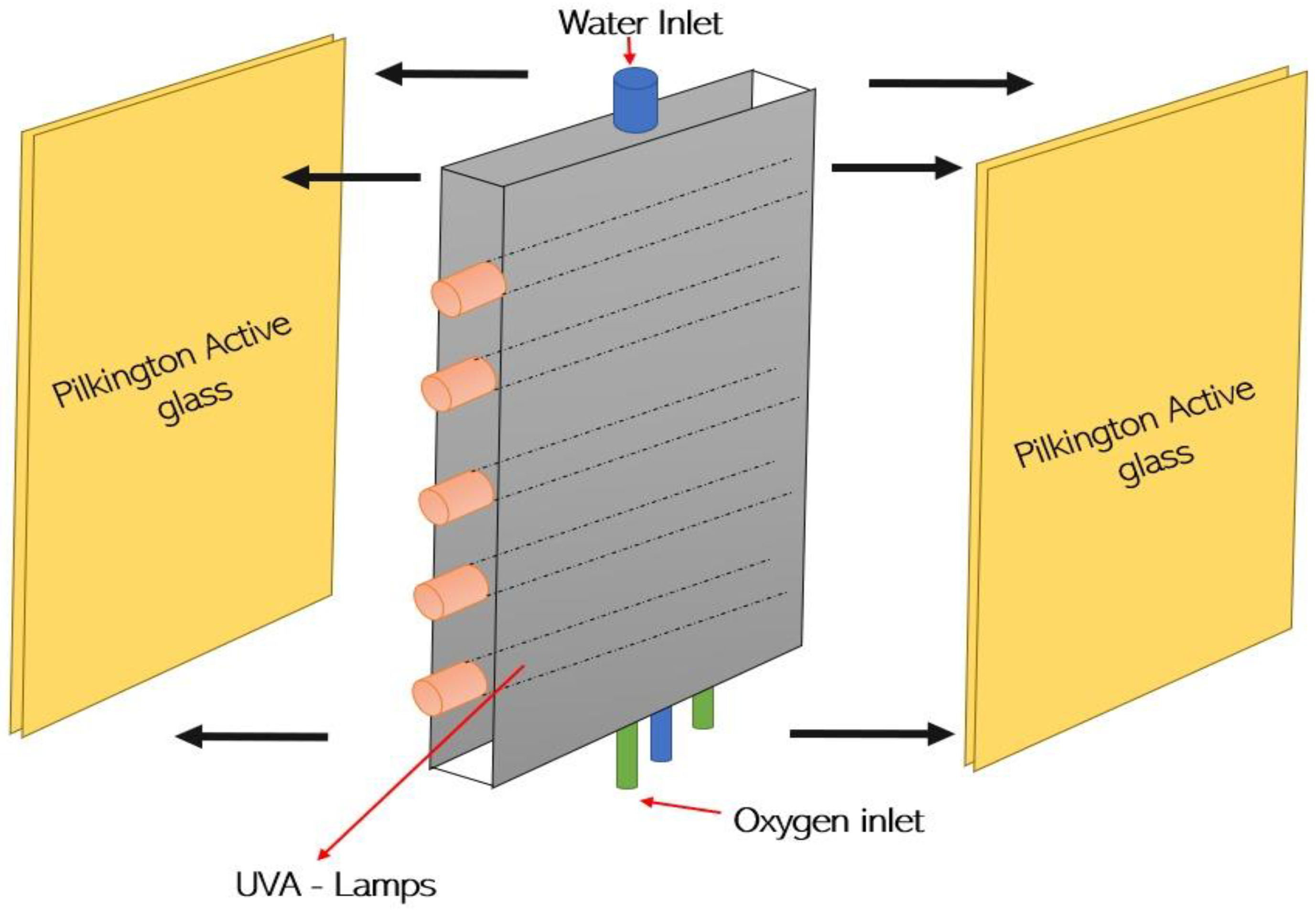
3.5. Photocatalytic Degradation Using Illuminated TIO2
3.6. UV Disinfection
3.7. Modified Saaty Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jones, E.R.; van Vliet, M.T.H.; Qadir, M.; Bierkens, M.F.P. Country-level and gridded estimates of wastewater production, collection, treatment and reuse. Earth Syst. Sci. Data 2021, 13, 237–254. [Google Scholar] [CrossRef]
- Ruiz-Rosa, I.; García-Rodríguez, F.J.; Jimenez, J.M. Development and application of a cost management model for wastewater treatment and reuse processes. J. Clean. Prod. 2016, 113, 299–310. [Google Scholar] [CrossRef]
- Ahmed, S.; Mofijur, M.; Nuzhat, S.; Chowdhury, A.T.; Rafa, N.; Uddin, A.; Inayat, A.; Mahlia, T.; Ong, H.C.; Chia, W.Y.; et al. Recent developments in physical, biological, chemical, and hybrid treatment techniques for removing emerging contaminants from wastewater. J. Hazard. Mater. 2021, 416, 125912. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Mozaz, S.; Vaz-Moreira, I.; Della Giustina, S.V.; Llorca, M.; Barceló, D.; Schubert, S.; Berendonk, T.U.; Michael-Kordatou, I.; Fatta-Kassinos, D.; Martinez, J.L.; et al. Antibiotic residues in final effluents of European wastewater treatment plants and their impact on the aquatic environment. Environ. Int. 2020, 140, 105733. [Google Scholar] [CrossRef] [PubMed]
- Rout, P.R.; Zhang, T.C.; Bhunia, P.; Surampalli, R.Y. Treatment technologies for emerging contaminants in wastewater treatment plants: A review. Sci. Total Environ. 2021, 753, 141990. [Google Scholar] [CrossRef]
- Sandoval, J.A.; Granados, M.A.M.; Rubio, D. A Brief Review About the Use of Microalgae for Degrading Emerging Pollutants in Wastewater. Gest. Ambiente 2020, 23, 127–137. [Google Scholar] [CrossRef]
- Hale, S.E.; Arp, H.P.H.; Schliebner, I.; Neumann, M. Persistent, mobile and toxic (PMT) and very persistent and very mobile (vPvM) substances pose an equivalent level of concern to persistent, bioaccumulative and toxic (PBT) and very persistent and very bioaccumulative (vPvB) substances under REACH. Environ. Sci. Eur. 2020, 32, 155. [Google Scholar] [CrossRef]
- Sivaranjanee, R.; Kumar, P.S. A review on remedial measures for effective separation of emerging contaminants from wastewater. Environ. Technol. Innov. 2021, 23, 101741. [Google Scholar] [CrossRef]
- Mohan, H.; Rajput, S.S.; Jadhav, E.B.; Sankhla, M.S. Ecotoxicity, Occurrence, and Removal of Pharmaceuticals and Illicit Drugs from Aquatic Systems. Biointerface Res. Appl. Chem. 2021, 11, 12530–12546. [Google Scholar] [CrossRef]
- Couto, C.F.; Lange, L.; Amaral, M.C. Occurrence, fate and removal of pharmaceutically active compounds (PhACs) in water and wastewater treatment plants—A review. J. Water Process Eng. 2019, 32, 100927. [Google Scholar] [CrossRef]
- Yadav, M.K.; Short, M.D.; Gerber, C.; Awad, J.; van den Akker, B.; Saint, C.P. Removal of emerging drugs of addiction by wastewater treatment and water recycling processes and impacts on effluent-associated environmental risk. Sci. Total. Environ. 2019, 680, 13–22. [Google Scholar] [CrossRef]
- Archer, E.; Castrignanò, E.; Kasprzyk-Hordern, B.; Wolfaardt, G. Wastewater-based epidemiology and enantiomeric profiling for drugs of abuse in South African wastewaters. Sci. Total Environ. 2018, 625, 792–800. [Google Scholar] [CrossRef]
- Causanilles, A.; Ruepert, C.; Ibañez, M.; Emke, E.; Hernández, F.; de Voogt, P. Occurrence and fate of illicit drugs and pharmaceuticals in wastewater from two wastewater treatment plants in Costa Rica. Sci. Total Environ. 2017, 599–600, 98–107. [Google Scholar] [CrossRef]
- Watanabe, K.; Batikian, C.M.; Pelley, D.; Carlson, B.; Pitt, J.; Gersberg, R.M. Occurrence of Stimulant Drugs of Abuse in a San Diego, CA, Stream and their Consumption Rates in the Neighboring Community. Water Air Soil Pollut. 2020, 231, 202. [Google Scholar] [CrossRef]
- Zheng, Q.; Tscharke, B.J.; Krapp, C.; O’Brien, J.W.; Mackie, R.S.; Connor, J.; Mueller, J.F.; Thomas, K.V.; Thai, P.K. New approach for the measurement of long-term alcohol consumption trends: Application of wastewater-based epidemiology in an Australian regional city. Drug Alcohol Depend. 2020, 207, 107795. [Google Scholar] [CrossRef]
- Driver, E.M.; Gushgari, A.; Chen, J.; Halden, R.U. Alcohol, nicotine, and caffeine consumption on a public U.S. university campus determined by wastewater-based epidemiology. Sci. Total Environ. 2020, 727, 138492. [Google Scholar] [CrossRef]
- Deng, Y.; Guo, C.; Zhang, H.; Yin, X.; Chen, L.; Wu, D.; Xu, J. Occurrence and removal of illicit drugs in different wastewater treatment plants with different treatment techniques. Environ. Sci. Eur. 2020, 32, 28. [Google Scholar] [CrossRef]
- Amin, M.T.; Alazba, A.A.; Manzoor, U. A review of removal of pollutants from water/wastewater using different types of nanomaterials. Adv. Mater. Sci. Eng. 2014, 2014, 825910. [Google Scholar] [CrossRef] [Green Version]
- Asimakopoulos, A.G.; Kannan, K. Neuropsychiatric pharmaceuticals and illicit drugs in wastewater treatment plants: A review. Environ. Chem. 2016, 13, 541–576. [Google Scholar] [CrossRef]
- Diamanti, K.; Aalizadeh, R.; Alygizakis, N.; Galani, A.; Mardal, M.; Thomaidis, N.S. Wide-scope target and suspect screening methodologies to investigate the occurrence of new psychoactive substances in influent wastewater from Athens. Sci. Total Environ. 2019, 685, 1058–1065. [Google Scholar] [CrossRef]
- Ferreira, A.P. Drugs of Abuse and Metabolites in Urban Wastewater: A Case Study, Rio De Janeiro Municipality, Brazil. World J. Res. Rev. 2019, 9, 14–19. [Google Scholar] [CrossRef]
- Styszko, K.; Proctor, K.; Castrignanò, E.; Kasprzyk-Hordern, B. Occurrence of pharmaceutical residues, personal care products, lifestyle chemicals, illicit drugs and metabolites in wastewater and receiving surface waters of Krakow agglomeration in South Poland. Sci. Total Environ. 2021, 768, 144360. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.K.; Gerber, C.; Saint, C.P.; Akker, B.V.D.; Short, M. Understanding the Removal and Fate of Selected Drugs of Abuse in Sludge and Biosolids from Australian Wastewater Treatment Operations. Engineering 2019, 5, 872–879. [Google Scholar] [CrossRef]
- Boni, M.R.; Chiavola, A.; Di Marcantonio, C.; Sbaffoni, S.; Biagioli, S.; Cecchini, G.; Frugis, A. A study through batch tests on the analytical determination and the fate and removal of methamphetamine in the biological treatment of domestic wastewater. Environ. Sci. Pollut. Res. 2018, 25, 27756–27767. [Google Scholar] [CrossRef]
- Boles, T.H.; Wells, M.J.M. Analysis of amphetamine and methamphetamine in municipal wastewater influent and effluent using weak cation-exchange SPE and LC-MS/MS. Electrophoresis 2016, 37, 3101–3108. [Google Scholar] [CrossRef]
- Carvalho, I.T.; Santos, L. Antibiotics in the aquatic environments: A review of the European scenario. Environ. Int. 2016, 94, 736–757. [Google Scholar] [CrossRef]
- Kim, K.Y.; Lai, F.Y.; Kim, H.-Y.; Thai, P.K.; Mueller, J.F.; Oh, J.-E. The first application of wastewater-based drug epidemiology in five South Korean cities. Sci. Total Environ. 2015, 524-525, 440–446. [Google Scholar] [CrossRef]
- Rostam, A.B.; Taghizadeh, M. Advanced oxidation processes integrated by membrane reactors and bioreactors for various wastewater treatments: A critical review. J. Environ. Chem. Eng. 2020, 8, 104566. [Google Scholar] [CrossRef]
- Bhatt, P.; Bhandari, G.; Bilal, M. Occurrence, toxicity impacts and mitigation of emerging micropollutants in the aquatic environments: Recent tendencies and perspectives. J. Environ. Chem. Eng. 2022, 10, 107598. [Google Scholar] [CrossRef]
- Rosman, N.; Salleh, W.; Mohamed, M.A.; Jaafar, J.; Ismail, A.; Harun, Z. Hybrid membrane filtration-advanced oxidation processes for removal of pharmaceutical residue. J. Colloid Interface Sci. 2018, 532, 236–260. [Google Scholar] [CrossRef]
- Ghafoori, S.; Omar, M.; Koutahzadeh, N.; Zendehboudi, S.; Malhas, R.N.; Mohamed, M.; Al-Zubaidi, S.; Redha, K.; Baraki, F.; Mehrvar, M. New advancements, challenges, and future needs on treatment of oilfield produced water: A state-of-the-art review. Sep. Purif. Technol. 2022, 289, 120652. [Google Scholar] [CrossRef]
- Chabukdhara, M.; Gogoi, M.; Gupta, S.K. Potential and Feasibility of the Microalgal System in Removal of Pharmaceutical Compounds from Wastewater. In Application of Microalgae in Wastewater Treatment; Springer: Cham, Switzerland, 2019; Volume 1, pp. 177–206. [Google Scholar]
- Liu, L.; Wheeler, S.; Venkataramanan, R.; A Rymer, J.; Pizon, A.F.; Lynch, M.J.; Tamama, K. Newly Emerging Drugs of Abuse and Their Detection Methods: An ACLPS Critical Review. Am. J. Clin. Pathol. 2018, 149, 105–116. [Google Scholar] [CrossRef]
- Saaty, T.L. Relative measurement and its generalization in decision making why pairwise comparisons are central in mathematics for the measurement of intangible factors the analytic hierarchy/network process. RACSAM-Rev. Real Acad. Cienc. Exactas Fis. Nat. Ser. A Mat. 2008, 102, 251–318. [Google Scholar] [CrossRef]
- Saaty, T.L. The Modern Science of Multicriteria Decision Making and Its Practical Applications: The AHP/ANP Approach. Oper. Res. 2013, 61, 1101–1118. [Google Scholar] [CrossRef]
- Leiva, A.M.; Piña, B.; Vidal, G. Antibiotic resistance dissemination in wastewater treatment plants: A challenge for the reuse of treated wastewater in agriculture. Rev. Environ. Sci. Bio/Technol. 2021, 20, 1043–1072. [Google Scholar] [CrossRef]
- Božanić, D.; Pamučar, D.; Bojanić, D. Modification of the analytic hierarchy process (AHP) method using fuzzy logic: Fuzzy AHP approach as a support to the decision making process concerning engagement of the group for additional hindering. Serb. J. Manag. 2015, 10, 151–171. [Google Scholar] [CrossRef] [Green Version]
- Shah, S.A.A.; Solangi, Y.A.; Ikram, M. Analysis of barriers to the adoption of cleaner energy technologies in Pakistan using Modified Delphi and Fuzzy Analytical Hierarchy Process. J. Clean. Prod. 2019, 235, 1037–1050. [Google Scholar] [CrossRef]
- Orejuela, I.P.; Toulkeridis, T. Evaluation of the susceptibility to landslides through diffuse logic and analytical hierarchy process (AHP) between Macas and Riobamba in Central Ecuador. In Proceedings of the 7th International Conference on eDemocracy and eGovernment, ICEDEG, Buenos Aires, Argentina, 22–24 April 2020; pp. 201–207. [Google Scholar] [CrossRef]
- Salcedo, D.; Almeida, O.P.; Morales, B.; Toulkeridis, T. Smart City Planning Based on Landslide Susceptibility Mapping Using Fuzzy Logic and Multi-criteria Evaluation Techniques in the City of Quito, Ecuador. Lect. Notes Electr. Eng. 2022, 846, 89–103. [Google Scholar] [CrossRef]
- Dong, Q.; Saaty, T.L. An analytic hierarchy process model of group consensus. J. Syst. Sci. Syst. Eng. 2014, 23, 362–374. [Google Scholar] [CrossRef]
- Van Tran, V.; Park, D.; Lee, Y.-C. Hydrogel applications for adsorption of contaminants in water and wastewater treatment. Environ. Sci. Pollut. Res. 2018, 25, 24569–24599. [Google Scholar] [CrossRef]
- Sodré, F.; Souza, G.; Feitosa, R.; Pereira, C.; Maldaner, A. Illicit Drugs, Metabolites and Adulterants in Wastewater: Monitoring Community Drug Abuse in the Brazilian Federal District during the 2014 Soccer World Cup. J. Braz. Chem. Soc. 2017, 28, 2146–2154. [Google Scholar] [CrossRef]
- Kim, M.-K.; Zoh, K.-D. Occurrence and removals of micropollutants in water environment. Environ. Eng. Res. 2016, 21, 319–332. [Google Scholar] [CrossRef] [Green Version]
- Voulvoulis, N.; Barceló, D.; Verlicchi, P. Pharmaceutical residues in sewage treatment works and their fate in the receiving environment. In Issues in Environmental Science and Technology; Hester, R.E., Harrison, R.M., Eds.; Royal Society of Chemistry: London, UK, 2015; Volume 41, pp. 120–179. [Google Scholar]
- Robin, T.; Barnes, A.; Dulaurent, S.; Loftus, N.; Baumgarten, S.; Moreau, S.; Marquet, P.; El Balkhi, S.; Saint-Marcoux, F. Fully automated sample preparation procedure to measure drugs of abuse in plasma by liquid chromatography tandem mass spectrometry. Anal. Bioanal. Chem. 2018, 410, 5071–5083. [Google Scholar] [CrossRef]
- Rizzo, L.; Malato, S.; Antakyali, D.; Beretsou, V.G.; Đolić, M.; Gernjak, W.; Heath, E.; Ivancev-Tumbas, I.; Karaolia, P.; Ribeiro, A.R.; et al. Consolidated vs new advanced treatment methods for the removal of contaminants of emerging concern from urban wastewater. Sci. Total Environ. 2019, 655, 986–1008. [Google Scholar] [CrossRef]
- Miao, L.; Wang, K.; Wang, S.; Zhu, R.; Li, B.; Peng, Y.; Weng, D. Advanced nitrogen removal from landfill leachate using real-time controlled three-stage sequence batch reactor (SBR) system. Bioresour. Technol. 2014, 159, 258–265. [Google Scholar] [CrossRef]
- Soliman, M.; Eldyasti, A. Development of partial nitrification as a first step of nitrite shunt process in a Sequential Batch Reactor (SBR) using Ammonium Oxidizing Bacteria (AOB) controlled by mixing regime. Bioresour. Technol. 2016, 221, 85–95. [Google Scholar] [CrossRef]
- Esteves, B.M.; Rodrigues, C.S.; Boaventura, R.A.; Maldonado-Hódar, F.; Madeira, L.M. Coupling of acrylic dyeing wastewater treatment by heterogeneous Fenton oxidation in a continuous stirred tank reactor with biological degradation in a sequential batch reactor. J. Environ. Manag. 2016, 166, 193–203. [Google Scholar] [CrossRef]
- Rajasimman, M.; Babu, S.V.; Rajamohan, N. Biodegradation of textile dyeing industry wastewater using modified anaerobic sequential batch reactor—Start-up, parameter optimization and performance analysis. J. Taiwan Inst. Chem. Eng. 2017, 72, 171–181. [Google Scholar] [CrossRef]
- Moreira, I.; Amorim, C.L.; Ribeiro, A.R.; Mesquita, R.; Rangel, A.; van Loosdrecht, M.; Tiritan, M.E.; Castro, P.M. Removal of fluoxetine and its effects in the performance of an aerobic granular sludge sequential batch reactor. J. Hazard. Mater. 2015, 287, 93–101. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, Y.; Lv, D.; Wang, M.; Peng, Y.; Li, B. Advanced nitrogen and phosphorus removal in the pre-denitrification anaerobic/anoxic/aerobic nitrification sequence batch reactor (pre-A 2 NSBR) treating low carbon/nitrogen (C/N) wastewater. Chem. Eng. J. 2016, 302, 296–304. [Google Scholar] [CrossRef]
- Soliman, M.; Eldyasti, A. Long-term dynamic and pseudo-state modeling of complete partial nitrification process at high nitrogen loading rates in a sequential batch reactor (SBR). Bioresour. Technol. 2017, 233, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Grehs, B.W.N.; Linton, M.A.O.; Clasen, B.; Silveira, A.D.O.; Carissimi, E. Antibiotic resistance in wastewater treatment plants: Understanding the problem and future perspectives. Arch. Microbiol. 2020, 203, 1009–1020. [Google Scholar] [CrossRef] [PubMed]
- Dutta, A.; Sarkar, S. Sequencing Batch Reactor for Wastewater Treatment: Recent Advances. Curr. Pollut. Rep. 2015, 1, 177–190. [Google Scholar] [CrossRef]
- Świątczak, P.; Cydzik-Kwiatkowska, A. Performance and microbial characteristics of biomass in a full-scale aerobic granular sludge wastewater treatment plant. Environ. Sci. Pollut. Res. 2018, 25, 1655–1669. [Google Scholar] [CrossRef] [Green Version]
- Pronk, M.; de Kreuk, M.; de Bruin, B.; Kamminga, P.; Kleerebezem, R.; van Loosdrecht, M. Full scale performance of the aerobic granular sludge process for sewage treatment. Water Res. 2015, 84, 207–217. [Google Scholar] [CrossRef]
- Osman, M.; Awaleh, M.O.; Soubaneh, Y.D. Waste Water Treatment in Chemical Industries: The Concept and Current Technologies. J. Waste Water Treat. Anal. 2014, 5, 164. [Google Scholar] [CrossRef]
- Alemu, T.; Lemma, E.; Mekonnen, A.; Leta, S. Performance of Pilot Scale Anaerobic-SBR System Integrated with Constructed Wetlands for the Treatment of Tannery Wastewater. Environ. Process. 2016, 3, 815–827. [Google Scholar] [CrossRef]
- Huang, A.; Yan, M.; Lin, J.; Xu, L.; Gong, H.; Gong, H. A Review of Processes for Removing Antibiotics from Breeding Wastewater. Int. J. Environ. Res. Public Health 2021, 18, 4909. [Google Scholar] [CrossRef]
- Jagaba, A.; Kutty, S.; Lawal, I.; Abubakar, S.; Hassan, I.; Zubairu, I.; Umaru, I.; Abdurrasheed, A.; Adam, A.; Ghaleb, A.; et al. Sequencing batch reactor technology for landfill leachate treatment: A state-of-the-art review. J. Environ. Manag. 2021, 282, 111946. [Google Scholar] [CrossRef]
- Lin, Z.; Li, J.; Shen, W.; Corriou, J.-P.; Chen, X.; Xi, H. Different photocatalytic levels of organics in papermaking wastewater by flocculation-photocatalysis and SBR-photocatalysis: Degradation and GC–MS experiments, adsorption and photocatalysis simulations. Chem. Eng. J. 2021, 412, 128715. [Google Scholar] [CrossRef]
- Deng, Y.; Zhao, R. Advanced Oxidation Processes (AOPs) in Wastewater Treatment. Curr. Pollut. Rep. 2015, 1, 167–176. [Google Scholar] [CrossRef] [Green Version]
- Sivagami, K.; Sakthivel, K.; Nambi, I.M. Advanced oxidation processes for the treatment of tannery wastewater. J. Environ. Chem. Eng. 2018, 6, 3656–3663. [Google Scholar] [CrossRef]
- Mousset, E.; Loh, W.H.; Lim, W.S.; Jarry, L.; Wang, Z.; Lefebvre, O. Cost comparison of advanced oxidation processes for wastewater treatment using accumulated oxygen-equivalent criteria. Water Res. 2021, 200, 117234. [Google Scholar] [CrossRef]
- Elmobarak, W.; Hameed, B.; Almomani, F.; Abdullah, A. A Review on the Treatment of Petroleum Refinery Wastewater Using Advanced Oxidation Processes. Catalysts 2021, 11, 782. [Google Scholar] [CrossRef]
- Giannakis, S.; Vives, F.A.G.; Grandjean, D.; Magnet, A.; De Alencastro, L.F.; Pulgarin, C. Effect of advanced oxidation processes on the micropollutants and the effluent organic matter contained in municipal wastewater previously treated by three different secondary methods. Water Res. 2015, 84, 295–306. [Google Scholar] [CrossRef]
- Bokare, A.D.; Choi, W. Review of iron-free Fenton-like systems for activating H2O2 in advanced oxidation processes. J. Hazard. Mater. 2014, 275, 121–135. [Google Scholar] [CrossRef]
- An, J.; Li, N.; Wu, Y.; Wang, S.; Liao, C.; Zhao, Q.; Zhou, L.; Li, T.; Wang, X.; Feng, Y. Revealing Decay Mechanisms of H2O2-Based Electrochemical Advanced Oxidation Processes after Long-Term Operation for Phenol Degradation. Environ. Sci. Technol. 2020, 54, 10916–10925. [Google Scholar] [CrossRef]
- Mackuľak, T.; Mosný, M.; Grabic, R.; Golovko, O.; Koba, O.; Birošová, L. Fenton-like reaction: A possible way to efficiently remove illicit drugs and pharmaceuticals from wastewater. Environ. Toxicol. Pharmacol. 2015, 39, 483–488. [Google Scholar] [CrossRef]
- Giannakis, S.; Watts, S.; Rtimi, S.; Pulgarin, C. Solar light and the photo-Fenton process against antibiotic resistant bacteria in wastewater: A kinetic study with a Streptomycin-resistant strain. Catal. Today 2018, 313, 86–93. [Google Scholar] [CrossRef]
- Guzman, P.V.; Giannakis, S.; Rtimi, S.; Grandjean, D.; Bensimon, M.; de Alencastro, L.F.; Torres-Palma, R.; Pulgarin, C. A green solar photo-Fenton process for the elimination of bacteria and micropollutants in municipal wastewater treatment using mineral iron and natural organic acids. Appl. Catal. B Environ. 2017, 219, 538–549. [Google Scholar] [CrossRef]
- Ahmed, M.M.; Chiron, S. Solar photo-Fenton like using persulphate for carbamazepine removal from domestic wastewater. Water Res. 2014, 48, 229–236. [Google Scholar] [CrossRef]
- Catalá, M.; Domínguez-Morueco, N.; Migens, A.; Molina, R.; Martínez, F.; Valcárcel, Y.; Mastroianni, N.; de Alda, M.L.; Barceló, D.; Segura, Y. Elimination of drugs of abuse and their toxicity from natural waters by photo-Fenton treatment. Sci. Total Environ. 2015, 520, 198–205. [Google Scholar] [CrossRef]
- Duan, L.; Zhang, Y.; Wang, B.; Deng, S.; Huang, J.; Wang, Y.; Yu, G. Occurrence, elimination, enantiomeric distribution and intra-day variations of chiral pharmaceuticals in major wastewater treatment plants in Beijing, China. Environ. Pollut. 2018, 239, 473–482. [Google Scholar] [CrossRef]
- Carolin, C.F.; Kumar, P.S.; Joshiba, G.J.; Kumar, V.V. Analysis and removal of pharmaceutical residues from wastewater using membrane bioreactors: A review. Environ. Chem. Lett. 2020, 19, 329–343. [Google Scholar] [CrossRef]
- Archer, E.; Volschenk, M.; Brocker, L.; Wolfaardt, G.M. A two-year study of emerging micro-pollutants and drugs of abuse in two Western Cape wastewater treatment works (South Africa). Chemosphere 2021, 285, 131460. [Google Scholar] [CrossRef]
- Singh, S.; Shikha. Treatment and Recycling of Wastewater from Oil Refinery/Petroleum Industry. In Advances in Biological Treatment of Industrial Waste Water and Their Recycling for a Sustainable Future; Springer: Heidelberg/Berlin, Germany, 2019; pp. 303–332. [Google Scholar] [CrossRef]
- Mahat, S.B.; Omar, R.; Idris, A.; Kamal, S.M.M.; Idris, A.I.M. Dynamic membrane applications in anaerobic and aerobic digestion for industrial wastewater: A mini review. Food Bioprod. Process. 2018, 112, 150–168. [Google Scholar] [CrossRef]
- Yang, X.; López-Grimau, V.; Vilaseca, M.; Crespi, M.; Ribera-Pi, J.; Calderer, M.; Martínez-Lladó, X. Reuse of textile wastewater treated by moving bed biofilm reactor coupled with membrane bioreactor. Color. Technol. 2021, 137, 484–492. [Google Scholar] [CrossRef]
- Hernández, F.H.; Ibañez, M.; Botero-Coy, A.M.; Bade, R.; Bustos-López, M.C.; Rincón, J.; Moncayo-Lasso, A.; Bijlsma, L. LC-QTOF MS screening of more than 1,000 licit and illicit drugs and their metabolites in wastewater and surface waters from the area of Bogotá, Colombia. Anal. Bioanal. Chem. 2015, 407, 6405–6416. [Google Scholar] [CrossRef] [Green Version]
- Muter, O.; Pērkons, I.; Selga, T.; Berzins, A.; Gudra, D.; Radovica-Spalvina, I.; Fridmanis, D.; Bartkevics, V. Removal of pharmaceuticals from municipal wastewaters at laboratory scale by treatment with activated sludge and biostimulation. Sci. Total Environ. 2017, 584-585, 402–413. [Google Scholar] [CrossRef]
- Kuyukina, M.S.; Krivoruchko, A.V.; Ivshina, I.B. Advanced Bioreactor Treatments of Hydrocarbon-Containing Wastewater. Appl. Sci. 2020, 10, 831. [Google Scholar] [CrossRef] [Green Version]
- Kanaujiya, D.K.; Paul, T.; Sinharoy, A.; Pakshirajan, K. Biological Treatment Processes for the Removal of Organic Micropollutants from Wastewater: A Review. Curr. Pollut. Rep. 2019, 5, 112–128. [Google Scholar] [CrossRef]
- Ezugbe, E.O.; Rathilal, S. Membrane Technologies in Wastewater Treatment: A Review. Membranes 2020, 10, 89. [Google Scholar] [CrossRef] [PubMed]
- Rodayan, A.; Segura, P.A.; Yargeau, V. Ozonation of wastewater: Removal and transformation products of drugs of abuse. Sci. Total Environ. 2014, 487, 763–770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, P.; Zhang, H.; Feng, Y.; Yang, F.; Zhang, J. Removal of trace antibiotics from wastewater: A systematic study of nanofiltration combined with ozone-based advanced oxidation processes. Chem. Eng. J. 2014, 240, 211–220. [Google Scholar] [CrossRef]
- Hassaan, M.; el Nemr, A.; Hassaan, M.A. Advanced Oxidation Processes for Textile Wastewater Treatment. Int. J. Photochem. Photobiol. 2017, 2, 85–93. [Google Scholar]
- Yasar, A.; Ghaffar, A.; Mahfooz, Y.; Tabinda, A.B.; Mehmood, A. Comparative performance evaluation of ozone oxidation and coagulation for the treatment of electroplating wastewater. Desalination Water Treat. 2021, 230, 268–275. Available online: https://www.deswater.com/DWT_articles/vol_230_papers/230_2021_268.pdf (accessed on 25 April 2022). [CrossRef]
- Rekhate, C.V.; Srivastava, J.K. Recent advances in ozone-based advanced oxidation processes for treatment of wastewater- A review. Chem. Eng. J. Adv. 2020, 3, 100031. [Google Scholar] [CrossRef]
- Aziz, K.H.H.; Omer, K.M.; Mahyar, A.; Miessner, H.; Mueller, S.; Moeller, D. Application of Photocatalytic Falling Film Reactor to Elucidate the Degradation Pathways of Pharmaceutical Diclofenac and Ibuprofen in Aqueous Solutions. Coatings 2019, 9, 465. [Google Scholar] [CrossRef] [Green Version]
- Wen, J.; Li, X.; Liu, W.; Fang, Y.; Xie, J.; Xu, Y. Photocatalysis fundamentals and surface modification of TiO2 nanomaterials. Chin. J. Catal. 2015, 36, 2049–2070. [Google Scholar] [CrossRef]
- Wang, Z.; Thuy, G.N.S.T.; Srivastava, V.; Ambat, I.; Sillanpää, M. Photocatalytic degradation of an artificial sweetener (Acesulfame-K) from synthetic wastewater under UV-LED controlled illumination. Process Saf. Environ. Prot. 2019, 123, 206–214. [Google Scholar] [CrossRef]
- Farzadkia, M.; Bazrafshan, E.; Esrafili, A.; Yang, J.-K.; Shirzad-Siboni, M. Photocatalytic degradation of Metronidazole with illuminated TiO2 nanoparticles. J. Environ. Heal. Sci. Eng. 2015, 13, 35. [Google Scholar] [CrossRef] [Green Version]
- Jonidi-Jafari, A.; Shirzad-Siboni, M.; Yang, J.-K.; Naimi-Joubani, M.; Farrokhi, M. Photocatalytic degradation of diazinon with illuminated ZnO–TiO2 composite. J. Taiwan Inst. Chem. Eng. 2015, 50, 100–107. [Google Scholar] [CrossRef]
- Hunge, Y.; Yadav, A.; Dhodamani, A.; Suzuki, N.; Terashima, C.; Fujishima, A.; Mathe, V. Enhanced photocatalytic performance of ultrasound treated GO/TiO2 composite for photocatalytic degradation of salicylic acid under sunlight illumination. Ultrason. Sonochemistry 2020, 61, 104849. [Google Scholar] [CrossRef]
- AlAbduljabbar, F.A.; Haider, S.; Ali, F.A.A.; Alghyamah, A.A.; Almasry, W.A.; Patel, R.; Mujtaba, I.M. TiO2 nanostructured coated functionally modified and composite electrospun chitosan nanofibers membrane for efficient photocatalytic degradation of organic pollutant in wastewater. J. Mater. Res. Technol. 2021, 15, 5197–5212. [Google Scholar] [CrossRef]
- Hernández Rodríguez, M.J.; Pulido Melián, E.; González Díaz, O.; Araña, J.; Macías, M.; González Orive, A.; Doña Rodríguez, J.M. Comparison of supported TiO2 catalysts in the photocatalytic degradation of NOx. J. Mol. Catal. A Chem. 2016, 413, 56–66. [Google Scholar] [CrossRef]
- Chen, J.; Loeb, S.; Kim, J.-H. LED revolution: Fundamentals and prospects for UV disinfection applications. Environ. Sci. Water Res. Technol. 2017, 3, 188–202. [Google Scholar] [CrossRef]
- Sun, Z.; Li, M.; Li, W.; Qiang, Z. A review of the fluence determination methods for UV reactors: Ensuring the reliability of UV disinfection. Chemosphere 2022, 286, 131488. [Google Scholar] [CrossRef]
- Li, H.; Osman, H.; Kang, C.; Ba, T. Numerical and experimental investigation of UV disinfection for water treatment. Appl. Therm. Eng. 2017, 111, 280–291. [Google Scholar] [CrossRef]
- Afonso-Olivares, C.; Fernández-Rodríguez, C.; Ojeda-González, R.; Sosa-Ferrera, Z.; Rodríguez, J.M.D. Estimation of kinetic parameters and UV doses necessary to remove twenty-three pharmaceuticals from pre-treated urban wastewater by UV/H2O2. J. Photochem. Photobiol. A Chem. 2016, 329, 130–138. [Google Scholar] [CrossRef]
- Sarkar, S.; Chakraborty, S.; Bhattacharjee, C. Photocatalytic degradation of pharmaceutical wastes by alginate supported TiO2 nanoparticles in packed bed photo reactor (PBPR). Ecotoxicol. Environ. Saf. 2015, 121, 263–270. [Google Scholar] [CrossRef]
- Iervolino, G.; Zammit, I.; Vaiano, V.; Rizzo, L. Limitations and Prospects for Wastewater Treatment by UV and Visible-Light-Active Heterogeneous Photocatalysis: A Critical Review. Heterog. Photocatal. 2020, 378, 225–264. [Google Scholar] [CrossRef]
- Gimeno, O.; García-Araya, J.; Beltrán, F.; Rivas, F.; Espejo, A. Removal of emerging contaminants from a primary effluent of municipal wastewater by means of sequential biological degradation-solar photocatalytic oxidation processes. Chem. Eng. J. 2016, 290, 12–20. [Google Scholar] [CrossRef]
- Murgolo, S.; Yargeau, V.; Gerbasi, R.; Visentin, F.; EL Habra, N.; Ricco, G.; Lacchetti, I.; Carere, M.; Curri, M.; Mascolo, G. A new supported TiO 2 film deposited on stainless steel for the photocatalytic degradation of contaminants of emerging concern. Chem. Eng. J. 2017, 318, 103–111. [Google Scholar] [CrossRef]
| Variable | Category | Values/Data | Weighting |
|---|---|---|---|
| Removal efficiency | Evil | 70–75% | 1 |
| Regular | 76–80% | 3 | |
| Well | 81–85% | 5 | |
| Very good | 86–92% | 7 | |
| Excellent | 93–100% | 9 | |
| Construction cost | Down | - | 9 |
| Medium | - | 5 | |
| High | - | 1 | |
| Development stage | Regular | Laboratory | 1 |
| Well | Pilot Planpt | 5 | |
| Very good | Big scale | 9 | |
| Waste generation | Does not generate | Does not imply | 9 |
| Low detrimental | Carbon Diocid | 7 | |
| Slightly detrimental | Sludge and microbial biomass | 5 | |
| Moderately detrimental | Transformation products resulting from incomplete mineralization | 3 | |
| Highly detrimental | Free radicals, peroxides, and oxidative by products | 1 |
| Removal Efficiency | Construction Cost | Development Stage | Waste Generation | = | wi | |
|---|---|---|---|---|---|---|
| Removal efficiency | 1 | 0.5555 | 0.7142 | 1.6666 | = | 0.2083 |
| Construction cost | 1.8 | 1 | 1.2857 | 3 | = | 0.3750 |
| Development stage | 1.4 | 0.7777 | 1 | 2.3333 | = | 0.2917 |
| Waste generation | 0.6 | 0.3333 | 0.4285 | 1 | = | 0.1250 |
| Treatment/Type | Drug | Efficiency | Advantages | Disadvantages |
|---|---|---|---|---|
| Sequential Batch Reactor (SBR)/ Biological Activated Sludge Process | METH | 70–95% |
|
|
| Cocaine | 40–50% | |||
| Benzoylecgonine | >90% | |||
| Amphetamine | >40% | |||
| Methadone (MET) | >90% | |||
| Codeine (COD) | >90% | |||
| Ketamine (KET) | >75% | |||
| 3,4-Methylenedioxyamphetamine (MDA) | >60% | |||
| Caffeine | 99% | |||
| Fenton Reaction (FR)/Chemical | Amphetamine | 80% |
|
|
| METH | 87% | |||
| Benzoylecgonine | 50–70% | |||
| Cocaine | 50–70% | |||
| THC-COOH | 80% | |||
| Codeine | 50–70% | |||
| Methadone | 10% | |||
| Mixed-flow bioreactor (anaerobic and aerobic)/Biological | Codeine | 95% |
|
|
| Caffeine | 91% | |||
| Benzoylecgonine | 85% | |||
| Codeine | 90% | |||
| METH | 85% | |||
| Methaqualone | 60% | |||
| Ozonation/ Chemical | Benzoylecgonine (BE) | 96% |
|
|
| Amphetamine (AMP) | >25% | |||
| Ketamine (KET) | >25% | |||
| Cocaine (COC) | 70% | |||
| METH (METH) | 70% | |||
| Extasis (MDMA) | 50% | |||
| Photocatalytic degradation using illuminated TiO2/Chemical | Ketamine | 99.9% |
|
|
| METH | 99.9% | |||
| Morphine | 99.9% | |||
| UV Disinfection/ Physical | Benzoylecgonine | 83% |
|
|
| METH | 92% | |||
| MDMA | 73% | |||
| Codeine | 89% | |||
| Morphine | 99% |
| Technique = | 0.2083 x Efficiency | + | 0.3750 x Cost | + | 0.2917 x Stage | + | 0.1250 * Waste | = | Results |
|---|---|---|---|---|---|---|---|---|---|
| SBR= | 0.0000 | + | 0.2652 | + | 0.2062 | + | 0.0884 | = | 0.5598 |
| Ozonation = | 0.0000 | + | 0.0000 | + | 0.0000 | + | 0.1155 | = | 0.1155 |
| Fenton = | 0.1925 | + | 0.3750 | + | 0.0000 | + | 0.1250 | = | 0.6925 |
| TiO2 = | 0.2083 | + | 0.0000 | + | 0.2062 | + | 0.0478 | = | 0.4624 |
| Bioreactor = | 0.1473 | + | 0.0000 | + | 0.2917 | + | 0.0884 | = | 0.5274 |
| UV disinfection = | 0.1925 | + | 0.3750 | + | 0.2917 | + | 0.0000 | = | 0.8591 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ponce-Arguello, M.; Abad-Sarango, V.; Crisanto-Perrazo, T.; Toulkeridis, T. Removal of METH through Tertiary or Advanced Treatment in a WWTP. Water 2022, 14, 1807. https://doi.org/10.3390/w14111807
Ponce-Arguello M, Abad-Sarango V, Crisanto-Perrazo T, Toulkeridis T. Removal of METH through Tertiary or Advanced Treatment in a WWTP. Water. 2022; 14(11):1807. https://doi.org/10.3390/w14111807
Chicago/Turabian StylePonce-Arguello, Mariuxi, Viviana Abad-Sarango, Tania Crisanto-Perrazo, and Theofilos Toulkeridis. 2022. "Removal of METH through Tertiary or Advanced Treatment in a WWTP" Water 14, no. 11: 1807. https://doi.org/10.3390/w14111807
APA StylePonce-Arguello, M., Abad-Sarango, V., Crisanto-Perrazo, T., & Toulkeridis, T. (2022). Removal of METH through Tertiary or Advanced Treatment in a WWTP. Water, 14(11), 1807. https://doi.org/10.3390/w14111807







