Side-Stream Phosphorus Recovery in Activated Sludge Processes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Aerobic Mixed Liquor Characteristics
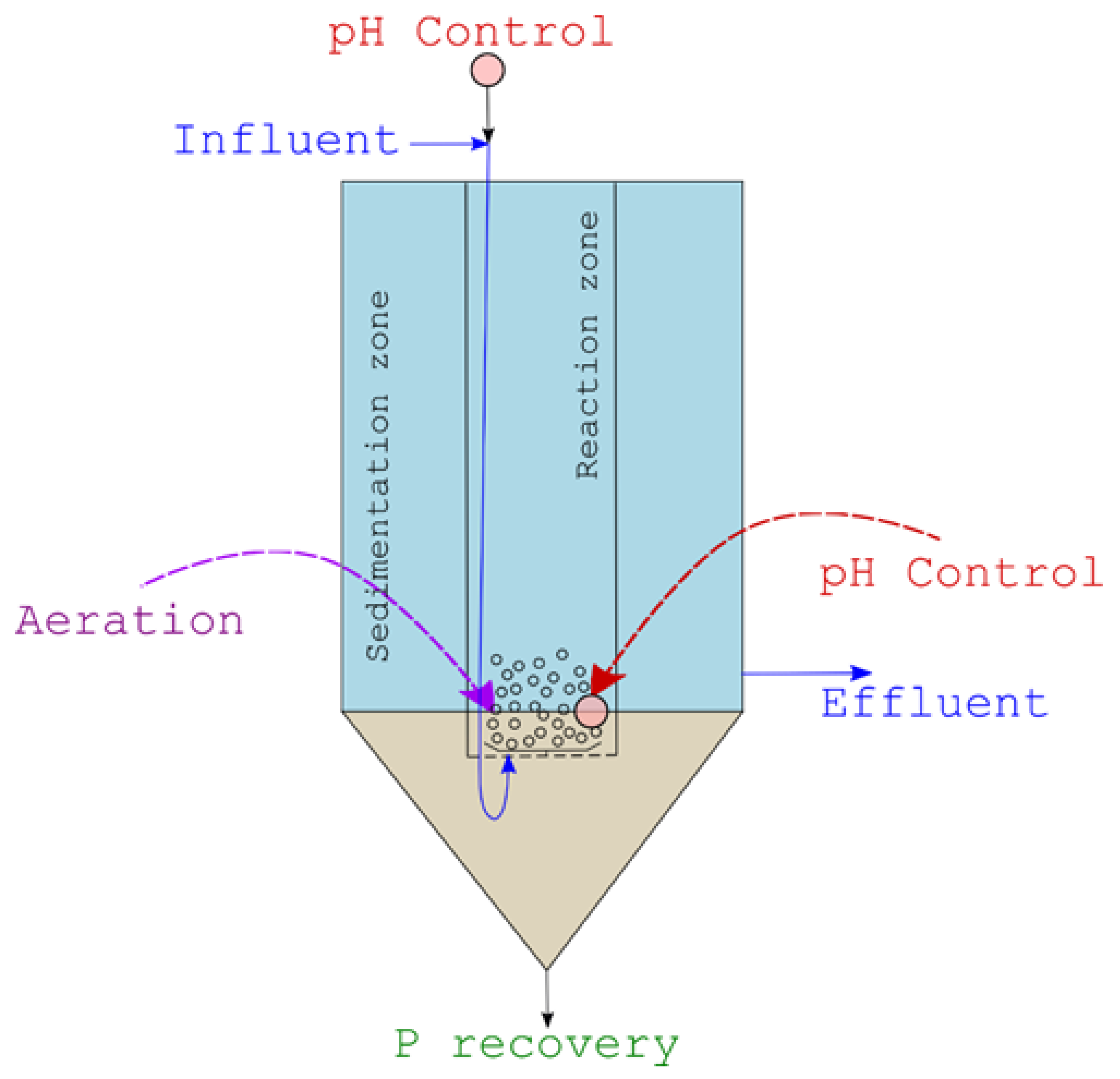
2.2. Pilot Reactor
2.3. Instrumentation and Analytics
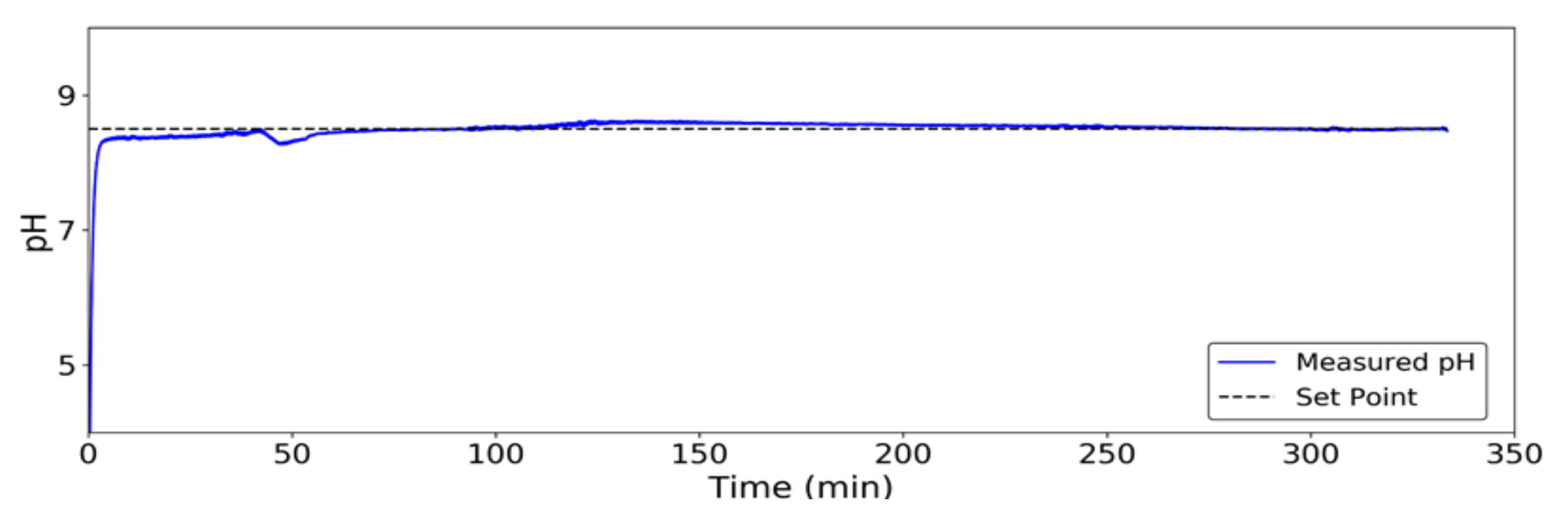
2.4. pH Control
2.5. Experimental Design
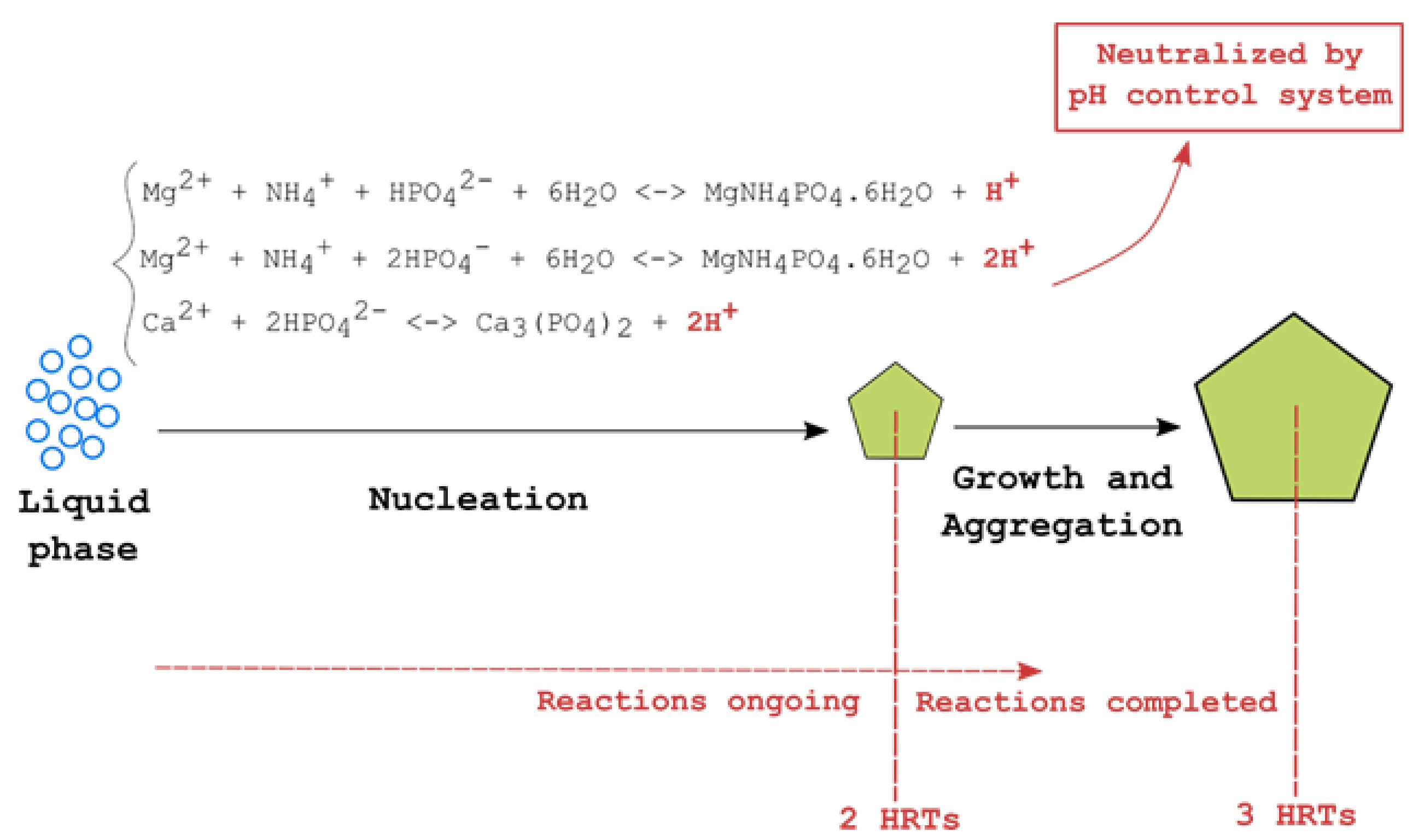
2.6. Chemical Equilibrium Modeling
3. Results
3.1. Performance of the pH Control System
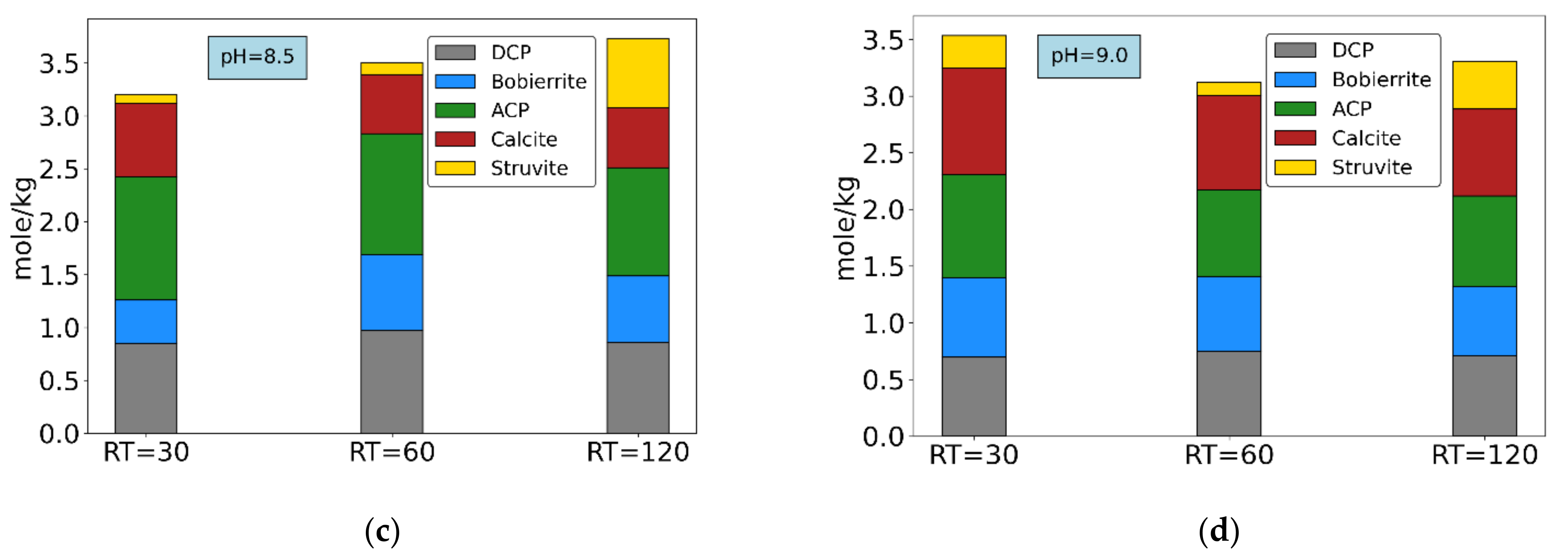
3.2. Determination of Mineral Precipitation Likelihood
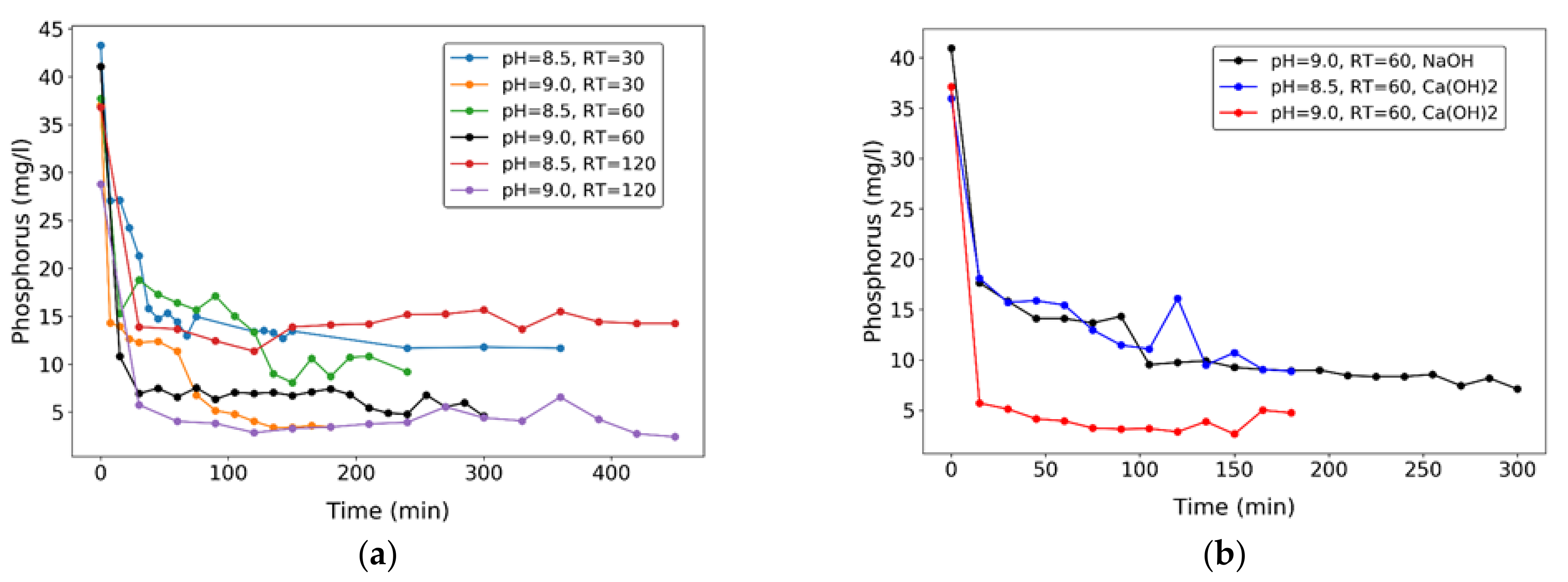
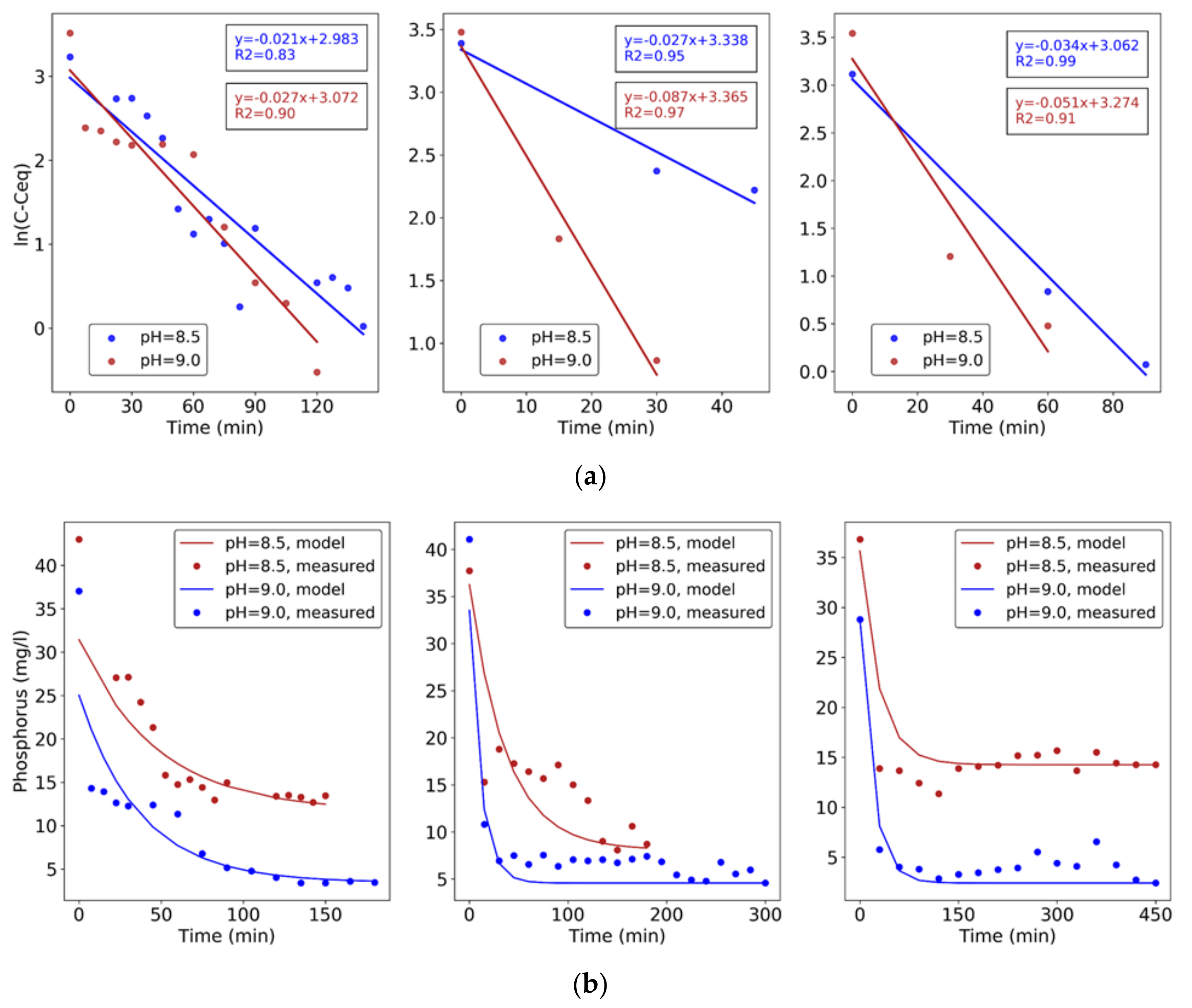
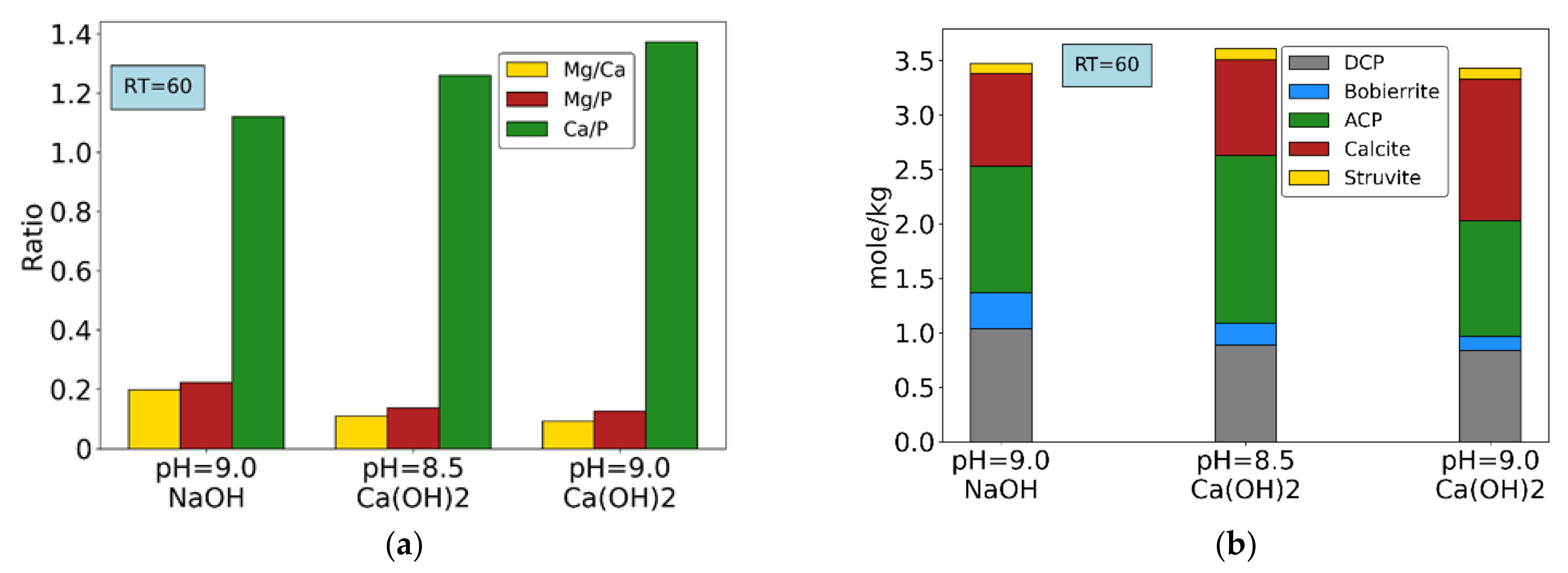
3.3. Synthetic Wastewater P-Recovery Tests
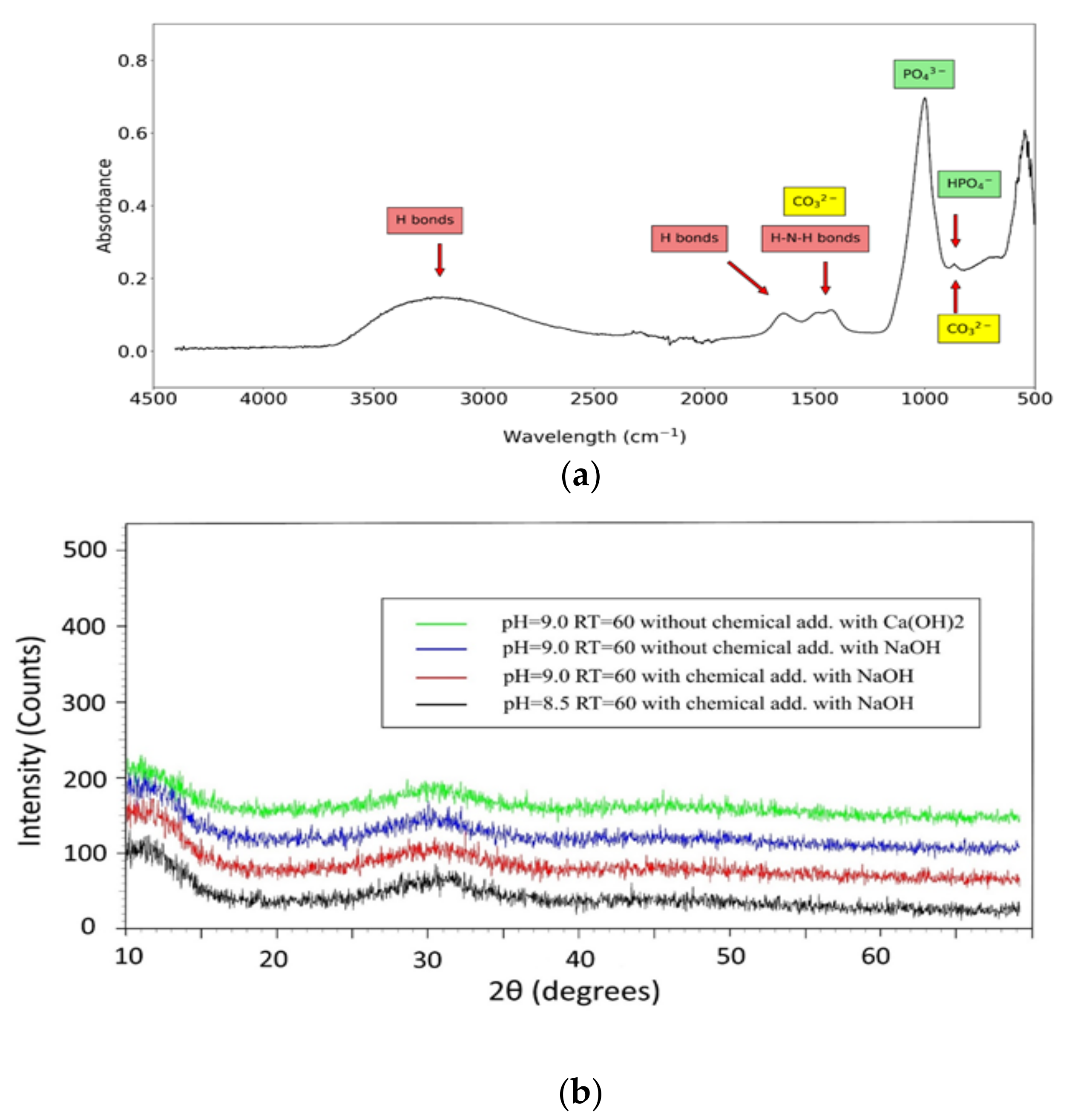
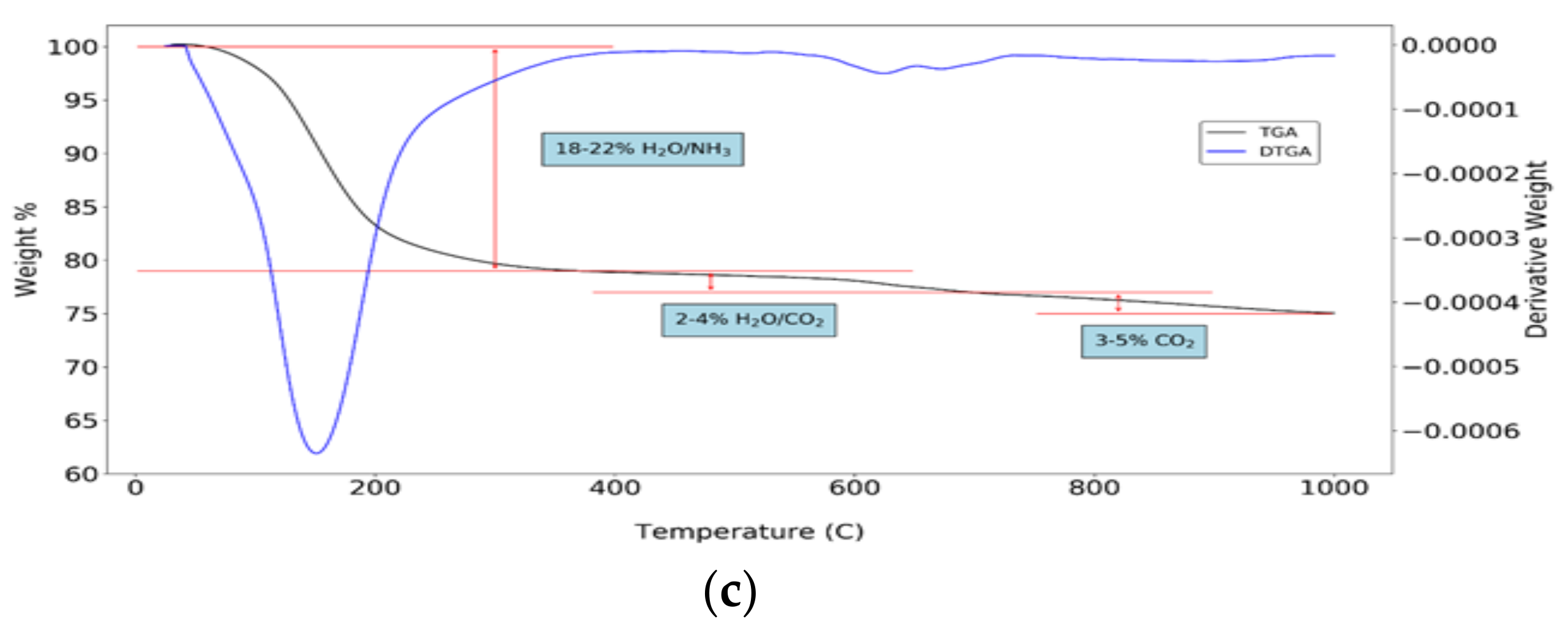
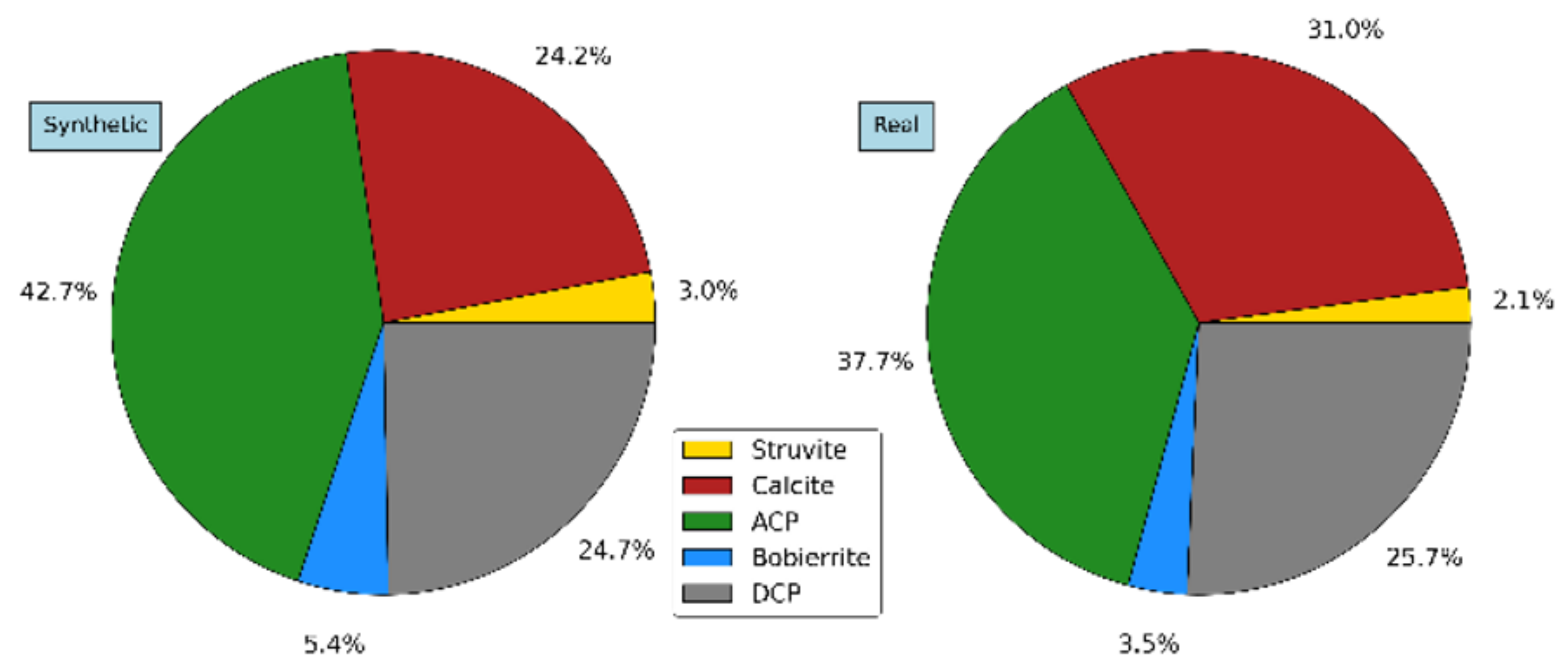
3.4. Precipitates Analysis
3.5. Second Stage: Process Application with Real Wastewater
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Copetti, D.; Valsecchi, L.; Capodaglio, A.G.; Tartari, G. Direct measurement of nutrient concentrations in freshwaters with a miniaturized analytical probe: Evaluation and validation. Environ. Monit. Assess 2017, 189, 144. [Google Scholar] [CrossRef] [PubMed]
- Lewis, W.M.; Wurtsbaugh, W.A.; Paerl, H.W. Rationale for Control of Anthropogenic Nitrogen and Phosphorus to Reduce Eutrophication of Inland Waters. Environ. Sci. Technol. 2011, 45, 10300–10305. [Google Scholar] [CrossRef] [PubMed]
- Barnard, J. A review of biological phosphorus removal in the activated sludge process. Water SA 1976, 2, 136–144. [Google Scholar]
- Capodaglio, A.G.; Hlavínek, P.; Raboni, M. Physico-chemical technologies for nitrogen removal from wastewaters: A review. Rev. Ambiente Agua 2015, 10, 481–498. [Google Scholar]
- Capodaglio, A.G.; Hlavínek, P.; Raboni, M. Advances in wastewater nitrogen removal by biological processes: State of the art review. Rev. Ambiente Agua 2016, 11, 250–267. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, S.; Lobanov, S.; Lo, V.K. An overview of technologies to recover phosphorus as struvite from wastewater: Advantages and shortcomings. Environ. Sci. Pollut. Res. 2019, 26, 19063–19077. [Google Scholar] [CrossRef]
- EEC. Council Directive 91/271/EEC, Concerning Urban Waste-Water Treatment. Off. J. Eur. Communities 1991, 34, 40. Available online: https://eurlex.europa.eu/legalcontent/EN/TXT/PDF/?uri=CELEX:31991L0271&from=EN (accessed on 20 November 2021).
- Daneshgar, S.; Callegari, A.; Capodaglio, A.G.; Vaccari, D. The Potential Phosphorus Crisis: Resource Conservation and Possible Escape Technologies: A Review. Resources 2018, 7, 37. [Google Scholar] [CrossRef] [Green Version]
- Monetti, J.; Ledezma, P.; Freguia, S. Optimised operational parameters for improved nutrient recovery from hydrolysed urine by bio-electroconcentration. Separ. Purific. Technol. 2021, 27915, 119793. [Google Scholar] [CrossRef]
- Capodaglio, A.G.; Olsson, G. Energy Issues in Sustainable Urban Wastewater Management: Use, Demand Reduction and Recovery in the Urban Water Cycle. Sustainability 2020, 12, 266. [Google Scholar] [CrossRef] [Green Version]
- Capodaglio, A.G.; Bolognesi, S.; Cecconet, D. Sustainable, decentralized sanitation and reuse with hybrid nature-based systems. Water 2021, 13, 1583. [Google Scholar] [CrossRef]
- Capodaglio, A.G.; Callegari, A.; Cecconet, D.; Molognoni, D. Sustainability of decentralized wastewater treatment technologies. Water Pract. Technol. 2017, 12, 463–477. [Google Scholar] [CrossRef]
- Novotny, V. Integrated Sustainable Urban Water, Energy, and Solids Management: Achieving Triple Net-Zero Adverse Impact Goals and Resiliency of Future Communities; Wiley: Hoboken, NJ, USA, 2020. [Google Scholar]
- Mainardis, M.; Cecconet, D.; Moretti, A.; Callegari, A.; Goi, D.; Freguia, S.; Capodaglio, A.G. Wastewater fertigation in agriculture: Issues and opportunities for improved water management and circular economy. Environ. Pollut. 2022, 296, 118755. [Google Scholar] [CrossRef]
- Larriba, O.; Rovira-Cal, E.; Juznic-Zonta, Z.; Guisasola, A.; Baeza, J.A. Evaluation of the integration of P recovery, polyhydroxyalkanoate production and short cut nitrogen removal in a mainstream wastewater treatment process. Water Res. 2020, 172, 115474. [Google Scholar] [CrossRef]
- Desmidt, E.; Ghyselbrecht, K.; Zhang, Y.; Pinoy, L.; Van Der Bruggen, B.; Verstraete, W.; Meesschaert, B. Global phosphorus scarcity and full-scale P-recovery techniques: A review. Crit. Rev. Environ. Sci. Technol. 2015, 45, 336–384. [Google Scholar] [CrossRef]
- Morse, G.K.; Brett, S.W.; Guy, J.A.; Lester, J.N. Phosphorus removal and recovery technologies. Sci. Total Environ. 1998, 212, 69–81. [Google Scholar] [CrossRef]
- Parsons, S.A.; Doyle, D. Struvite scale formation and control. Water Sci. Technol. 2004, 49, 177–182. [Google Scholar] [CrossRef]
- Le Corre, K.S.; Valsami-Jones, E.; Hobbs, P.; Parsons, S.A. Phosphorus Recovery from Wastewater by Struvite Crystallization: A Review. Crit. Rev. Environ. Sci. Technol. 2009, 39, 433–477. [Google Scholar] [CrossRef] [Green Version]
- Rahaman, M.S.; Mavinic, D.S.; Meikleham, A.; Ellis, N. Modeling phosphorus removal and recovery from anaerobic digester supernatant through struvite crystallization in a fluidized bed reactor. Water Res. 2014, 51, 1–10. [Google Scholar] [CrossRef]
- Doyle, J.D.; Parsons, S.A. Struvite formation, control and recovery. Water Res. 2002, 36, 3925–3940. [Google Scholar] [CrossRef]
- Jaffer, Y.; Clark, T.A.; Pearce, P.; Parsons, S.A. Potential phosphorus recovery by struvite formation. Water Res. 2002, 36, 1834–1842. [Google Scholar] [CrossRef]
- Nelson, N.O.; Mikkelsen, R.L.; Hesterberg, D.L. Struvite precipitation in anaerobic swine lagoon liquid: Effect of pH and Mg:P ratio and determination of rate constant. Bioresour. Technol. 2003, 89, 229–236. [Google Scholar] [CrossRef]
- Daneshgar, S.; Buttafava, A.; Capsoni, D.; Callegari, A.; Capodaglio, A.G. Impact of pH and Ionic Molar Ratios on Phosphorous Forms Precipitation and Recovery from Different Wastewater Sludges. Resources 2018, 7, 71. [Google Scholar] [CrossRef] [Green Version]
- Çelen, I.; Buchanan, J.R.; Burns, R.T.; Bruce Robinson, R.; Raj Raman, D. Using a chemical equilibrium model to predict amendments required to precipitate phosphorus as struvite in liquid swine manure. Water Res. 2007, 41, 1689–1696. [Google Scholar] [CrossRef]
- Daneshgar, S.; Buttafava, A.; Callegari, A.; Capodaglio, A.G. Simulations and laboratory tests for assessing phosphorus recovery efficiency from sewage sludge. Resources 2018, 7, 54. [Google Scholar] [CrossRef] [Green Version]
- Musvoto, E.V.; Wentzel, M.C.M.; Ekama, G.A.M. Integrated Chemical-Physical Processes Modelling II. Simulating Aeration Treatment of Anaerobic Digester Supernatants. Water Res. 2000, 34, 1868–1880. [Google Scholar] [CrossRef]
- Muster, T.H.; Douglas, G.B.; Sherman, N.; Seeber, A.; Wright, N.; Guzukara, Y. Towards effective phosphorus recycling from wastewater: Quantity and quality. Chemosphere 2013, 91, 676–684. [Google Scholar] [CrossRef]
- Johnston, A.E.; Richards, I.R. Effectiveness of different precipitated phosphate as phosphorus source for plants. Soil Use Manag. 2003, 19, 45–49. [Google Scholar] [CrossRef]
- Egle, L.; Rechberger, H.; Krampe, J.; Zessner, M. Phosphorus recovery from municipal wastewater: An integrated comparative technological, environmental and economic assessment of P recovery technologies. Sci. Total Environ. 2016, 571, 522–542. [Google Scholar] [CrossRef] [Green Version]
- Zeeman, G.; Kujawa, K.; de Mes, T.; Hernandez, L.; de Graaf, M.; Abu-Ghunmi, L.; Mels, A.; Meulman, B.; Temmink, H.; Buisman, C.; et al. Anaerobic treatment as a core technology for energy, nutrients and water recovery from source-separated domestic waste(water). Water Sci. Technol. 2008, 57, 1207–1212. [Google Scholar] [CrossRef] [Green Version]
- Cecconet, D.; Callegari, A.; Capodaglio, A.G. UASB Performance and Perspectives in Urban Wastewater Treatment at Sub-Mesophilic Operating Temperature. Water 2022, 14, 115. [Google Scholar] [CrossRef]
- Cecconet, D.; Mainardis, M.; Callegari, A.; Capodaglio, A.G. Psychrophilic treatment of municipal wastewater with a combined UASB/ASD system, and perspectives for improving urban WWTP sustainability. Chemosphere 2022, 297, 134228. [Google Scholar] [CrossRef]
- Daneshgar, S.; Vanrolleghem, P.A.; Vaneeckhaute, C.; Buttafava, A.; Capodaglio, A.G. Optimization of P compounds recovery from aerobic sludge by chemical modeling and response surface methodology combination. Sci. Total Environ. 2019, 668, 668–677. [Google Scholar] [CrossRef]
- Hallas, J.F.; Mackowiak, C.L.; Wilkie, A.C.; Harris, W.G. Struvite Phosphorus Recovery from Aerobically Digested Municipal Wastewater. Sustainability 2019, 11, 376. [Google Scholar] [CrossRef] [Green Version]
- Levin, G.; Shapiro, J. Metabolic uptake of phosphorus by wastewater organisms. J. Water Pollut. Control Fed. 1965, 37, 800–821. [Google Scholar]
- Tomei, M.C.; Stazi, V.; Daneshgar, S.; Capodaglio, A.G. Holistic Approach to Phosphorus Recovery from Urban Wastewater: Enhanced Biological Removal Combined with Precipitation. Sustainability 2020, 12, 575. [Google Scholar] [CrossRef] [Green Version]
- EPA. Method 365.3: Phosphorus, All Forms (Colorimetric, Ascorbic Acid, Two Reagent), United States Environmental Protection Agency. 1978. Available online: https://www.epa.gov/sites/production/files/2015-08/.../method_365-3_1978.pdf (accessed on 15 February 2016).
- Astrom, K.; Hagglund, T. PID Controllers: Theory, Design, and Tuning, 2nd ed.; Instrument Society of America: Research Triangle, NC, USA, 1995. [Google Scholar]
- Ziegler, J.G.; Nichols, N.B. Optimum settings for automatic controllers. Trans. ASME 1942, 64, 759–768. [Google Scholar] [CrossRef]
- Comeau, Y.; Rabionwitz, B.; Hall, K.J.; Oldham, W.K. Phosphate Release and Uptake in Enhanced Biological Phosphorus Removal from Wastewater. J. Water Pollut. Control Fed. 1987, 59, 707–715. [Google Scholar]
- Tykesson, E.; Jansen, J.L.C. Evaluation of laboratory batch tests for enhanced biological phosphorus removal. Vatten 2005, 61, 43–50. [Google Scholar]
- USGS. User’s Guide to PHREEQC (Version 2): A Computer Program for Speciation, Batch-Reaction, One-Dimensional Transport, and Inverse Geochemical Calculations. Water-Resour. Investig. Rep. 1999, 99, 312. [Google Scholar]
- Liu, X.; Wang, J. Impact of calcium on struvite crystallization in the wastewater and its competition with magnesium. Chem. Eng. 2019, 378, 122121. [Google Scholar] [CrossRef]
- Soptrajanov, B.; Stefov, V.; Lutz, H.D.; Engelen, B. Infrared and Raman Spectra of Magnesium Ammonium Phosphate Hexahydrate (struvite) and its Isomorphous Analogues. Spectrosc. Emerg. Mater. 2004, 165, 299–308. [Google Scholar]
- Berzina-Cimdina, L.; Borodajenko, N. Research of Calcium Phosphates Using Fourier Transform Infrared Spectroscopy. In Infrared Spectroscopy—Materials Science, Engineering and Technology; Theophile Theophanides: London, UK, 2012; pp. 123–148. [Google Scholar]
- Lei, Y.; Song, B.; Saakes, M.; van der Weijden, R.D.; Buisman, C.J.N. Interaction of calcium, phosphorus and natural organic matter in electrochemical recovery of phosphate. Water Res. 2018, 142, 10–17. [Google Scholar] [CrossRef]
- Lam, E.; Gu, Q.; Swedlund, P.J.; Marchesseau, S.; Hemar, Y. X-ray diffraction investigation of amorphous calcium phosphate and hydroxyapatite under ultra-high hydrostatic pressure. Int. J. Min. Met. Mater. 2015, 22, 1225–1231. [Google Scholar] [CrossRef]
- Tõnsuaadu, K.; Gross, K.A.; Pluduma, L.; Veiderma, M. A review on the thermal stability of calcium apatites. J. Therm. Anal. Calorim 2012, 110, 647–659. [Google Scholar] [CrossRef]
- Vecstaudza, J.; Gasik, M.; Locs, J. Amorphous calcium phosphate materials: Formation, structure and thermal behavior. J. Eur. Ceram. 2019, 39, 1642–1649. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Amorphous calcium (ortho)phosphates. Acta Biomater. 2010, 6, 4457–4475. [Google Scholar] [CrossRef]
- Lee, S.H.; Yoo, B.H.; Kim, S.K.; Lim, S.J.; Kim, J.Y.; Kim, T.H. Enhancement of struvite purity by re-dissolution of calcium ions in synthetic wastewaters. J. Hazard. Mater. 2013, 261, 29–37. [Google Scholar] [CrossRef]
- Lee, S.H.; Kumar, R.; Jeon, B.H. Struvite precipitation under changing ionic conditions in synthetic wastewater: Experiment and modeling. J. Colloid Interface Sci. 2016, 474, 93–102. [Google Scholar] [CrossRef]
- Wei, L.; Hong, T.; Liu, H.; Chen, T. The effect of sodium alginate on struvite crystallization in aqueous solution: A kinetics study. J. Cryst. Growth 2017, 473, 60–65. [Google Scholar] [CrossRef]
- Wei, L.; Hong, T.; Cui, K.; Chen, T.; Zhou, Y.; Zhao, Y.; Yin, Y.; Wang, J.; Zhang, Q. Probing the effect of humic acid on the nucleation and growth kinetics of struvite by constant composition technique. Chem. Eng. 2019, 378, 122130. [Google Scholar] [CrossRef]
- Zeng, F.; Zhao, Q.; Jin, W.; Liu, Y.; Wang, K.; Lee, D.J. Struvite precipitation from anaerobic sludge supernatant and mixed fresh/stale human urine. Chem. Eng. 2018, 344, 254–261. [Google Scholar] [CrossRef]
- Daneshgar, S.; Buttafava, A.; Callegari, A.; Capodaglio, A.G. Economic and energetic assessment of different phosphorus recovery options from aerobic sludge. J. Clean. Prod. 2019, 223, 729–738. [Google Scholar] [CrossRef]
- Romer, W.; Steingrobe, B. Fertilizer effect of phosphorus recycling products. Sustainability 2018, 10, 1166. [Google Scholar] [CrossRef] [Green Version]
- Likosova, E.M.; Keller, J.; Poussade, Y.; Freguia, S. A novel electrochemical process for the recovery and recycling of ferric chloride from precipitation sludge. Water Res. 2014, 51, 96–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kato, F.; Kitakoji, H.; Oshita, K.; Takaoka, M.; Takeda, N.; Matsumoto, T. Extraction efficiency of phosphate from pre-coagulated sludge with NaHS. Water Sci. Technol. 2006, 54, 119–129. [Google Scholar] [CrossRef] [Green Version]


| Ion | Concentration (mg/L) |
|---|---|
| Ca2+ | 101 |
| Mg2+ | 26.4 |
| P | 40.0 * |
| NH4+ | 32.6 |
| Run No. | pH | HRT (min) | pH Buffer | Chemical Addition (Mg, NH4) |
|---|---|---|---|---|
| 1 | 8.5 | 30 | NaOH | Yes |
| 2 | 9.0 | 30 | NaOH | Yes |
| 3 | 8.5 | 60 | NaOH | Yes |
| 4 | 9.0 | 60 | NaOH | Yes |
| 5 | 8.5 | 120 | NaOH | Yes |
| 6 | 9.0 | 120 | NaOH | Yes |
| 7 | 9.0 | 60 | NaOH | No |
| 8 | 8.5 | 60 | Ca(OH)2 | No |
| 9 | 9.0 | 60 | Ca(OH)2 | No |
| Struvite | ACP | DCP | DCPD | HAP | Calcite | Magnesite | Bobierrite | Newberyte | K-Struvite | Na-Struvite | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 8 | 0.26 | 1.79 | 0.36 | 0.08 | 13.66 | 0.79 | 0.89 | 1.58 | −12.75 | −1.83 | −1.37 |
| 8.25 | 0.46 | 2.21 | 0.32 | 0.04 | 14.53 | 0.99 | 1.08 | 2 | −12.78 | −1.62 | −1.16 |
| 8.5 | 0.61 | 2.57 | 0.25 | −0.03 | 15.32 | 1.16 | 1.25 | 2.37 | −12.85 | −1.44 | −0.98 |
| 8.75 | 0.72 | 2.87 | 0.15 | −0.13 | 16.02 | 1.27 | 1.37 | 2.66 | −12.95 | −1.29 | −0.83 |
| 9 | 0.77 | 3.09 | 0.01 | −0.27 | 16.61 | 1.3 | 1.4 | 2.89 | −13.09 | −1.17 | −0.71 |
| 9.25 | 0.75 | 3.27 | −0.16 | −0.44 | 17.12 | 1.18 | 1.26 | 3.05 | −13.26 | −1.09 | −0.63 |
| 9.5 | 0.66 | 3.4 | −0.35 | −0.63 | 17.58 | 0.19 | 0.27 | 3.16 | −13.46 | −1.04 | −0.58 |
| HRT (min) | pH | k (h−1) |
|---|---|---|
| 30 | 8.5 | 1.28 |
| 9.0 | 1.62 | |
| 60 | 8.5 | 1.63 |
| 9.0 | 5.23 | |
| 120 | 8.5 | 2.06 |
| 9.0 | 3.07 |
| Experiment | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Unit | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
| K+ | g/kg | 0.61 | 0.21 | 0.33 | 0.26 | 0.59 | 0.49 | 0.33 | 0.32 | 0.25 |
| PO43− | g/kg | 443.06 | 453.79 | 518.18 | 400.13 | 512.05 | 420.06 | 456.86 | 481.39 | 367.94 |
| NH4+ | g/kg | 5.15 | 1.55 | 2.06 | 2.19 | 11.85 | 7.60 | 1.67 | 1.93 | 1.80 |
| H2O | g/kg | 292.69 | 271.29 | 272.94 | 278.94 | 293.61 | 300.31 | 231.71 | 199.91 | 215.37 |
| CO32− | g/kg | 56.46 | 41.47 | 33.47 | 49.96 | 33.97 | 45.97 | 50.96 | 52.46 | 77.94 |
| Mg2+ | g/kg | 58.00 | 36.40 | 55.00 | 51.00 | 62.00 | 55.00 | 26.00 | 17.00 | 12.00 |
| Ca2+ | g/kg | 175.00 | 202.00 | 199.00 | 155.00 | 180.00 | 155.00 | 216.00 | 256.00 | 213.00 |
| Total | g | 1030.96 | 1006.71 | 1080.99 | 937.49 | 1094.08 | 984.43 | 983.53 | 1009.01 | 888.30 |
| Error | % | 3.1+ | 0.7+ | 8.1+ | 6.3− | 9.4+ | 1.6− | 1.6− | 0.9+ | 11.2− |
| Unit | Synthetic ww | Real ww | |
|---|---|---|---|
| K+ | g/kg | 0.32 | 0.28 |
| PO43− | g/kg | 481.39 | 436.43 |
| NH4+ | g/kg | 1.93 | 1.36 |
| H2O | g/kg | 199.91 | 184.92 |
| CO32− | g/kg | 52.46 | 67.13 |
| Mg2+ | g/kg | 17.00 | 11.05 |
| Ca2+ | g/kg | 256.00 | 245.61 |
| Total | g | 1009.01 | 946.81 |
| Error | % | 0.9+ | 5.3− |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daneshgar, S.; Cecconet, D.; Capsoni, D.; Capodaglio, A.G. Side-Stream Phosphorus Recovery in Activated Sludge Processes. Water 2022, 14, 1861. https://doi.org/10.3390/w14121861
Daneshgar S, Cecconet D, Capsoni D, Capodaglio AG. Side-Stream Phosphorus Recovery in Activated Sludge Processes. Water. 2022; 14(12):1861. https://doi.org/10.3390/w14121861
Chicago/Turabian StyleDaneshgar, Saba, Daniele Cecconet, Doretta Capsoni, and Andrea G. Capodaglio. 2022. "Side-Stream Phosphorus Recovery in Activated Sludge Processes" Water 14, no. 12: 1861. https://doi.org/10.3390/w14121861
APA StyleDaneshgar, S., Cecconet, D., Capsoni, D., & Capodaglio, A. G. (2022). Side-Stream Phosphorus Recovery in Activated Sludge Processes. Water, 14(12), 1861. https://doi.org/10.3390/w14121861









