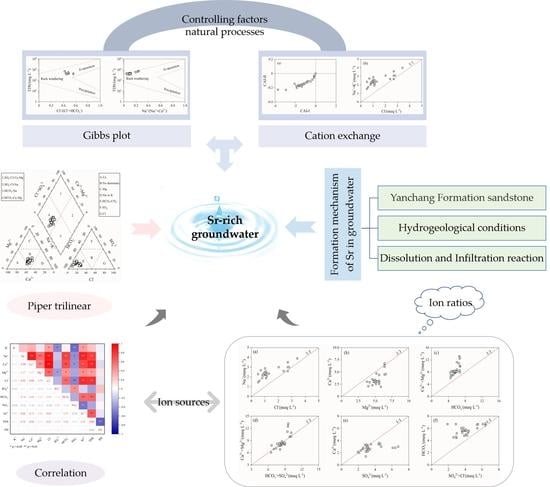
Hydrochemical Characteristics and Formation Mechanism of Strontium-Rich Groundwater in Tianjiazhai, Fugu, China
Abstract
:1. Introduction
2. Materials and Methods
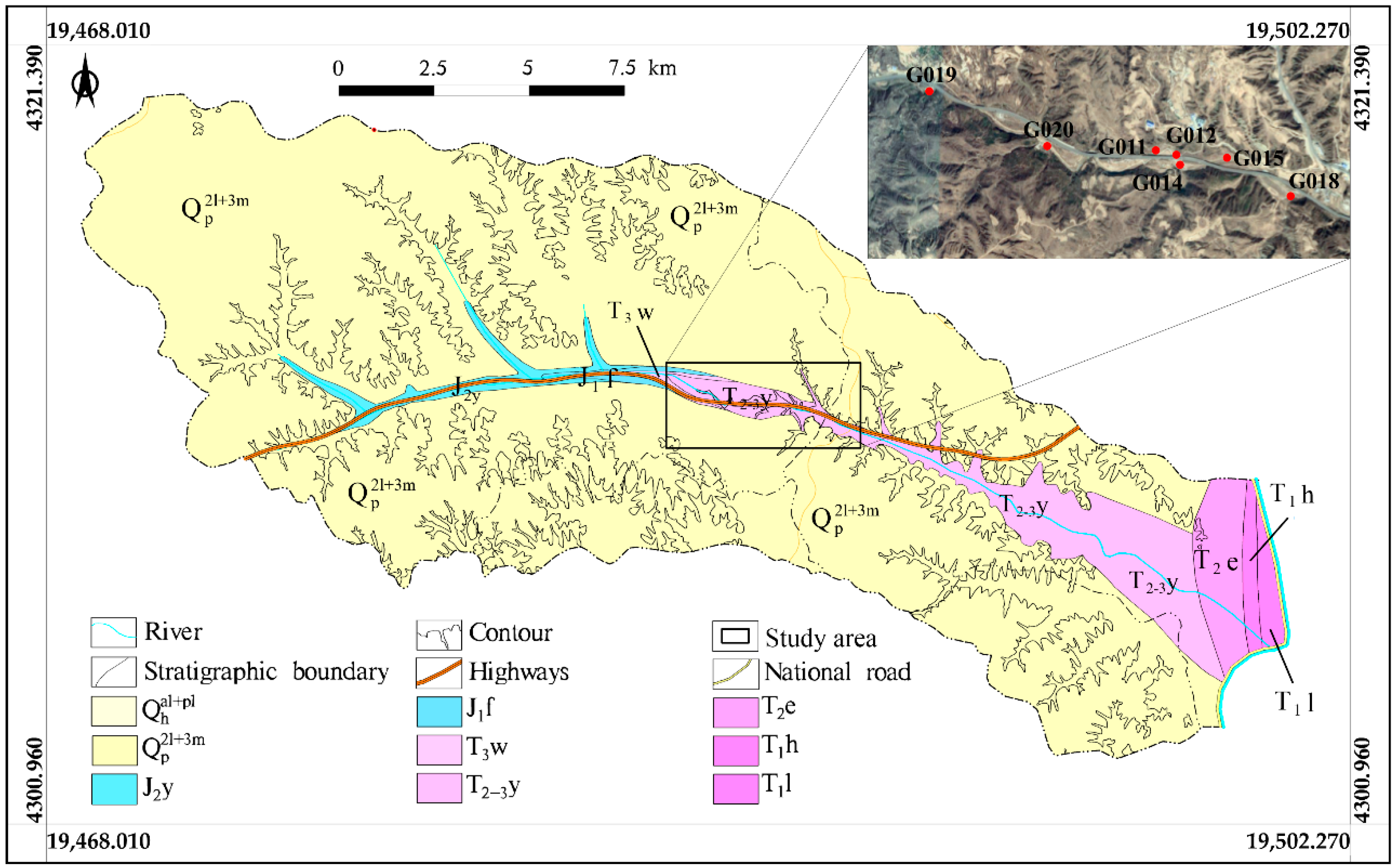
2.1. Study Area
2.2. Sampling
2.3. Methods
3. Results and Discussion
3.1. Hydrochemical Characteristics
3.1.1. Descriptive Analysis of Hydrochemical Characteristics
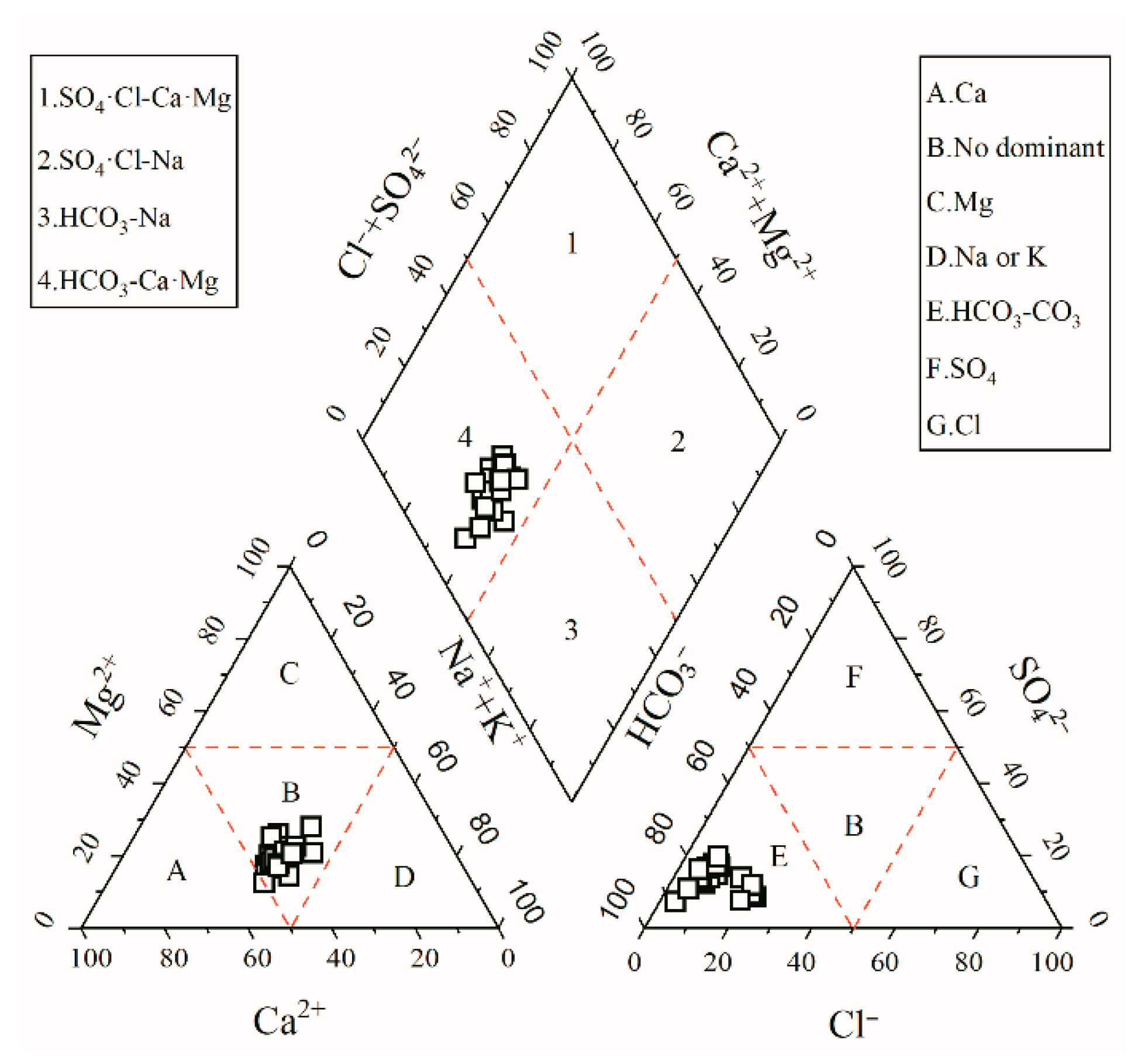
3.1.2. Hydrochemical Facies
3.1.3. Correlation Analysis of Hydrochemical Compositions
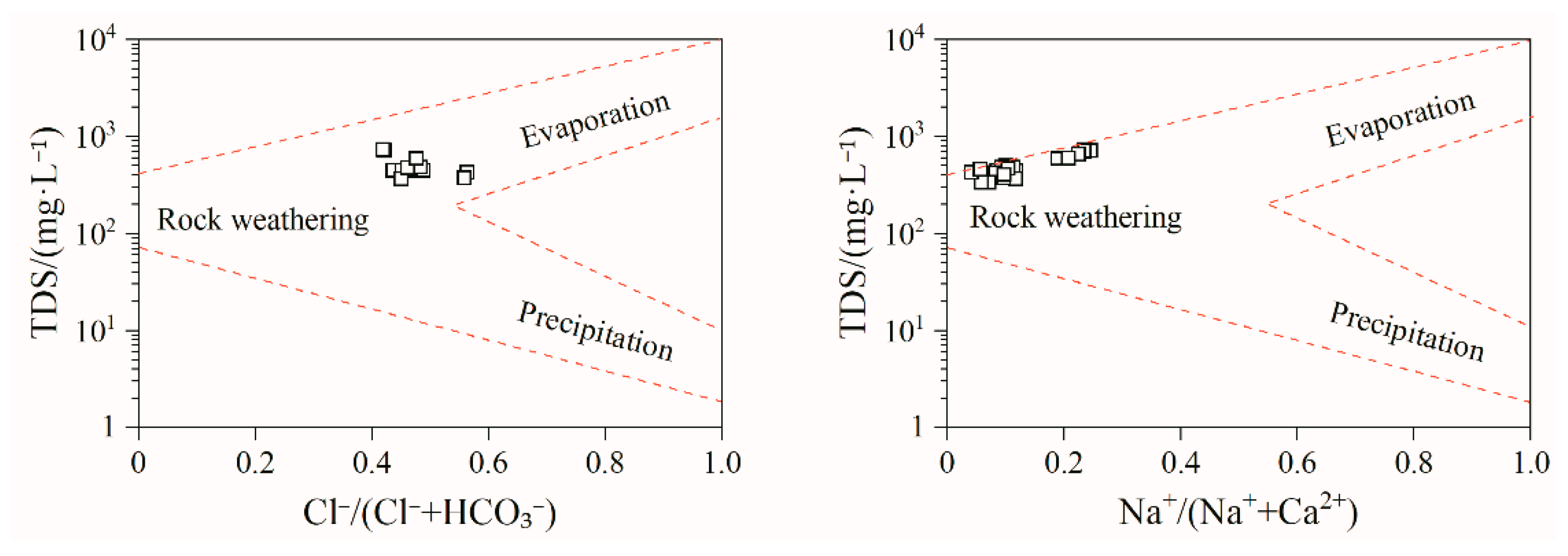
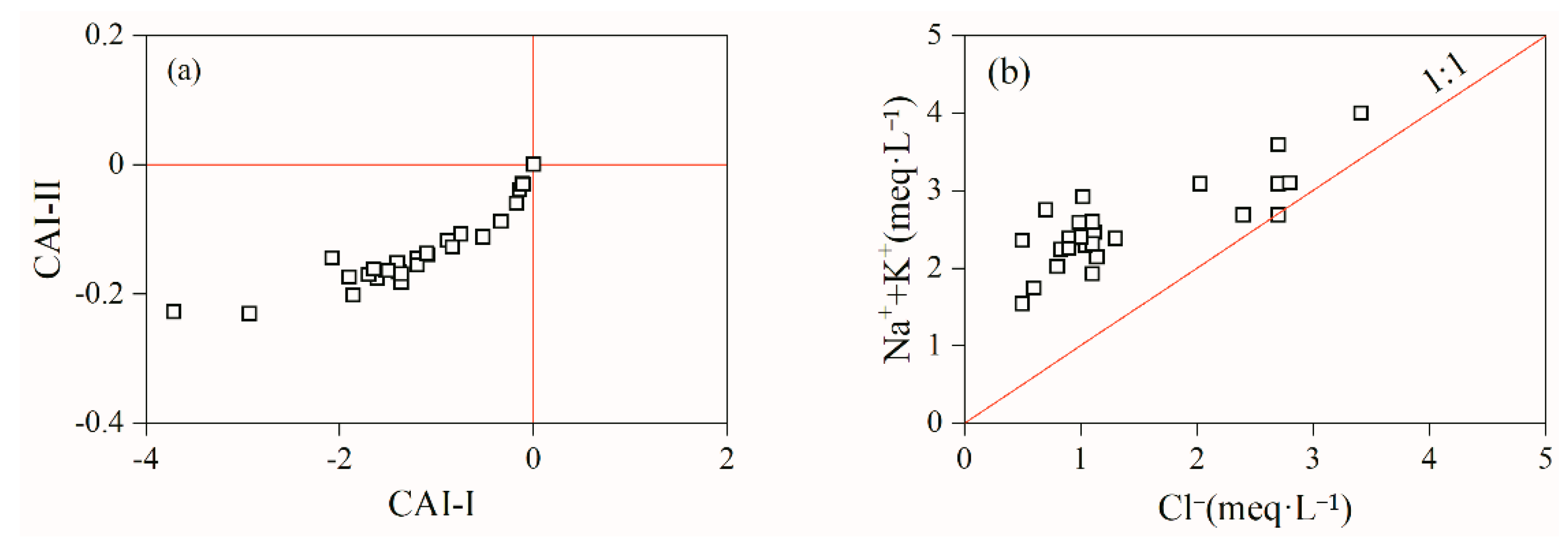
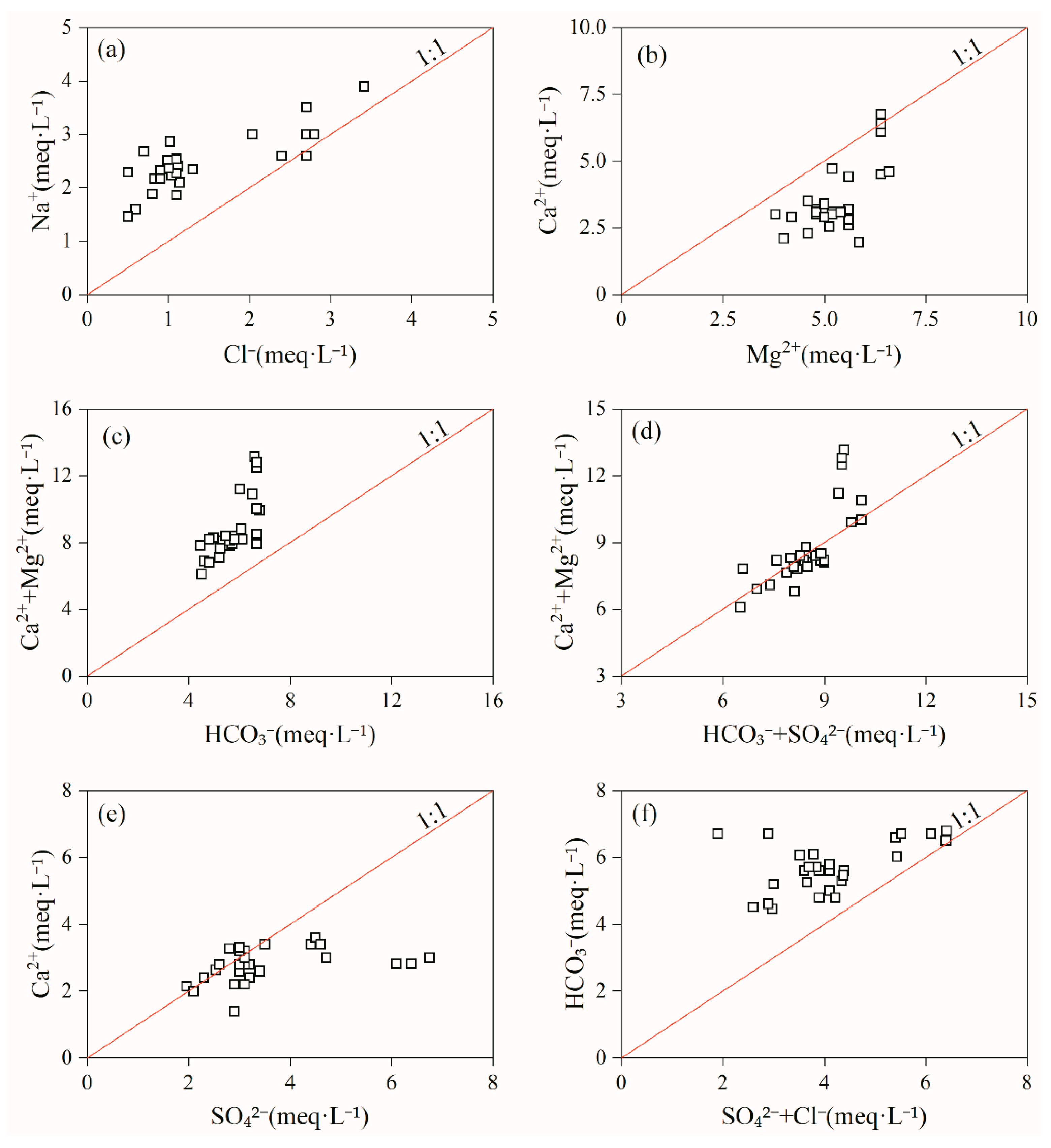
3.1.4. Controlling Factors and Natural Processes
3.1.5. Qualitative Analysis of Ion Sources
3.2. Formation Mechanism of Sr in Groundwater
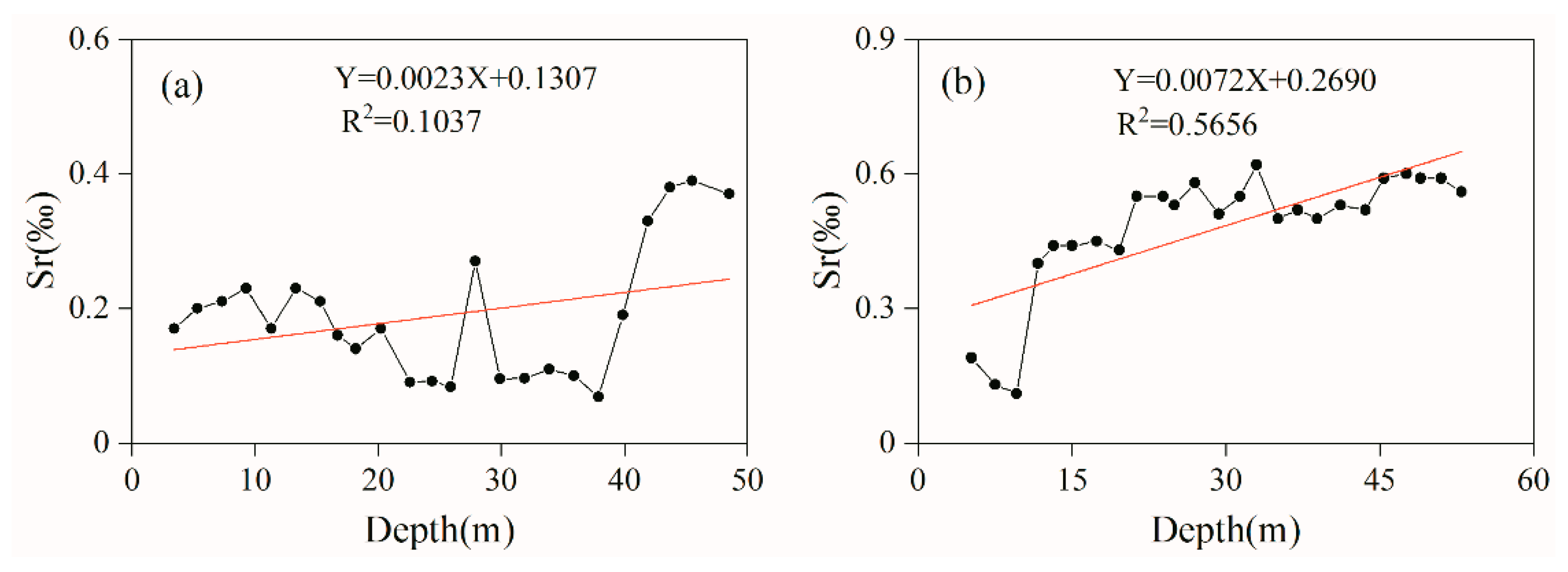
3.2.1. Source Conditions
3.2.2. Hydrodynamic Conditions
3.2.3. Hydrochemical Conditions
4. Conclusions
- (1)
- Sr2+ content in Sr-rich groundwater ranged from 0.85 to 2.99 mg·L−1, with an average value of 1.45 mg·L−1, which was 7.25 times higher than the national limit value of Sr2+ content in natural mineral water for drinking (0.20 mg·L−1). In addition, the pH and TDS values ranged from 7.50 to 8.30 and from 332.00 to 718.00 mg·L−1, respectively, indicating that Sr-rich groundwater is generally slightly alkaline. The dominant cation and anion were Ca2+ and HCO3−, respectively. On the other hand, HCO3-Ca·Mg·Na was the main facies type of Sr-rich groundwater in the Tianjiazhai area. The coefficients of variation of the hydrochemical parameters of Sr-rich groundwater were less than 1, indicating that the contents of all hydrochemical parameters indicators were relatively stable.
- (2)
- The correlation coefficients of Sr2+ with Ca2+ and HCO3− were 0.73 and 0.59, respectively, indicating that the source of Sr may be the same as that of these two ions. The correlation coefficients of TDS with Cl− and Na+ were 0.81 and 0.76, respectively, mainly because that the groundwater in the Tianjiazhai area originated from atmospheric precipitation, whereas the correlation coefficients of TDS with Ca2+ and HCO3− were 0.84 and 0.60, respectively, indicating the important role of Ca2+ and HCO3− in the groundwater chemistry in the study area. The hydrochemical characteristics of Sr-rich groundwater in the Tianjiazhai area are controlled by water–rock interactions and influenced by cation exchange. In addition, water–rock interactions in Sr-rich groundwater are dominated by the dissolution of carbonate rocks.
- (3)
- Weathering, dissolution, and leaching in the sandstone of the Yanchang Formation are the main processes controlling Sr content in groundwater. Indeed, the Sr-rich groundwater is mainly stored in the weathering fracture unconfined aquifer of the Yanchang Formation. In addition, the higher the degree of weathering of Sr-bearing minerals (rocks) and the longer the reaction time of water–rock interaction, the more favorable the dissolution and enrichment of Sr in groundwater. The hydrogeological conditions and geological and tectonic environment of the Tianjiazhai area provide favorable conditions for the formation of Sr-rich groundwater.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Karunanidhi, D.; Aravinthasamy, P.; Deepali, M.; Subramani, T.; Bellows, B.C.; Li, P. Groundwater quality evolution based on geochemical modeling and aptness testing for ingestion using entropy water quality and total hazard indexes in an urban-industrial area (Tiruppur) of Southern India. Environ. Sci. Pollut. Res. 2021, 28, 18523–18538. [Google Scholar] [CrossRef]
- Li, P.; He, X.; Guo, W. Spatial groundwater quality and potential health risks due to nitrate ingestion through drinking water: A case study in Yan’an City on the Loess Plateau of northwest China. Hum. Ecol. Risk Assess. Int. J. 2019, 25, 11–31. [Google Scholar] [CrossRef]
- Alvarez, M.d.P.; Funes, D.; Dapeña, C.; Bouza, P.J. Origin and hydrochemical characteristics of groundwater in the Northeastern Patagonia, Argentina: The relationship with geomorphology and soils. Environ. Earth Sci. 2020, 79, 503. [Google Scholar] [CrossRef]
- Li, Y.; Li, P.; Cui, X.; He, S. Groundwater quality, health risk, and major influencing factors in the lower Beiluo River watershed of northwest China. Hum. Ecol. Risk Assess. Int. J. 2021, 27, 1987–2013. [Google Scholar] [CrossRef]
- Srinivas, Y.; Aghil, T.B.; Hudson Oliver, D.; Nithya Nair, C.; Chandrasekar, N. Hydrochemical characteristics and quality assessment of groundwater along the Manavalakurichi coast, Tamil Nadu, India. Appl. Water Sci. 2017, 7, 1429–1438. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Gao, Z.; Wang, M.; Li, Y.; Shi, M.; Zhang, H.; Ma, Y. Hydrochemical characteristics and possible controls in the groundwater of the Yarlung Zangbo River Valley, China. Environ. Earth Sci. 2019, 78, 76. [Google Scholar] [CrossRef]
- Chotpantarat, S.; Thamrongsrisakul, J. Natural and anthropogenic factors influencing hydrochemical characteristics and heavy metals in groundwater surrounding a gold mine, Thailand. J. Asian Earth Sci. 2021, 211, 104692. [Google Scholar] [CrossRef]
- Feng, W.; Qian, H.; Xu, P.; Hou, K. Hydrochemical Characteristic of Groundwater and Its Impact on Crop Yields in the Baojixia Irrigation Area, China. Water 2020, 12, 1443. [Google Scholar] [CrossRef]
- Bowman, R.S. Aqueous Environmental Geochemistry. Eos Trans. Am. Geophys. Union 1997, 78, 586. [Google Scholar] [CrossRef]
- Fuoco, I.; De Rosa, R.; Barca, D.; Figoli, A.; Gabriele, B.; Apollaro, C. Arsenic polluted waters: Application of geochemical modelling as a tool to understand the release and fate of the pollutant in crystalline aquifers. J. Environ. Manag. 2022, 301, 113796. [Google Scholar] [CrossRef]
- Fuoco, I.; Marini, L.; De Rosa, R.; Figoli, A.; Gabriele, B.; Apollaro, C. Use of reaction path modelling to investigate the evolution of water chemistry in shallow to deep crystalline aquifers with a special focus on fluoride. Sci. Total Environ. 2022, 830, 154566. [Google Scholar] [CrossRef]
- Yang, F.; Liu, S.; Jia, C.; Gao, M.; Chang, W.; Wang, Y. Hydrochemical characteristics and functions of groundwater in southern Laizhou Bay based on the multivariate statistical analysis approach. Estuar. Coast. Shelf Sci. 2021, 250, 107153. [Google Scholar] [CrossRef]
- Van Geldern, R.; Schulte, P.; Mader, M.; Baier, A.; Barth, J.A.C.; Juhlke, T.R.; Lee, K. Insights into agricultural influences and weathering processes from major ion patterns. Hydrol. Processes 2018, 32, 891–903. [Google Scholar] [CrossRef] [Green Version]
- Ismail, E.; Abdelhalim, A.; Heleika, M.A. Hydrochemical characteristics and quality assessment of groundwater aquifers northwest of Assiut district, Egypt. J. Afr. Earth Sci. 2021, 181, 104260. [Google Scholar] [CrossRef]
- Zhang, B.; Zhao, D.; Zhou, P.; Qu, S.; Liao, F.; Wang, G. Hydrochemical Characteristics of Groundwater and Dominant Water–Rock Interactions in the Delingha Area, Qaidam Basin, Northwest China. Water 2020, 12, 836. [Google Scholar] [CrossRef] [Green Version]
- Dai, L.L.; Mei, M.L.; Chu, C.H.; Lo, E.C.M. Remineralizing effect of a new strontium-doped bioactive glass and fluoride on demineralized enamel and dentine. J. Dent. 2021, 108, 103633. [Google Scholar] [CrossRef]
- Neves, N.; Campos, B.B.; Almeida, I.F.; Costa, P.C.; Cabral, A.T.; Barbosa, M.A.; Ribeiro, C.C. Strontium-rich injectable hybrid system for bone regeneration. Mater. Sci. Eng. C 2016, 59, 818–827. [Google Scholar] [CrossRef]
- Haixue, L.; Xuxue, C.; Yuekun, M.; Weipo, L.; Bin, Z. Characteristics and formation mechanism of strontium-rich groundwater in Malian River drainage basin, Southern Ordos Basin. Geoscience 2021, 35, 682. [Google Scholar]
- Wang, R.; Wu, X.; Zhai, Y.; Su, Y.; Liu, C. An Experimental Study on the Sources of Strontium in Mineral Water and General Rules of Its Dissolution—A Case Study of Chengde, Hebei. Water 2021, 13, 699. [Google Scholar] [CrossRef]
- Cao, Y.; Song, L.; Liu, L.; Zhu, W.; Cui, S.; Wang, Y.; Guo, P. Preliminary study on strontium-rich characteristics of shallow groundwater in Dingtao area, China. J. Groundw. Sci. Eng. 2020, 8, 244–258. [Google Scholar]
- Pit’eva, K.E.; Fortygina, M.A.; Mikheev, A.V. Formation of the composition and quality of carbonate mineral water of the Elburgan water bearing complex (Essentuki Town). Mosc. Univ. Geol. Bull. 2007, 62, 277–285. [Google Scholar] [CrossRef]
- Lu, S.; Zhou, H.; Liu, W.; Chen, Q.; Yan, Z.; Chen, L. Distribution and enrichment of strontium in the Zigui karst watershed. Geol. China 2021, 48, 1865–1874. [Google Scholar]
- Zuo, R.; Teng, Y.; Wang, J. Modeling migration of strontium in sand and gravel aquifer in the candidate VLLW disposal site. J. Radioanal. Nucl. Chem. 2009, 281, 653. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, W.; Li, Z.; Chen, T.; Ren, Y.; Shao, H.; Wang, L. Distribution and characterization analyses of strontium-bearing mineral spring water in the Chengde region. Hydrogeol. Eng. Geol. 2020, 47, 65–73. [Google Scholar]
- Li, D.; Gan, S.; Li, J.; Dong, Z.; Long, Q.; Qiu, S.; Zhou, Y.; Lu, C. Hydrochemical characteristics and formation mechanism of strontium-rich groundwater in Shijiazhuang, north China plain. J. Chem. 2021, 2021, 5547924. [Google Scholar] [CrossRef]
- Yang, Y.; Shao, C.; Lu, T.; Zhang, A. Discovery of Strontium-rich mineral water in Tingsiqiao and Guantangyi Town, Xianning. Geol. China 2021, 48, 1661–1662. [Google Scholar]
- Li, P.; He, S.; Yang, N.; Xiang, G. Groundwater quality assessment for domestic and agricultural purposes in Yan’an City, northwest China: Implications to sustainable groundwater quality management on the Loess Plateau. Environ. Earth Sci. 2018, 77, 775. [Google Scholar] [CrossRef]
- Xu, P.; Zhang, Q.; Qian, H.; Li, M.; Hou, K. Characterization of geothermal water in the piedmont region of Qinling Mountains and Lantian-Bahe Group in Guanzhong Basin, China. Environ. Earth Sci. 2019, 78, 442. [Google Scholar] [CrossRef]
- Chung, S.Y.; Rajendran, R.; Senapathi, V.; Sekar, S.; Ranganathan, P.C.; Oh, Y.Y.; Elzain, H.E. Processes and characteristics of hydrogeochemical variations between unconfined and confined aquifer systems: A case study of the Nakdong River Basin in Busan City, Korea. Environ. Sci. Pollut. Res. 2020, 27, 10087–10102. [Google Scholar] [CrossRef]
- Li, P.; He, S.; He, X.; Tian, R. Seasonal Hydrochemical Characterization and Groundwater Quality Delineation Based on Matter Element Extension Analysis in a Paper Wastewater Irrigation Area, Northwest China. Expo. Health 2018, 10, 241–258. [Google Scholar] [CrossRef]
- Kwon, E.; Park, J.; Lee, J.M.; Kim, Y.-T.; Woo, N.C. Spatiotemporal changes in hydrogeochemistry of coastal groundwater through the construction of underground disposal facility for low and intermediate level radioactive wastes in Korea. J. Hydrol. 2020, 584, 124750. [Google Scholar] [CrossRef]
- Su, C.; Huang, C.; Zou, S.; Xie, D.; Zhao, G.; Tang, J.; Luo, F.; Yang, Y. Enrichm entenvironment and sources of strontium of groundwater in Xintian county, Hunan Province. Carsologica Sin. 2017, 36, 678–683. [Google Scholar]
- Zhou, C.; Zou, S.; Xia, R.; Xue, Q.; Zhu, D.; Li, L.; Cao, J.; Li, J.; Xie, H. Extreme enrichment of strontium discovered in surface water and groundwater in the Guanling area of Guizhou Province. Geol. China 2021, 48, 961–962. [Google Scholar]
- Zhang, Q.; Qian, H.; Xu, P.; Li, W.; Feng, W.; Liu, R. Effect of hydrogeological conditions on groundwater nitrate pollution and human health risk assessment of nitrate in Jiaokou Irrigation District. J. Clean. Prod. 2021, 298, 126783. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, Z.; Wang, G.; Jiang, J.; Yang, B. Hydrochemical characteristics and evolution of groundwater in the Dachaidan area, Qaidam Basin. Earth Sci. Front. 2021, 28, 194–205. [Google Scholar]
- Piper, A. A Graphic Procedure in the Geochemical Interpretation of Water-Analyses. Eos Trans. Am. Geophys. Union 1944, 25, 914–923. [Google Scholar] [CrossRef]
- He, X.; Li, P.; Wu, J.; Wei, M.; Ren, X.; Wang, D. Poor groundwater quality and high potential health risks in the Datong Basin, northern China: Research from published data. Environ. Geochem. Health 2021, 43, 791–812. [Google Scholar] [CrossRef]
- Apollaro, C.; Di Curzio, D.; Fuoco, I.; Buccianti, A.; Dinelli, E.; Vespasiano, G.; Castrignanò, A.; Rusi, S.; Barca, D.; Figoli, A.; et al. A multivariate non-parametric approach for estimating probability of exceeding the local natural background level of arsenic in the aquifers of Calabria region (Southern Italy). Sci. Total Environ. 2022, 806, 150345. [Google Scholar] [CrossRef]
- Marghade, D.; Malpe, D.B.; Duraisamy, K.; Patil, P.D.; Li, P. Hydrogeochemical evaluation, suitability, and health risk assessment of groundwater in the watershed of Godavari basin, Maharashtra, Central India. Environ. Sci. Pollut. Res. 2021, 28, 18471–18494. [Google Scholar] [CrossRef]
- Gibbs Ronald, J. Mechanisms Controlling World Water Chemistry. Science 1970, 170, 1088–1090. [Google Scholar] [CrossRef]
- Schoeller, H. Qualitative Evaluation of Groundwater Resources. In Methods and Techniques of Groundwater Investigation and Development; UNESCO: Paris, France, 1967. [Google Scholar]
- Xing, L.; Guo, H.; Zhan, Y. Groundwater hydrochemical characteristics and processes along flow paths in the North China Plain. J. Asian Earth Sci. 2013, 70–71, 250–264. [Google Scholar] [CrossRef]
- Zhao, Q.; Su, X.; Kang, B.; Zhang, Y.; Wu, X.; Liu, M. A hydrogeochemistry and multi-isotope (Sr, O, H, and C) study of groundwater salinity origin and hydrogeochemcial processes in the shallow confined aquifer of northern Yangtze River downstream coastal plain, China. Appl. Geochem. 2017, 86, 49–58. [Google Scholar] [CrossRef]
- Ma, R.; Wang, Y.; Sun, Z.; Zheng, C.; Ma, T.; Prommer, H. Geochemical evolution of groundwater in carbonate aquifers in Taiyuan, northern China. Appl. Geochem. 2011, 26, 884–897. [Google Scholar] [CrossRef]
- Li, C.; Gao, X.; Wang, Y. Hydrogeochemistry of high-fluoride groundwater at Yuncheng Basin, northern China. Sci. Total Environ. 2015, 508, 155–165. [Google Scholar] [CrossRef]
- Fan, B.-L.; Zhao, Z.-Q.; Tao, F.-X.; Liu, B.-J.; Tao, Z.-H.; Gao, S.; Zhang, L.-H. Characteristics of carbonate, evaporite and silicate weathering in Huanghe River basin: A comparison among the upstream, midstream and downstream. J. Asian Earth Sci. 2014, 96, 17–26. [Google Scholar] [CrossRef]
- Ehya, F.; Shakouri, B.; Rafi, M. Geology, mineralogy, and isotope (Sr, S) geochemistry of the Likak celestite deposit, SW Iran. Carbonates Evaporites 2013, 28, 419–431. [Google Scholar] [CrossRef]
- Parisi, S.; Paternoster, M.; Perri, F.; Mongelli, G. Source and mobility of minor and trace elements in a volcanic aquifer system: Mt. Vulture (southern Italy). J. Geochem. Explor. 2011, 110, 233–244. [Google Scholar] [CrossRef]
- Zhang, Y.; Ding, H.; Fu, D.; Huang, Z.; Li, A.; Yan, C.; Jin, X. A study of enrichment environment and formation mechanism of strontium mineral water in Gansu Province. Geol. China 2020, 47, 1688–1701. [Google Scholar]
- Su, C.; Nie, F.; Zou, S.; Zhao, G.; Luo, F.; Huang, Q.; Ba, J.; Li, X.; Liang, J.; Yang, Y. Hydrochemical Characteristics and Formation Mechanism of Strontium-rich Groundwater in Xintian County, Hunan Province. Geoscience 2018, 32, 554–564. [Google Scholar]
- Sun, Q.; Sun, Z.; Jia, L.; Tian, H.; Guo, X.; Du, J.; Li, X.; Li, X.; Jia, L. Formation mechanism of the strontium-rich and metasilicic acid groundwater in the Lianhuashan area, Changchun, Jilin Province. Geol. China 2020, 44, 1031–1032. [Google Scholar]


| Projects | mg·L−1 | pH | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| K+ | Na+ | Ca2+ | Mg2+ | Cl− | SO42− | HCO3− | NO3− | Sr2+ | TH * | TDS | ||
| Min | 1.63 | 33.6 | 39.2 | 23.1 | 17.7 | 33.6 | 272 | 1.98 | 0.85 | 205 | 332 | 7.5 |
| Max | 5.59 | 89.6 | 122 | 40.4 | 126 | 81.7 | 415 | 30.3 | 2.99 | 825 | 718 | 8.3 |
| Mean | 2.92 | 60.57 | 71.09 | 31.77 | 56.72 | 61.16 | 348.8 | 7.76 | 1.45 | 324.9 | 492.57 | 7.92 |
| SD | 1.01 | 15.36 | 22.27 | 4.02 | 36.79 | 13.08 | 40.94 | 5.74 | 0.51 | 115.25 | 112.14 | 0.21 |
| CV/% | 34.53 | 25.36 | 31.33 | 12.66 | 64.86 | 21.38 | 11.74 | 73.97 | 34.92 | 35.47 | 22.77 | 2.64 |
| CS 1 | / | 200 | / | / | 250 | 250 | / | 20 | / | / | 1000 | 6.5–8.5 |
| WG 2 | / | 200 | / | / | 250 | 500 | / | 50 | / | / | 1000 | 6.5–8.5 |
| SI | Min | Max | Mean | SD |
|---|---|---|---|---|
| Dolomite | 5.24 | 5.98 | 5.55 | 0.29 |
| Gypsum | 1.97 | 2.95 | 2.36 | 0.37 |
| rock salt | −5.73 | −3.92 | −5.13 | 0.75 |
| Calcite | 4.53 | 4.92 | 4.71 | 0.14 |
| Rock | Lithology | Strontium (‰) | Rock | Lithology | Strontium (‰) |
|---|---|---|---|---|---|
| pm-1 | Medium-grained sandstone | 0.126 | pm-12 | Fine-grained sandstone | 0.080 |
| pm-2 | Conglomerate | 0.103 | pm-13 | Mudstone | 0.143 |
| pm-3 | Fine-grained sandstone | 0.101 | pm-14 | Fine-grained sandstone | 0.111 |
| pm-4 | Fine-grained sandstone | 0.076 | pm-15 | Mudstone | 0.110 |
| pm-5 | Coarse-grained sandstone | 0.098 | pm-16 | Mudstone | 0.074 |
| pm-6 | Coarse-grained sandstone | 0.147 | pm-17 | Mudstone | 0.128 |
| pm-7 | Coarse-grained sandstone | 0.120 | pm-18 | Mudstone | 0.119 |
| pm-8 | Fine-grained sandstone | 0.116 | pm-19 | Fine-grained sandstone | 0.126 |
| pm-9 | Fine-grained sandstone | 0.145 | pm-20 | Fine-grained sandstone | 0.121 |
| pm-10 | Fine-grained sandstone | 0.111 | pm-21 | Loess | 0.255 |
| pm-11 | Fine-grained sandstone | 0.116 | pm-22 | Loess | 0.266 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, C.; Wang, W.; Ke, X.; Ou, A.; Wang, D. Hydrochemical Characteristics and Formation Mechanism of Strontium-Rich Groundwater in Tianjiazhai, Fugu, China. Water 2022, 14, 1874. https://doi.org/10.3390/w14121874
Liang C, Wang W, Ke X, Ou A, Wang D. Hydrochemical Characteristics and Formation Mechanism of Strontium-Rich Groundwater in Tianjiazhai, Fugu, China. Water. 2022; 14(12):1874. https://doi.org/10.3390/w14121874
Chicago/Turabian StyleLiang, Chengcheng, Wei Wang, Xianmin Ke, Anfeng Ou, and Dahao Wang. 2022. "Hydrochemical Characteristics and Formation Mechanism of Strontium-Rich Groundwater in Tianjiazhai, Fugu, China" Water 14, no. 12: 1874. https://doi.org/10.3390/w14121874
APA StyleLiang, C., Wang, W., Ke, X., Ou, A., & Wang, D. (2022). Hydrochemical Characteristics and Formation Mechanism of Strontium-Rich Groundwater in Tianjiazhai, Fugu, China. Water, 14(12), 1874. https://doi.org/10.3390/w14121874