Chemical Oxidation and Reduction Pathways of Mercury Relevant to Natural Waters: A Review
Abstract
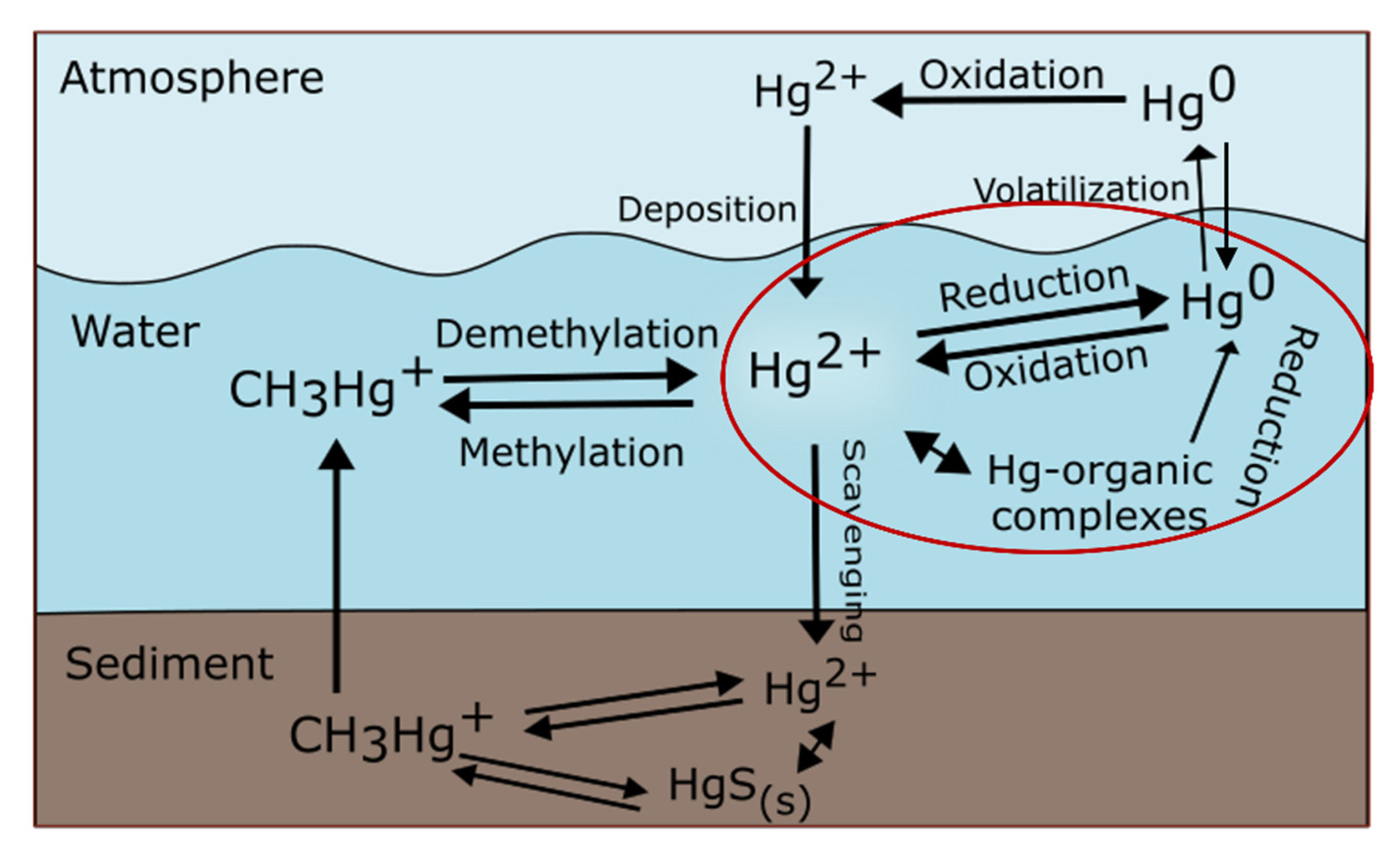
:1. Introduction
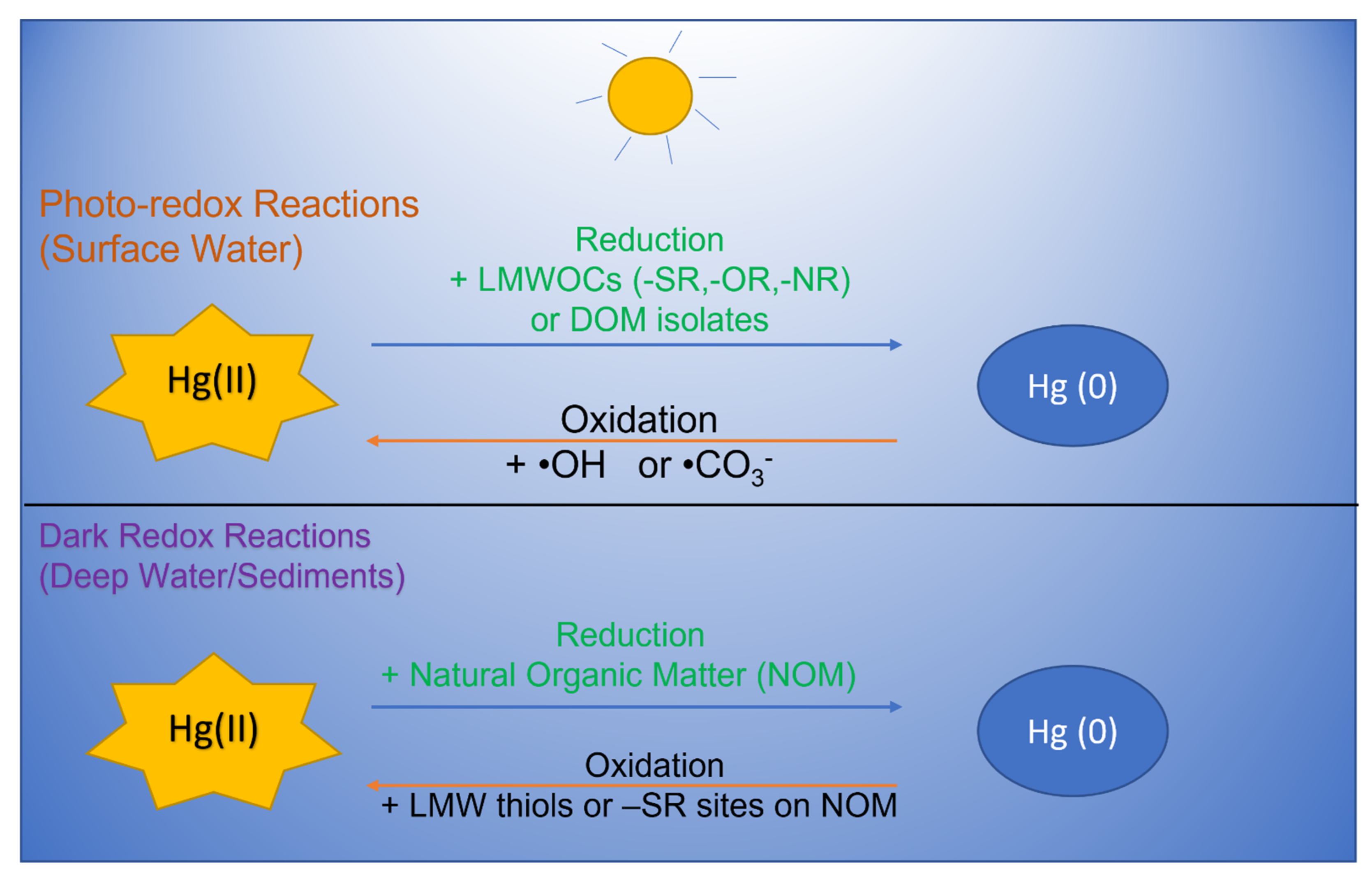
2. Abiotic Dark Reduction of Hg(II) Relevant to Natural Waters
| Reductant | Rate Constants | pH | Factors Affecting Kinetics 1 | Reaction Mechanism | References |
|---|---|---|---|---|---|
| Tropical River HS 2 | ~0.1 h−1 & 0.02 h−1 | 2–11 | +/−: pH (max. at 8) +: [HS] N: Hg(II):HS ratio. | slow two-step, 1st-order reaction. | [22] |
| Tropical River HS | 5 | +: the ratio of phenolic/carboxylic groups; −: the amount of S in the HS, N: molecular sizes. | slow two-step, 1st-order reaction. | [23] | |
| Nordic Reservoir DOM 3 (IHSS) 4 | 0.0290 h−1 & 0.0005 h−1 | ~6 | Binding of DOM to Hg(II). | N/A | [24] |
| Reduced Elliott soil HA 5 | ~7 | +/−: DOM:Hg(II) ratios. | Binding of HA to Hg(II) at hight DOM:Hg(II) ratios may inhibit Hg(II) reduction. | [25] | |
| Four reduced NOM 6 isolates | Initial Rates: 0.4–5.5 h−1 | ~7 | +/−: DOM:Hg(II) ratios. | Reduction of Hg(II) by the reduced quinones and Hg(0) oxidized by thiols in NOMs. | [26] |
| Three different HAs | 3.6, 6.8, 7.2, 8.1 | +/−: pH, initial [Hg(II)] or [HA] Y: the HA types (structures) +: temperatures, light or aqueous phase. | N/A | [27] | |
| Suwannee River HA, Suwannee River FA 7 and Eliot Soil HA from IHSS | 4.0–9.0 | −: pH, salinity ([Cl−]), S content in HS. | Hg(II) reduction suppressed by complexation of Hg(II) with S groups in HS or with Cl−, and by the back oxidation of Hg(0) by thiols in HS. | [28] | |
| +: the −COOH/−OH ratio in HS. | Hg(II) reduction impacted by both the types and positioning of the functional groups in HS. | ||||
| Peat HA Coal HA Soil HA Peat HA Coal HA Soil HA | 0.18 h−1 8 0.22 h−1 8 0.35 h−1 8 0.003 h−1 9 0.0003 h−1 9 (biased) 0.006 h−1 9 | 6.8 | +: initial Hg(II), or [HA] at an identical Hg(II)/DOC 10 ratio Y: Hg(II)/DOC ratio. | Structure-specific kinetics of the proposed Hg(II)-NOM complexes (Hg(OR)2 and RSHgOR). | [18] |
| Suwannee River HA, Suwannee River FA and Eliot Soil HA from IHSS | 8 | +: Hg(II)/HS ratio, Y: the sources of HS and −OH/−COOH ratio in HS; −: [Ca2+]. | Hg(II) reduction mediated via the complexes of Hg(II) with HS. Ca2+ may impede the intermolecular reaction between Hg(II) and HS. | [29] | |
| Reduced NOM | 2.42 ± 0.53 h−1 (no Cl−) 0.14 ± 0.01 h−1 (with Cl−) | 7 | −: [Cl−]. | Both the formation of Hg(II)-chloride complexes and the re-oxidation of Hg(0) to Hg(II), may contribute to the retarding effect of Cl− on the Hg(II) reduction. | [30] |
2.1. The Rate Constants
2.2. Factors Affecting Dark Hg(II) Reduction Rates by NOMs
2.2.1. Effect of pH
2.2.2. Effect of Hg(II)/NOM Ratio, the Concentration of Hg(II) ([Hg(II)]) or NOM ([NOM])
2.2.3. Effect of NOM Structure
2.2.4. Effect of Cl−
2.2.5. Effect of Temperature and Ca2+ Ion
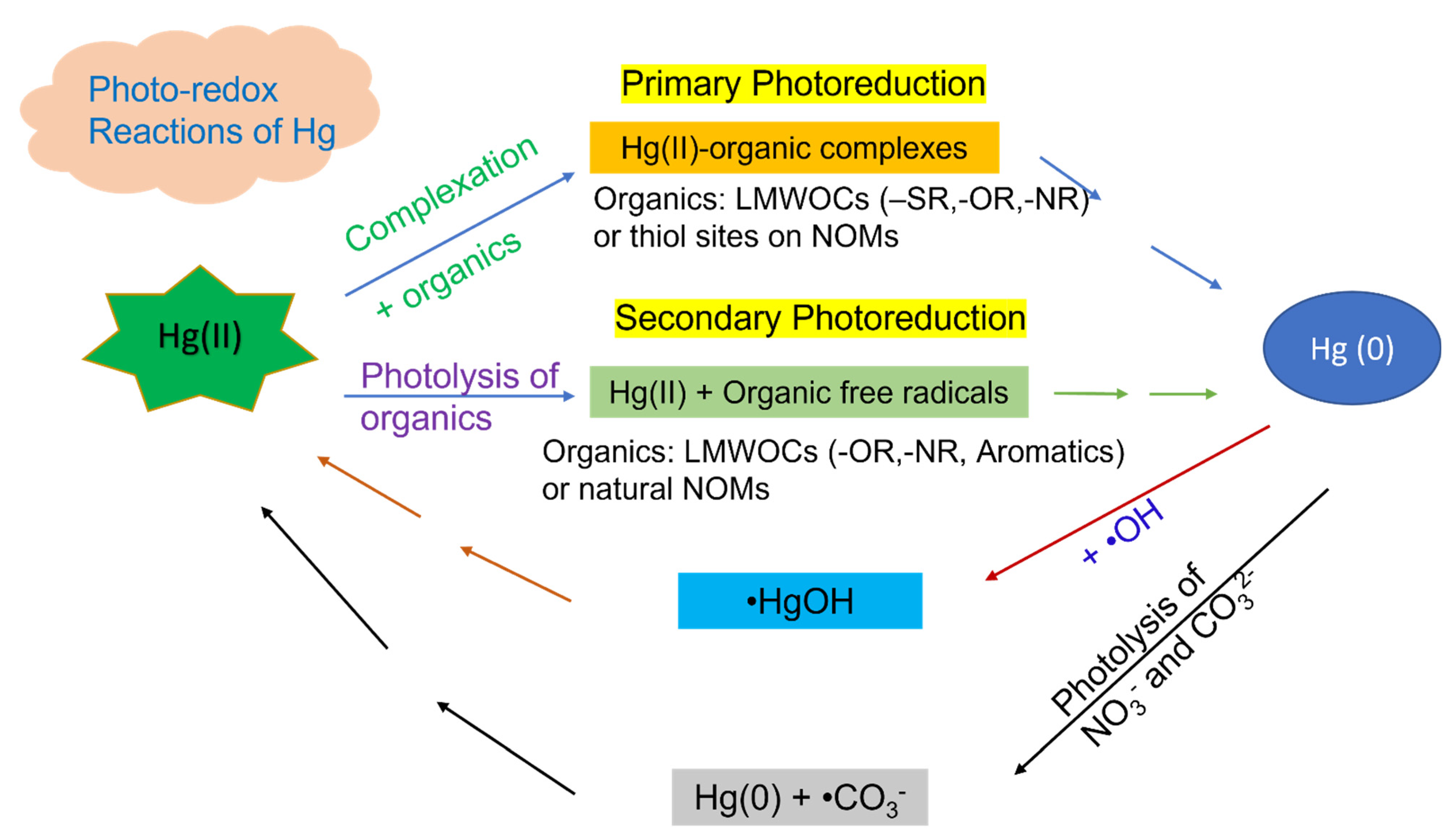
3. Photochemical Reduction of Hg(II) Relevant to Natural Waters
| Reductant | Rate Constants | Light Source | pH | Factors Affecting Kinetics 1 | Reaction Mechanism | References |
|---|---|---|---|---|---|---|
| Hg(OH)2 | 3 × 10−7 s−1 | simulated sunlight | 7 | N/A | Primary reaction | [37] |
| HgS22− | ~10−7 s−1 | simulated sunlight | 7 | N/A | [37] | |
| Oxalic acid | 1.7 × 104 M−1 s−1 (no Cl−) 1.1 × 104 M−1 s−1 (with Cl−) | simulated sunlight | 3.9 or 7 | −: Cl− | Secondary Mechanism by HO2 radical | [38] |
| Oxalic acid | N/A | N/A | Primary reaction | [39] | ||
| Dicarboxylic Acids (C2–C4) | 1.2 × 104 M−1 s−1 (oxalic) 4.9 × 103 M−1 s−1 (malonic) 2.8 ×103 M−1 s−1 (succinic) | UV or visible lamps | 3.0 | −: Cl− or O2 | Both primary and secondary reactions | [40] |
| Serine | 0.640 h−1 | simulated sunlight | 3.6 or 3.8 | −: reduced sulfur groups on LMWOCs | Both primary and secondary reactions | [41] |
| cysteine | 0.047 h−1 | |||||
| Salicylic acid | 2.1–8.3 h−1 | UV-B | 4.2 | Y: both the substituent functional groups and their positions on the benzene ring | Secondary reactions by organic free radicals | [42] |
| Anthranilic acid | 2.0–8.8 h−1 | |||||
| phthalic acid | 3.4 h−1 | |||||
| 4-hydrobenzoic acid | 2.4 h−1 | |||||
| 4-aminobenzoic acid | 7.6 h−1 | |||||
| Salicylic acid | 0.60 h−1 | UV-A | 4.2 | |||
| Anthranilic acid | 8.4 h−1 | |||||
| 4-hydrobenzoic acid | 0.33 h−1 | |||||
| 4-aminobenzoic acid | 1.8 h−1 | |||||
| Thioglycolic acid (TGA) | (2.3 ± 0.4) × 10−5 s−1 | UV | 4.0 | Y: pH | Hg(II) reduction may be mediated via Hg(II)-TGA complex Heterogeneous processes may be involved | [43] |
| cysteine (Cys) | 1.13 ± 0.21 day−1 (highest, pH 7, oxic) | Natural sunlight | 7 or 3.2 | Y: pH, light wavelength or dissolved O2 Y: functional groups on LMWOCs | [44] | |
| serine (Ser) | 29.94 ± 4.23 day−1 (highest, pH 7, oxic) | |||||
| ethylenediamine (en) | 2.80 ± 0.36 day−1 (pH 7, oxic) |
3.1. Photoreduction of Hg(II) by LMWOCs
3.1.1. Reduced Sulfur Functional Groups (−SR)
3.1.2. Aromatics
3.1.3. Oxygenated (−OR) and/or Amine (−NR) Functional Groups
3.2. Photoreduction of Hg(II) in the Presence of Natural DOM Isolates
3.3. Effect of Light Wavelength and Intensity on Hg(II) Photoreduction
4. Chemical Oxidation Pathways of Hg(0) in the Dark
| Oxidant | Rate Constants | pH | Factors Affecting Kinetics 1 | Reaction Mechanism | References |
|---|---|---|---|---|---|
| Reduced Elliott soil HA 2 from IHSS 2 | N/A | 7 | +: [reduced HA] | ligand-induced oxidative complexation of Hg(0) by thiol groups in HA | [25] |
| Reduced NOMs 2 | Initial Rates: 2.21–5.42 h−1 | 7 | +: DOM:Hg(II) ratios 2 | Same as above | [26,63] |
| Various thiol organic ligands | 0.01–2.18 h−1 | 7 | Y: thiol species and thoil to Hg ratios | Same as above | [64] |
| Reduced NOM | 0.14 ± 0.01 h−1 (no Cl−) 0.25 ± 0.07 h−1 (with Cl−) | 7 | −: Cl− | N/A | [30] |
5. Photochemical Oxidation of Hg(II) by DOM in Natural Waters
6. Concluding Remarks and Future Research Needs
- To reduce the uncertainties in the determination of the rate constants under a wide range of environmental conditions.
- To study how the reaction kinetics and mechanisms varied with the functional groups on NOMs.
- To decipher the mechanisms on how various environmental variables (e.g., Cl−) impact the chemical redox reactions of Hg.
- To characterize NOM composition at a molecular level.
- To identify more abiotic redox reactions of Hg that contribute to the observed field data.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- O’Connor, D.; Hou, D.; Ok, Y.S.; Mulder, J.; Duan, L.; Wu, Q.; Wang, S.; Tack, F.M.G.; Rinklebe, J. Mercury Speciation, Transformation, and Transportation in Soils, Atmospheric Flux, and Implications for Risk Management: A Critical Review. Environ. Int. 2019, 126, 747–761. [Google Scholar] [CrossRef] [PubMed]
- Vost, E.; Amyot, M.; O’Driscoll, N.J. Photoreactions of Mercury in Aquatic Systems. In Environmental Chemistry and Toxicology of Mercury; Liu, G., Cai, Y., Nelson J. O’Driscoll, Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2012; pp. 193–218. [Google Scholar]
- Ullrich, S.M.; Tanton, T.W.; Abdrashitova, S.A. Mercury in the Aquatic Environment: A Review of Factors Affecting Methylation. Crit. Rev. Environ. Sci. Technol. 2001, 31, 241–293. [Google Scholar] [CrossRef]
- Ariya, P.A.; Amyot, M.; Dastoor, A.; Deeds, D.; Feinberg, A.; Kos, G.; Poulain, A.; Ryjkov, A.; Semeniuk, K.; Subir, M.; et al. Mercury Physicochemical and Biogeochemical Transformation in the Atmosphere and at Atmospheric Interfaces: A Review and Future Directions. Chem. Rev. 2015, 115, 3760–3802. [Google Scholar] [CrossRef]
- Obrist, D.; Agnan, Y.; Jiskra, M.; Olson, C.L.; Colegrove, D.P.; Hueber, J.; Moore, C.W.; Sonke, J.E.; Helmig, D. Tundra Uptake of Atmospheric Elemental Mercury Drives Arctic Mercury Pollution. Nature 2017, 547, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, M. Interactions between Mercury and Dissolved Organic Matter—A Review. Chemosphere 2004, 55, 319–331. [Google Scholar] [CrossRef]
- Bjørklund, G.; Dadar, M.; Mutter, J.; Aaseth, J. The Toxicology of Mercury: Current Research and Emerging Trends. Environ. Res. 2017, 159, 545–554. [Google Scholar] [CrossRef]
- Rose, C.H.; Ghosh, S.; Blum, J.D.; Bergquist, B.A. Effects of Ultraviolet Radiation on Mercury Isotope Fractionation during Photo-Reduction for Inorganic and Organic Mercury Species. Chem. Geol. 2015, 405, 102–111. [Google Scholar] [CrossRef]
- Borch, T.; Kretzschmar, R.; Skappler, A.; Van Cappellen, P.; Ginder-Vogel, M.; Voegelin, A.; Campbell, K. Biogeochemical Redox Processes and Their Impact on Contaminant Dynamics. Environ. Sci. Technol. 2010, 44, 15–23. [Google Scholar] [CrossRef]
- Branfireun, B.A.; Cosio, C.; Poulain, A.J.; Riise, G.; Bravo, A.G. Mercury Cycling in Freshwater Systems - An Updated Conceptual Model. Sci. Total Environ. 2020, 745, 140906. [Google Scholar] [CrossRef]
- O’Driscoll, N.J.; Siciliano, S.D.; Lean, D.R.S. Continuous Analysis of Dissolved Gaseous Mercury in Freshwater Lakes. Sci. Total Environ. 2003, 304, 285–294. [Google Scholar] [CrossRef]
- O’Driscoll, N.J.; Poissant, L.; Canário, J.; Lean, D.R.S. Dissolved Gaseous Mercury Concentrations and Mercury Volatilization in a Frozen Freshwater Fluvial Lake. Environ. Sci. Technol. 2008, 42, 5125–5130. [Google Scholar] [CrossRef] [PubMed]
- Mason, R.P.; Choi, A.L.; Fitzgerald, W.F.; Hammerschmidt, C.R.; Lamborg, C.H.; Soerensen, A.L.; Sunderland, E.M. Mercury Biogeochemical Cycling in the Ocean and Policy Implications. Environ. Res. 2012, 119, 101–117. [Google Scholar] [CrossRef] [PubMed]
- Orihel, D.M.; Paterson, M.J.; Blanchfield, P.J.; Bodaly, R.A.; Hintelmann, H. Experimental Evidence of a Linear Relationship between Inorganic Mercury Loading and Methylmercury Accumulation by Aquatic Biota. Environ. Sci. Technol. 2007, 41, 4952–4958. [Google Scholar] [CrossRef]
- Luo, H.; Yin, X.; Jubb, A.M.; Chen, H.; Lu, X.; Zhang, W.; Lin, H.; Yu, H.-Q.; Liang, L.; Sheng, G.-P.; et al. Photochemical Reactions between Mercury (Hg) and Dissolved Organic Matter Decrease Hg Bioavailability and Methylation. Environ. Pollut. 2017, 220, 1359–1365. [Google Scholar] [CrossRef]
- Corbitt, E.S.; Jacob, D.J.; Holmes, C.D.; Streets, D.G.; Sunderland, E.M. Global Source-Receptor Relationships for Mercury Deposition under Present-Day and 2050 Emissions Scenarios. Environ. Sci. Technol. 2011, 45, 10477–10484. [Google Scholar] [CrossRef] [PubMed]
- Lamborg, C.H.; Hansel, C.M.; Bowman, K.L.; Voelker, B.M.; Marsico, R.M.; Oldham, V.E.; Swarr, G.J.; Zhang, T.; Ganguli, P.M. Dark Reduction Drives Evasion of Mercury From the Ocean. Front. Environ. Chem. 2021, 2, 4. [Google Scholar] [CrossRef]
- Jiang, T.; Skyllberg, U.; Wei, S.; Wang, D.; Lu, S.; Jiang, Z.; Flanagan, D.C. Modeling of the Structure-Specific Kinetics of Abiotic, Dark Reduction of Hg(II) Complexed by O/N and S Functional Groups in Humic Acids While Accounting for Time-Dependent Structural Rearrangement. Geochim. Cosmochim. Acta 2015, 154, 151–167. [Google Scholar] [CrossRef]
- Zhang, H.; Lindberg, S.E. Sunlight and Iron(III)-Induced Photochemical Production of Dissolved Gaseous Mercury in Freshwater. Environ. Sci. Technol. 2001, 35, 928–935. [Google Scholar] [CrossRef]
- Zhu, S.; Zhang, Z.; Žagar, D. Mercury Transport and Fate Models in Aquatic Systems: A Review and Synthesis. Sci. Total Environ. 2018, 639, 538–549. [Google Scholar] [CrossRef]
- Zhang, H. Photochemical Redox Reactions of Mercury. Struct. Bond. 2006, 120, 37–79. [Google Scholar] [CrossRef]
- Rocha, J.C.; Junior, É.S.; Zara, L.F.; Rosa, A.H.; Dos Santos, A.; Burba, P. Reduction of Mercury(II) by Tropical River Humic Substances (Rio Negro) - A Possible Process of the Mercury Cycle in Brazil. Talanta 2000, 53, 551–559. [Google Scholar] [CrossRef]
- Rocha, J.C.; Sargentini, É.; Zara, L.F.; Rosa, A.H.; Dos Santos, A.; Burba, P. Reduction of Mercury(II) by Tropical River Humic Substances (Rio Negro) - Part II. Influence of Structural Features (Molecular Size, Aromaticity, Phenolic Groups, Organically Bound Sulfur). Talanta 2003, 61, 699–707. [Google Scholar] [CrossRef]
- Zheng, W.; Hintelmann, H. Nuclear Field Shift Effect in Isotope Fractionation of Mercury during Abiotic Reduction in the Absence of Light. J. Phys. Chem. A 2010, 114, 4238–4245. [Google Scholar] [CrossRef] [PubMed]
- Gu, B.; Bian, Y.; Miller, C.L.; Dong, W.; Jiang, X.; Liang, L. Mercury Reduction and Complexation by Natural Organic Matter in Anoxic Environments. Proc. Natl. Acad. Sci. USA 2011, 108, 1479–1483. [Google Scholar] [CrossRef]
- Zheng, W.; Liang, L.; Gu, B. Mercury Reduction and Oxidation by Reduced Natural Organic Matter in Anoxic Environments. Environ. Sci. Technol. 2012, 46, 292–299. [Google Scholar] [CrossRef]
- Jiang, T.; Wei, S.-Q.; Flanagan, D.C.; Li, M.J.; Li, X.M.; Wang, Q.; Chang, L. Effect of Abiotic Factors on the Mercury Reduction Process by Humic Acids in Aqueous Systems. Pedosphere 2014, 24, 125–136. [Google Scholar] [CrossRef]
- Chakraborty, P.; Vudamala, K.; Coulibaly, M.; Ramteke, D.; Chennuri, K.; Lean, D. Reduction of Mercury (II) by Humic Substances. Environ. Sci. Pollut. Res. 2015, 22, 10529–10538. [Google Scholar] [CrossRef]
- Vudamala, K.; Chakraborty, P.; Sailaja, B.B.V. An Insight into Mercury Reduction Process by Humic Substances in Aqueous Medium under Dark Condition. Environ. Sci. Pollut. Res. 2017, 24, 14499–14507. [Google Scholar] [CrossRef]
- Lee, S.; Roh, Y.; Kim, K.-W. Influence of Chloride Ions on the Reduction of Mercury Species in the Presence of Dissolved Organic Matter. Environ. Geochem. Health 2019, 41, 71–79. [Google Scholar] [CrossRef]
- Skyllberg, U. Competition among Thiols and Inorganic Sulfides and Polysulfides for Hg and MeHg in Wetland Soils and Sediments under Suboxic Conditions: Illumination of Controversies and Implications for MeHg Net Production. J. Geophys. Res. Biogeosciences 2008, 113, G00C03. [Google Scholar] [CrossRef]
- Zheng, W.; Hintelmann, H. Mercury Isotope Fractionation during Photoreduction in Natural Water Is Controlled by Its Hg/DOC Ratio. Geochim. Cosmochim. Acta 2009, 73, 6704–6715. [Google Scholar] [CrossRef]
- Jiang, T.; Wang, D.; Meng, B.; Chi, J.; Laudon, H.; Liu, J. The Concentrations and Characteristics of Dissolved Organic Matter in High-Latitude Lakes Determine Its Ambient Reducing Capacity. Water Res. 2020, 169, 115217. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.I.; Yang, J.H.; Kim, P.R.; Han, Y.J. Effects of Organic Carbon and UV Wavelength on the Formation of Dissolved Gaseous Mercury in Water under a Controlled Environment. Environ. Eng. Res. 2019, 24, 54–62. [Google Scholar] [CrossRef]
- Lalonde, J.D.; Amyot, M.; Kraepiel, A.M.L.; Morel, F.M. Photooxidation of Hg(0) in Artificial and Natural Waters. Environ. Sci. Technol. 2001, 35, 1367–1372. [Google Scholar] [CrossRef] [PubMed]
- Lalonde, J.D.; Amyot, M.; Orvoine, J.; Morel, F.M.M.; Auclair, J.-C.; Ariya, P.A. Photoinduced Oxidation of Hg0(Aq) in the Waters from the St. Lawrence Estuary. Enviromental Sci. Technol. 2004, 38, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.F.; Munthe, J.; Strömberg, D.; Lindqvist, O. Photochemical Behavior of Inorganic Mercury Compounds in Aqueous Solution. In Mercury Pollution Intergration and Synthesis; Watras, C.J., Huckabee, J.W., Eds.; Lewis Publishers: Boca Raton, FL, USA, 1994; pp. 581–591. [Google Scholar]
- Pehkonen, S.O.; Lin, C.-J. Aqueous Photochemistry of Mercury with Organic Acids. J. Air Waste Manage. Assoc. 1998, 48, 144–150. [Google Scholar] [CrossRef]
- Gårdfeldt, K.; Jonsson, M. Is Bimolecular Reduction of Hg(II) Complexes Possible in Aqueous Systems of Environmental Importance. J. Phys. Chem. A 2003, 107, 4478–4482. [Google Scholar] [CrossRef]
- Si, L.; Ariya, P.A. Reduction of Oxidized Mercury Species by Dicarboxylic Acids (C 2-C4): Kinetic and Product Studies. Environ. Sci. Technol. 2008, 42, 5150–5155. [Google Scholar] [CrossRef]
- Zheng, W.; Hintelmann, H. Isotope Fractionation of Mercury during Its Photochemical Reduction by Low-Molecular-Weight Organic Compounds. J. Phys. Chem. A 2010, 114, 4246–4253. [Google Scholar] [CrossRef]
- He, F.; Zheng, W.; Liang, L.; Gu, B. Mercury Photolytic Transformation Affected by Low-Molecular-Weight Natural Organics in Water. Sci. Total Environ. 2012, 416, 429–435. [Google Scholar] [CrossRef]
- Si, L.; Ariya, P.A. Photochemical Reactions of Divalent Mercury with Thioglycolic Acid: Formation of Mercuric Sulfide Particles. Chemosphere 2015, 119, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Motta, L.C.; Kritee, K.; Blum, J.D.; Tsz-Ki Tsui, M.; Reinfelder, J.R. Mercury Isotope Fractionation during the Photochemical Reduction of Hg(II) Coordinated with Organic Ligands. J. Phys. Chem. A 2020, 124, 2842–2853. [Google Scholar] [CrossRef] [PubMed]
- Jeremiason, J.D.; Portner, J.C.; Aiken, G.R.; Hiranaka, A.J.; Dvorak, M.T.; Tran, K.T.; Latch, D.E. Photoreduction of Hg(II) and Photodemethylation of Methylmercury: The Key Role of Thiol Sites on Dissolved Organic Matter. Environ. Sci. Process. Impacts 2015, 17, 1892–1903. [Google Scholar] [CrossRef] [PubMed]
- Si, L.; Ariya, P.A. Aqueous Photoreduction of Oxidized Mercury Species in Presence of Selected Alkanethiols. Chemosphere 2011, 84, 1079–1084. [Google Scholar] [CrossRef]
- Garcia, E.; Amyot, M.; Ariya, P.A. Relationship between DOC Photochemistry and Mercury Redox Transformations in Temperate Lakes and Wetlands. Geochim. Cosmochim. Acta 2005, 69, 1917–1924. [Google Scholar] [CrossRef]
- O’Driscoll, N.J.; Vost, E.; Mann, E.; Klapstein, S.; Tordon, R.; Lukeman, M. Mercury Photoreduction and Photooxidation in Lakes: Effects of Filtration and Dissolved Organic Carbon Concentration. J. Environ. Sci. 2018, 103, 151–159. [Google Scholar] [CrossRef]
- O’Driscoll, N.J.; Siciliano, S.D.; Lean, D.R.S.; Amyot, M. Gross Photoreduction Kinetics of Mercury in Temperate Freshwater Lakes and Rivers: Application to a General Model of DGM Dynamics. Environ. Sci. Technol. 2006, 40, 837–843. [Google Scholar] [CrossRef]
- Oh, S.; Kim, M.-K.; Lee, Y.-M.; Zoh, K.-D. Effect of Abiotic and Biotic Factors on the Photo-Induced Production of Dissolved Gaseous Mercury. Water. Air. Soil Pollut. 2011, 220, 353–363. [Google Scholar] [CrossRef]
- Wollenberg, J.L.; Peters, S.C. Mercury Emission from a Temperate Lake during Autumn Turnover. Sci. Total Environ. 2009, 407, 2909–2918. [Google Scholar] [CrossRef]
- O’Driscoll, N.J.; Siciliano, S.D.; Peak, D.; Carignan, R.; Lean, D.R.S. The Influence of Forestry Activity on the Structure of Dissolved Organic Matter in Lakes: Implications for Mercury Photoreactions. Sci. Total Environ. 2006, 366, 880–893. [Google Scholar] [CrossRef]
- Luo, H.; Cheng, Q.; Pan, X. Photochemical Behaviors of Mercury (Hg) Species in Aquatic Systems: A Systematic Review on Reaction Process, Mechanism, and Influencing Factor. Sci. Total Environ. 2020, 720, 137540. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Kim, J.; Soerensen, A.L.; Lee, W.; Han, S. The Role of Fluorescent Dissolved Organic Matter on Mercury Photoreduction Rates: A Case Study of Three Temperate Lakes. Geochim. Cosmochim. Acta 2020, 277, 192–205. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, X.; Yang, Y.; Wang, D.; Liu, X. Effect of Dissolved Organic Matter on Mercury Release from Water Body. J. Environ. Sci. 2011, 23, 912–917. [Google Scholar] [CrossRef]
- Chen, H.; Johnston, R.C.; Mann, B.F.; Chu, R.K.; Tolic, N.; Parks, J.M.; Gu, B. Identification of Mercury and Dissolved Organic Matter Complexes Using Ultrahigh Resolution Mass Spectrometry. Environ. Sci. Technol. Lett. 2017, 4, 59–65. [Google Scholar] [CrossRef]
- Jiang, T.; Chen, X.; Wang, D.; Liang, J.; Bai, W.; Zhang, C.; Wang, Q.; Wei, S. Dynamics of Dissolved Organic Matter (DOM) in a Typical Inland Lake of the Three Gorges Reservoir Area: Fluorescent Properties and Their Implications for Dissolved Mercury Species. J. Environ. Manag. 2018, 206, 418–429. [Google Scholar] [CrossRef]
- Wang, Z.; Fei, Z.; Wu, Q.; Yin, R. Evaluation of the Effects of Hg/DOC Ratios on the Reduction of Hg(II) in Lake Water. Chemosphere 2020, 253, 126634. [Google Scholar] [CrossRef]
- O’Driscoll, N.J.; Lean, D.R.S.; Loseto, L.L.; Carignan, R.; Siciliano, S.D. Effect of Dissolved Organic Carbon on the Photoproduction of Dissolved Gaseous Mercury in Lakes: Potential Impacts of Forestry. Environ. Sci. Technol. 2004, 38, 2664–2672. [Google Scholar] [CrossRef]
- Qureshi, A.; O’Driscoll, N.J.; Macleod, M.; Neuhold, Y.M.; Hungerbuhler, K. Photoreactions of Mercury in Surface Ocean Water: Gross Reaction Kinetics and Possible Pathways. Environ. Sci. Technol. 2010, 44, 644–649. [Google Scholar] [CrossRef]
- Gårdfeldt, K.; Sommar, J.; Strömberg, D.; Feng, X. Oxidation of Atomic Mercury by Hydroxyl Radicals and Photoinduced Decomposition of Methylmercury in the Aqueous Phase. Atmos. Environ. 2001, 35, 3039–3047. [Google Scholar] [CrossRef]
- Hines, N.A.; Brezonik, P.L. Mercury Dynamics in a Small Northern Minnesota Lake: Water to Air Exchange and Photoreactions of Mercury. Mar. Chem. 2004, 90, 137–149. [Google Scholar] [CrossRef]
- Zheng, W.; Demers, J.D.; Lu, X.; Bergquist, B.A.; Anbar, A.D.; Blum, J.D.; Gu, B. Mercury Stable Isotope Fractionation during Abiotic Dark Oxidation in the Presence of Thiols and Natural Organic Matter. Environ. Sci. Technol. 2019, 53, 1853–1862. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Lin, H.; Mann, B.F.; Liang, L.; Gu, B. Oxidation of Dissolved Elemental Mercury by Thiol Compounds under Anoxic Conditions. Environ. Sci. Technol. 2013, 47, 12827–12834. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Zhao, W.; Liang, L.; Gu, B. Photochemical Oxidation of Dissolved Elemental Mercury by Carbonate Radicals in Water. Environ. Sci. Technol. Lett. 2014, 1, 499–503. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Si, L.; Branfireun, B.A.; Fierro, J. Chemical Oxidation and Reduction Pathways of Mercury Relevant to Natural Waters: A Review. Water 2022, 14, 1891. https://doi.org/10.3390/w14121891
Si L, Branfireun BA, Fierro J. Chemical Oxidation and Reduction Pathways of Mercury Relevant to Natural Waters: A Review. Water. 2022; 14(12):1891. https://doi.org/10.3390/w14121891
Chicago/Turabian StyleSi, Lin, Brian A. Branfireun, and Jessica Fierro. 2022. "Chemical Oxidation and Reduction Pathways of Mercury Relevant to Natural Waters: A Review" Water 14, no. 12: 1891. https://doi.org/10.3390/w14121891
APA StyleSi, L., Branfireun, B. A., & Fierro, J. (2022). Chemical Oxidation and Reduction Pathways of Mercury Relevant to Natural Waters: A Review. Water, 14(12), 1891. https://doi.org/10.3390/w14121891





