Comparative Life-Cycle Cost Analysis of Alternative Technologies for the Removal of Emerging Contaminants from Urban Wastewater
Abstract
:1. Introduction
2. Materials and Methods
2.1. Goal and Scope Descriptions
2.2. Life Cycle Cost Model Development
2.3. Eco-Efficiency Assessment
2.4. Life Cycle Impact Assessment
2.5. Sensitivity Analysis
2.6. Uncertainty Analysis
3. Results and Discussion
3.1. Life Cycle Costs
3.2. Sensitivity Analysis
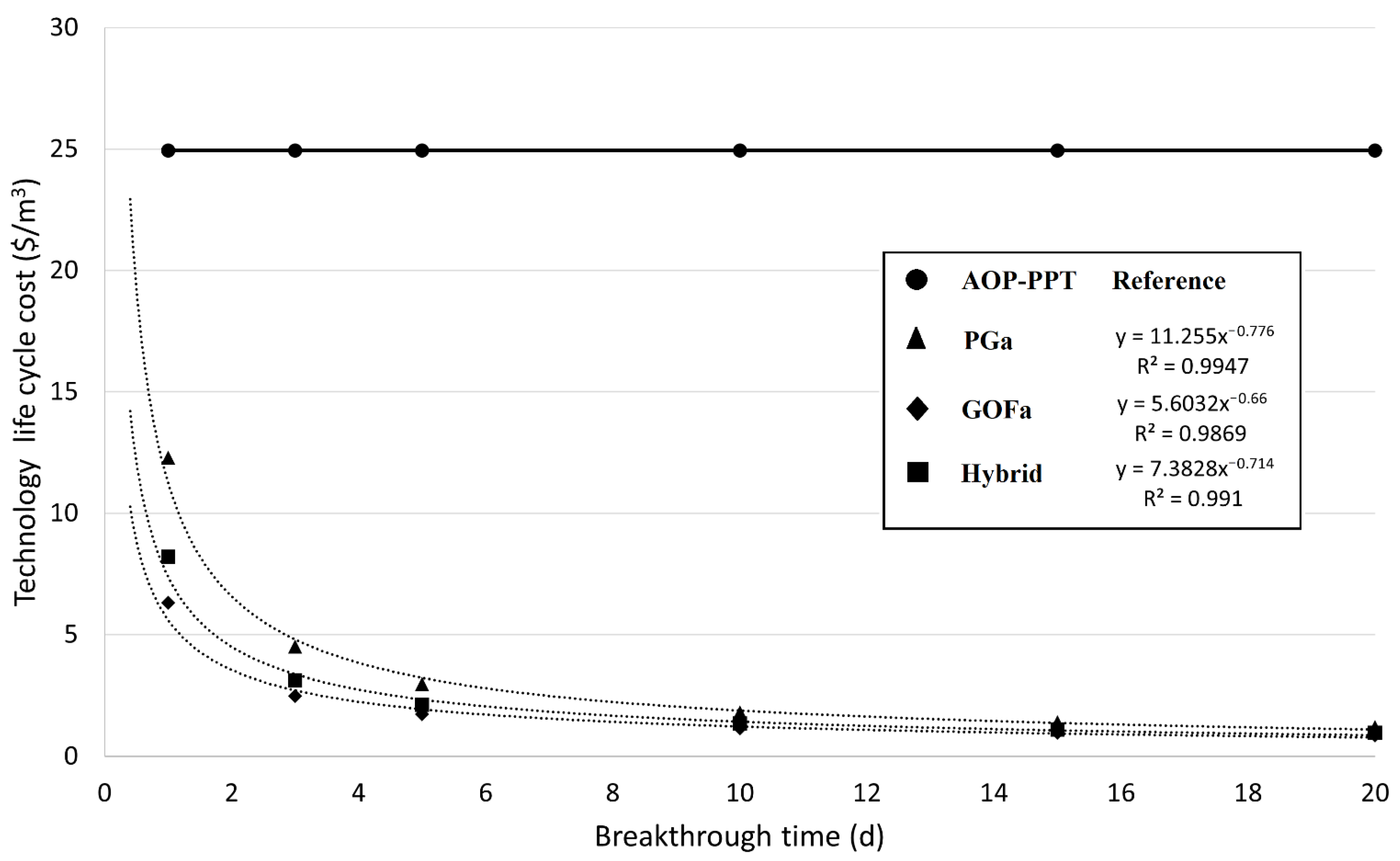
3.3. Breakthrough Times
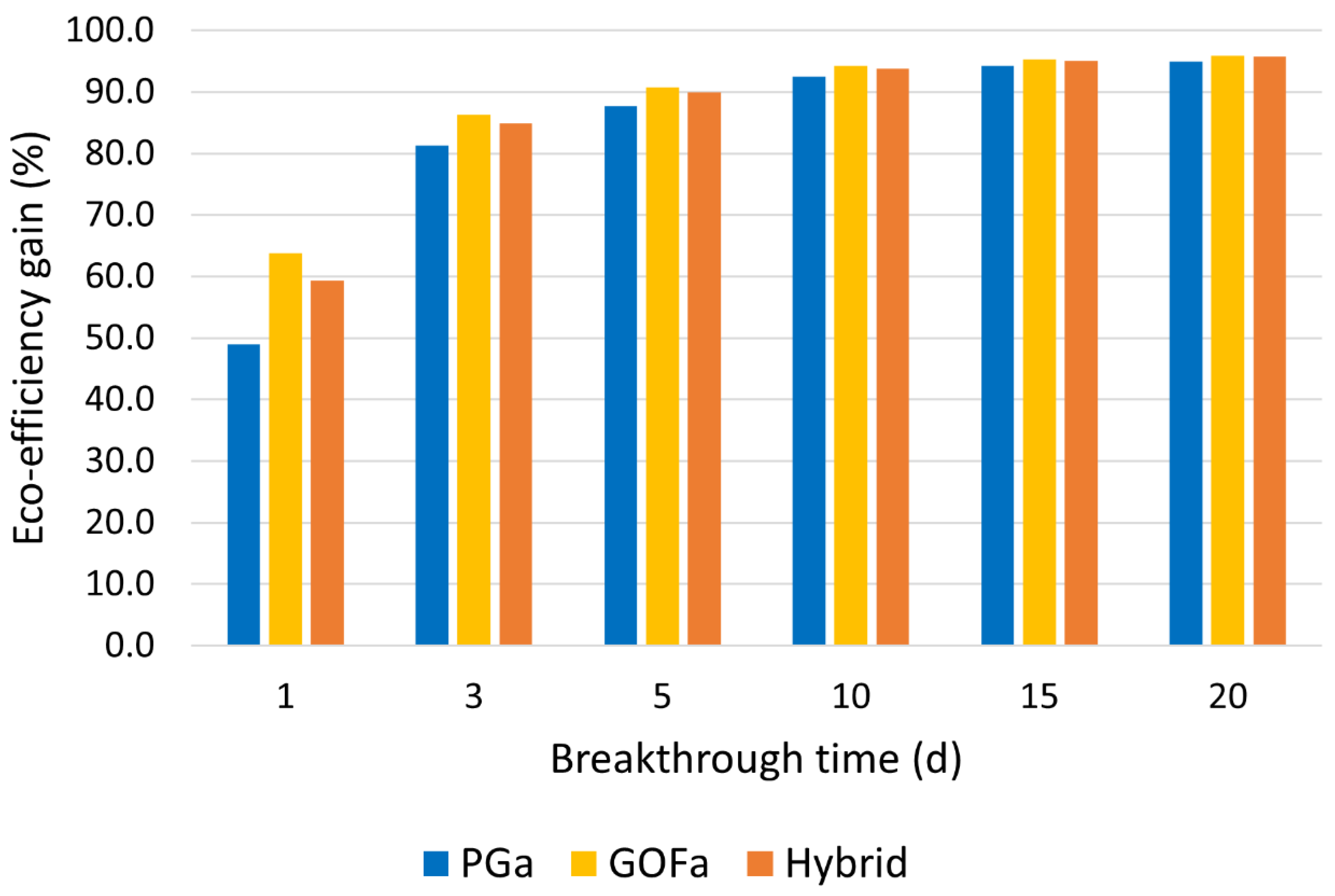
3.4. Eco-Efficiency Assessment
4. Synthesis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Naidu, R.; Espana, V.A.A.; Liu, Y.; Jit, J. Emerging contaminants in the environment: Risk-based analysis for better management. Chemosphere 2016, 154, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Rokhina, E.; Singh, T.; Curran, M.; Suri, R. Combined Life Cycle Assessment and Life Cycle Costing of UV-based Treatment of Emerging Contaminants. UV Sol. Mag. 2012, 14, 23–32. [Google Scholar]
- Ahmed, S.F.; Mofijur, M.; Nuzhat, S.; Chowdhury, A.T.; Rafa, N.; Uddin, A.; Inayat, A.; Mahlia, T.M.I.; Ong, H.C.; Chia, W.Y.; et al. Recent developments in physical, biological, chemical, and hybrid treatment techniques for removing emerging contaminants from wastewater. J. Hazard. Mater. 2021, 416, 125912. [Google Scholar] [CrossRef] [PubMed]
- Rout, P.R.; Zhang, T.C.; Bhunia, P.; Surampalli, R.Y. Treatment technologies for emerging contaminants in wastewater treatment plants: A review. Sci. Total Environ. 2021, 753, 141990. [Google Scholar] [CrossRef] [PubMed]
- Wen, D.; Fu, R.; Li, Q. Removal of inorganic contaminants in soil by electrokinetic remediation technologies: A review. J. Hazard. Mater. 2021, 401, 123345. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Du, X.; Huang, S. The economic and environmental implications of wastewater management policy in China: From the LCA perspective. J. Clean. Prod. 2017, 142, 3544–3557. [Google Scholar] [CrossRef]
- Sophia, A.C.; Lima, E.C. Removal of emerging contaminants from the environment by adsorption. Ecotoxicol. Environ. Saf. 2018, 150, 1–17. [Google Scholar] [CrossRef]
- Siyal, A.A.; Shamsuddin, M.R.; Low, A.; Rabat, N.E. A review on recent developments in the adsorption of surfactants from wastewater. J. Environ. Manag. 2020, 254, 109797. [Google Scholar] [CrossRef]
- Rathi, B.S.; Kumar, P.S. Application of adsorption process for effective removal of emerging contaminants from water and wastewater. Environ. Pollut. 2021, 280, 116995. [Google Scholar] [CrossRef]
- Pryce, D.; Khalil, A.M.; Memon, F.A. Investigating the environmental costs of utilizing graphene-based adsorbents and pulsed power oxidation for the removal of emerging contaminants from urban wastewater. Sci. Total Environ. 2022, 817, 152985. [Google Scholar] [CrossRef]
- Thu, K.; Chakraborty, A.; Saha, B.; Chun, W.G.; Ng, K.C. Life-cycle cost analysis of adsorption cycles for desalination. Desalination Water Treat. 2010, 20, 1–10. [Google Scholar] [CrossRef]
- Ng, K.C.; Thu, K.; Kim, Y.; Chakraborty, A.; Amy, G. Adsorption desalination: An emerging low-cost thermal desalination method. Desalination 2013, 308, 161–179. [Google Scholar] [CrossRef]
- Behrooz, H.A.; Hoseini, M.; Mahamadzade, M.; Ranjbaran, N. Economic Comparison between Membrane and Adsorption Processes for Separation of CO2 and CH4 Mixture. In Proceedings of the 5th National Conference on New Researches in Chemistry and Chemical Engineering, Tehran, Iran, 13 February 2019. [Google Scholar]
- Toor, M.; Jin, B.; Dai, S.; Vimonses, V. Activating natural bentonite as a cost-effective adsorbent for removal of Congo-red in wastewater. J. Ind. Eng. Chem. 2015, 21, 653–661. [Google Scholar] [CrossRef]
- Zhu, S.; Xia, M.; Chu, Y.; Khan, M.A.; Lei, W.; Wang, F.; Muhmood, T.; Wang, A. Adsorption and Desorption of Pb(II) on l-Lysine Modified Montmorillonite and the simulation of Interlayer Structure. Appl. Clay Sci. 2019, 169, 40–47. [Google Scholar] [CrossRef]
- Zhu, S.; Chen, Y.; Khan, M.A.; Xu, H.; Wang, F.; Xia, M. In-Depth Study of Heavy Metal Removal by an Etidronic Acid-Functionalized Layered Double Hydroxide. ACS Appl. Mater. Interfaces 2022, 14, 7450–7463. [Google Scholar] [CrossRef]
- Zhu, S.; Khan, M.A.; Kameda, T.; Xu, H.; Wang, F.; Xia, M.; Yoshioka, T. New insights into the capture performance and mechanism of hazardous metals Cr3+ and Cd2+ onto an effective layered double hydroxide based material. J. Hazard. Mater. 2022, 426, 128062. [Google Scholar] [CrossRef]
- Othman, N.; Abd-Kadir, A.; Zayadi, N. Waste fish scale as cost effective adsorbent in removing zinc and ferum ion in wastewater. J. Eng. Appl. Sci. 2016, 11, 1584–1592. [Google Scholar]
- Zhang, C.; Li, J.; Cheng, F. Recycling of powder coke to cost effective adsorbent material and its application for tertiary treatment of coking wastewater. J. Clean. Prod. 2020, 261, 121114. [Google Scholar] [CrossRef]
- Rahim, A.R.A.; Mohsin, H.M.; Thanabalan, M.; Rabat, N.E.; Saman, N.; Mat, H.; Johari, K. Effective carbonaceous desiccated coconut waste adsorbent for application of heavy metal uptakes by adsorption: Equilibrium, kinetic and thermodynamics analysis. Biomass Bioenergy 2020, 142, 105805. [Google Scholar] [CrossRef]
- Cheng, N.; Wang, B.; Wu, P.; Lee, X.; Xing, Y.; Chen, M.; Gao, B. Adsorption of emerging contaminants from water and wastewater by modified biochar: A review. Environ. Pollut. 2021, 273, 116448. [Google Scholar] [CrossRef]
- Delgado-Moreno, L.; Bazhari, S.; Gasco, G.; Méndez, A.; El Azzouzi, M.; Romero, E. New insights into the efficient removal of emerging contaminants by biochars and hydrochars derived from olive oil wastes. Sci. Total Environ. 2021, 752, 141838. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, D.L.; Ralph, P.J. Microalgal bioremediation of emerging contaminants—Opportunities and challenges. Water Res. 2019, 164, 114921. [Google Scholar] [CrossRef] [PubMed]
- Varsha, M.; Kumar, P.S.; Rathi, B.S. A review on recent trends in the removal of emerging contaminants from aquatic environment using low-cost adsorbents. Chemosphere 2022, 287, 132270. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.A.; Kirchain, R.; Novoa, H.; Araujo, A. Cost of quality: Evaluating cost-quality trade-offs for inspection strategies of manufacturing processes. Int. J. Prod. Econ. 2017, 188, 156–166. [Google Scholar] [CrossRef]
- Molinos-Senante, M.; Hernández-Sancho, F.; Sala-Garrido, R. Economic feasibility study for wastewater treatment: A cost–benefit analysis. Sci. Total Environ. 2010, 408, 4396–4402. [Google Scholar] [CrossRef]
- Quesada, H.B.; Baptista, A.T.A.; Cusioli, L.F.; Seibert, D.; de Oliveira Bezerra, C.; Bergamasco, R. Surface water pollution by pharmaceuticals and an alternative of removal by low-cost adsorbents: A review. Chemosphere 2019, 222, 766–780. [Google Scholar] [CrossRef]
- Hu, M.; Mi, B. Enabling Graphene Oxide Nanosheets as Water Separation Membranes. Environ. Sci. Technol. 2013, 47, 3715–3723. [Google Scholar] [CrossRef]
- Yeh, C.-N.; Raidongia, K.; Shao, J.; Yang, Q.-H.; Huang, J. On the origin of the stability of graphene oxide membranes in water. Nat. Chem. 2015, 7, 166–170. [Google Scholar] [CrossRef]
- Dai, J.; Huang, T.; Tian, S.-Q.; Xiao, Y.-J.; Yang, J.-H.; Zhang, N.; Wang, Y.; Zhou, Z.-W. High structure stability and outstanding adsorption performance of graphene oxide aerogel supported by polyvinyl alcohol for waste water treatment. Mater. Des. 2016, 107, 187–197. [Google Scholar] [CrossRef]
- Khalil, A.M.; Memon, F.A.; Tabish, T.A.; Salmon, D.; Zhang, S.; Butler, D. Nanostructured porous graphene for efficient removal of emerging contaminants (pharmaceuticals) from water. Chem. Eng. J. 2020, 398, 125440. [Google Scholar] [CrossRef]
- Khalil, A.M.E.; Memon, F.A.; Tabish, T.A.; Fenton, B.; Salmon, D.; Zhang, S.; Butler, D. Performance Evaluation of Porous Graphene as Filter Media for the Removal of Pharmaceutical/Emerging Contaminants from Water and Wastewater. Nanomaterials 2021, 11, 79. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Du, Q.; Liu, T.; Peng, X.; Wang, J.; Sun, J.; Wang, Y.; Wu, S.; Wang, Z.; Xia, Y.; et al. Comparative study of methylene blue dye adsorption onto activated carbon, graphene oxide, and carbon nanotubes. Chem. Eng. Res. Des. 2013, 91, 361–368. [Google Scholar] [CrossRef]
- Ren, X.; Li, J.; Tan, X.; Wang, X. Comparative study of graphene oxide, activated carbon and carbon nanotubes as adsorbents for copper decontamination. Dalton Trans. 2013, 42, 5266–5274. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Liu, Y.-G.; Liu, S.-B.; Zeng, G.-M.; Jiang, L.-H.; Tan, X.F.; Zhou, L.; Zeng, W.; Li, T.-T.; Yang, C.-P. Adsorption of emerging contaminant metformin using graphene oxide. Chemosphere 2017, 179, 20–28. [Google Scholar] [CrossRef]
- Catherine, H.N.; Ou, M.-H.; Manu, B.; Shih, Y.-H. Adsorption mechanism of emerging and conventional phenolic compounds on graphene oxide nanoflakes in water. Sci. Total Environ. 2018, 635, 629–638. [Google Scholar] [CrossRef]
- Galunin, E.; Burakova, I.; Neskoromnaya, E.; Babkin, A.; Melezhik, A.; Burakov, A.; Tkachev, A. Adsorption of heavy metals from aqueous media on graphene-based nanomaterials. AIP Conf. Proc. 2018, 2041, 020007. [Google Scholar] [CrossRef]
- Yu, L.; Wang, L.; Xu, W.; Chen, L.; Fu, M.; Wu, J.; Ye, D. Adsorption of VOCs on reduced graphene oxide. J. Environ. Sci. 2018, 67, 171–178. [Google Scholar] [CrossRef]
- Allgayer, R.; Yousefi, N.; Tufenkji, N. Graphene oxide sponge as adsorbent for organic contaminants: Comparison with granular activated carbon and influence of water chemistry. Environ. Sci. Nano 2020, 7, 2669–2680. [Google Scholar] [CrossRef]
- Park, S. The puzzle of graphene commercialization. Nat. Rev. Mater. 2016, 1, 16085. [Google Scholar] [CrossRef]
- Ali, I.; Basheer, A.A.; Mbianda, X.Y.; Burakov, A.; Galunin, E.; Burakova, I.; Mkrtchyan, E.; Tkachev, A.; Grachev, V. Graphene based adsorbents for remediation of noxious pollutants from wastewater. Environ. Int. 2019, 127, 160–180. [Google Scholar] [CrossRef]
- Khalil, A.M.E.; Memon, F.A.; Tabish, T.A.; Zhang, S.; Butler, D. Porous graphene–A potential sorbent for removing emerging contaminants from water. In Proceedings of the International Conference on Green Energy and Environmental Technology, Paris, France, 24–26 July 2019. [Google Scholar]
- Rebitzer, G.; Hunkeler, D. Life cycle costing in LCM: Ambitions, opportunities, and limitations. Int. J. Life Cycle Assess. 2003, 8, 253–256. [Google Scholar] [CrossRef]
- Ilyas, M.; Kassa, F.M.; Darun, M.R. Life cycle cost analysis of wastewater treatment: A systematic review of literature. J. Clean. Prod. 2021, 310, 127549. [Google Scholar] [CrossRef]
- Akhoundi, A.; Nazif, S. Sustainability assessment of wastewater reuse alternatives using the evidential reasoning approach. J. Clean. Prod. 2018, 195, 1350–1376. [Google Scholar] [CrossRef]
- Alhashimi, H.A.; Aktas, C.B. Life cycle environmental and economic performance of biochar compared with activated carbon: A meta-analysis. Resour. Conserv. Recycl. 2017, 118, 13–26. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.H.; Yang, W.N.; Ngo, H.H.; Guo, W.S.; Jin, P.K.; Dzakpasu, M.; Yang, S.J.; Wang, Q.; Wang, X.C.; Ao, D. Current status of urban wastewater treatment plants in China. Environ. Int. 2016, 92–93, 11–22. [Google Scholar] [CrossRef]
- Rashid, R.; Shafiq, I.; Akhter, P.; Iqbal, M.J.; Hussain, M. A state-of-the-art review on wastewater treatment techniques: The effectiveness of adsorption method. Environ. Sci. Pollut. Res. 2021, 28, 9050–9066. [Google Scholar] [CrossRef]
- Shi, J.; Bian, W.; Yin, X. Organic contaminants removal by the technique of pulsed high-voltage discharge in water. J. Hazard. Mater. 2009, 171, 924–931. [Google Scholar] [CrossRef]
- Valente, A.; Iribarren, D.; Gálvez-Martos, J.-L.; Dufour, J. Robust eco-efficiency assessment of hydrogen from biomass gasification as an alternative to conventional hydrogen: A life-cycle study with and without external costs. Sci. Total Environ. 2019, 650, 1465–1475. [Google Scholar] [CrossRef]
- Rupp, M.; Rieke, C.; Handschuh, N.; Kuperjans, I. Economic and ecological optimization of electric bus charging considering variable electricity prices and CO2eq intensities. Transp. Res. Part D Transp. Environ. 2020, 81, 102293. [Google Scholar] [CrossRef]
- Lorenzo-Toja, Y.; Vázquez-Rowe, I.; Chenel, S.; Marín-Navarro, D.; Moreira, M.T.; Feijoo, G. Eco-efficiency analysis of Spanish WWTPs using the LCA + DEA method. Water Res. 2015, 68, 651–666. [Google Scholar] [CrossRef]
- Little, J.C.; Hester, E.T.; Carey, C.C. Assessing and Enhancing Environmental Sustainability: A Conceptual Review. Environ. Sci. Technol. 2016, 50, 6830–6845. [Google Scholar] [CrossRef] [PubMed]
- Canaj, K.; Morrone, D.; Roma, R.; Boari, F.; Cantore, V.; Todorovic, M. Reclaimed Water for Vineyard Irrigation in a Mediterranean Context: Life Cycle Environmental Impacts, Life Cycle Costs, and Eco-Efficiency. Water 2021, 13, 2242. [Google Scholar] [CrossRef]
- Younis, A.; Ebead, U.; Judd, S. Life cycle cost analysis of structural concrete using seawater, recycled concrete aggregate, and GFRP reinforcement. Constr. Build. Mater. 2018, 175, 152–160. [Google Scholar] [CrossRef]
- Jawad, D. Life Cycle Cost Optimization Model for Infrastructures. Ph.D. Thesis, Rutgers University, New Brunswick, NJ, USA, 2003. [Google Scholar]
- World Bank Data. Inflation, Consumer Prices (Annual %—India). Available online: https://data.worldbank.org/indicator/FP.CPI.TOTL.ZG?locations=IN (accessed on 3 December 2021).
- World Bank Data. Real Interest Rate (Annual %—India). Available online: https://data.worldbank.org/indicator/FR.INR.RINR?locations=IN (accessed on 3 December 2021).
- Ortiz, I.M. Life Cycle Assessment as a Tool for Green Chemistry: Application to Different Advanced Oxidation Processes for Wastewater Treatment. Ph.D. Thesis, Universitat Autònoma de Barcelona, Barcelona, Spain, 2006. [Google Scholar]
- Jolliet, O.; Margni, M.; Charles, R.; Humbert, S.; Payet, J.; Rebitzer, G.; Rosenbaum, R. IMPACT 2002+: A new life cycle impact assessment methodology. Int. J. Life Cycle Assess. 2003, 8, 324–330. [Google Scholar] [CrossRef] [Green Version]
- Saltelli, A.; Aleksankina, K.; Becker, W.; Fennell, P.; Ferretti, F.; Holst, N.; Li, S.; Wu, Q. Why so many published sensitivity analyses are false: A systematic review of sensitivity analysis practices. Environ. Model. Softw. 2019, 114, 29–39. [Google Scholar] [CrossRef]
- Saltelli, A.; Annoni, P. How to avoid a perfunctory sensitivity analysis. Environ. Model. Softw. 2010, 25, 1508–1517. [Google Scholar] [CrossRef]
- Ingalls, B. Sensitivity analysis: From model parameters to system behaviour. Essays Biochem. 2008, 45, 177–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macdonald, I.; Strachan, P. Practical application of uncertainty analysis. Energy Build. 2001, 33, 219–227. [Google Scholar] [CrossRef]
- Farrance, I.; Frenkel, R. Uncertainty in measurement: A review of monte carlo simulation using microsoft excel for the calculation of uncertainties through functional relationships, including uncertainties in empirically derived constants. Clin. Biochem. Rev. 2014, 35, 37–61. [Google Scholar]
- Nikhitha, P.; Saibabu, K.B.S. Techno-Economic Analysis of Hydrazine Hydrate Technologies. Chem. Eng. Technol. 2010, 33, 1543–1551. [Google Scholar] [CrossRef]
- Leader, A.; Gaustad, G.; Babbitt, C. The effect of critical material prices on the competitiveness of clean energy technologies. Mater. Renew. Sustain. Energy 2019, 8, 8. [Google Scholar] [CrossRef] [Green Version]
- Johnstone, D.; Havyatt, D. Sophistry and high electricity prices in Australia. Crit. Perspect. Account. 2021, 102298. [Google Scholar] [CrossRef]
- Jamasb, T.; Pollitt, M. Electricity Market Reform in the European Union: Review of Progress toward Liberalization and Integration. Energy J. 2005, 26, 11–42. [Google Scholar] [CrossRef] [Green Version]
- da Silva, P.P.; Cerqueira, P.A. Assessing the determinants of household electricity prices in the EU: A system-GMM panel data approach. Renew. Sustain. Energy Rev. 2017, 73, 1131–1137. [Google Scholar] [CrossRef] [Green Version]
- Adom, P.K.; Minlah, M.K.; Adams, S. Impact of renewable energy (hydro) on electricity prices in Ghana: A tale of the short- and long-run. Energy Strat. Rev. 2018, 20, 163–178. [Google Scholar] [CrossRef]
- Wen, L.; Suomalainen, K.; Sharp, B.; Yi, M.; Sheng, M.S. Impact of wind-hydro dynamics on electricity price: A seasonal spatial econometric analysis. Energy 2022, 238, 122076. [Google Scholar] [CrossRef]
- Wong, J.B.; Zhang, Q. Impact of carbon tax on electricity prices and behaviour. Finance Res. Lett. 2022, 44, 102098. [Google Scholar] [CrossRef]
- Liu, T.; He, X.; Nakajima, T.; Hamori, S. Influence of Fluctuations in Fossil Fuel Commodities on Electricity Markets: Evidence from Spot and Futures Markets in Europe. Energies 2020, 13, 1900. [Google Scholar] [CrossRef] [Green Version]
- Pollitt, M.G. The European Single Market in Electricity: An Economic Assessment. Rev. Ind. Organ. 2019, 55, 63–87. [Google Scholar] [CrossRef] [Green Version]
- Khan, K.; Su, C.W.; Khurshid, A. Do booms and busts identify bubbles in energy prices? Resour. Policy 2022, 76, 102556. [Google Scholar] [CrossRef]
- Escribano, G. The geopolitics of renewable and electricity cooperation between Morocco and Spain. Mediterr. Politics 2019, 24, 674–681. [Google Scholar] [CrossRef]
- Hickey, S.M.; Malkawi, S.; Khalil, A. Nuclear power in the Middle East: Financing and geopolitics in the state nuclear power programs of Turkey, Egypt, Jordan and the United Arab Emirates. Energy Res. Soc. Sci. 2021, 74, 101961. [Google Scholar] [CrossRef]
- Du, R.; Wu, Q.; Dong, G.; Tian, L.; Vilela, A.L.M.; Zhao, L.; Zheng, X. Natural Gas Scarcity Risk for Countries along the Belt and Road. Energies 2022, 15, 1053. [Google Scholar] [CrossRef]
- Khan, F. Borders and pipelines. Nat. Energy 2022, 7, 213. [Google Scholar] [CrossRef]
- Gielen, D.; Boshell, F.; Saygin, D.; Bazilian, M.D.; Wagner, N.; Gorini, R. The role of renewable energy in the global energy transformation. Energy Strategy Rev. 2019, 24, 38–50. [Google Scholar] [CrossRef]
- Ahmadi, A.; Tiruta-Barna, L.; Benetto, E.; Capitanescu, F.; Marvuglia, A. On the importance of integrating alternative renewable energy resources and their life cycle networks in the eco-design of conventional drinking water plants. J. Clean. Prod. 2016, 135, 872–883. [Google Scholar] [CrossRef]
- Luderer, G.; Madeddu, S.; Merfort, L.; Ueckerdt, F.; Pehl, M.; Pietzcker, R.; Rottoli, M.; Schreyer, F.; Bauer, N.; Baumstark, L.; et al. Impact of declining renewable energy costs on electrification in low-emission scenarios. Nat. Energy 2022, 7, 32–42. [Google Scholar] [CrossRef]
- Jain, S.; Babu, S.; Sawle, Y. Prefeasibility Economic Scrutiny of the Off-grid Hybrid Renewable System for Remote Area Electrification. In Proceedings of the International Conference on Paradigms of Communication, Computing and Data Sciences, Kurukshetra, India, 7–9 May 2021; Springer: Singapore, 2022; pp. 73–84. [Google Scholar] [CrossRef]
- Topalović, Z.; Haas, R.; Ajanović, A.; Hiesl, A. Economics of electric energy storage. The case of Western Balkans. Energy 2022, 238, 121669. [Google Scholar] [CrossRef]
- Egli, F.; Steffen, B.; Schmidt, T.S. A dynamic analysis of financing conditions for renewable energy technologies. Nat. Energy 2018, 3, 1084–1092. [Google Scholar] [CrossRef]
- Child, M.; Kemfert, C.; Bogdanov, D.; Breyer, C. Flexible electricity generation, grid exchange and storage for the transition to a 100% renewable energy system in Europe. Renew. Energy 2019, 139, 80–101. [Google Scholar] [CrossRef]
- Tarpani, R.R.Z.; Azapagic, A. Life cycle costs of advanced treatment techniques for wastewater reuse and resource recovery from sewage sludge. J. Clean. Prod. 2018, 204, 832–847. [Google Scholar] [CrossRef] [Green Version]
- Al-Qodah, Z.; Shawabkah, R. Production and characterization of granular activated carbon from activated sludge. Braz. J. Chem. Eng. 2009, 26, 127–136. [Google Scholar] [CrossRef]
- Ciriminna, R.; Zhang, N.; Yang, M.-Q.; Meneguzzo, F.; Xu, Y.-J.; Pagliaro, M. Commercialization of graphene-based technologies: A critical insight. Chem. Commun. 2015, 51, 7090–7095. [Google Scholar] [CrossRef] [PubMed]
- Jose, J.; Philip, L. Continuous flow pulsed power plasma reactor for the treatment of aqueous solution containing volatile organic compounds and real pharmaceutical wastewater. J. Environ. Manag. 2021, 286, 112202. [Google Scholar] [CrossRef] [PubMed]
- Khalil, A.M.; (University of Exeter, Exeter, Devon, UK). Personal communication, 2022.
- Haldi, J.; Whitcomb, D. Economies of Scale in Industrial Plants. J. Political Econ. 1967, 75, 373–385. [Google Scholar] [CrossRef]
- Kawachale, N.; Kumar, A. Simulation, scale-up and economics of adsorption and membrane based processes for isoflavones recovery. Chem. Eng. Res. Des. 2011, 89, 428–435. [Google Scholar] [CrossRef] [Green Version]
- Nippatla, N.; Philip, L. Electrocoagulation-floatation assisted pulsed power plasma technology for the complete mineralization of potentially toxic dyes and real textile wastewater. Process Saf. Environ. Prot. 2019, 125, 143–156. [Google Scholar] [CrossRef]
- Singh, R.K.; Philip, L.; Ramanujam, S. Continuous flow pulse corona discharge reactor for the tertiary treatment of drinking water: Insights on disinfection and emerging contaminants removal. Chem. Eng. J. 2019, 355, 269–278. [Google Scholar] [CrossRef]
- Central Electrical Authority. All India Installed Generation Capacity, Ministry of Power, Government of India. Updated 16 Mar 2022. Available online: https://powermin.gov.in/en/content/power-sector-glance-all-india (accessed on 25 March 2022).
- Pryce, D.; Memon, F.A.; Kapelan, Z. Life cycle analysis approach to comparing environmental impacts of alternative materials used in the construction of small wastewater treatment plants. Environ. Adv. 2021, 4, 100065. [Google Scholar] [CrossRef]
- Xu, X.; Wei, Z.; Ji, Q.; Wang, C.; Gao, G. Global renewable energy development: Influencing factors, trend predictions and countermeasures. Resour. Policy 2019, 63, 101470. [Google Scholar] [CrossRef]
- Mikhaylov, A. Geothermal Energy Development in Iceland. Int. J. Energy Econ. Policy 2020, 10, 31–35. Available online: https://econpapers.repec.org/RePEc:eco:journ2:2020-04-5 (accessed on 13 April 2022). [CrossRef]
- Mayer, K.; Trück, S. Electricity markets around the world. J. Commod. Mark. 2018, 9, 77–100. [Google Scholar] [CrossRef]
- Ragain, L. Risk communication and media coverage of emerging contaminants. J. Am. Water Works Assoc. 2009, 101, 100–105. [Google Scholar] [CrossRef]
- Molinos-Senante, M.; Hernández-Sancho, F.; Sala-Garrido, R. Cost–benefit analysis of water-reuse projects for environmental purposes: A case study for Spanish wastewater treatment plants. J. Environ. Manag. 2011, 92, 3091–3097. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pryce, D.; Alsharrah, F.; Khalil, A.M.E.; Kapelan, Z.; Memon, F.A. Comparative Life-Cycle Cost Analysis of Alternative Technologies for the Removal of Emerging Contaminants from Urban Wastewater. Water 2022, 14, 1919. https://doi.org/10.3390/w14121919
Pryce D, Alsharrah F, Khalil AME, Kapelan Z, Memon FA. Comparative Life-Cycle Cost Analysis of Alternative Technologies for the Removal of Emerging Contaminants from Urban Wastewater. Water. 2022; 14(12):1919. https://doi.org/10.3390/w14121919
Chicago/Turabian StylePryce, David, Fatemah Alsharrah, Ahmed M. E. Khalil, Zoran Kapelan, and Fayyaz A. Memon. 2022. "Comparative Life-Cycle Cost Analysis of Alternative Technologies for the Removal of Emerging Contaminants from Urban Wastewater" Water 14, no. 12: 1919. https://doi.org/10.3390/w14121919
APA StylePryce, D., Alsharrah, F., Khalil, A. M. E., Kapelan, Z., & Memon, F. A. (2022). Comparative Life-Cycle Cost Analysis of Alternative Technologies for the Removal of Emerging Contaminants from Urban Wastewater. Water, 14(12), 1919. https://doi.org/10.3390/w14121919







