An Overview to Technical Solutions for Molybdenum Removal: Perspective from the Analysis of the Scientific Literature on Molybdenum and Drinking Water (1990–2019)
Abstract
:1. Introduction
2. Data Sources and Methodology
3. Results and discussion
3.1. Bibliometric Analysis of Research Trends on Molybdenum and Drinking Water (1990–2019)
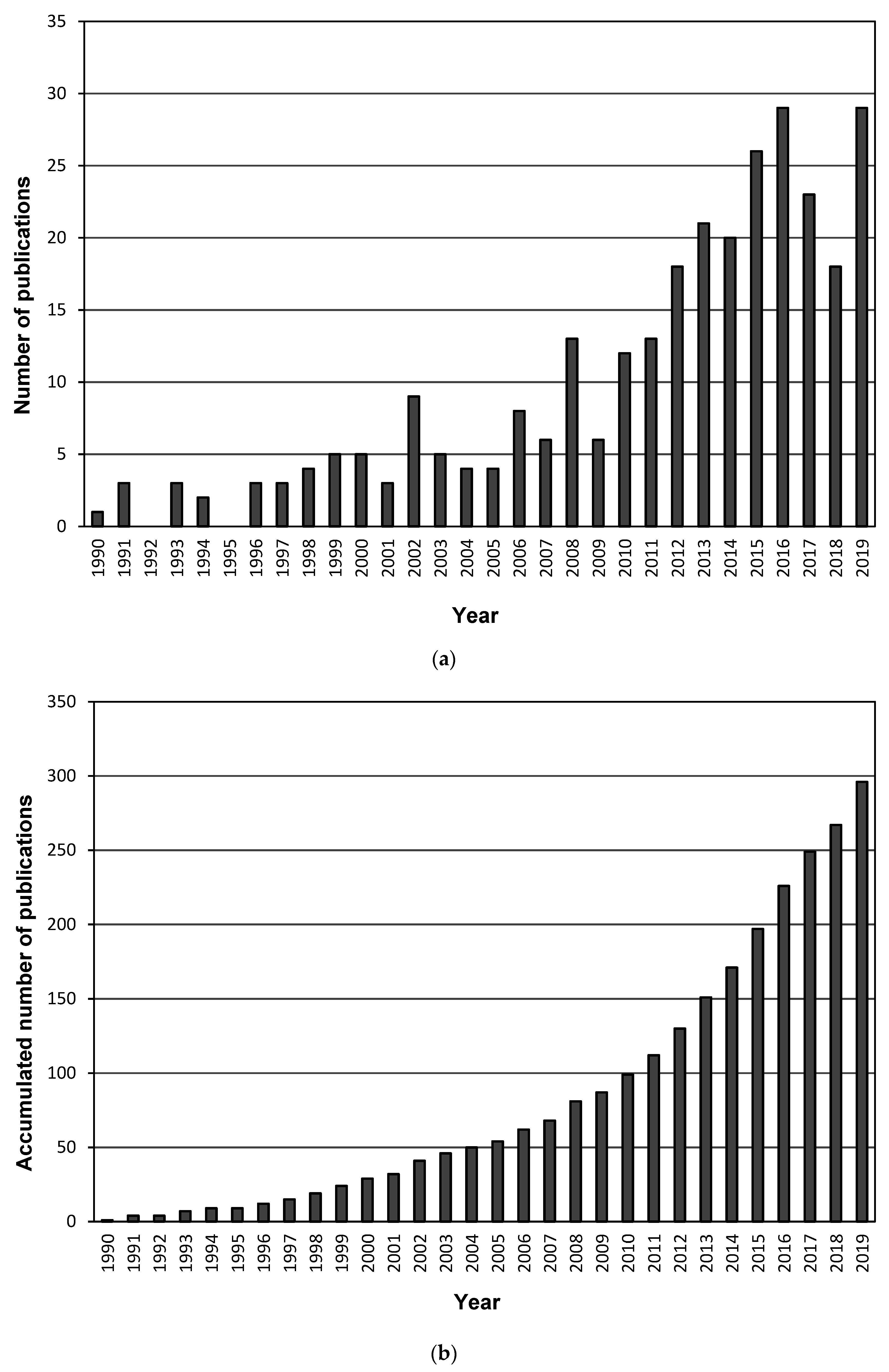
3.1.1. Publication Year, Document Type and Language of Publications
3.1.2. Publication Distribution of Countries and Institutions
3.1.3. Distribution of Output in Subject Categories and Journals
3.1.4. Most Frequently Cited Papers
3.1.5. Distribution Analysis of Author Keywords and Trending Topics of the Research
3.2. Review of Current Treatment Alternatives for Molybdenum Removal from Drinking Water
- Adsorption and ion exchange.
- Coagulation–flocculation–precipitation.
- Membrane technologies.
- Biological processes.
3.2.1. Adsorption and Ion Exchange
3.2.2. Coagulation–Flocculation–Precipitation
| Treated Water | Precipitate | pH | Initial [Mo] (µg/L) | Removal (%) | Reference |
|---|---|---|---|---|---|
| Synthetic solution | PbMoO4 | - | 4,800,000 | 43 | [114] |
| Synthetic solution | CoMoO6·0.9 H2O | 7 | 23,000,000 | >90 | [115] |
| Synthetic alkali leaching solution | (NH4)2Mo4O13 | 2.5 | 22,100,000 | 99 | [116] |
| Acidic leachate of mineral sludge | MoO3 | 2 | 10,160,000 | 50 | [118] |
| Acidic leachate of catalyst | MoS | 2 | 17,300,000 | 98 | [119] |
| Synthetic leachate of catalyst | Mo sulfides and oxides | 2/6 | 38,000 | 72/87 | [120] |
3.2.3. Membrane-Based Processes
3.2.4. Biological Treatments
| Treated Water | Technology | Species | Initial [Mo] (µg/L) | Removal (%) | Sorption Capacity (mg/g) | Reference |
|---|---|---|---|---|---|---|
| Urban wastewater | Membrane bioreactor | Activated sludge | 3.5 | 70 | - | [130] |
| Synthetic solution | Column bioreactor | Desulfovibrio desulfuricans | 10,000 | 99 | - | [131] |
| Spent catalyst pulp | Bioleaching | Cupriavidus metallidurans | 530,000 | 18 | - | [132] |
| Synthetic solution | Biosorbent | Petalonia fascia | 120,000 | - | 1376 | [133] |
| Synthetic solution | Biosorbent | Cystoseria indica | 95,000 | - | 30 | [134] |
| Synthetic solution | Biosorbent | Posidonia oceanica | 40,000 | - | 18 | [135] |
| Synthetic solution | Biosorbent | Spongomorpha pacifica | 3,200,000 | - | 1280 | [136] |
| Oil sands tailings pond water | Biosorption | Parachlorella kessleri | 50–125 | 2–27 | - | [137] |
| Tap water doped with Mo | Constructed wetland | Phragmites australis and Typha latifolia | 40,000 | 88 | - | [138] |
| Municipal wastewater | Constructed wetland | Phragmites australis | 1 | 59 | - | [140] |
4. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stiefel, E. Molybdenum compounds. In Kirk-Othmer Encyclopedia of Chemical Technology; Wiley: Hoboken, NJ, USA, 2011. [Google Scholar]
- Enghag, P. Encyclopedia of the Elements; Wiley-Blackwell: Hoboken, NJ, USA, 2004; ISBN 3527307176. [Google Scholar]
- Considine, G.D. Van Nostrand’s Encyclopedia of Chemistry; Wiley: Hoboken, NJ, USA, 2005. [Google Scholar]
- Kaiser, B.N.; Gridley, K.L.; Brady, J.N.; Phillips, T.; Tyerman, S.D. The role of molybdenum in agricultural plant production. Ann. Bot. 2005, 96, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Gupta, U.C.; Srivastava, P.C.; Gupta, S.C. Role of micronutrients: Boron and molybdenum in crops and in human health and nutrition. Curr. Nutr. Food Sci. 2011, 7, 126–136. [Google Scholar] [CrossRef]
- Chan, S.; Gerson, B.; Subramanian, S. The role of copper, molybdenum, selenium, and zinc in nutrition and health. Clin. Lab. Med. 1998, 18, 673–685. [Google Scholar] [CrossRef]
- Chappell, W.R.; Meglen, R.R.; Moure-Eraso, R.; Solomons, C.C.; Tsongas, T.A.; Walravens, P.A.; Winston, P.W. Human Health Effects of Molybdenum in Drinking Water; University of Colorado Boulder: Boulder, CO, USA, 1979. [Google Scholar]
- Albin, M.; Oskarsson, A. Molybdenum. In Handbook on the Toxicology of Metals; Elsevier: Amsterdam, The Netherlands, 2021; pp. 601–614. [Google Scholar]
- Ott, H.C.; Prior, C.; Herold, M.; Riha, M.; Laufer, G.; Ott, G. Respiratory symptoms and bronchoalveolar lavage abnormalities in molybdenum exposed workers. Wien. Klin. Wochenschr. 2004, S116, 25–30. [Google Scholar]
- Meeker, J.D.; Rossano, M.G.; Protas, B.; Diamond, M.P.; Puscheck, E.; Daly, D.; Paneth, N.; Wirth, J.J. Cadmium, lead, and other metals in relation to semen quality: Human evidence for molybdenum as a male reproductive toxicant. Environ. Health Perspect. 2008, 116, 1473–1479. [Google Scholar] [CrossRef] [Green Version]
- Meeker, J.D.; Rossano, M.G.; Protas, B.; Padmanahban, V.; Diamond, M.P.; Puscheck, E.; Daly, D.; Paneth, N.; Wirth, J.J. Environmental exposure to metals and male reproductive hormones: Circulating testosterone is inversely associated with blood molybdenum. Fertil. Steril. 2010, 93, 130–140. [Google Scholar] [CrossRef] [Green Version]
- National Academy of Sciences. Recommended Dietary Allowances, 10th ed.; National Research Council-National Academy Press: Washington, DC, USA, 1989; ISBN 0-309-04633-5. [Google Scholar]
- U.S. Environmental Protection Agency. 2018 Edition of the Drinking Water Standards and Health Advisories Tables; U.S. Environmental Protection Agency: Washington, DC, USA, 2018.
- WHO. Guidelines for Drinking-Water Quality; Organization of the United Nations: Geneva, Switzerland, 2011. [Google Scholar]
- European Union. 1829/2003 del Parlamento Europeo y del Consejo de 22 de Septiembre de 2003 Reglamento (CE) n; European Union: Brussels, Belgium, 1998. [Google Scholar]
- Pichler, T.; Koopmann, S. Should Monitoring of Molybdenum (Mo) in Groundwater, Drinking Water and Well Permitting Made Mandatory? Environ. Sci. Technol. 2020, 54, 1–2. [Google Scholar] [CrossRef] [Green Version]
- Smedley, P.L.; Cooper, D.M.; Ander, E.L.; Milne, C.J.; Lapworth, D.J. Occurrence of molybdenum in British surface water and groundwater: Distributions, controls and implications for water supply. Appl. Geochem. 2014, 40, 144–154. [Google Scholar] [CrossRef]
- Smedley, P.L.; Cooper, D.M.; Lapworth, D.J. Molybdenum distributions and variability in drinking water from England and Wales. Environ. Monit. Assess. 2014, 186, 6403–6416. [Google Scholar] [CrossRef] [Green Version]
- Jarrell, W.M.; Page, A.L.; Elseewi, A.A. Molybdenum in the environment. Residue Rev. 1980, 74, 1–43. [Google Scholar] [CrossRef]
- Hays, S.M.; Macey, K.; Poddalgoda, D.; Lu, M.; Nong, A.; Aylward, L.L. Biomonitoring Equivalents for molybdenum. Regul. Toxicol. Pharmacol. 2016, 77, 223–229. [Google Scholar] [CrossRef]
- Pichler, T.; Mozaffari, A. Distribution and mobility of geogenic molybdenum and arsenic in a limestone aquifer matrix. Appl. Geochem. 2015, 63, 623–633. [Google Scholar] [CrossRef]
- Harkness, J.S.; Darrah, T.H.; Moore, M.T.; Whyte, C.J.; Mathewson, P.D.; Cook, T.; Vengosh, A. Naturally Occurring versus Anthropogenic Sources of Elevated Molybdenum in Groundwater: Evidence for Geogenic Contamination from Southeast Wisconsin, United States. Environ. Sci. Technol. 2017, 51, 12190–12199. [Google Scholar] [CrossRef] [PubMed]
- Smedley, P.L.; Nicolli, H.B. Molybdenum distributions and controls in groundwater from the Pampean Aquifer of La Pampa Province, Argentina. In Molybdenum and Its Compounds: Applications, Electrochemical Properties and Geological Implications; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2014; pp. 399–416. [Google Scholar]
- Leybourne, M.I.; Cameron, E.M. Source, transport, and fate of rhenium, selenium, molybdenum, arsenic, and copper in groundwater associated with porphyry-Cu deposits, Atacama Desert, Chile. Chem. Geol. 2008, 247, 208–228. [Google Scholar] [CrossRef]
- Johannesson, K.H.; Lyons, W.B.; Graham, E.Y.; Welch, K.A. Oxyanion concentrations in Eastern Sierra Nevada Rivers—3. Boron, Molybdenum, Vanadium, and Tungsten. Aquat. Geochem. 2000, 6, 19–46. [Google Scholar] [CrossRef]
- Song, Z.; Song, G.; Tang, W.; Zhao, Y.; Yan, D.; Zhang, W. Spatial and temporal distribution of Mo in the overlying water of a reservoir downstream from mining area. J. Environ. Sci. 2021, 102, 256–262. [Google Scholar] [CrossRef]
- Rango, T.; Vengosh, A.; Dwyer, G.; Bianchini, G. Mobilization of arsenic and other naturally occurring contaminants in groundwater of the main ethiopian rift aquifers. Water Res. 2013, 47, 5801–5818. [Google Scholar] [CrossRef]
- Hiasat, T.H.; Rimawi, O.A.; Makhlouf, I.M. Hydrochemical Evaluation of Molybdenum Content of the Groundwater Aquifer System in Northern Jordan. J. Water Resour. Prot. 2020, 12, 223–239. [Google Scholar] [CrossRef] [Green Version]
- Smedley, P.L.; Kinniburgh, D.G. Molybdenum in natural waters: A review of occurrence, distributions and controls. Appl. Geochem. 2017, 84, 387–432. [Google Scholar] [CrossRef] [Green Version]
- Al Kuisi, M.; Al-Hwaiti, M.; Mashal, K.; Abed, A.M. Spatial distribution patterns of molybdenum (Mo) concentrations in potable groundwater in Northern Jordan. Environ. Monit. Assess. 2015, 187, 148. [Google Scholar] [CrossRef]
- Archana; Jaitly, A.K. Analysis of trace metals in underground drinking water of Bareilly. Invertis J. Renew. Energy 2016, 6, 106–111. [Google Scholar] [CrossRef]
- Sebenik, R.F.; Burkin, A.R.; Dorfler, R.R.; Laferty, J.M.; Leichtfried, G.; Meyer-Grunow, H.; Mitchell, P.C.H.; Vukasovich, M.S.; Church, D.A.; van Riper, G.G.; et al. Molybdenum and Molybdenum Compounds. Ullmann’s Encycl. Ind. Chem. 2000. [Google Scholar] [CrossRef]
- Xu, N.; Braida, W.; Christodoulatos, C.; Chen, J. A Review of Molybdenum Adsorption in Soils/Bed Sediments: Speciation, Mechanism, and Model Applications. Soil Sediment Contam. 2013, 22, 912–929. [Google Scholar] [CrossRef]
- Mendel, R.R. Molybdenum: Biological activity and metabolism. Dalt. Trans. 2005, 21, 3404–3409. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Cheng, J.; Sprik, M.; Lu, X. Solution structures and acidity constants of molybdic acid. J. Phys. Chem. Lett. 2013, 4, 2926–2930. [Google Scholar] [CrossRef]
- Wang, D. Redox chemistry of molybdenum in natural waters and its involvement in biological evolution. Front. Microbiol. 2012, 3, 427. [Google Scholar] [CrossRef] [Green Version]
- Pritchard, A. Statistical bibliography or Bibliometrics? J. Doc. 1969, 25, 348–349. [Google Scholar]
- Abejón, R. A Bibliometric Study of Scientific Publications regarding Hemicellulose Valorization during the 2000–2016 Period: Identification of Alternatives and Hot Topics. ChemEngineering 2018, 2, 7. [Google Scholar] [CrossRef] [Green Version]
- van Raan, A.F.J. For Your Citations Only? Hot Topics in Bibliometric Analysis. Interdiscip. Res. Perspect. 2005, 3, 50–62. [Google Scholar] [CrossRef]
- Glänzel, W. Bibliometrics-aided retrieval: Where information retrieval meets scientometrics. Scientometrics 2015, 102, 2215–2222. [Google Scholar] [CrossRef]
- Abejón, R.; Pérez-Acebo, H.; Garea, A. A Bibliometric Analysis of Research on Supported Ionic Liquid Membranes during the 1995–2015 Period: Study of the Main Applications and Trending Topics. Membranes 2017, 7, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, N.; Han, R.; Lu, X. Bibliometric analysis of research trends on solid waste reuse and recycling during 1992–2016. Resour. Conserv. Recycl. 2018, 130, 109–117. [Google Scholar] [CrossRef]
- Pennells, J.; Godwin, I.D.; Amiralian, N.; Martin, D.J. Trends in the production of cellulose nanofibers from non-wood sources. Cellulose 2020, 27, 575–593. [Google Scholar] [CrossRef]
- Patience, G.S.; Patience, C.A.; Bertrand, F. Chemical engineering research synergies across scientific categories. Can. J. Chem. Eng. 2018, 96, 1684–1690. [Google Scholar] [CrossRef]
- Mesdaghinia, A.; Mahvi, A.H.; Nasseri, S.; Nodehi, R.N.; Hadi, M. A bibliometric analysis on the solid waste-related research from 1982 to 2013 in Iran. Int. J. Recycl. Org. Waste Agric. 2015, 4, 185–195. [Google Scholar] [CrossRef] [Green Version]
- Blettler, M.C.M.; Abrial, E.; Khan, F.R.; Sivri, N.; Espinola, L.A. Freshwater plastic pollution: Recognizing research biases and identifying knowledge gaps. Water Res. 2018, 143, 416–424. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Chen, X.; Xie, L.; Liu, Z.; Xiong, X. Bioelectrochemical systems for groundwater remediation: The development trend and research front revealed by bibliometric analysis. Water 2019, 11, 1532. [Google Scholar] [CrossRef] [Green Version]
- Abejón, R.; Garea, A. A bibliometric analysis of research on arsenic in drinking water during the 1992-2012 period: An outlook to treatment alternatives for arsenic removal. J. Water Process Eng. 2015, 6, 105–119. [Google Scholar] [CrossRef]
- Hu, J.; Ma, Y.; Zhang, L.; Gan, F.; Ho, Y.S. A historical review and bibliometric analysis of research on lead in drinking water field from 1991 to 2007. Sci. Total Environ. 2010, 408, 1738–1744. [Google Scholar] [CrossRef]
- Colares, G.S.; Dell’Osbel, N.; Wiesel, P.G.; Oliveira, G.A.; Lemos, P.H.Z.; da Silva, F.P.; Lutterbeck, C.A.; Kist, L.T.; Machado, Ê.L. Floating treatment wetlands: A review and bibliometric analysis. Sci. Total Environ. 2020, 714, 136776. [Google Scholar] [CrossRef]
- Scopus Content Coverage Guide (Version 08.17). 2017. Available online: http://library.mephi.ru/files/research_support/scopus_content_coverage_guide_august_2017.pdf (accessed on 25 June 2022).
- Yi, H.; Jie, W. A bibliometric study of the trend in articles related to eutrophication published in Science Citation Index. Scientometrics 2011, 89, 919–927. [Google Scholar] [CrossRef]
- Abejón, R.; Moya, L. Cross-laminated timber: Perspectives from a bibliometric analysis (2006–2018). Wood Mater. Sci. Eng. 2021. [Google Scholar] [CrossRef]
- Hamel, R.E. The dominance of English in the international scientific periodical literature and the future of language use in science. AILA Rev. 2007, 20, 53–71. [Google Scholar] [CrossRef]
- Abejón, R. A Bibliometric Analysis of Research on Selenium in Drinking Water during the 1990–2021 Period: Treatment Options for Selenium Removal. Int. J. Environ. Res. Public Health 2022, 19, 5834. [Google Scholar] [CrossRef]
- Demirel, Z.; Özer, O.; Özpinar, Z. Investigation of groundwater pollution in a protected area in turkey, The Göksu Delta. Gazi Univ. J. Sci. 2011, 24, 17–27. [Google Scholar]
- Arslan, H.; Ayyildiz Turan, N. Estimation of spatial distribution of heavy metals in groundwater using interpolation methods and multivariate statistical techniques; its suitability for drinking and irrigation purposes in the Middle Black Sea Region of Turkey. Environ. Monit. Assess. 2015, 187, 516. [Google Scholar] [CrossRef]
- Al-Harbi, N.A. Physico-chemical properties of well waters in Al-Yanfa village, Asir region, Saudi Arabia. J. Food Agric. Environ. 2010, 8, 965–967. [Google Scholar]
- Al-Saleh, I.A. Trace elements in drinking water coolers collected from primary schools, Riyadh, Saudi Arabia. Sci. Total Environ. 1996, 181, 215–221. [Google Scholar] [CrossRef]
- Leybourne, M.I.; Cameron, E.M. Composition of groundwaters associated with porphyry-Cu deposits, Atacama Desert, Chile: Elemental and isotopic constraints on water sources and water-rock reactions. Geochim. Cosmochim. Acta 2006, 70, 1616–1635. [Google Scholar] [CrossRef]
- Tapia, J.; Davenport, J.; Townley, B.; Dorador, C.; Schneider, B.; Tolorza, V.; von Tümpling, W. Sources, enrichment, and redistribution of As, Cd, Cu, Li, Mo, and Sb in the Northern Atacama Region, Chile: Implications for arid watersheds affected by mining. J. Geochem. Explor. 2018, 185, 33–51. [Google Scholar] [CrossRef]
- Liu, C.; Kong, D.; Hsu, P.-C.; Yuan, H.; Lee, H.-W.; Liu, Y.; Wang, H.; Wang, S.; Yan, K.; Lin, D.; et al. Rapid water disinfection using vertically aligned MoS2 nanofilms and visible light. Nat. Nanotechnol. 2016, 11, 1098–1104. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yin, Y.; Liu, E.; Ma, Y.; Wan, J.; Fan, J.; Hu, X. In situ growing Bi2MoO6 on g-C3N4 nanosheets with enhanced photocatalytic hydrogen evolution and disinfection of bacteria under visible light irradiation. J. Hazard. Mater. 2017, 321, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Ai, K.; Ruan, C.; Shen, M.; Lu, L. MoS2 Nanosheets with Widened Interlayer Spacing for High-Efficiency Removal of Mercury in Aquatic Systems. Adv. Funct. Mater. 2016, 26, 5542–5549. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, W.; Yue, X.; Yang, Q.; Liu, F.; Wang, Y.; Zhang, D.; Li, Z.; Wang, J. One-pot synthesis of multifunctional magnetic ferrite-MoS2-carbon dot nanohybrid adsorbent for efficient Pb(II) removal. J. Mater. Chem. A 2016, 4, 3893–3900. [Google Scholar] [CrossRef]
- Dambies, L.; Guibal, E.; Roze, A. Arsenic(V) sorption on molybdate-impregnated chitosan beads. Colloids Surfaces A Physicochem. Eng. Asp. 2000, 170, 19–31. [Google Scholar] [CrossRef]
- Dambies, L.; Vincent, T.; Guibal, E. Treatment of arsenic-containing solutions using chitosan derivatives: Uptake mechanism and sorption performances. Water Res. 2002, 36, 3699–3710. [Google Scholar] [CrossRef]
- Vyskočil, A.; Viau, C. Assessment of molybdenum toxicity in humans. J. Appl. Toxicol. 1999, 19, 185–192. [Google Scholar] [CrossRef]
- Praharaj, T.; Powell, M.A.; Hart, B.R.; Tripathy, S. Leachability of elements from sub-bituminous coal fly ash from India. Environ. Int. 2002, 27, 609–615. [Google Scholar] [CrossRef]
- Bundschuh, J.; Litter, M.I.; Parvez, F.; Román-Ross, G.; Nicolli, H.B.; Jean, J.S.; Liu, C.W.; López, D.; Armienta, M.A.; Guilherme, L.R.G.; et al. One century of arsenic exposure in Latin America: A review of history and occurrence from 14 countries. Sci. Total Environ. 2012, 429, 2–35. [Google Scholar] [CrossRef]
- Zhang, M.; Reardon, E.J. Removal of B, Cr, Mo, and Se from wastewater by incorporation into hydrocalumite and ettringite. Environ. Sci. Technol. 2003, 37, 2947–2952. [Google Scholar] [CrossRef]
- Fischler, C.; Ldle, M. A Review of Selected Florida Aquifer Storage and Recovery (ASR) Sites and Their Geochemical Characteristics Florida Geological Survey Report of Investigation No. 112; Florida Geological Survey: Tallahassee, FL, USA, 2015.
- Ćurković, M.; Sipos, L.; Puntarić, D.; Dodig-Ćurković, K.; Pivac, N.; Kralik, K. Arsenic, copper, molybdenum, and selenium exposure through drinking water in rural eastern Croatia. Pol. J. Environ. Stud. 2016, 25, 981–992. [Google Scholar] [CrossRef]
- Pichler, T.; Renshaw, C.E.; Sültenfuß, J. Geogenic As and Mo groundwater contamination caused by an abundance of domestic supply wells. Appl. Geochem. 2017, 77, 68–79. [Google Scholar] [CrossRef]
- Su, C.; Puls, R.W. Arsenate and Arsenite Removal by Zerovalent Iron: Effects of Phosphate, Silicate, Carbonate, Borate, Sulfate, Chromate, Molybdate, and Nitrate, Relative to Chloride. Environ. Sci. Technol. 2001, 35, 4562–4568. [Google Scholar] [CrossRef] [PubMed]
- Lazaridis, N.K.; Jekel, M.; Zouboulis, A.I. Removal of Cr(VI), Mo(VI), and V(V) ions from single metal aqueous solutions by sorption or nanofiltration. Sep. Sci. Technol. 2003, 38, 2201–2219. [Google Scholar] [CrossRef]
- Bostick, B.C.; Fendorf, S.; Helz, G.R. Differential adsorption of molybdate and tetrathiomolybdate on pyrite (FeS2). Environ. Sci. Technol. 2003, 37, 285–291. [Google Scholar] [CrossRef]
- Das, S.; Jim Hendry, M. Adsorption of molybdate by synthetic hematite under alkaline conditions: Effects of aging. Appl. Geochem. 2013, 28, 194–201. [Google Scholar] [CrossRef]
- Xu, N.; Christodoulatos, C.; Braida, W. Adsorption of molybdate and tetrathiomolybdate onto pyrite and goethite: Effect of pH and competitive anions. Chemosphere 2006, 62, 1726–1735. [Google Scholar] [CrossRef]
- Li, J.; Chen, D.; Liao, X.; Pan, B. Selective adsorption of molybdate from water by polystyrene anion exchanger-supporting nanocomposite of hydrous ferric oxides. Sci. Total Environ. 2019, 691, 64–70. [Google Scholar] [CrossRef]
- Xu, N.; Christodoulatos, C.; Braida, W. Modeling the competitive effect of phosphate, sulfate, silicate, and tungstate anions on the adsorption of molybdate onto goethite. Chemosphere 2006, 64, 1325–1333. [Google Scholar] [CrossRef]
- Lang, F.; Kaupenjohann, M. Immobilisation of molybdate by iron oxides: Effects of organic coatings. Geoderma 2003, 113, 31–46. [Google Scholar] [CrossRef]
- Wu, C.H.; Lo, S.L.; Lin, C.F. Competitive adsorption of molybdate, chromate, sulfate, selenate, and selenite on γ-Al2O3. Colloids Surfaces A Physicochem. Eng. Asp. 2000, 166, 251–259. [Google Scholar] [CrossRef]
- Wu, C.H.; Lo, S.L.; Lin, C.F.; Kuo, C.Y. Modeling competitive adsorption of molybdate, sulfate, and selenate on γ-Al2O3 by the triple-layer model. J. Colloid Interface Sci. 2001, 233, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Bourikas, K.; Hiemstra, T.; Van Riemsdijk, W.H. Adsorption of molybdate monomers and polymers on Titania with a multisite approach. J. Phys. Chem. B 2001, 105, 2393–2403. [Google Scholar] [CrossRef]
- Vissenberg, M.J.; Joosten, L.J.M.; Heffels, M.M.E.H.; Van Welsenes, A.J.; De Beer, V.H.J.; Van Santen, R.A.; Rob Van Veen, J.A. Tungstate versus molybdate adsorption on oxidic surfaces: A chemical approach. J. Phys. Chem. B 2000, 104, 8456–8461. [Google Scholar] [CrossRef]
- Namasivayam, C.; Prathap, K. Uptake of molybdate by adsorption onto industrial solid waste FE (III)/CR(III) hydroxide: Kinetic and equilibrium studies. Environ. Technol. 2006, 27, 923–932. [Google Scholar] [CrossRef] [PubMed]
- Ardau, C.; Frau, F.; Dore, E.; Lattanzi, P. Molybdate sorption by Zn-Al sulphate layered double hydroxides. Appl. Clay Sci. 2012, 65–66, 128–133. [Google Scholar] [CrossRef]
- Paikaray, S.; Hendry, M.J.; Essilfie-Dughan, J. Controls on arsenate, molybdate, and selenate uptake by hydrotalcite-like layered double hydroxides. Chem. Geol. 2013, 345, 130–138. [Google Scholar] [CrossRef]
- Peinemann, N.; Helmy, A.K. Molybdate sorption by hydroxy-aluminium treated montmorillonite. Appl. Clay Sci. 1994, 8, 389–396. [Google Scholar] [CrossRef]
- Faghihian, H.; Malekpour, A.; Maragheh, M.G. Adsorption of molybdate ion by natrolite and clinoptilolite-rich tuffs. Int. J. Environ. Pollut. 2002, 18, 181–189. [Google Scholar] [CrossRef]
- Bonifacio-Martínez, J.; Serrano-Gómez, J.; Del Carmen López-Reyes, M.; Granados-Correa, F. Mechano-chemical effects on surface properties and molybdate exchange on hydrotalcite. Clay Miner. 2009, 44, 311–317. [Google Scholar] [CrossRef]
- Namasivayam, C.; Sangeetha, D. Removal of molybdate from water by adsorption onto ZnCl2 activated coir pith carbon. Bioresour. Technol. 2006, 97, 1194–1200. [Google Scholar] [CrossRef] [PubMed]
- Bertoni, F.A.; González, J.C.; García, S.I.; Sala, L.F.; Bellú, S.E. Application of chitosan in removal of molybdate ions from contaminated water and groundwater. Carbohydr. Polym. 2018, 180, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Milot, C.; Mcbrien, J.; Allen, S.; Guibal, E. Influence of physicochemical and structural characteristics of chitosan flakes on molybdate sorption. J. Appl. Polym. Sci. 1998, 68, 571–580. [Google Scholar] [CrossRef]
- Guibal, E.; Milot, C.; Roussy, J. Influence of hydrolysis mechanisms on molybdate sorption isotherms using chitosan. Sep. Sci. Technol. 2000, 35, 1021–1038. [Google Scholar] [CrossRef]
- Guibal, E.; Milot, C.; Eterradossi, O.; Gauffier, C.; Domard, A. Study of molybdate ion sorption on chitosan gel beads by different spectrometric analyses. Int. J. Biol. Macromol. 1999, 24, 49–59. [Google Scholar] [CrossRef]
- Zhang, L.; Xue, J.; Zhou, X.; Fei, X.; Wang, Y.; Zhou, Y.; Zhong, L.; Han, X. Adsorption of molybdate on molybdate-imprinted chitosan/triethanolamine gel beads. Carbohydr. Polym. 2014, 114, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Xiao, Q.; Gao, Y.; Ning, P.; Xu, H.; Zhang, Y. Direct extraction of Mo(VI) from acidic leach solution of molybdenite ore by ion exchange resin: Batch and column adsorption studies. Trans. Nonferrous Met. Soc. China Engl. Ed. 2018, 28, 1660–1669. [Google Scholar] [CrossRef]
- Huo, G.S.; Song, Q.; Liao, C.H. Selection of ion exchange resins for tungsten and molybdenum separation. Adv. Mater. Res. 2014, 997, 363–367. [Google Scholar] [CrossRef]
- Joo, S.H.; Kim, Y.U.; Kang, J.G.; Kumar, J.R.; Yoon, H.S.; Parhi, P.K.; Shin, S.M. Recovery of rhenium and molybdenum from molybdenite roasting dust leaching solution by ion exchange resins. Mater. Trans. 2012, 53, 2034–2037. [Google Scholar] [CrossRef] [Green Version]
- Kůs, P.; Parschová, H.; Novotná, M.; Mištová, E.; Jelínek, L. Molybdate Sorption onto Ion Exchange Resin with Multiple Hydroxyl Groups. Sep. Sci. Technol. 2013, 48, 581–586. [Google Scholar] [CrossRef]
- Lee, M.; Sohn, S.; Lee, M. Ionic equilibria and ion exchange of molybdenum(VI) from strong acid solution. Bull. Korean Chem. Soc. 2011, 32, 3687–3691. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.H.; Sohn, S.H.; Lee, M.S. Removal of Mo and Fe from the cobalt chloride solution by ion exchange during the recovery process from spent hydrodesulfurization catalysts. Ind. Eng. Chem. Res. 2013, 52, 10028–10032. [Google Scholar] [CrossRef]
- Orrego, P.; Hernández, J.; Reyes, A. Uranium and molybdenum recovery from copper leaching solutions using ion exchange. Hydrometallurgy 2019, 184, 116–122. [Google Scholar] [CrossRef]
- Xiao, L.S.; Zhang, Q.X.; Gong, B.F.; Huang, S.Y. Separation of molybdenum from tungstate solution by a combination of moving packed bed and fluid bed ion exchange technique. Kuangye Gongcheng/Min. Metall. Eng. 2001, 21, 66–68+71. [Google Scholar]
- Kotov, S.V.; Boneva, S.; Kolev, T. Some molybdenum-containing chelating ion-exchange resins (polyampholites) as catalysts for the epoxidation of alkenes by organic hydroperoxides. J. Mol. Catal. A Chem. 2000, 154, 121–129. [Google Scholar] [CrossRef]
- Guo, W.; Shen, Y.H. Recovery of Molybdenum and Vanadium from Acidic Sulfate Leach Solution of Blue Sludge by Ion Exchange. Environ. Prog. Sustain. Energy 2016, 35, 156–160. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, J.; Lu, X.; Huangfu, X.; Zou, J. High efficient removal of molybdenum from water by Fe2(SO4)3: Effects of pH and affecting factors in the presence of co-existing background constituents. J. Hazard. Mater. 2015, 300, 823–829. [Google Scholar] [CrossRef]
- Ma, W.; Sha, X.; Gao, L.; Cheng, Z.; Meng, F.; Cai, J.; Tan, D.; Wang, R. Effect of iron oxide nanocluster on enhanced removal of molybdate from surface water and pilot scale test. Colloids Surfaces A Physicochem. Eng. Asp. 2015, 478, 45–53. [Google Scholar] [CrossRef]
- Zhao, Y.H.; Jin, Z.Y.; Yan, X.; Wang, X.; Wang, Z.P. Research on molybdenum removal from water polluted for drinking. Appl. Mech. Mater. 2013, 361–363, 670–673. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, X.; Liu, F.; Yang, X.; Wang, F. Study on Coagulation Settlement Test of Mineral Processing Wastewater from a Tungsten-Molybdenum Mine. IOP Conf. Ser. Earth Environ. Sci. 2019, 384, 012021. [Google Scholar] [CrossRef]
- Shalchian, H.; Ferella, F.; Birloaga, I.; De Michelis, I.; Vegliò, F. Recovery of molybdenum from leach solution using polyelectrolyte extraction. Hydrometallurgy 2019, 190, 105167. [Google Scholar] [CrossRef]
- Cao, C.; Zhao, Z.; Chen, X. Selective precipitation of tungstate from molybdate-containing solution using divalent ions. Hydrometallurgy 2011, 110, 115–119. [Google Scholar] [CrossRef]
- Huang, J.H.; Kargl-Simard, C.; Oliazadeh, M.; Alfantazi, A.M. pH-controlled precipitation of cobalt and molybdenum from industrial waste effluents of a cobalt electrodeposition process. Hydrometallurgy 2004, 75, 77–90. [Google Scholar] [CrossRef]
- Joo, S.H.; Kim, Y.U.; Kang, J.G.; Yoon, H.S.; Kim, D.S.; Shin, S.M. Recovery of molybdenum and rhenium using selective precipitation method from molybdenite roasting dust in alkali leaching solution. Mater. Trans. 2012, 53, 2038–2042. [Google Scholar] [CrossRef] [Green Version]
- Niinae, M.; Suzuki, T.; Fuji, A.; Matsunaga, N.; Shibata, J. A Study on Precipitation Recovery of Molybdenum from Aqueous Solutions of Ammonium Chloride. Resour. Process. 2013, 60, 151–156. [Google Scholar] [CrossRef] [Green Version]
- Vemic, M.; Bordas, F.; Comte, S.; Guibaud, G.; Lens, P.N.L.; van Hullebusch, E.D. Recovery of molybdenum, nickel and cobalt by precipitation from the acidic leachate of a mineral sludge. Environ. Technol. 2016, 37, 2231–2242. [Google Scholar] [CrossRef]
- Hamza, M.F.; Roux, J.C.; Guibal, E. Metal valorization from the waste produced in the manufacturing of Co/Mo catalysts: Leaching and selective precipitation. J. Mater. Cycles Waste Manag. 2019, 21, 525–538. [Google Scholar] [CrossRef]
- Cibati, A.; Cheng, K.Y.; Morris, C.; Ginige, M.P.; Sahinkaya, E.; Pagnanelli, F.; Kaksonen, A.H. Selective precipitation of metals from synthetic spent refinery catalyst leach liquor with biogenic H2S produced in a lactate-fed anaerobic baffled reactor. Hydrometallurgy 2013, 139, 154–161. [Google Scholar] [CrossRef]
- Zeng, L.; Zhao, Z.; Huo, G.; Wang, X.; Pu, H. Mechanism of Selective Precipitation of Molybdenum from Tungstate Solution. JOM 2020, 72, 800–805. [Google Scholar] [CrossRef]
- Yobilishetty, S.M.; Marathe, K.V. Removal of molybdenum (VI) from effluent waste water streams by cross flow micellar enhanced ultrafiltration (MEUF) using anionic, non-ionic and mixed surfactants. Indian J. Chem. Technol. 2014, 21, 321–327. [Google Scholar]
- Sadaoui, Z.; Azoug, C.; Charbit, G.; Charbit, F. Surfactants for Separation Processes: Enhanced Ultrafiltration. J. Environ. Eng. 1998, 124, 695–700. [Google Scholar] [CrossRef]
- Sánchez, J.; Riffo, L.; Salazar, P.; Rivas, B.L. Removal of molybdate and vanadate ions by a copolymer adsorbent in a ultrafiltration system. J. Appl. Polym. Sci. 2019, 136, 48184. [Google Scholar] [CrossRef]
- Chellam, S.; Clifford, D.A. Physical-chemical treatment of groundwater contaminated by leachate from surface disposal of uranium tailings. J. Environ. Eng. 2002, 128, 942–952. [Google Scholar] [CrossRef]
- Alzahrani, S.; Mohammad, A.W.; Hilal, N.; Abdullah, P.; Jaafar, O. Comparative study of NF and RO membranes in the treatment of produced water-Part I: Assessing water quality. Desalination 2013, 315, 18–26. [Google Scholar] [CrossRef]
- Meschke, K.; Hansen, N.; Hofmann, R.; Haseneder, R.; Repke, J.U. Characterization and performance evaluation of polymeric nanofiltration membranes for the separation of strategic elements from aqueous solutions. J. Memb. Sci. 2018, 546, 246–257. [Google Scholar] [CrossRef]
- Richards, L.A.; Richards, B.S.; Schäfer, A.I. Renewable energy powered membrane technology: Salt and inorganic contaminant removal by nanofiltration/reverse osmosis. J. Memb. Sci. 2011, 369, 188–195. [Google Scholar] [CrossRef]
- Choubert, J.M.; Pomiès, M.; Martin Ruel, S.; Coquery, M. Influent concentrations and removal performances of metals through municipal wastewater treatment processes. Water Sci. Technol. 2011, 63, 1967–1973. [Google Scholar] [CrossRef]
- Arévalo, J.; Ruiz, L.M.; Pérez, J.; Moreno, B.; Gómez, M.Á. Removal performance of heavy metals in MBR systems and their influence in water reuse. Water Sci. Technol. 2013, 67, 894–900. [Google Scholar] [CrossRef]
- Tucker, M.D.; Barton, L.L.; Thomson, B.M. Removal of U and Mo from water by immobilized Desulfovibrio desulfuricans in column reactors. Biotechnol. Bioeng. 1998, 60, 88–96. [Google Scholar] [CrossRef]
- Rivas-Castillo, A.M.; Monges-Rojas, T.L.; Rojas-Avelizapa, N.G. Specificity of Mo and V Removal from a Spent Catalyst by Cupriavidus metallidurans CH34. Waste Biomass Valorization 2019, 10, 1037–1042. [Google Scholar] [CrossRef]
- Carnevale, B.; Blanes, P.; Sala, L.F.; Bellú, S.E. Removal of molybdate anions from contaminated waters by brown algae biomass in batch and continuous processes. J. Chem. Technol. Biotechnol. 2017, 92, 1298–1305. [Google Scholar] [CrossRef]
- Kafshgari, F.; Keshtkar, A.R.; Mousavian, M.A. Study of Mo (Vi) removal from aqueous solution: Application of different mathematical models to continuous biosorption data. Iran. J. Environ. Health Sci. Eng. 2013, 10, 14. [Google Scholar] [CrossRef] [Green Version]
- Pennesi, C.; Totti, C.; Beolchini, F. Removal of Vanadium(III) and Molybdenum(V) from Wastewater Using Posidonia oceanica (Tracheophyta) Biomass. PLoS ONE 2013, 8, e76870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertoni, F.A.; Medeot, A.C.; González, J.C.; Sala, L.F.; Bellú, S.E. Application of green seaweed biomass for MoVI sorption from contaminated waters. Kinetic, thermodynamic and continuous sorption studies. J. Colloid Interface Sci. 2015, 446, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Mahdavi, H.; Ulrich, A.C.; Liu, Y. Metal removal from oil sands tailings pond water by indigenous micro-alga. Chemosphere 2012, 89, 350–354. [Google Scholar] [CrossRef]
- Lian, J.J.; Xu, S.G.; Zhang, Y.M.; Han, C.W. Molybdenum(VI) removal by using constructed wetlands with different filter media and plants. Water Sci. Technol. 2013, 67, 1859–1866. [Google Scholar] [CrossRef]
- Šíma, J.; Svoboda, L.; Šeda, M.; Krejsa, J.; Jahodová, J. Removal of selected risk elements from wastewater in a horizontal subsurface flow constructed wetland. Water Environ. J. 2017, 31, 486–491. [Google Scholar] [CrossRef]
- Šíma, J.; Svoboda, L.; Pomijová, Z. Removal of Selected Metals from Wastewater Using a Constructed Wetland. Chem. Biodivers. 2016, 13, 582–590. [Google Scholar] [CrossRef]

| Language | Publications | Contribution (%) |
|---|---|---|
| English | 277 | 93.6 |
| Russian | 5 | 1.7 |
| Chinese | 4 | 1.4 |
| French | 2 | 0.7 |
| Japanese | 2 | 0.7 |
| Turkish | 2 | 0.7 |
| German | 1 | 0.3 |
| Hungarian | 1 | 0.3 |
| Polish | 1 | 0.3 |
| Spanish | 1 | 0.3 |
| Ranking | Country | Publications | Contribution (%) |
|---|---|---|---|
| 1 | United States | 71 | 24.0 |
| 2 | China | 49 | 16.6 |
| 3 | India | 25 | 8.4 |
| 4 | Turkey | 23 | 7.8 |
| 5 | Canada | 20 | 6.8 |
| 6 | United Kingdom | 18 | 6.1 |
| 7 | France | 14 | 4.7 |
| 8 | Germany | 13 | 4.4 |
| 8 | Japan | 13 | 4.4 |
| 10 | Iran | 10 | 3.4 |
| 11 | Argentina | 9 | 3.0 |
| 11 | Italy | 9 | 3.0 |
| 11 | Sweden | 9 | 3.0 |
| 14 | Australia | 8 | 2.7 |
| 15 | Bangladesh | 6 | 2.0 |
| 15 | Russian Federation | 6 | 2.0 |
| 17 | Saudi Arabia | 5 | 1.7 |
| Ranking | Institution | Publications | Contribution (%) |
|---|---|---|---|
| 1 | Chinese Academy of Sciences (CHINA) | 10 | 3.4 |
| 2 | Pamukkale Üniversitesi (TURKEY) | 9 | 3.0 |
| 3 | Ministry of Education (CHINA) | 8 | 2.7 |
| 4 | Akdeniz Üniversitesi (TURKEY) | 8 | 2.7 |
| 4 | University of Toronto (CANADA) | 6 | 2.0 |
| 6 | Beijing Normal University (CHINA) | 6 | 2.0 |
| 7 | University of Chinese Academy of Sciences (CHINA) | 6 | 2.0 |
| 7 | Hospital for Sick Children-University of Toronto (CANADA) | 6 | 2.0 |
| 9 | Consejo Nacional de Investigaciones Científicas y Técnicas (ARGENTINA) | 5 | 1.7 |
| 9 | United States Geological Survey (USA) | 5 | 1.7 |
| 9 | Norwich University (USA) | 5 | 1.7 |
| 9 | British Geological Survey (UNITED KINGDOM) | 5 | 1.7 |
| Ranking | Subject | Publications | Contribution (%) |
|---|---|---|---|
| 1 | Environmental Science | 152 | 51.4 |
| 2 | Chemistry | 79 | 26.7 |
| 3 | Medicine | 61 | 20.6 |
| 4 | Biochemistry, Genetics and Molecular Biology | 46 | 15.5 |
| 5 | Pharmacology, Toxicology and Pharmaceutics | 29 | 9.8 |
| 6 | Agricultural and Biological Sciences | 28 | 9.5 |
| 6 | Chemical Engineering | 28 | 9.5 |
| 8 | Engineering | 24 | 8.1 |
| 9 | Earth and Planetary Sciences | 21 | 7.1 |
| Ranking | Source | IF 2018 (WoS) | SJR 2018 (Scopus) | Publications | Contribution (%) |
|---|---|---|---|---|---|
| 1 | Environmental Monitoring and Assessment | 1.959 | 0.623 | 15 | 5.1 |
| 2 | Science of the Total Environment | 5.589 | 1.536 | 11 | 3.7 |
| 3 | Biological Trace Element Research | 2.431 | 0.693 | 9 | 3.0 |
| 4 | Water Research | 7.913 | 2.721 | 8 | 2.7 |
| 5 | Journal of Environmental Science and Health: Part A Toxic Hazardous Substances and Environmental Engineering | 1.536 | 0.480 | 7 | 2.4 |
| 6 | Environmental Science and Technology | 7.149 | 2.514 | 5 | 1.7 |
| 6 | Food Chemistry | 5.399 | 1.768 | 5 | 1.7 |
| Ranking | Articles | Times Cited |
|---|---|---|
| 1 | Title: Rapid water disinfection using vertically aligned MoS2 nanofilms and visible light Authors: Liu, C., Kong, D., Hsu, P.-C., (…), Boehm, A.B., Cui, Y. Source: Nature Nanotechnology Published: 2016 | 284 |
| 2 | Title: One century of arsenic exposure in Latin America: A review of history and occurrence from 14 countries Authors: Bundschuh, J., Litter, M.I., Parvez, F., (…), Cumbal, L., Toujaguez, R. Source: Science of the Total Environment Published: 2012 | 261 |
| 3 | Title: Removal of B, Cr, Mo, and Se from wastewater by incorporation into hydrocalumite and ettringite Authors: Zhang, M., Reardon, E.J. Source: Environmental Science and Technology Published: 2003 | 187 |
| 4 | Title: In situ growing Bi2MoO6 on g-C3N4 nanosheets with enhanced photocatalytic hydrogen evolution and disinfection of bacteria under visible light irradiation Authors: Li, J., Yin, Y., Liu, E., (…), Fan, J., Hu, X. Source: Journal of Hazardous Materials Published: 2017 | 140 |
| 5 | Title: MoS2 nanosheets with widened interlayer spacing for high-efficiency removal of mercury in aquatic systems Authors: Ai, K., Ruan, C., Shen, M., Lu, L. Source: Advanced Functional Materials Published: 2016 | 133 |
| 6 | Title: Leachability of elements from sub-bituminous coal fly ash from India Authors: Praharaj, T., Powell, M.A., Hart, B.R., Tripathy, S. Source: Environment International Published: 2002 | 124 |
| 7 | Title: Treatment of arsenic-containing solutions using chitosan derivatives: Uptake mechanism and sorption performances Authors: Dambies, L., Vincent, T., Guibal, E. Source: Water Research Published: 2002 | 119 |
| 8 | Title: Assessment of molybdenum toxicity in humans Authors: Vyskočil, A., Viau, C. Source: Journal of Applied Toxicology Published: 1999 | 116 |
| 9 | Title: One-pot synthesis of multifunctional magnetic ferrite-MoS2-carbon dot nanohybrid adsorbent for efficient Pb(II) removal Authors: Wang, J., Zhang, W., Yue, X., (…), Li, Z., Wang, J. Source: Journal of Materials Chemistry A Published: 2016 | 114 |
| 10 | Title: Arsenic(V) sorption on molybdate-impregnated chitosan beads Authors: Dambies, L., Guibal, E., Roze, A. Source: Colloids and Surfaces A: Physicochemical and Engineering Aspects Published: 2000 | 99 |
| Treated Water | Adsorbent | Sorption Capacity (mg/g) | Reference |
|---|---|---|---|
| Synthetic solution | Akaganeite (β-FeOOH) | 400 | [76] |
| Synthetic solution | Pyrite (FeS2) | 21 | [77] |
| Synthetic solution | Synthetic hematite (α-Fe2O3) | 6 | [78] |
| Synthetic solution | Goethite (α-FeO(OH)) | 26 | [81] |
| Synthetic solution | Titania (TiO2) | 7 | [85] |
| Synthetic solution | Alumina (Al2O3) | 125 | [86] |
| Synthetic solution | Fe(III)/Cr(III) hydroxide | 12 | [87] |
| Synthetic solution | Zn–Al sulphate layered double hydroxide | 154 | [88] |
| Synthetic solution | Hydrotalcite-like layered double hydroxide | 10 | [89] |
| Synthetic solution | Hydroxy-aluminum treated montmorillonite | 5 | [90] |
| Synthetic solution | Ag-exchanged clinoptilolite | 100 | [91] |
| Synthetic solution | ZnCl2 activated coir pith carbon | 17 | [93] |
| Synthetic solution | Chitosan | 265 | [94] |
| Synthetic solution | Chitosan | 750 | [95] |
| Synthetic solution | Chitosan | 820 | [96] |
| Synthetic solution | Imprinted chitosan/triethanolamine gel | 350 | [98] |
| Resin | Manufacturer | Functional Group |
|---|---|---|
| D301 | Tianjin Nankai Hecheng | Tertiary ammonium |
| D201 | Hangzhou Zhengguang Chemical | Quaternary ammonium |
| D213 | Hangzhou Zhengguang Chemical | Quaternary ammonium |
| D308 | Hangzhou Zhengguang Chemical | Tertiary amine |
| D319 | Hangzhou Zhengguang Chemical | Tertiary amine |
| D303 | Hangzhou Zhengguang Chemical | Primary amine |
| D309 | Hangzhou Zhengguang Chemical | Primary amine |
| D320 | Hangzhou Zhengguang Chemical | Quaternary ammonium and tertiary amine |
| A-170 | Purolite | Complex amine |
| A-172 | Purolite | Complex amine |
| D3411 | Purolite | Diethanol amine |
| AG 1 X-8 | Bio-Rad | Quaternary ammonium |
| D290 | Bengbu Dongli Chemical | Quaternary ammonium |
| VP OC 1065 | Lewatit | Primary amine |
| MP 800 | Lewatit | Quaternary ammonium |
| MP 62 | Lewatit | Tertiary amine |
| M-43 | Dowex | Tertiary amine |
| Treated Water | Coagulant | Dose (mg/L) | pH | Initial [Mo] (µg/L) | Removal (%) | Reference |
|---|---|---|---|---|---|---|
| Synthetic solution | FeCl3/Fe2(SO4)3 | 10 | 6 | 700 | 90/89 | [109] |
| River reservoir | Ferromagnetic nanoparticles + FeCl3 | 35 + 97 | 7 | 500 | 97 | [110] |
| Synthetic solution | Aluminum polychloride + polyacrylamide | 40 + 0.8 | - | 1100 | 92 | [111] |
| Mineral processing wastewater | BK-A (commercial formulation) | 25 | 9 | - | - | [112] |
| Nitric acid media doped with molybdenite | KlarAid products (commercial formulation) | 20,000 | - | 615,000 | 88 | [113] |
| Treated Water | Membrane | ΔP (Bar) | Permeate Flux (m3/m2·s) | Initial [Mo] (µg/L) | Removal (%) | Reference |
|---|---|---|---|---|---|---|
| Synthetic solution | NF270-2540 (Filmtec) | 5 | 1.0 × 10−3 | 1000 | 98 | [76] |
| Groundwater contaminated by uranium mill tailings | PAC1 RO (Ionics) TW30 LE RO (FilmTec) TFC-S NF (Koch) | 7 | - | 33,400 | 96–98 | [125] |
| Produced water | NF1 and BW30 (Filmtec) | 6 and 20 | - | 815 | 28 and 76 | [126] |
| Synthetic solution | NF99HF (Alfa Laval) and UTC-60 (Toray) | 20 | 1.7–3.7 × 10−5 | 500 | 60–70 | [127] |
| Brackish groundwater | BW30 (Filmtec) | 14 | 1.4 × 10−5 | 5 | >95 | [128] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abejón, R. An Overview to Technical Solutions for Molybdenum Removal: Perspective from the Analysis of the Scientific Literature on Molybdenum and Drinking Water (1990–2019). Water 2022, 14, 2108. https://doi.org/10.3390/w14132108
Abejón R. An Overview to Technical Solutions for Molybdenum Removal: Perspective from the Analysis of the Scientific Literature on Molybdenum and Drinking Water (1990–2019). Water. 2022; 14(13):2108. https://doi.org/10.3390/w14132108
Chicago/Turabian StyleAbejón, Ricardo. 2022. "An Overview to Technical Solutions for Molybdenum Removal: Perspective from the Analysis of the Scientific Literature on Molybdenum and Drinking Water (1990–2019)" Water 14, no. 13: 2108. https://doi.org/10.3390/w14132108
APA StyleAbejón, R. (2022). An Overview to Technical Solutions for Molybdenum Removal: Perspective from the Analysis of the Scientific Literature on Molybdenum and Drinking Water (1990–2019). Water, 14(13), 2108. https://doi.org/10.3390/w14132108






