Adsorption of Tannic Acid and Macromolecular Humic/Fulvic Acid onto Polystyrene Microplastics: A Comparison Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Materials Characterization
2.3. Adsorption Experiments
2.4. Adsorption Models
2.5. Approximate Site Energy Distribution Analysis
3. Results and Discussion
3.1. Characterization of PS MPs
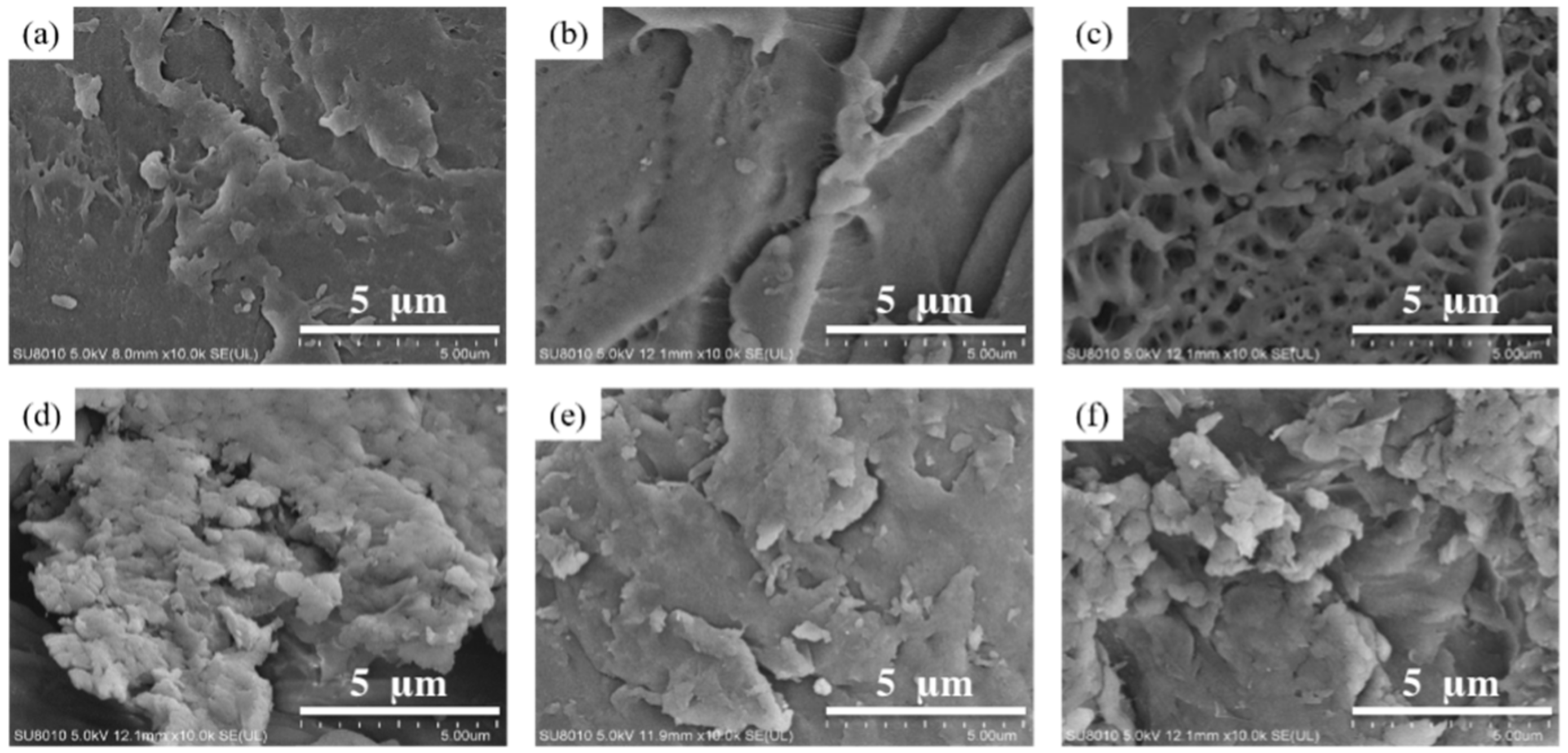
3.1.1. SEM Observation
3.1.2. FT-IR Analysis
3.1.3. Zeta Potentials Analysis
3.2. Effect of pH on HA, FA, and TA Adsorption by PS MPs
3.3. Effect of Salinity on HA, FA, and TA Adsorption by PS MPs
3.4. Adsorption Kinetics Analysis
3.5. Adsorption Isotherms Analysis
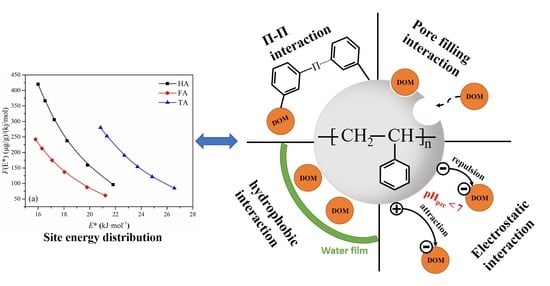
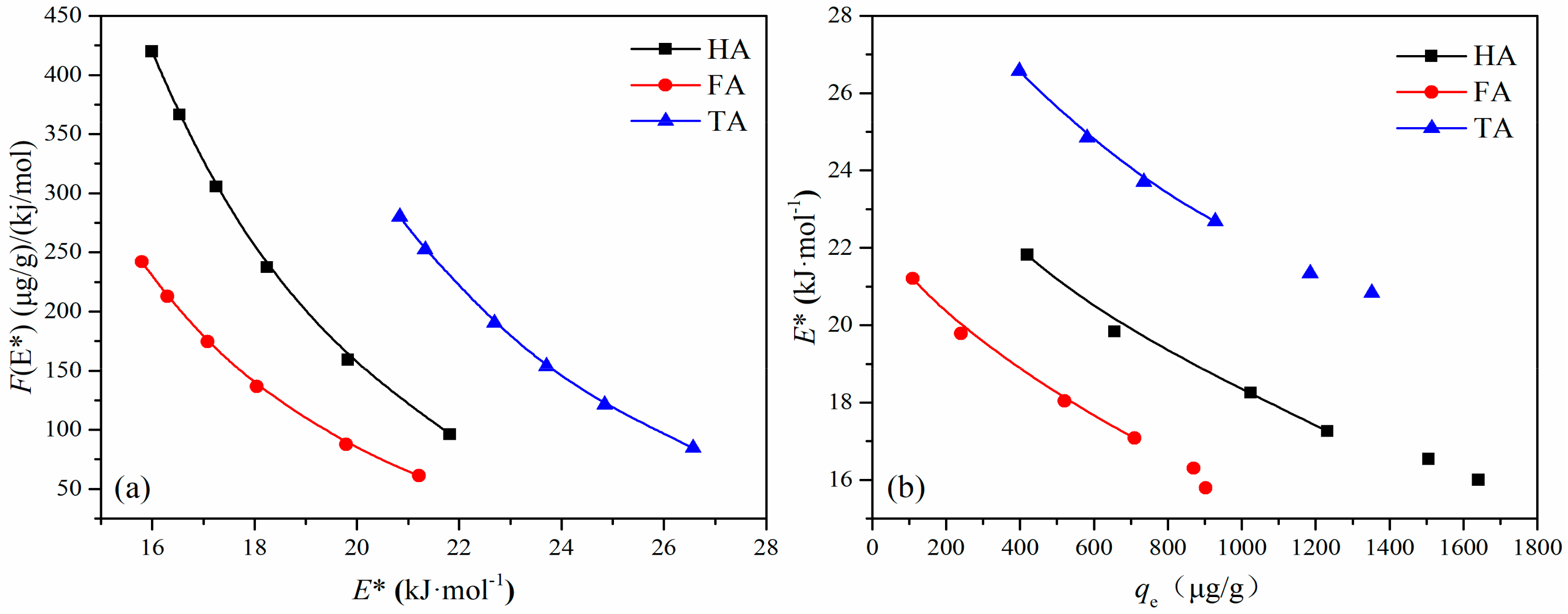
3.6. Adsorption Site Energy Distribution Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Q.; Xu, E.G.; Li, J.; Chen, Q.; Ma, L.; Zeng, E.Y.; Shi, H. A review of microplastics in table salt, drinking water, and air: Direct human exposure. Environ. Sci. Technol. 2020, 54, 3740–3751. [Google Scholar] [CrossRef] [PubMed]
- Okoffo, E.D.; Donner, E.; McGrath, S.P.; Tscharke, B.J.; O’Brien, J.W.; O’Brien, S.; Ribeiro, F.; Burrows, S.D.; Toapanta, T.; Rauert, C.; et al. Plastics in biosolids from 1950 to 2016: A function of global plastic production and consumption. Water Res. 2021, 201, 117367. [Google Scholar] [CrossRef] [PubMed]
- Plastics Europe. Plastics: The Facts. Brussels, Belgium: Plastics Europe. 2021. Available online: https://plasticseurope.org/wp-content/uploads/2021/12/Plastics-the-Facts-2021-web-final.pdf (accessed on 8 July 2022).
- Meyers, N.; Catarino, A.I.; Declercq, A.M.; Brenan, A.; Devriese, L.; Vandegehuchte, M.; De Witte, B.; Janssen, C.; Everaert, G. Microplastic detection and identification by Nile red staining: Towards a semi-automated, cost- and time-effective technique. Sci. Total Environ. 2022, 823, 153441. [Google Scholar] [CrossRef] [PubMed]
- Lebreton, L.; Andrady, A. Future scenarios of global plastic waste generation and disposal. Palgrave Commun. 2019, 5, 6. [Google Scholar] [CrossRef] [Green Version]
- Plastic Waste Statistics. 2022. Available online: https://seedscientific.com/plastic-waste-statistics/ (accessed on 8 July 2022).
- Zhang, K.; Hamidian, A.H.; Tubic, A.; Zhang, Y.; Fang, J.K.H.; Wu, C.; Lam, P.K.S. Understanding plastic degradation and microplastic formation in the environment: A review. Environ. Pollut. 2021, 274, 116554. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, Y.; Liu, G.; He, G.; Liu, W. Adsorption mechanism of cadmium on microplastics and their desorption behavior in sediment and gut environments: The roles of water pH, lead ions, natural organic matter and phenanthrene. Water Res. 2020, 184, 116209. [Google Scholar] [CrossRef]
- Rillig, M.C.; de Souza Machado, A.A.; Lehmann, A.; Klumper, U. Evolutionary implications of microplastics for soil biota. Environ. Chem. 2019, 16, 3–7. [Google Scholar] [CrossRef] [Green Version]
- de Souza Machado, A.A.; Kloas, W.; Zarfl, C.; Hempel, S.; Rillig, M.C. Microplastics as an emerging threat to terrestrial ecosystems. Glob. Chang. Biol. 2018, 24, 1405–1416. [Google Scholar] [CrossRef] [Green Version]
- Galloway, T.S.; Lewis, C.N. Marine microplastics spell big problems for future generations. Proc. Natl. Acad. Sci. USA 2016, 113, 2331–2333. [Google Scholar] [CrossRef] [Green Version]
- Alimi, O.S.; Farner Budarz, J.; Hernandez, L.M.; Tufenkji, N. Microplastics and nanoplastics in aquatic environments: Aggregation, deposition, and enhanced contaminant transport. Environ. Sci. Technol. 2018, 52, 1704–1724. [Google Scholar] [CrossRef]
- de Souza Machado, A.A.; Lau, C.W.; Kloas, W.; Bergmann, J.; Bachelier, J.B.; Faltin, E.; Becker, R.; Görlich, A.S.; Rillig, M.C. Microplastics can change soil properties and affect plant performance. Environ. Sci. Technol. 2019, 53, 6044–6052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pannetier, P.; Morin, B.; Le Bihanic, F.; Dubreil, L.; Clerandeau, C.; Chouvellon, F.; Van Arkel, K.; Danion, M.; Cachot, J. Environmental samples of microplastics induce significant toxic effects in fish larvae. Environ. Int. 2020, 134, 105047. [Google Scholar] [CrossRef]
- Gu, L.; Tian, L.; Gao, G.; Peng, S.; Zhang, J.; Wu, D.; Huang, J.; Hua, Q.; Lu, T.; Zhong, L.; et al. Inhibitory effects of polystyrene microplastics on caudal fin regeneration in zebrafish larvae. Environ. Pollut. 2020, 266, 114664. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Song, B.; Liang, J.; Niu, Q.; Zeng, G.; Shen, M.; Deng, J.; Luo, Y.; Wen, X.; Zhang, Y. Microplastics and associated contaminants in the aquatic environment: A review on their ecotoxicological effects, trophic transfer, and potential impacts to human health. J. Hazard. Mater. 2021, 405, 124187. [Google Scholar] [CrossRef] [PubMed]
- Okeke, E.S.; Okoye, C.O.; Atakpa, E.O.; Ita, R.E.; Nyaruaba, R.; Mgbechidinma, C.L.; Akan, O.D. Microplastics in agroecosystems-impacts on ecosystem functions and food chain. Resour. Conserv. Recycl. 2022, 177, 105961. [Google Scholar] [CrossRef]
- Munoz, M.; Ortiz, D.; Nieto-Sandoval, J.; de Pedro, Z.M.; Casas, J.A. Adsorption of micropollutants onto realistic microplastics: Role of microplastic nature, size, age, and NOM fouling. Chemosphere 2021, 283, 131085. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Ding, J.; Zhang, G.; Liu, S.; Zou, H.; Wang, Z.; Zhu, W.; Geng, J. Interactive effects of microplastics and selected pharmaceuticals on red tilapia: Role of microplastic aging. Sci. Total Environ. 2021, 752, 142256. [Google Scholar] [CrossRef]
- Yu, Y.; Mo, W.Y.; Luukkonen, T. Adsorption behaviour and interaction of organic micropollutants with nano and microplastics-A review. Sci. Total Environ. 2021, 797, 149140. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Zhang, Y.; Wei, W. Effects of macromolecular humic/fulvic acid on Cd (II) adsorption onto reed-derived biochar as compared with tannic acid. Int. J. Biol. Macromol. 2019, 134, 43–55. [Google Scholar] [CrossRef]
- Chen, C.-S.; Le, C.; Chiu, M.-H.; Chin, W.-C. The impact of nanoplastics on marine dissolved organic matter assembly. Sci. Total Environ. 2018, 634, 316–320. [Google Scholar] [CrossRef]
- Kinigopoulou, V.; Pashalidis, I.; Kalderis, D.; Anastopoulos, I. Microplastics as carriers of inorganic and organic contaminants in the environment: A review of recent progress. J. Mol. Liq. 2022, 350, 118580. [Google Scholar] [CrossRef]
- Caruso, G. Microplastics as vectors of contaminants. Mar. Pollut. Bull. 2019, 146, 921–924. [Google Scholar] [CrossRef] [PubMed]
- Hakim, A.; Kobayashi, M. Aggregation and aggregate strength of microscale plastic particles in the presence of natural organic matter: Effects of ionic valence. J. Polym. Environ. 2021, 29, 1921–1929. [Google Scholar] [CrossRef]
- Corinaldesi, C.; Canensi, S.; Carugati, L.; Lo Martire, M.; Marcellini, F.; Nepote, E.; Sabbatini, S.; Danovaro, R. Organic enrichment can increase the impact of microplastics on meiofaunal assemblages in tropical beach systems. Environ. Pollut. 2022, 292, 118415. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Ouyang, Z.-Y.; Qian, C.; Yu, H.-Q. Induced structural changes of humic acid by exposure of polystyrene microplastics: A spectroscopic insight. Environ. Pollut. 2018, 233, 1–7. [Google Scholar] [CrossRef]
- Qiao, R.; Lu, K.; Deng, Y.; Ren, H.; Zhang, Y. Combined effects of polystyrene microplastics and natural organic matter on the accumulation and toxicity of copper in zebrafish. Sci. Total Environ. 2019, 682, 128–137. [Google Scholar] [CrossRef]
- Dong, Y.; Gao, M.; Song, Z.; Qiu, W. As(III) adsorption onto different-sized polystyrene microplastic particles and its mechanism. Chemosphere 2020, 239, 124792. [Google Scholar] [CrossRef]
- Sighicelli, M.; Pietrelli, L.; Lecce, F.; Iannilli, V.; Falconieri, M.; Coscia, L.; Di Vito, S.; Nuglio, S.; Zampetti, G. Microplastic pollution in the surface waters of Italian Subalpine Lakes. Environ. Pollut. 2018, 236, 645–651. [Google Scholar] [CrossRef]
- Kukkola, A.; Krause, S.; Lynch, I.; Sambrook Smith, G.H.; Nel, H. Nano and microplastic interactions with freshwater biota–Current knowledge, challenges and future solutions. Environ. Int. 2021, 152, 106504. [Google Scholar] [CrossRef]
- Lu, S.; Zhu, K.; Song, W.; Song, G.; Chen, D.; Hayat, T.; Alharbi, N.S.; Chen, C.; Sun, Y. Impact of water chemistry on surface charge and aggregation of polystyrene microspheres suspensions. Sci. Total Environ. 2018, 630, 951–959. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, Z.; Wang, S.; Fang, H.; Wang, D. Aquatic behavior and toxicity of polystyrene nanoplastic particles with different functional groups: Complex roles of pH, dissolved organic carbon and divalent cations. Chemosphere 2019, 228, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Li, J.; Wang, G.; Luan, Y.; Dai, W. Adsorption behavior of organic pollutants on microplastics. Ecotox. Environ. Saf. 2021, 217, 112207. [Google Scholar] [CrossRef] [PubMed]
- Lagergren, S.K. About the theory of so-called adsorption of soluble substances. Kung Svenska Vetenskap 1898, 24, 1–39. [Google Scholar]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process. Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Wu, F.C.; Tseng, R.L.; Juang, R.S. Characteristics of Elovich equation used for the analysis of adsorption kinetics in dye-chitosan systems. Chem. Eng. J. 2009, 150, 366–373. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1916, 40, 1361–1368. [Google Scholar]
- Freundlich, H.M.F. Over the adsorption in solution. Z. Phys. Chem. 1906, A57, 358–471. [Google Scholar]
- Sips, R. On the structure of a catalyst surface. J. Chem. Phys. 1948, 16, 490–495. [Google Scholar] [CrossRef]
- Carter, M.C.; Kilduff, J.E.; Weber, W.J. Site energy distribution analysis of preloaded adsorbents. Environ. Sci. Technol. 1995, 29, 1773–1780. [Google Scholar] [CrossRef]
- Cerofolini, G.F. Localized adsorption on heterogeneous surfaces. Thin Solid Film. 1974, 23, 129–152. [Google Scholar] [CrossRef]
- Lang, M.; Yu, X.; Liu, J.; Xia, T.; Wang, T.; Jia, H.; Guo, X. Fenton aging significantly affects the heavy metal adsorption capacity of polystyrene microplastics. Sci. Total Environ. 2020, 722, 137762. [Google Scholar] [CrossRef]
- Ding, L.; Mao, R.; Ma, S.; Guo, X.; Zhu, L. High temperature depended on the ageing mechanism of microplastics under different environmental conditions and its effect on the distribution of organic pollutants. Water Res. 2020, 174, 115634. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Gao, M.; Qiu, W.; Song, Z. Adsorption of arsenite to polystyrene microplastics in the presence of humus. Environ. Sci. Proc. Imp. 2020, 22, 2388–2397. [Google Scholar] [CrossRef] [PubMed]
- Nel, H.A.; Chetwynd, A.J.; Kelly, C.A.; Stark, C.; Valsami-Jones, E.; Krause, S.; Lynch, I. An untargeted thermogravimetric analysis-fourier transform infrared-gas chromatography-mass spectrometry approach for plastic polymer identification. Environ. Sci. Technol. 2021, 55, 8721–8729. [Google Scholar] [CrossRef]
- Yao, Y.; Mi, N.; He, C.; He, H.; Zhang, Y.; Zhang, Y.; Yin, L.; Li, J.; Yang, S.; Li, S.; et al. Humic acid modified nano-ferrous sulfide enhances the removal efficiency of Cr(VI). Sep. Purif. Technol. 2020, 240, 116623. [Google Scholar] [CrossRef]
- Yang, R.; Li, Z.; Huang, M.; Luo, N.; Wen, J.; Zeng, G. Characteristics of fulvic acid during coprecipitation and adsorption to iron oxides-copper aqueous system. J. Mol. Liq. 2019, 274, 664–672. [Google Scholar] [CrossRef]
- Ding, L.; Luo, Y.; Yu, X.; Ouyang, Z.; Liu, P.; Guo, X. Insight into interactions of polystyrene microplastics with different types and compositions of dissolved organic matter. Sci. Total Environ. 2022, 824, 153883. [Google Scholar] [CrossRef]
- Yin, L.; Mi, N.; Yao, Y.-R.; Li, J.; Zhang, Y.; Yang, S.-g.; He, H.; Hu, X.; Li, S.-Y.; Ni, L.-X. Efficient removal of Cr(VI) by tannic acid-modified FeS nanoparticles: Performance and mechanisms. Water Sci. Eng. 2021, 14, 210–218. [Google Scholar] [CrossRef]
- Zhu, B.; Zhang, Z.; Song, F.; Guo, Z.; Liu, B. Efficient removal of U(VI) ions from aqueous solutions by tannic acid/graphene oxide composites. Appl. Sci. 2020, 10, 8870. [Google Scholar] [CrossRef]
- Schmidt, P.; Dybal, J.; Trchová, M. Investigations of the hydrophobic and hydrophilic interactions in polymer–water systems by ATR FTIR and Raman spectroscopy. Vibrat. Spectr. 2006, 42, 278–283. [Google Scholar] [CrossRef]
- Dong, Y.; Gao, M.; Liu, X.; Qiu, W.; Song, Z. The mechanism of polystyrene microplastics to affect arsenic volatilization in arsenic-contaminated paddy soils. J. Hazard. Mater. 2020, 398, 122896. [Google Scholar] [CrossRef] [PubMed]
- Jayalath, S.; Wu, H.; Larsen, S.C.; Grassian, V.H. Surface adsorption of suwannee river humic acid on tio2 nanoparticles: A study of pH and particle size. Langmuir 2018, 34, 3136–3145. [Google Scholar] [CrossRef]
- Zhang, C.; Wei, S.; Hu, Y.; Tang, H.; Gao, J.; Yin, Z.; Guan, Q. Selective adsorption of tannic acid on calcite and implications for separation of fluorite minerals. J. Colloid Interf. Sci. 2018, 512, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Xu, Y.; Yu, X.; Ouyang, Z.; Guo, X. Effect of cadmium on the sorption of tylosin by polystyrene microplastics. Ecotox. Environ. Saf. 2021, 207, 111255. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Yang, L.; Zhong, W.; Cui, J.; Wei, Z. Poorly crystalline hydroxyapatite: A novel adsorbent for enhanced fulvic acid removal from aqueous solution. Appl. Surface Sci. 2015, 332, 328–339. [Google Scholar] [CrossRef]
- Doulia, D.; Leodopoulos, C.; Gimouhopoulos, K.; Rigas, F. Adsorption of humic acid on acid-activated Greek bentonite. J. Colloid Interf. Sci. 2009, 340, 131–141. [Google Scholar] [CrossRef]
- Tang, S.; Lin, L.; Wang, X.; Sun, X.; Yu, A. Adsorption of fulvic acid onto polyamide 6 microplastics: Influencing factors, kinetics modeling, site energy distribution and interaction mechanisms. Chemosphere 2021, 272, 129638. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, S.; Liu, J.; Xu, Z. Tannic acid adsorption on amino-functionalized magnetic mesoporous silica. Chem. Eng. J. 2010, 165, 10–16. [Google Scholar] [CrossRef]
- Yang, K.; Xing, B. Adsorption of fulvic acid by carbon nanotubes from water. Environ. Pollut. 2009, 157, 1095–1100. [Google Scholar] [CrossRef]
- Islam, M.A.; Morton, D.W.; Johnson, B.B.; Angove, M.J. Adsorption of humic and fulvic acids onto a range of adsorbents in aqueous systems, and their effect on the adsorption of other species: A review. Sep. Purif. Technol. 2020, 247, 116949. [Google Scholar] [CrossRef]
- Sun, C.; Xiong, B.; Pan, Y.; Cui, H. Adsorption removal of tannic acid from aqueous solution by polyaniline: Analysis of operating parameters and mechanism. J. Colloid Interf. Sci. 2017, 487, 175–181. [Google Scholar] [CrossRef]
- Wei, W.; Li, J.; Han, X.; Yao, Y.; Zhao, W.; Han, R.; Li, S.; Zhang, Y.; Zheng, C. Insights into the adsorption mechanism of tannic acid by a green synthesized nano-hydroxyapatite and its effect on aqueous Cu(II) removal. Sci. Total Environ. 2021, 778, 146189. [Google Scholar] [CrossRef] [PubMed]
- Brigante, M.; Zanini, G.; Avena, M. Effect of pH, anions and cations on the dissolution kinetics of humic acid particles. Colloids Surf. A Physicochem. Eng. Asp. 2009, 347, 180–186. [Google Scholar] [CrossRef]
- Capasso, S.; Salvestrini, S.; Coppola, E.; Buondonno, A.; Colella, C. Sorption of humic acid on zeolitic tuff: A preliminary investigation. Appl. Clay Sci. 2005, 28, 159–165. [Google Scholar] [CrossRef]
- Engel, M.; Chefetz, B. Adsorption and desorption of dissolved organic matter by carbon nanotubes: Effects of solution chemistry. Environ. Pollut. 2016, 213, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Abdurahman, A.; Cui, K.; Wu, J.; Li, S.; Gao, R.; Dai, J.; Liang, W.; Zeng, F. Adsorption of dissolved organic matter (DOM) on polystyrene microplastics in aquatic environments: Kinetic, isotherm and site energy distribution analysis. Ecotox. Environ. Saf. 2020, 198, 110658. [Google Scholar] [CrossRef]
- Li, S.C.; Liu, H.; Gao, R.; Abdurahman, A.; Dai, J.; Zeng, F. Aggregation kinetics of microplastics in aquatic environment: Complex roles of electrolytes, pH, and natural organic matter. Environ. Pollut. 2018, 237, 126–132. [Google Scholar] [CrossRef]
- Hyung, H.; Kim, J.-H. Natural organic matter (NOM) adsorption to multi-walled carbon nanotubes: Effect of nom characteristics and water quality parameters. Environ. Sci. Technol. 2008, 42, 4416–4421. [Google Scholar] [CrossRef]
- Liang, L.; Luo, L.; Zhang, S. Adsorption and desorption of humic and fulvic acids on SiO2 particles at nano- and micro-scales. Colloids Surf. A Physicochem. Eng. Aspects. 2011, 384, 126–130. [Google Scholar] [CrossRef]
- Fan, S.; Wang, Y.; Li, Y.; Tang, J.; Wang, Z.; Tang, J.; Li, X.; Hu, K. Facile synthesis of tea waste/Fe3O4 nanoparticle composite for hexavalent chromium removal from aqueous solution. RSC Adv. 2017, 7, 7576–7590. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Zhao, F.; Sang, Q.; Zhang, Y.; Chang, L.; Huang, D.; Mu, B. Investigation of 3-aminopropyltriethoxysilane modifying attapulgite for Congo red removal: Mechanisms and site energy distribution. Powder Technol. 2021, 383, 74–83. [Google Scholar] [CrossRef]
- Wang, T.; Yu, C.; Chu, Q.; Wang, F.; Lan, T.; Wang, J. Adsorption behavior and mechanism of five pesticides on microplastics from agricultural polyethylene films. Chemosphere 2020, 244, 125491. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Cai, Z.; Jin, H.; Tang, Y. Adsorption mechanisms of five bisphenol analogues on PVC microplastics. Sci. Total Environ. 2019, 650, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, H.; He, H.; Cheng, X.; Ma, T.; Hu, J.; Yang, S.; Li, S.; Zhang, L. Adsorption behavior and mechanism of 9-Nitroanthracene on typical microplastics in aqueous solutions. Chemosphere 2020, 245, 125628. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Guo, X.; Zhang, M.; Tao, S.; Wang, X. Sorption Mechanisms of Organic Compounds by Carbonaceous Materials: Site Energy Distribution Consideration. Environ. Sci. Technol. 2015, 49, 4894–4902. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.-K.; Jang, M.; Hwang, Y.S. Adsorption of benzalkonium chlorides onto polyethylene microplastics: Mechanism and toxicity evaluation. J. Hazard. Mater. 2022, 426, 128076. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Jin, Q.; Tandon, P.; Li, A.; Shan, A.; Du, J. High-resolution insight into the competitive adsorption of heavy metals on natural sediment by site energy distribution. Chemosphere 2018, 197, 411–419. [Google Scholar] [CrossRef]



| Systems | qe, Exp (μg/g) | Pseudo-First-Order Model | Pseudo-Second-Order Model | Elovich Model | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| qe, Cal (μg/g) | k1 (1/min) | R2 | qe, Cal (μg/g) | k2 (g/(μg·min)) | R2 | α (g/μg) | β (μg/(g·min)) | R2 | ||
| PS + HA | 1641 | 1550 | 0.435 | 0.7430 | 1614 | 3.914 × 10−4 | 0.9254 | 3.719 × 104 | 0.006 | 0.9308 |
| PS + FA | 918 | 910 | 0.037 | 0.8971 | 1003 | 5.751 × 10−5 | 0.9206 | 296.628 | 0.006 | 0.9238 |
| PS + TA | 1385 | 1297 | 0.164 | 0.8242 | 1378 | 1.7816 × 10−4 | 0.9440 | 2286.03 | 0.005 | 0.9583 |
| Systems | Langmuir | Freundlich | Sips | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| qm (μg/g) | KL (L/μg) | R2 | KF(μg/g (L/μg)1/n) | 1/n | R2 | qm (μg/g) | KS (L/μg) | 1/n | R2 | |
| PS + HA | 2813 | 2.848 × 10−5 | 0.9782 | 2.1594 | 0.617 | 0.9844 | 5810 | 3.826 × 10−6 | 0.671 | 0.9798 |
| PS + FA | 1654 | 2.607 × 10−5 | 0.9848 | 1.1785 | 0.619 | 0.9887 | 2603 | 6.497 × 10−6 | 0.715 | 0.9870 |
| PS + TA | 1885 | 4.601 × 10−5 | 0.9797 | 5.5315 | 0.508 | 0.9981 | 6908 | 9.154 × 10−7 | 0.519 | 0.9975 |
| HA | FA | TA | |||
|---|---|---|---|---|---|
| μ (E*) | σe* | μ (E*) | σe* | μ (E*) | σe* |
| 14.40 | 11.28 | 13.98 | 11.28 | 17.72 | 14.98 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Ma, S.; Li, X.; Wei, W. Adsorption of Tannic Acid and Macromolecular Humic/Fulvic Acid onto Polystyrene Microplastics: A Comparison Study. Water 2022, 14, 2201. https://doi.org/10.3390/w14142201
Li J, Ma S, Li X, Wei W. Adsorption of Tannic Acid and Macromolecular Humic/Fulvic Acid onto Polystyrene Microplastics: A Comparison Study. Water. 2022; 14(14):2201. https://doi.org/10.3390/w14142201
Chicago/Turabian StyleLi, Junsuo, Shoucheng Ma, Xinying Li, and Wei Wei. 2022. "Adsorption of Tannic Acid and Macromolecular Humic/Fulvic Acid onto Polystyrene Microplastics: A Comparison Study" Water 14, no. 14: 2201. https://doi.org/10.3390/w14142201
APA StyleLi, J., Ma, S., Li, X., & Wei, W. (2022). Adsorption of Tannic Acid and Macromolecular Humic/Fulvic Acid onto Polystyrene Microplastics: A Comparison Study. Water, 14(14), 2201. https://doi.org/10.3390/w14142201