Ecology and Distribution of Red King Crab Larvae in the Barents Sea: A Review
Abstract
:1. Introduction
2. Occurrence and General Biology of RCK in the Barents Sea
3. Larval Morphology of RCK in the Barents Sea
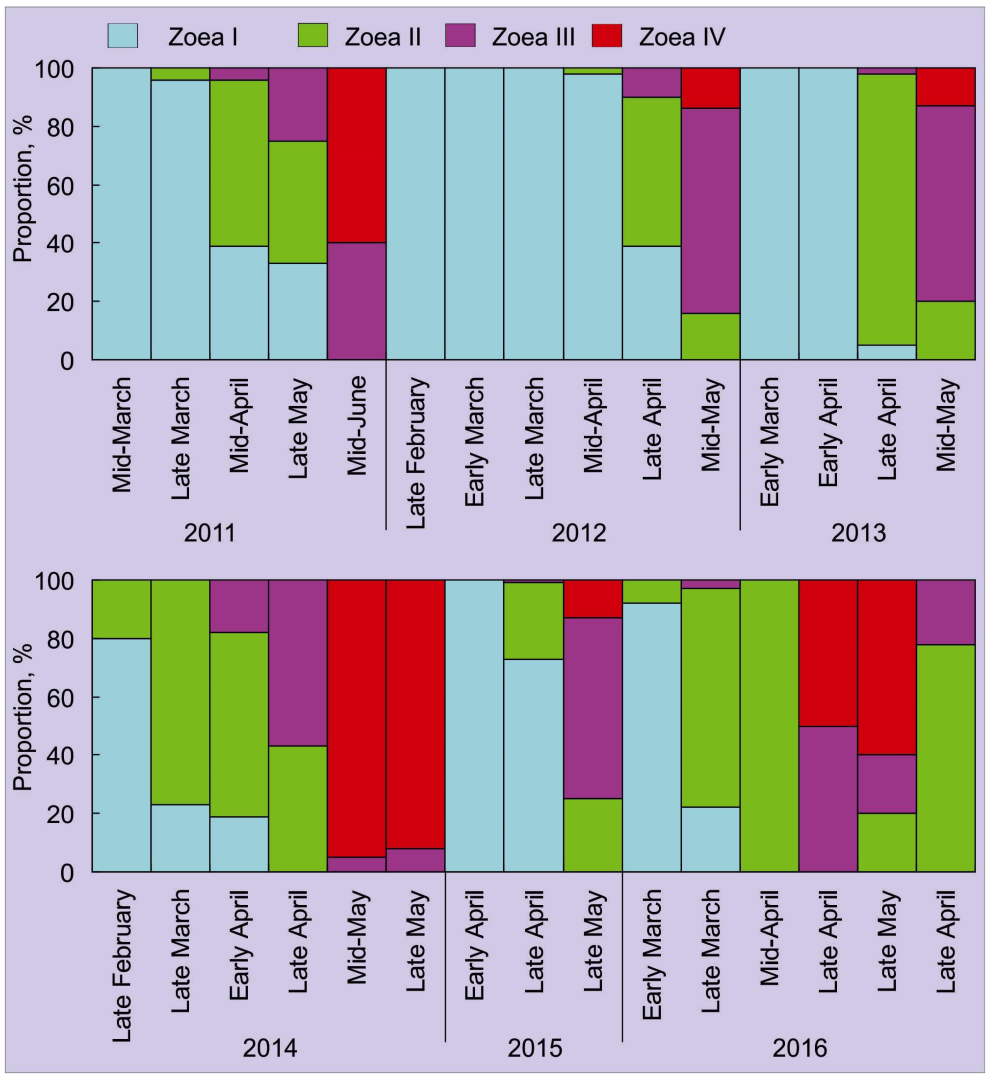
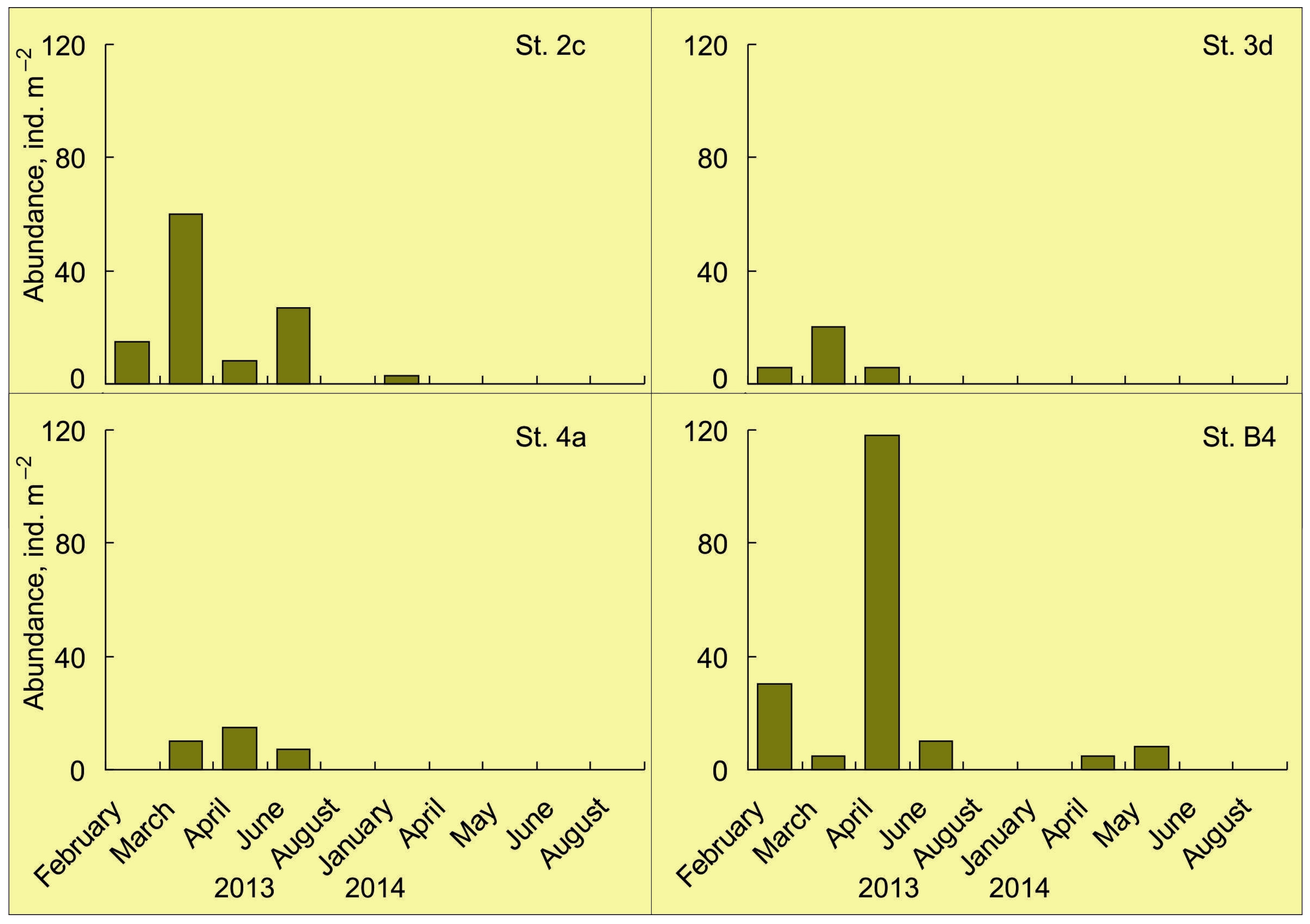
4. Abundance, Phenology, and Distribution of RCK Larvae in the Barents Sea
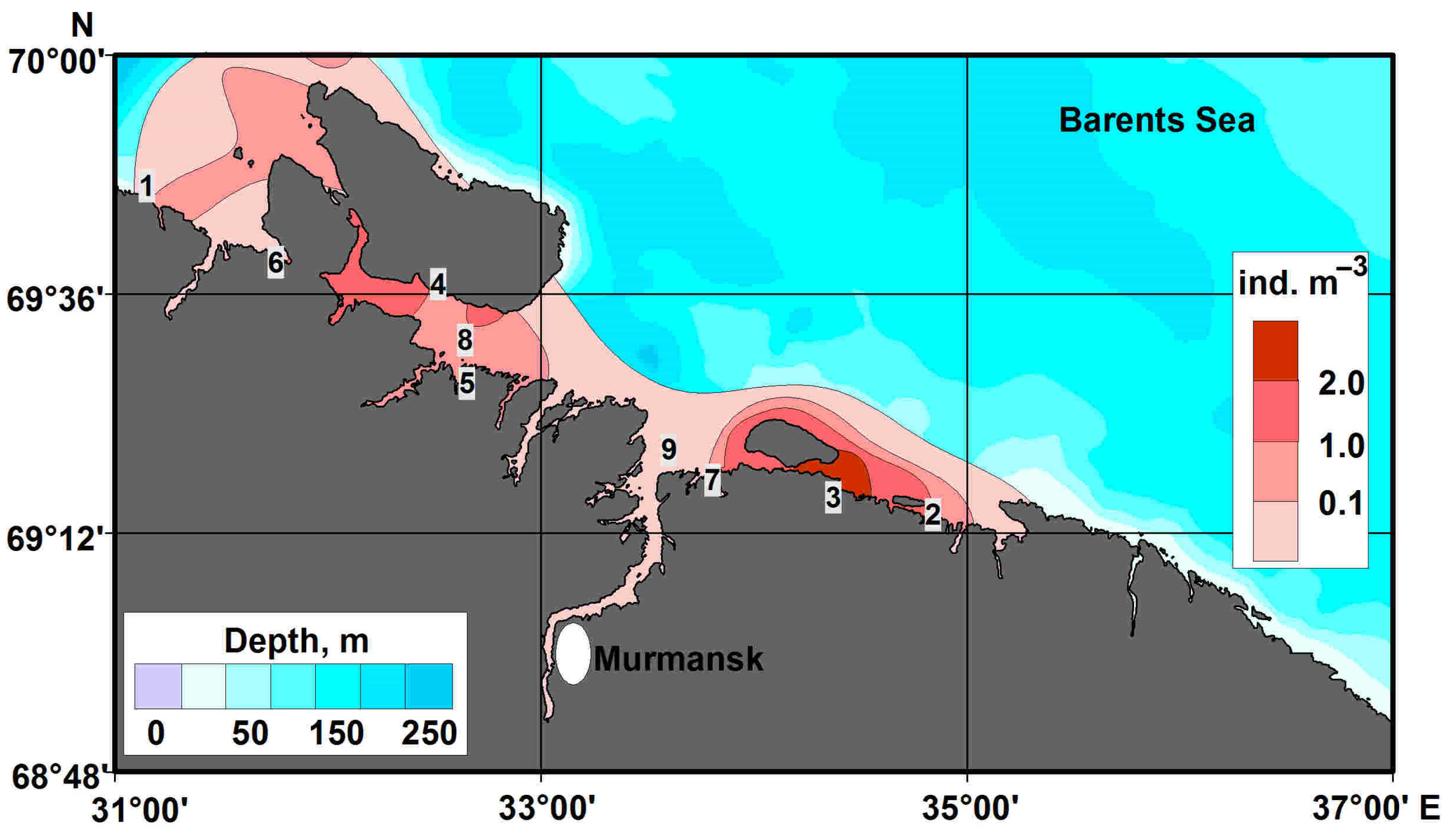
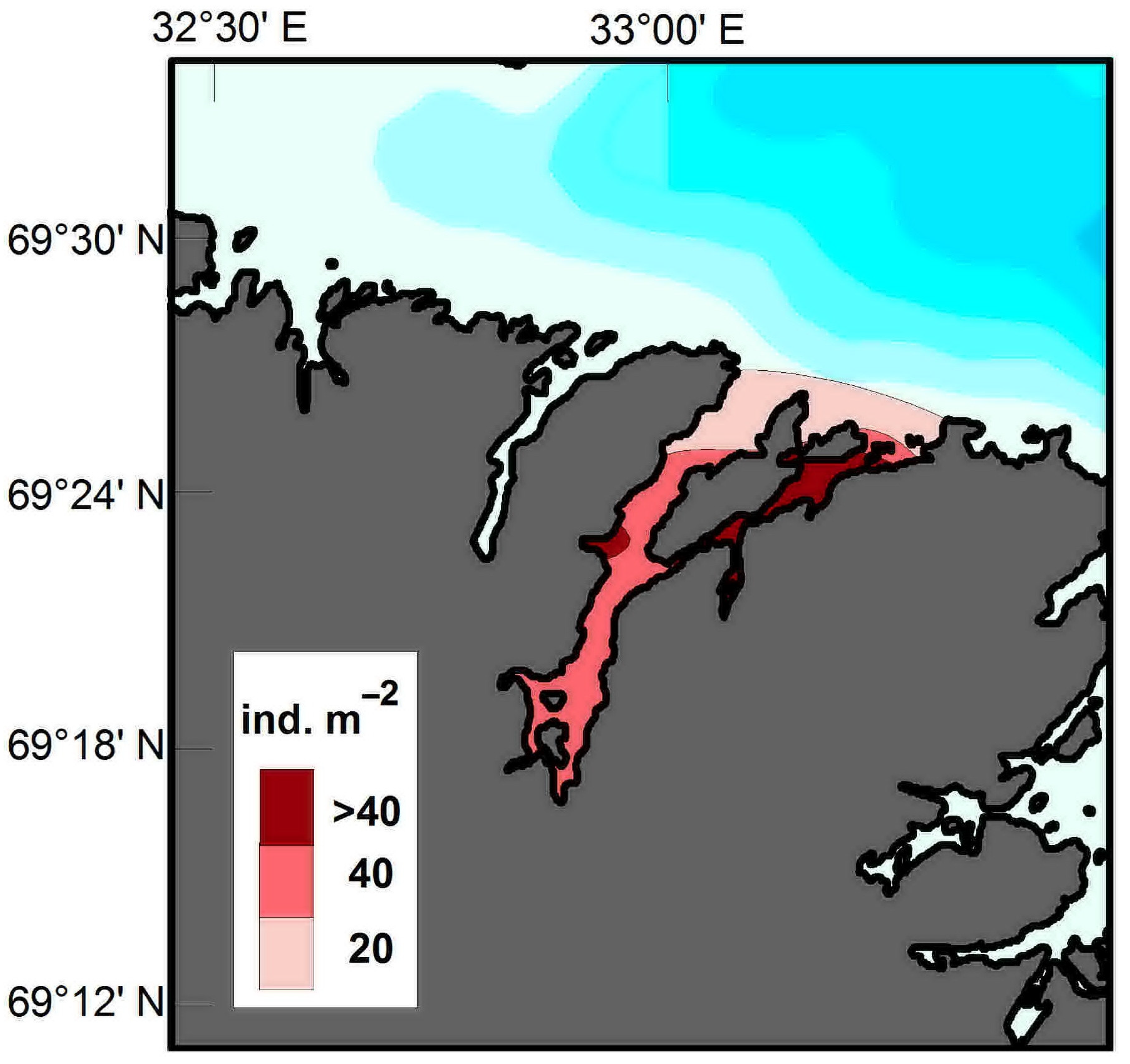
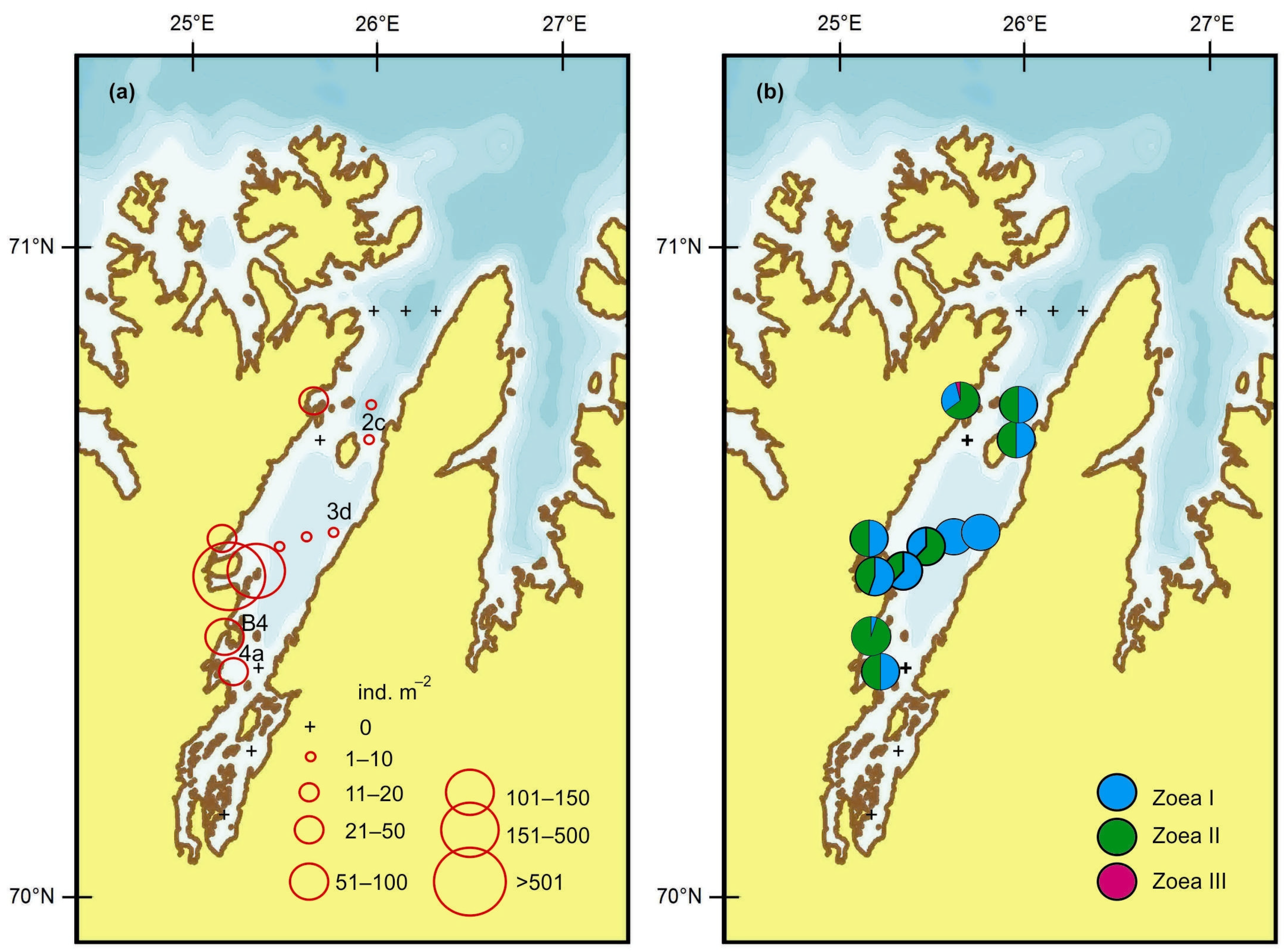
4.1. Horizontal Pattern
4.2. Vertical Pattern
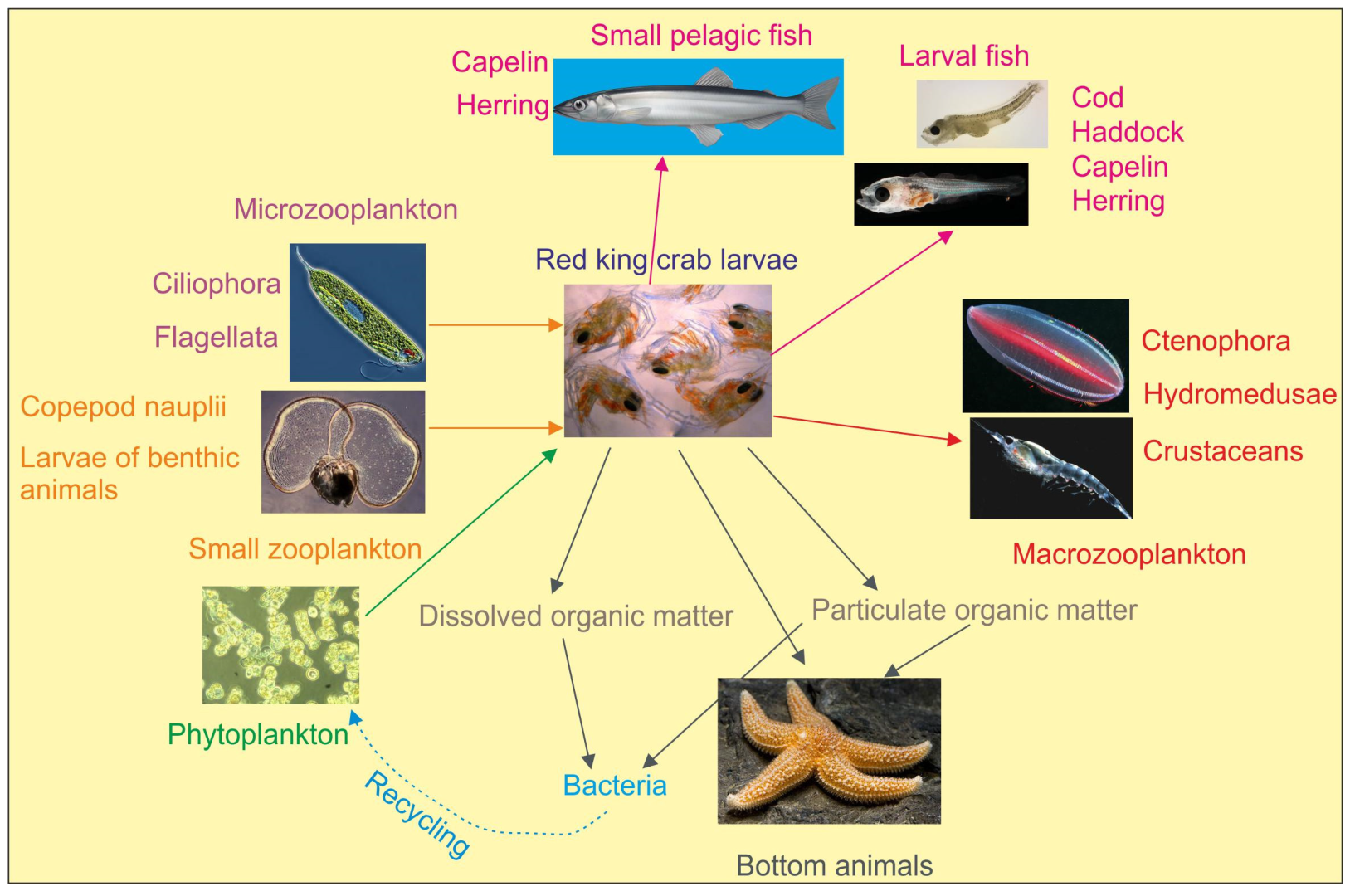
5. Role of RKC Larvae in Plankton Communities in the Barents Sea
6. Environmental Impact on RKC Larvae in the Barents Sea
6.1. Temperature and Salinity
6.2. Currents
6.3. Acidification
6.4. Overall Environmental Changes
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wassmann, P.; Reigstad, M.; Haug, T.; Rudels, B.; Carroll, M.L.; Hop, H.; Gabrielsen, G.W.; Falk-Petersen, S.; Denisenko, S.G.; Arashkevich, E.; et al. Food webs and carbon flux in the Barents Sea. Progr. Oceanogr. 2006, 71, 232–287. [Google Scholar] [CrossRef]
- Jakobsen, T.; Ozhigin, V.K. (Eds.) The Barents Sea: Ecosystem, Resources, Management: Half a Century of Russian-Norwegian Co-Operation; Tapir Academic Press: Trondheim, Norway, 2011. [Google Scholar]
- Dvoretsky, V.G.; Dvoretsky, A.G. Coastal mesozooplankton assemblages during spring bloom in the eastern Barents Sea. Biology 2022, 11, 204. [Google Scholar] [CrossRef] [PubMed]
- Meier, W.N.; Hovelsrud, G.K.; van Oort, B.E.H.; Key, J.R.; Kovacs, K.M.; Michel, C.; Haas, C.; Granskog, M.A.; Gerland, S.; Perovich, D.K.; et al. Arctic sea ice in transformation: A review of recent observed changes and impacts on biology and human activity. Rev. Geophys. 2014, 51, 185–217. [Google Scholar] [CrossRef]
- ICES. Working Group on the Integrated Assessments of the Barents Sea (WGIBAR); ICES Scientific Reports; Issue 30; International Council for the Exploration of the Sea: Copenhagen, Denmark, 2020; Volume 2. [Google Scholar]
- ICES. Working Group on the Integrated Assessments of the Barents Sea (WGIBAR); ICES Scientific Reports; Issue 77; International Council for the Exploration of the Sea: Copenhagen, Denmark, 2021; Volume 3. [Google Scholar]
- Dvoretsky, V.G.; Dvoretsky, A.G. Winter zooplankton in a small Arctic lake: Abundance and vertical distribution. Water 2021, 13, 912. [Google Scholar] [CrossRef]
- Bilge, T.A.; Fournier, N.; Mignac, D.; Hume-Wright, L.; Bertino, L.; Williams, T.; Tietsche, S. An evaluation of the performance of sea ice thickness forecasts to support Arctic marine transport. J. Mar. Sci. Eng. 2022, 10, 265. [Google Scholar] [CrossRef]
- Polyakov, I.V.; Alkire, M.B.; Bluhm, B.A.; Brown, K.A.; Carmack, E.C.; Chierici, M.; Danielson, S.L.; Ellingsen, I.; Ershova, E.A.; Gårdfeldt, K.; et al. Borealization of the Arctic Ocean in response to anomalous advection from sub-arctic seas. Front. Mar. Sci. 2020, 7, 491. [Google Scholar] [CrossRef]
- Polyakov, I.V.; Pnyushkov, A.; Alkire, M.; Ashik, I.M.; Baumann, T.M.; Carmack, E.C.; Goszczko, I.; Guthrie, J.D.; Ivanov, V.V.; Kanzow, T.; et al. Greater role for Atlantic inflows on sea-ice loss in the Eurasian Basin of the Arctic Ocean. Science 2017, 356, 285–291. [Google Scholar] [CrossRef] [Green Version]
- Sakshaug, E.; Johnsen, G.; Kovacs, K. (Eds.) Ecosystem Barents Sea; Tapir Academic Press: Trondheim, Norway, 2009. [Google Scholar]
- Makarevich, P.R.; Vodopianova, V.V.; Bulavina, A.S. Dynamics of the spatial chlorophyll-a distribution at the Polar Front in the marginal ice zone of the Barents Sea during spring. Water 2022, 14, 101. [Google Scholar] [CrossRef]
- Krause-Jensen, D.; Archambault, P.; Assis, J.; Bartsch, I.; Bischof, K.; Filbee-Dexter, K.; Dunton, K.H.; Maximova, O.; Ragnarsdóttir, S.B.; Sejr, M.K.; et al. Imprint of climate change on Pan-Arctic marine vegetation. Front. Mar. Sci. 2020, 7, 617324. [Google Scholar] [CrossRef]
- Pecuchet, L.; Blanchet, M.-A.; Frainer, A.; Husson, B.; Jørgensen, L.L.; Kortsch, S.; Primicerio, R. Novel feeding interactions amplify the impact of species redistribution on an Arctic food web. Glob Change Biol. 2020, 26, 4894–4906. [Google Scholar] [CrossRef] [PubMed]
- Evseeva, O.Y.; Ishkulova, T.G.; Dvoretsky, A.G. Environmental drivers of an intertidal bryozoan community in the Barents Sea: A case study. Animals 2022, 12, 552. [Google Scholar] [CrossRef] [PubMed]
- Johannesen, E.; Ingvaldsen, R.B.; Bogstad, B.; Dalpadado, P.; Eriksen, E.; Gjøsæter, H.; Knutsen, T.; Skern-Mauritzen, M.; Stiansen, J.E. Changes in Barents Sea ecosystem state, 1970–2009: Climate fluctuations, human impact, and trophic interac-tions. ICES J. Mar. Sci. 2012, 69, 880–889. [Google Scholar] [CrossRef] [Green Version]
- Chan, F.T.; Stanislawczyk, K.; Sneekes, A.C.; Dvoretsky, A.; Gollasch, S.; Minchin, D.; David, M.; Jelmert, A.; Albretsen, J.; Bailey, S.A. Climate change opens new frontiers for marine species in the Arctic: Current trends and future invasion risks. Glob. Change Biol. 2019, 25, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Dvoretsky, V.G.; Dvoretsky, A.G. Structure of mesozooplankton community in the Barents Sea and adjacent waters in August 2009. J. Nat. Hist. 2013, 47, 2095–2114. [Google Scholar] [CrossRef]
- Dvoretsky, V.G.; Dvoretsky, A.G. Estimated copepod production rate and structure of mesozooplankton communities in the coastal Barents Sea during summer–autumn 2007. Polar Biol. 2012, 35, 1321–1342. [Google Scholar] [CrossRef]
- Dvoretsky, V.G.; Dvoretsky, A.G. Epiplankton in the Barents Sea: Summer variations of mesozooplankton biomass, community structure and diversity. Contint Shelf Res. 2013, 52, 1–11. [Google Scholar] [CrossRef]
- Dvoretsky, V.G.; Dvoretsky, A.G. Arctic marine mesozooplankton at the beginning of the polar night: A case study for southern and south-western Svalbard waters. Polar Biol. 2020, 43, 71–79. [Google Scholar] [CrossRef]
- Dvoretsky, V.G.; Dvoretsky, A.G. Mesozooplankton in the Kola Transect (Barents Sea): Autumn and winter structure. J. Sea Res. 2018, 142, 125–131. [Google Scholar] [CrossRef]
- Dvoretsky, A.G.; Dvoretsky, V.G. Commercial fish and shellfish in the Barents Sea: Have introduced crab species affected the population trajectories of commercial fish? Rev. Fish Biol. Fish. 2015, 25, 297–322. [Google Scholar] [CrossRef]
- Dvoretsky, A.G.; Dvoretsky, V.G. Red king crab (Paralithodes camtschaticus) fisheries in Russian waters: Historical review and present status. Rev. Fish Biol. Fish. 2018, 28, 331–353. [Google Scholar] [CrossRef]
- Dvoretsky, A.G.; Dvoretsky, V.G. Ecology of Red King Crab in the Coastal Barents Sea; SSC RAS Publishers: Rostov-on-Don, Russia, 2018. (In Russian) [Google Scholar]
- Stevens, B.G.; Lovrich, G.A. King Crabs of the World: Species and Distributions. In King Crabs of the World: Biology and Fisheries Management; Stevens, B.G., Ed.; CRC Press (Taylor and Francis Group): Boca Raton, FL, USA, 2014; pp. 1–29. [Google Scholar]
- Dvoretsky, A.G.; Dvoretsky, V.G. Red king crab in Russia: Populations, fisheries, and symbionts. In King crabs of the World: Biology and Fisheries Management; Stevens, B.G., Ed.; CRC Press (Taylor and Francis Group): Boca Raton, FL, USA, 2014; pp. 501–518. [Google Scholar]
- Orlov, Y.I.; Ivanov, B.G. On the introduction of the Kamchatka king crab Paralithodes camtschatica (Decapoda: Anomura: Lithodidae) into the Barents Sea. Mar. Biol. 1978, 48, 373–375. [Google Scholar] [CrossRef]
- Kuzmin, S.A.; Gudimova, E.N. Introduction of the Kamchatka (Red King) Crab in the Barents Sea: Peculiarities of Biology, Perspectives of Fishery; KSC RAS Press: Apatity, Russia, 2002. (In Russian) [Google Scholar]
- Dvoretsky, A.G.; Dvoretsky, V.G. Inter-annual dynamics of the Barents Sea red king crab (Paralithodes camtschaticus) stock indices in relation to environmental factors. Polar Sci. 2016, 10, 541–552. [Google Scholar] [CrossRef]
- Dvoretsky, A.G.; Dvoretsky, V.G. Effects of environmental factors on the abundance, biomass, and individual weight of juvenile red king crabs in the Barents Sea. Front. Mar. Sci. 2020, 7, 726. [Google Scholar] [CrossRef]
- Dvoretsky, A.G.; Dvoretsky, V.G. New echinoderm-crab epibiotic associations from the coastal Barents Sea. Animals 2021, 11, 917. [Google Scholar] [CrossRef]
- Dvoretsky, A.G.; Dvoretsky, V.G. Epibiotic communities of common crab species in the coastal Barents Sea: Biodiversity and infestation patterns. Diversity 2022, 14, 6. [Google Scholar] [CrossRef]
- Dvoretsky, A.G.; Dvoretsky, V.G. Renewal of the recreational red king crab fishery in Russian waters of the Barents Sea: Potential benefits and costs. Mar. Policy 2022, 136, 104916. [Google Scholar] [CrossRef]
- Dvoretsky, A.G.; Bichkaeva, F.A.; Baranova, N.F.; Dvoretsky, V.G. Fatty acid composition of the Barents Sea red king crab (Paralithodes camtschaticus) leg meat. J. Food Compos. Anal. 2021, 98, 103826. [Google Scholar] [CrossRef]
- Ponomareva, T.; Timchenko, M.; Filippov, M.; Lapaev, S.; Sogorin, E. Prospects of red king crab hepatopancreas processing: Fundamental and applied biochemistry. Recycling 2021, 6, 3. [Google Scholar] [CrossRef]
- Dvoretsky, A.G.; Bichkaeva, F.A.; Baranova, N.F.; Dvoretsky, V.G. Fatty acid composition in the hepatopancreas of the Barents Sea red king crab. Biol. Bull. 2020, 47, 332–338. [Google Scholar] [CrossRef]
- Dvoretsky, A.G.; Bichkaeva, F.A.; Baranova, N.F.; Dvoretsky, V.G. Fatty acids in the circulatory system of an invasive king crab from the Barents Sea. J. Food Compos. Anal. 2022, 110, 104528. [Google Scholar] [CrossRef]
- Didham, R.K.; Hutchinson, M.A.; Ewers, R.M.; Gemmel, N.J. Are invasive species the drivers of ecological change? Trends Ecol. Evol. 2005, 20, 470–474. [Google Scholar] [CrossRef] [PubMed]
- Britayev, T.A.; Rzhavsky, A.V.; Pavlova, L.V.; Dvoretskij, A.G. Studies on impact of the alien Red King Crab (Paralithodes camtschaticus) on the shallow water benthic communities of the Barents Sea. J. Appl. Ichthyol. 2010, 26 (Suppl. S2), 66–73. [Google Scholar] [CrossRef]
- Oug, E.; Cochrane, S.; Sundet, J.; Norling, K.; Nilsson, H. Effects of the invasive red king crab (Paralithodes camtschaticus) on soft-bottom fauna in Varangerfjorden, northern Norway. Mar. Biodivers. 2011, 41, 467–479. [Google Scholar] [CrossRef]
- Oug, E.; Cochrane, S.K.J.; Sundet, J.H. Structural and functional changes of soft-bottom ecosystems in northern fjords invaded by the red king crab (Paralithodes camtschaticus). J. Mar. Syst. 2018, 180, 255–264. [Google Scholar] [CrossRef]
- Eriksen, E.; Benzik, A.N.; Dolgov, A.V.; Skjoldal, H.R.; Vihtakari, M.; Johannesen, E.; Prokhorova, T.A.; Keulder-Stenevik, F.; Prokopchuk, I.; Strand, E. Diet and trophic structure of fishes in the Barents Sea: The Norwegian-Russian program “Year of stomachs” 2015—Establishing a baseline. Progr. Oceanogr. 2020, 183, 102262. [Google Scholar] [CrossRef]
- Pavlova, L.V.; Dvoretsky, A.G. Prey selectivity in juvenile red king crabs from the coastal Barents Sea. Diversity 2022, 14, 568. [Google Scholar] [CrossRef]
- Dvoretsky, A.G.; Dvoretsky, V.G. Fouling community of the red king crab, Paralithodes camtschaticus (Tilesius 1815), in a subarctic fjord of the Barents Sea. Polar Biol. 2009, 32, 1047–1054. [Google Scholar] [CrossRef]
- Dvoretsky, A.G.; Dvoretsky, V.G. Distribution of amphipods Ischyrocerus on the red king crab, Paralithodes camtschaticus: Possible interactions with the host in the Barents Sea. Estuar. Coast. Shelf Sci. 2009, 82, 390–396. [Google Scholar] [CrossRef]
- Dvoretsky, A.G.; Dvoretsky, V.G. Limb autotomy patterns in Paralithodes camtschaticus (Tilesius, 1815), an invasive crab, in the coastal Barents Sea. J. Exp. Mar. Biol. Ecol. 2009, 377, 20–27. [Google Scholar] [CrossRef]
- Dvoretsky, A.G.; Dvoretsky, V.G. Some aspects of the biology of the amphipods Ischyrocerus anguipes associated with the red king crab, Paralithodes camtschaticus, in the Barents Sea. Polar Biol. 2009, 32, 463–469. [Google Scholar] [CrossRef]
- Dvoretsky, A.G.; Dvoretsky, V.G. Epifauna associated with an introduced crab in the Barents Sea: A 5-year study. ICES J. Mar. Sci. 2010, 67, 204–214. [Google Scholar] [CrossRef] [Green Version]
- Dvoretsky, A.G.; Dvoretsky, V.G. The amphipod Ischyrocerus commensalis on the eggs of the red king crab Paralithodes camtschaticus: Egg predator or scavenger? Aquaculture 2010, 298, 185–189. [Google Scholar] [CrossRef]
- Dvoretsky, A.G.; Dvoretsky, V.G. Does spine removal affect molting process in the king red crab (Paralithodes camtschaticus) in the Barents Sea? Aquaculture 2012, 326–329, 173–177. [Google Scholar] [CrossRef]
- Dvoretsky, A.G.; Dvoretsky, V.G. Copepods associated with the red king crab Paralithodes camtschaticus (Tilesius, 1815) in the Barents Sea. Zool. Stud. 2013, 52, 17. [Google Scholar] [CrossRef] [Green Version]
- Dvoretsky, A.G.; Dvoretsky, V.G. Population dynamics of the invasive lithodid crab, Paralithodes camtschaticus, in a typical bay of the Barents Sea. ICES J. Mar. Sci. 2013, 70, 1255–1262. [Google Scholar] [CrossRef]
- Dvoretsky, A.G.; Dvoretsky, V.G. Size-at-age of juvenile red king crab (Paralithodes camtschaticus) in the coastal Barents Sea. Cah. Biol. Mar. 2014, 55, 43–48. [Google Scholar]
- Dvoretsky, A.G.; Dvoretsky, V.G. Size at maturity of female red king crab, Paralithodes camtschaticus, from the costal zone of Kola Peninsula (southern Barents Sea). Cah. Biol. Mar. 2015, 56, 49–54. [Google Scholar]
- Falk-Petersen, J.; Renaud, P.; Anisimova, N. Establishment and ecosystem effects of the alien invasive red king crab (Paralithodes camtschaticus) in the Barents Sea—A review. ICES J. Mar. Sci. 2011, 68, 479–488. [Google Scholar] [CrossRef] [Green Version]
- Sundet, J.H. Red king crab in the Barents Sea. In King Crabs of the World: Biology and Fisheries Management; Stevens, B.G., Ed.; CRC Press (Taylor and Francis Group): Boca Raton, FL, USA, 2014; pp. 477–492. [Google Scholar]
- Windsland, K.; Hvingel, C.; Nilssen, E.M.; Sundet, J.H. Dispersal of the introduced red king crab (Paralithodes camtschaticus) in Norwegian waters: A tag-recapture study. ICES J. Mar. Sci. 2014, 71, 1966–1976. [Google Scholar] [CrossRef] [Green Version]
- Fuhrmann, M.M.; Pedersen, T.; Nilssen, E.M. Trophic niche of the invasive red king crab (Paralithodes camtschaticus) in a native benthic food web. Mar. Ecol. Prog. Ser. 2017, 565, 113–129. [Google Scholar] [CrossRef]
- Pedersen, T.; Fuhrmann, M.M.; Lindstrøm, U.; Nilssen, E.M.; Ivarjord, T.; Ramasco, V.; Jørgensen, L.L.; Sundet, J.H.; Sivertsen, K.; Källgren, E. Effects of the invasive red king crab on food web structure and ecosystem properties in an Atlantic fjord. Mar. Ecol. Prog. Ser. 2018, 596, 13–31. [Google Scholar] [CrossRef] [Green Version]
- Dvoretsky, A.G.; Tipisova, E.V.; Elfimova, A.E.; Alikina, V.A.; Dvoretsky, V.G. Sex hormones in hemolymph of red king crabs from the Barents Sea. Animals 2021, 11, 2149. [Google Scholar] [CrossRef] [PubMed]
- Dvoretsky, A.G.; Tipisova, E.V.; Alikina, V.A.; Elfimova, A.E.; Dvoretsky, V.G. Thyroid hormones in hemolymph of red king crabs from the Barents Sea. Animals 2022, 12, 379. [Google Scholar] [CrossRef] [PubMed]
- Stesko, A.V.; Bakanev, S.V. Bycatches of the red king crab in the bottom fish fishery in the Russian waters of the Barents Sea: Assessment and regulations. ICES J. Mar. Sci. 2021, 78, 575–583. [Google Scholar] [CrossRef]
- Anger, K. Contributions of larval biology to crustacean research: A review. Invert. Repr. Dev. 2006, 49, 175–205. [Google Scholar] [CrossRef] [Green Version]
- Manushin, I.; Anisimova, N. Selectivity in the red king crab feeding in the Barents Sea Research on the red king crab (Paralithodes camtschaticus) from the Barents Sea in 2005–2007. In IMR/PINRO Joint Report Series; Sundet, J.H., Berenboim, B., Eds.; Institute of Marine Research: Bergen, Norway, 2008; pp. 24–28. [Google Scholar]
- Bakanev, S.V. Fecundity and some other reproductive parameters of red king crab in the Barents Sea. In The Red King Crab in the Barents Sea; Berenboim, B.I., Ed.; PINRO Press: Murmansk, Russia, 2003; pp. 78–88. (In Russian) [Google Scholar]
- Stevens, B.G. Development and biology of king crab larvae. In King Crabs of the World: Biology and Fisheries Management; Stevens, B.G., Ed.; CRC Press (Taylor and Francis Group): Boca Raton, FL, USA, 2014; pp. 233–259. [Google Scholar]
- Bakanev, S.V. Larvae of red king crab in the coastal areas and large bays of Murman. In The Red King Crab in the Barents Sea; Berenboim, B.I., Ed.; PINRO Press: Murmansk, Russia, 2003; pp. 122–133. (In Russian) [Google Scholar]
- Dvoretsky, A.G.; Dvoretsky, V.G. Hemolymph molting hormone concentrations in red king crabs from the Barents Sea. Polar Biol. 2010, 33, 1293–1298. [Google Scholar] [CrossRef]
- Pinchukov, M.A.; Berenboim, B.I. Molting and growth of red king crab in the Barents Sea. In The Red King Crab in the Barents Sea; Berenboim, B.I., Ed.; PINRO Press: Murmansk, Russia, 2003; pp. 100–106. (In Russian) [Google Scholar]
- Dvoretsky, A.G.; Dvoretsky, V.G. Population biology of Ischyrocerus commensalis, a crab-associated amphipod, in the southern Barents Sea: A multi-annual summer study. Mar. Ecol. 2011, 32, 498–508. [Google Scholar] [CrossRef]
- Marukawa, H. Biology and fishery research on Japanese king crab Paralithodes camtschatica. J. Imper. Fish. Exper. Sta. Tokyo 1933, 37, 1–152. [Google Scholar]
- Epelbaum, A.B.; Borisov, R.R.; Kovatcheva, N.P. Early development of the red king crab Paralithodes camtschaticus from the Barents Sea reared under laboratory conditions: Morphology and behaviour. J. Mar. Biol. Assoc. UK 2006, 86, 317–333. [Google Scholar] [CrossRef]
- Sato, S.; Tanaka, S. Study on the larval stage of Paralithodes camtschatica (Tilesius) I. About morphological research. Bull. Hokkaido Reg. Fish. Res. Lab. 1949, 1, 7–24. [Google Scholar]
- Sato, S. Studies on larval development and fishery biology of king crab, Paralithodes camtschatica (Tilesius). Bull. Hokkaido Reg. Fish. Res. Lab. 1958, 17, 1–102. [Google Scholar]
- Nakanishi, T. Rearing condition of eggs, larvae and post-larvae of king crab. Bull. Japan Sea Reg. Fish. Lab. 1987, 37, 57–161. [Google Scholar]
- Jensen, G.C.; Andersen, H.B.; Armstrong, D.A. Differentiating Paralithodes larvae using telson spines: A tail of two species. Fish. Bull. 1992, 90, 778–783. [Google Scholar]
- Matyushkin, V.B.; Ushakova, M.F. Features of the larval cycle of red king crab (Paralithodes camtschaticus) and hermit crab (Pagurus pubescens) in the fjord waters of Western Murman. In Bioresources and Aquaculture in the Coastal Areas of the Barents and White Seas; PINRO Press: Murmansk, Russia, 2002; pp. 125–136. (In Russian) [Google Scholar]
- Matyushkin, V.B.; Ushakova, M.F. Larvae of red king crab in the fjords of Western Murman. In The Red King Crab in the Barents Sea; Berenboim, B.I., Ed.; PINRO Press: Murmansk, Russia, 2003; pp. 133–140. (In Russian) [Google Scholar]
- Dvoretskii, V.G. Distribution of euphausiid and decapod larvae in the spring plankton of the southern Barents Sea. Biol. Bull. 2011, 38, 393–399. [Google Scholar] [CrossRef]
- Shamray, T.V. Changes in the abundance and terms of presence in the plankton of the red king crab larvae within the Ura Bay (West Murman) in 2011–2016. Vestn. MGTU 2017, 20, 493–502. (In Russian) [Google Scholar] [CrossRef]
- Shamray, T.V. Distribution of pelagic larvae of some representatives of the Decapoda order in the coastal waters of Western Murman. In Biological Resources of Fishing off the Coast of Murmansk; Sokolov, V.M., Ed.; PINRO Press: Murmansk, Russia, 2013; pp. 129–140. (In Russian) [Google Scholar]
- Dvoretsky, V.G.; Dvoretsky, A.G. Ecology of Zooplankton Communities in the Barents Sea and Adjacent Waters; Renome: St. Petersburg, Russia, 2015. (In Russian) [Google Scholar]
- Shamray, T.V.; Matushkin, V.B. Larvae of the red king crab in the coastal waters of Western Murman. In The Red king Crab in the Barents Sea; Bizikov, V.A., Stesko, A.V., Alexeev, D.O., Buyanovsky, A.I., Dolgov, A.V., Novikov, M.A., Pereladov, M.V., Sentyabov, E.V., Sokolov, K.M., Eds.; VNIRO Publishing: Moscow, Russia, 2021; pp. 223–239. (In Russian) [Google Scholar]
- Michelsen, H.K.; Svensen, C.; Reigstad, M.; Nilssen, E.M.; Pedersen, T. Seasonal dynamics of meroplankton in a high-latitude fjord. J. Mar. Syst. 2017, 168, 17–30. [Google Scholar] [CrossRef] [Green Version]
- Michelsen, H.K.; Nilssen, E.M.; Pedersen, T.; Reigstad, M.; Svensen, C. Spatial patterns of spring meroplankton along environmental gradients in a sub-Arctic fjord. Aquat. Biol. 2017, 26, 185–197. [Google Scholar] [CrossRef] [Green Version]
- Michelsen, H.K.; Nilssen, E.M.; Pedersen, T.; Svensen, C. Temporal and spatial dynamics of the invasive red king crab and native brachyuran and anomuran larvae in Norwegian waters. Aquat. Biol. 2020, 29, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Stevens, B.G.; Swiney, K.M. Hatch timing, incubation period, and reproductive cycle for captive primiparous and multiparous red king crab, Paralithodes camtschaticus. J. Crust. Biol. 2007, 27, 37–48. [Google Scholar] [CrossRef] [Green Version]
- Ushakova, M.V. Distribution and abundance of larvae of some common crustacean species of in the coastal waters of the Western Murman. In Management of the Coastal Zone in the Northern Seas; Russian State Hydrometeorological University: St. Petersburg, Russia, 1999; pp. 184–188. (In Russian) [Google Scholar]
- Dvoretsky, V.G.; Dvoretsky, A.G. Zooplankton productivity in the coastal area of the southern Barents Sea in spring. Mar. Biol. J. 2020, 5, 3–14. [Google Scholar]
- Otto, R.S.; Macintosh, R.A.; Cummiskey, P.A. Fecundity and other reproductive parameters of female red king crab (Paralithodes camtschaticus) in Bristol Bay and Norton Sound, Alaska. In Proceedings of the International Symposium on King and Tanner Crabs. Univ. Alaska Sea Grant Rep., Fairbanks, AK, USA, 28–30 November 1989; pp. 65–90. [Google Scholar]
- Makarov, R.R. Larvae of Shrimps, Hermit Crabs and Crabs of the Western Kamchatka Shelf and Their Distribution; Nauka Publishing: Moscow, Russia, 1966. (In Russian) [Google Scholar]
- Paul, A.J.; Paul, J.M.; Coyle, K.O. Energy sources for first-feeding zoeae of king crab Paralithodes camtschatica (Tilesius). J. Exp. Mar. Biol. Ecol. 1989, 130, 55–69. [Google Scholar] [CrossRef]
- Paul, A.J.; Paul, J.M. Growth of stage I king crab larvae of Paralithodes camtschatica (Tilesius) (Decapoda:Lithodidae) in natural communities. J. Crust. Biol. 1990, 10, 175–183. [Google Scholar] [CrossRef]
- Klitin, A.K.; Samatov, A.D. Role of larvae dispersal in population dynamics of the red king crab in Tatar Strait. In Fisheries Investigations of the World’s Ocean; Dalrybvtuz Press: Valdivostok, Russia, 1999; pp. 140–142. (In Russian) [Google Scholar]
- McMurray, G.; Vogel, A.H.; Fishman, P.A.; Armstrong, D.A.; Jewett, S.C. Distribution of larval and juvenile red king crabs (Paralithodes camtschatica) in Bristol Bay. In Outer Continental Shelf Environmental Assessment Program. Report No. 53; NOAA Office of Marine Pollution Assessment; Alaska Office: Anchorage, AK, USA, 1986; pp. 267–477. [Google Scholar]
- Shirley, S.M.; Shirley, T.C. Interannual variability in density, timing and survival of Alaskan red king crab Paralithodes camtschatica larvae. Mar. Ecol. Prog. Ser. 1989, 54, 51–59. [Google Scholar] [CrossRef]
- Harms, J.; Seeger, B. Larval development and survival in seven decapod species (Crustacea) in relation to laboratory diet. J. Exp. Mar. Biol. Ecol. 1989, 133, 129–139. [Google Scholar] [CrossRef]
- Bright, D.B. Life Histories of the King Crab, Paralithodes Camtschatica, and the Tanner Crab, Chionoecetes Bairdi, in Cook Inlet, Alaska. Ph.D. Thesis, University of Southern California, Los Angeles, CA, USA, 1967. [Google Scholar]
- Sato, S.; Tanaka, S. Study on the larval stage of Paralithodes camtschatica (Tilesius) II. On the rearing. Sci. Pap. Hokkaido Fish. Sci. Inst. (Transl.) 1949, 3, 18–30. [Google Scholar]
- Kurata, H. Studies on the larva and post-larva of Paralithodes camtschatica II. Feeding habits of the zoea. Bull. Hokkaido Reg. Fish. Res. Lab. 1960, 21, 1–8. [Google Scholar]
- Epelbaum, A.B.; Kovatcheva, N.P. Daily food intakes and optimal food concentrations for red king crab (Paralithodes camtschaticus) larvae fed Artemia nauplii under laboratory conditions. Aquaculture Nutr. 2005, 11, 455–461. [Google Scholar] [CrossRef]
- Epelbaum, A.; Borisov, R.R. Feeding behavior and functional morphology of the feeding appendages of red king crab Paralithodes camtschaticus larvae. Mar. Biol. Res. 2006, 2, 77–88. [Google Scholar] [CrossRef]
- Paul, A.J.; Paul, J.M. The effect of early starvation on later feeding success of king crab zoeae. J. Exp. Mar. Biol. Ecol. 1980, 44, 247–251. [Google Scholar] [CrossRef]
- Makarevich, P.; Druzhkova, E.; Larionov, V. Primary producers of the Barents Sea. In Diversity of Ecosystems; Mahamane, A., Ed.; In Tech: Rijeka, Croatia, 2012; pp. 367–392. [Google Scholar]
- Makarevich, P.R.; Vodopianova, V.V.; Bulavina, A.S.; Vashchenko, P.S.; Ishkulova, T.G. Features of the distribution of chlorophyll-a concentration along the western coast of the Novaya Zemlya archipelago in spring. Water 2021, 13, 3648. [Google Scholar] [CrossRef]
- Dvoretsky, V.G.; Dvoretsky, A.G. Summer mesozooplankton structure in the Pechora Sea (south-eastern Barents Sea). Estuar. Coast. Shelf Sci. 2009, 84, 11–20. [Google Scholar] [CrossRef]
- Dvoretsky, V.G.; Dvoretsky, A.G. Summer mesozooplankton distribution near Novaya Zemlya (eastern Barents Sea). Polar Biol. 2009, 32, 719–731. [Google Scholar] [CrossRef]
- Dvoretsky, V.G.; Dvoretsky, A.G. Early winter mesozooplankton of the coastal south-eastern Barents Sea. Estuar. Coast. Shelf Sci. 2015, 152, 116–123. [Google Scholar] [CrossRef]
- Dvoretsky, V.G.; Dvoretsky, A.G. Macrozooplankton of the Arctic—The Kara Sea in relation to environmental conditions. Estuar. Coast. Shelf Sci. 2017, 188, 38–55. [Google Scholar] [CrossRef]
- Dvoretsky, V.G.; Dvoretsky, A.G. Summer macrozooplankton assemblages of Arctic shelf: A latitudinal study. Cont. Shelf Res. 2019, 188, 103967. [Google Scholar] [CrossRef]
- Dvoretsky, V.G.; Dvoretsky, A.G. Summer-fall macrozooplankton assemblages in a large Arctic estuarine zone (south-eastern Barents Sea): Environmental drivers of spatial distribution. Mar. Environ. Res. 2022, 173, 105498. [Google Scholar] [CrossRef]
- Paul, A.J.; Paul, J.M. Growth assays with first-feeding zoeae of king crab (Paralithodes camtschaticus, Decapoda: Lithodidae) in a plankton community of a deep fjord. In High Latitude Crabs: Biology, Management, and Economics. Report No. 96-02; University of Alaska Sea Grant: Anchorage, AK, USA, 1996; pp. 479–488. [Google Scholar]
- Straty, R.R. Ecology and behavior of juvenile sockeye salmon (Oncorhynchus nerka) in Bristol Bay and the eastern Bering Sea. In Oceanography of the Bering Sea with Emphasis on Renewable Resources; Hood, D.W., Kelley, E.J., Eds.; Institute of Marine Sciences Occasional Publication 2, University of Alaska: Fairbanks, AK, USA, 1974; pp. 285–320. [Google Scholar]
- Healey, M.C. The ecology of juvenile salmon in Georgia Strait, British Columbia. In Salmonid Ecosystems of the North Pacific; McNeil, W.J., Himsworth, D.C., Eds.; Oregon State University Press: Corvallis, OR, USA, 1980; pp. 203–229. [Google Scholar]
- Wespestad, V.G.; Livingston, P.A.; Reeves, J.E. Juvenile Sockeye Salmon (Oncorhynchus Nerka) Predation on Bering Sea Red King Crab (Paralithodes Camtschaticus) Larvae as a Cause of Recruitment Variation; ICES CM 1994/R: Copengagen, Denmark, 1994; Volume 10. [Google Scholar]
- Shirley, T.C.; Shirley, S.M.; Korn, S. Incubation period, molting, and growth of female red king crabs: Effects of temperature. In Proceedings of the International Symposium on King and Tanner Crabs; Meltef, B., Ed.; University of Alaska Sea Grant Program: Anchorage, AK, USA, 1990; pp. 51–64. [Google Scholar]
- Long, W.C.; Swiney, K.M.; Foy, R.J. Effects of ocean acidification on the embryos and larvae of red king crab, Paralithodes camtschaticus. Mar. Poll. Bull. 2013, 69, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Kurata, H. Studies on the larvae and postlarvae of Paralithodes camtschatica. III. The influence of temperature and salinity on the survival and growth of the larvae. Bull. Hokkaido Reg. Fish. Res. Lab. 1960, 21, 9–14. [Google Scholar]
- Shirley, S.M.; Shirley, T.C. Temperature and salinity tolerances and preferences of red king crab larvae. Mar. Behav. Physiol. 1989, 16, 19–30. [Google Scholar] [CrossRef]
- Swingle, J.S.; Daly, B.; Hetrick, J. Temperature effects on larval survival, larval period, and health of hatchery-reared red king crab, Paralithodes camtschaticus. Aquaculture 2013, 384, 13–18. [Google Scholar] [CrossRef]
- Sparboe, M.; Christiansen, J.S. Preliminary results from experimental studies of temperature preference and tolerance in Barents Sea red king crab (Paralithodes camtschaticus). In IMR/PINRO Joint Report Series; Sundet, J.H., Berenboim, B., Eds.; Institute of Marine Research: Bergen, Norway, 2008; pp. 57–58. [Google Scholar]
- Larsen, L. Temperature-Dependent Development, Growth and Mortality of Red King Crab (Paralithodes Camtschatica Tilesius) Larvae in Experimental Conditions. Ph.D. Thesis, Norwegian College of Fishery Science, University of Tromsø, Tromsø, Norway, 1996. (In Danish). [Google Scholar]
- Nizyaev, S.A.; Fedoseev, V.Y.; Myasoedov, V.I.; Rodin, V.E. To the Formation of the Yield of Generations of Kamchatka Crab Paralithodes Camtschaticus on the Shelf of Western Kamchatka. In Commercial and Biological Studies of Marine Invertebrates; VNIRO Publishing: Moscow, Russia, 1992; pp. 4–14. (In Russian) [Google Scholar]
- Grigoryeva, N.I. Spatial distribution of the crab larvae (Decapoda: Anomura et Brachyura) in Possyet Bay (Peter the Great Bay of the Sea of Japan) in 2000–2001. Oceanology 2009, 49, 663–671. [Google Scholar] [CrossRef]
- Mileikovsky, S.A. Types of larval development in marine bottom invertebrates, their distribution and ecological significance: A re-evaluation. Mar. Biol. 1971, 10, 193–213. [Google Scholar] [CrossRef]
- Thorson, G. Reproductive and larval ecology of marine bottom invertebrates. Biol. Rev. 1950, 25, 1–45. [Google Scholar] [CrossRef]
- Nizyaev, S.A.; Fedoseev, V.Y. Causes for the reduction in the number of crab generation and their reflection in its reproductive strategy. In Fisheries Research in the Sakhalin-Kurilsky District and Adjacent Water Areas; Yuzhno-Sakhalinsk Publishing House: Yuzhno-Sakhalinsk, Russia, 1994; pp. 57–67. (In Russian) [Google Scholar]
- Loher, T. Modeling larval advection and spatial population structure in king crabs: Interactions among life-history requirements, extrinsic forcing, and source–sink dynamics. In King Crabs of the World: Biology and Fisheries Management; Stevens, B.G., Ed.; CRC Press (Taylor and Francis Group): Boca Raton, FL, USA, 2014; pp. 539–581. [Google Scholar]
- Daly, B.; Parada, C.; Loher, T.; Hinckley, S.; Hermann, A.J.; Armstrong, D. Red king crab larval advection in Bristol Bay: Implications for recruitment variability. Fish. Oceanogr. 2020, 29, 505–525. [Google Scholar] [CrossRef]
- Pedersen, O.; Nilssen, E.M.; Jørgensen, L.L.; Slagstad, D. Advection of the red king crab larvae on the coast of North Norway—A Lagrangian model study. Fish. Res. 2006, 79, 325–336. [Google Scholar] [CrossRef]
- Findlay, H.S.; Kendall, M.A.; Spicer, J.I.; Widdicombe, S. Future high CO2 in the intertidal may compromise adult barnacle Semibalanus balanoides survival and embryonic development rate. Mar. Ecol. Prog. Ser. 2009, 389, 193–202. [Google Scholar] [CrossRef] [Green Version]
- Rato, L.D.; Novais, S.C.; Lemos, M.F.L.; Alves, L.M.F.; Leandro, S.M. Homarus gammarus (Crustacea: Decapoda) larvae under an ocean acidi fi cation scenario: Responses across different levels of biological organization. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2017, 203, 29–38. [Google Scholar] [CrossRef]
- Page, H.N.; Hewett, C.; Tompkins, H.; Hall, E.R. Ocean acidification and direct interactions affect coral, macroalga, and sponge growth in the Florida keys. J. Mar. Sci. Eng. 2021, 9, 739. [Google Scholar] [CrossRef]
- Barruffo, A.; Ciaralli, L.; Ardizzone, G.; Gambi, M.C.; Casoli, E. Ocean acidification and mollusc settlement in posidonia oceanica meadows: Does the seagrass buffer lower ph effects at CO2 vents? Diversity 2021, 13, 311. [Google Scholar] [CrossRef]
- Asnicar, D.; Marin, M.G. Effects of seawater acidification on echinoid adult stage: A review. J. Mar. Sci. Eng. 2022, 10, 477. [Google Scholar] [CrossRef]
- Wood, H.L.; Spicer, J.I.; Widdicombe, S. Ocean acidification may increase calcification rates, but at a cost. Proc. Roy. Soc. B–Biol. Sci. 2008, 275, 1767–1773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stevens, B.G. Embryo development and morphometry in the blue king crab Paralithodes platypus studied by using image and cluster analysis. J. Shellfish Res. 2006, 25, 569–576. [Google Scholar] [CrossRef]
- Ross, P.M.; Parker, L.; O’Connor, W.A.; Bailey, E.A. The impact of ocean acidification on reproduction, early development and settlement of marine organisms. Water 2011, 3, 1005–1030. [Google Scholar] [CrossRef] [Green Version]
- Espinel-Velasco, N.; Hoffmann, L.; Agüera, A.; Byrne, M.; Dupont, S.; Uthicke, S.; Webster, N.S.; Lamare, M. Effects of ocean acidification on the settlement and metamorphosis of marine invertebrate and fish larvae: A review. Mar. Ecol. Prog. Ser. 2018, 606, 237–257. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Latorre, C.; Triay-Portella, R.; Cosme, M.; Tuya, F.; Otero-Ferrer, F. Brachyuran crabs (Decapoda) associated with rhodolith beds: Spatio-temporal variability at Gran Canaria Island. Diversity 2020, 12, 223. [Google Scholar] [CrossRef]
- Bianchi, C.N.; Gerovasileiou, V.; Morri, C.; Froglia, C. Distribution and ecology of decapod crustaceans in Mediterranean marine caves: A review. Diversity 2022, 14, 176. [Google Scholar] [CrossRef]
- Otto, R.S. History of king crab fisheries with special reference to the North Pacific Ocean: Development, Maturity, and Senescence. In King Crabs of the World: Biology and Fisheries Management; Stevens, B.G., Ed.; CRC Press (Taylor and Francis Group): Boca Raton, CA, USA, 2014; pp. 81–138. [Google Scholar]
- Kuris, A.M.; Blau, S.F.; Paul, A.J.; Shields, J.D.; Wickham, D.E. Infestation by brood symbionts and their impact on egg mortality in the red king crab, Paralithodes camtschatica, in Alaska: Geographic and temporal variation. Can. J. Fish. Aquat. Sci. 1991, 48, 559–568. [Google Scholar] [CrossRef]





| Stage | Duration, Days | Carapace Length, mm | Rostrum Length, mm | Abdomen Length, mm | Wet Mass, mg | Dry Mass, mg |
|---|---|---|---|---|---|---|
| T = 7–8 °C | Barents Sea | |||||
| Zoea I | 10 | 1.39 | 1.29 | nd | 0.86 | 0.110 |
| Zoea II | 10 | 1.63 | 1.52 | nd | 1.41 | 0.165 |
| Zoea III | 9 | 1.83 | 1.53 | nd | 2.00 | 0.250 |
| Zoea IV | 10 | 2.07 | 1.63 | nd | 2.67 | 0.300 |
| T = 8 °C | North Pacific | |||||
| Zoea I | 12 | 1.18 | 1.45 | 2.63 | nd | 0.045 |
| Zoea II | 15 | 1.38 | 1.5 | 2.83 | nd | 0.084 |
| Zoea III | 26 | 1.45 | 1.6 | 3.25 | nd | 0.109 |
| Zoea IV | 33 | 1.53 | 1.3 | 3.63 | nd | 0.191 |
| Year | Late February | Early-March | Late March | Early April | Late April | Late May |
|---|---|---|---|---|---|---|
| 2011 | - | 1.0 | 1.0 | - | 7.0 | 4.0 |
| 2012 | 2.0 | 4.0 | 6.5 | 24.0 | 20.0 | 2.0 |
| 2013 | - | 4.0 | - | 2.0 | 3.5 | 16.0 |
| 2014 | 3.3 | - | 33.1 | 13.0 | 1.8 | 1.5 |
| 2015 | - | - | - | 1.5 | 6.5 | 1.0 |
| 2016 | - | 25.0 | 6.0 | 0.0 | - | 0.0 |
| Stage | Region | Period | Reference |
|---|---|---|---|
| Barents Sea | |||
| Zoea I | Ura Bay | Early March–May | [78,79,89] |
| Ura Bay | February–May | [81,82,84] | |
| Coastal waters | Mid–April–May | [68] | |
| Coastal waters | May | [80,83] | |
| Porsangerfjord | January–April | [87] | |
| Zoea II | Ura Bay | March–May | [78,79,89] |
| Ura Bay | February–May | [81,82,84] | |
| Coastal waters | Mid–April–May | [68] | |
| Coastal waters | May | [80,83] | |
| Porsangerfjord | April | [87] | |
| Zoea III | Ura Bay | March–June | [78,79,89] |
| Ura Bay | April–June | [81,82,84] | |
| Coastal waters | May | [68] | |
| Coastal waters | May | [80,83] | |
| Porsangerfjord | April | [87] | |
| Zoea IV | Ura Bay | April–June | [78,79,89] |
| Ura Bay | May–June | [81,82,84] | |
| Coastal waters | May | [80,83] | |
| Open waters | May | [90] | |
| Porsangerfjord | May–June | [87] | |
| North Pacific | |||
| Zoea I | Bristol Bay | March–July | [91] |
| Western Sakhalin waters | March–April | [92] | |
| Western Sakhalin waters | May–June | [75] | |
| Western Kamchatka waters | March–April | [75] | |
| Kamchatka waters | April–July | [92] | |
| Gulf of Alaska | Early April–late May | [93,94] | |
| South–eastern Bering Sea | Mid–April–late June | [92] | |
| Aniva Bay, Sea of Japan | April | [95] | |
| The Peter Great Bay, Sea of Japan | Late April–late May | [75] | |
| Sea of Japan | Late April–late May | 75] | |
| Zoea II | Gulf of Alaska | April–June | [94] |
| Kamchatka waters | May–July | [92] | |
| Zoea III | Gulf of Alaska | Mid–April–July | [94] |
| Kamchatka waters | June–early July | [92] | |
| Zoea IV | Gulf of Alaska | Mid–April–July | [93,94] |
| Tartar Strait | Early May | [95] | |
| Kamchatka waters | June–early July | [92] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dvoretsky, V.G.; Dvoretsky, A.G. Ecology and Distribution of Red King Crab Larvae in the Barents Sea: A Review. Water 2022, 14, 2328. https://doi.org/10.3390/w14152328
Dvoretsky VG, Dvoretsky AG. Ecology and Distribution of Red King Crab Larvae in the Barents Sea: A Review. Water. 2022; 14(15):2328. https://doi.org/10.3390/w14152328
Chicago/Turabian StyleDvoretsky, Vladimir G., and Alexander G. Dvoretsky. 2022. "Ecology and Distribution of Red King Crab Larvae in the Barents Sea: A Review" Water 14, no. 15: 2328. https://doi.org/10.3390/w14152328
APA StyleDvoretsky, V. G., & Dvoretsky, A. G. (2022). Ecology and Distribution of Red King Crab Larvae in the Barents Sea: A Review. Water, 14(15), 2328. https://doi.org/10.3390/w14152328







