Development of a Process for Domestic Wastewater Treatment Using Moringa oleifera for Pathogens and Antibiotic-Resistant Bacteria Inhibition under Tropical Conditions
Abstract
:1. Introduction
2. Materials and Methods
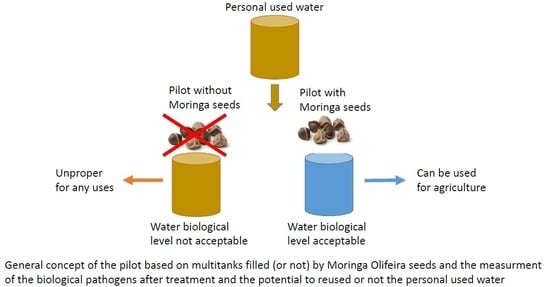
2.1. Conception of Wastewater Treatment Plant Prototype
2.2. Sampling Procedure
2.3. Sample Treatment with Moringa oleifera Seeds
- –
- 32 mg/L for enterobacteria, in particular faecal coliforms and E. coli
- –
- 16 mg/L for enterococcus, vibrio cholera and total flora.
2.4. Microbial Analysis of Water Samples
- –
- C = average of colonies counted for each dilution
- –
- D = dilution rate of the first dilution considered
| Marker | Nutritive Agar | Brand | Reference | LOT | Incubation |
|---|---|---|---|---|---|
| E. coli | TBX | Biolife Italiana | 402 1562 | ML 5507 | 18 to 24 h at 44 °C |
| Faecal coliform | VRBL | Biolife Italiana | 402 1852 | LD7103 | 18 to 24 h at 44 °C |
| Faecal streptococcus | BEA | Biolife Italiana | 401 0182 | FP5303 | 24 h at 44 °C |
| Vibrio cholera | TCBS | Oxoid Ltd. | 190 0511 | CM0333 | 24 h at 37 °C |
| Total flora | R2A | Biolife Italiana | 401 9962 | MC2801 | 5 days at room T° |
2.5. Data Analysis
3. Results and Discussion
3.1. Abatement of Bacteria in WWTP Prototype
3.2. Impact of Moringa oleifera Seeds on Bacteria Abatement
3.3. Prevalence and Abatement of Antibiotic-Resistant Bacteria in WWTP Prototype
3.4. Effect of Moringa oleifera Seeds on Antibiotic-Resistant Bacteria Abatement
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Legal Entity
Appendix A

References
- UN-Water. Integrated Monitoring Guide for Sustainable Development Goal 6 on Water and Sanitation—Targets and Global Indicators—AR, EN, FR, RU, SP, ZH. UN-Water 2017. Available online: https://www.unwater.org/publications/sdg-6-targets-indicators/ (accessed on 1 June 2021).
- FAO. The State of Food and Agriculture 2020: Overcoming Water Challenges in Agriculture; FAO: Rome, Italy, 2020. [Google Scholar] [CrossRef]
- Lyimo, B.; Buza, J.; Subbiah, M.; Smith, W.; Call, D.R. Comparison of antibiotic resistant Escherichia coli obtained from drinking water sources in northern Tanzania: A cross-sectional study. BMC Microbiol. 2016, 16, 254. [Google Scholar] [CrossRef] [Green Version]
- Nishiyama, M.; Praise, S.; Tsurumaki, K.; Baba, H.; Kanamori, H.; Watanabe, T. Prevalence of Antibiotic-Resistant Bacteria ESKAPE among Healthy People Estimated by Monitoring of Municipal Wastewater. Antibiotics 2021, 10, 495. [Google Scholar] [CrossRef]
- Paśmionka, I.B.; Bulski, K.; Herbut, P.; Boligłowa, E.; Vieira, F.M.C.; Bonassa, G.; Prá, M.C.D.; Bortoli, M. Evaluation of the Effectiveness of the Activated Sludge Process in the Elimination Both ATB-Resistant and ATB-Susceptible E. coli Strains. Energies 2021, 14, 5868. [Google Scholar] [CrossRef]
- Pandey, P.; Khan, F.; Mishra, R.; Singh, S.K. Elucidation of the potential of Moringa oleifera leaves extract as a novel alternate to the chemical coagulant in water treatment process. Water Environ. Res. 2020, 92, 1051–1056. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.; Bachmann, R.T. A contemporary review on plant-based coagulants for applications in water treatment. J. Ind. Eng. Chem. 2019, 72, 281–297. [Google Scholar] [CrossRef]
- Kurniawan, S.B.; Abdullah, S.R.S.; Imron, M.F.; Said, N.S.M.; Ismail, N.I.; Hasan, H.A.; Othman, A.R.; Purwanti, I.F. Challenges and Opportunities of Biocoagulant/Bioflocculant Application for Drinking Water and Wastewater Treatment and Its Potential for Sludge Recovery. Int. J. Environ. Res. Public Health 2020, 17, 9312. [Google Scholar] [CrossRef]
- Marzougui, N.; Guasmi, F.; Dhouioui, S.; Bouhlel, M.; Hachicha, M.; Berndtsson, R.; Sleimi, N. Efficiency of Different Moringa oleifera (Lam.) Varieties as Natural Coagulants for Urban Wastewater Treatment. Sustainability 2021, 13, 13500. [Google Scholar] [CrossRef]
- Desta, W.M.; Bote, M.E. Wastewater treatment using a natural coagulant (Moringa oleifera seeds): Optimization through response surface methodology. Heliyon 2021, 7, e08451. [Google Scholar] [CrossRef]
- Bichi, M. A Review of the applications of Moringa oleifera seeds extract in water treatment. Civ. Environ. Res. 2013, 3, 1–11. [Google Scholar]
- Shan, T.C.; Matar, M.A.; Makky, E.A.; Ali, E.N. The use of Moringa oleifera seed as a natural coagulant for wastewater treatment and heavy metals removal. Appl. Water Sci. 2017, 7, 1369–1376. [Google Scholar] [CrossRef]
- Vunain, E.; Masoamphambe, E.F.; Mpeketula, P.M.G.; Monjerezi, M.; Etale, A. Evaluation of coagulating efficiency and water borne pathogens reduction capacity of Moringa oleifera seed powder for treatment of domestic wastewater from Zomba, Malawi. J. Environ. Chem. Eng. 2019, 7, 103118. [Google Scholar] [CrossRef]
- Tesemma, M.; Adane, L.; Tariku, Y.; Muleta, D.; Demise, S. Isolation of Compounds from Acetone Extract of Root Wood of Moringa stenopetala and Evaluation of their Antibacterial Activities. Science Alert. Res. J. Med. Plant 2013, 7, 32–47. [Google Scholar] [CrossRef]
- Eze, V.C.; Ananso, J.D. Assessment of Water Purification Potential of Moringa oleifera Seeds. Int. J. Microbiol. Appl. 2014, 1, 23. [Google Scholar]
- Poumaye, N.; Mabingui, J.; Lutgen, P.; Bigan, M. Contribution to the clarification of surface water from the Moringa oleifera: Case M’Poko River to Bangui, Central African Republic. Chem. Eng. Res. Des. 2012, 90, 2346–2352. [Google Scholar] [CrossRef]
- Pritchard, M.; Craven, T.; Mkandawire, T.; Edmondson, A.S.; O’Neill, J.G. A comparison between Moringa oleifera and chemical coagulants in the purification of drinking water—An alternative sustainable solution for developing countries. Phys. Chem. Earth Parts A/B/C 2010, 35, 798–805. [Google Scholar] [CrossRef]
- Villaseñor-Basulto, D.L.; Astudillo-Sánchez, P.D.; del Real-Olvera, J.; Bandala, E.R. Wastewater treatment using Moringa oleifera Lam seeds: A review. J. Water Process Eng. 2018, 23, 151–164. [Google Scholar] [CrossRef]
- Jałowiecki, Ł.; Żur, J.; Chojniak, J.; Ejhed, H.; Płaza, G. Properties of Antibiotic-Resistant Bacteria Isolated from Onsite Wastewater Treatment Plant in Relation to Biofilm Formation. Curr. Microbiol. 2018, 75, 639–649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wade, A.; Ndiaye, S.N. Code de l’assainissement. Journal Officiel. 2009. Available online: http://www.jo.gouv.sn/spip.php?article7712 (accessed on 23 June 2022).
- Bachelot-Narquin, R.; Borloo, J.-L.; Le Maire, B.; Jouanno, C. Arrêté du 2 août 2010 relatif à l’utilisation d’eaux issues du traitement d’épuration des eaux résiduaires urbaines pour l’irrigation de cultures ou d’espaces verts. 2010. Available online: https://www.legifrance.gouv.fr/loda/id/JORFTEXT000022753522/ (accessed on 30 May 2021).
- Formation des Installateurs et des, Concepteurs en ANC. Assainissement Non Collectif: Règles et Bonne Pratiques à L’attention des Installateurs. France. PANANC. 2016. Available online: http://www.assainissement-non-collectif.developpement-durable.gouv.fr/IMG/pdf/Guide_des_regles_et_bonnes_pratiques-v2.pdf (accessed on 23 June 2021).
- USEPA. Improved Enumeration Methods for the Recreational Water Quality Indicators: Enterococci and Escherichia coli. 2000. Available online: https://nepis.epa.gov/Exe/ZyNET.exe/200036RZ.txt?ZyActionD=ZyDocument&Client=EPA&Index=1995%20Thru%201999&Docs=&Query=&Time=&EndTime=&SearchMethod=1&TocRestrict=n&Toc=&TocEntry=&QField=&QFieldYear=&QFieldMonth=&QFieldDay=&UseQField=&IntQFieldOp=0&ExtQFieldOp=0&XmlQuery=&File=D%3A%5CZYFILES%5CINDEX%20DATA%5C95THRU99%5CTXT%5C00000008%5C200036RZ.txt&User=ANONYMOUS&Password=anonymous&SortMethod=h%7C-&MaximumDocuments=1&FuzzyDegree=0&ImageQuality=r75g8/r75g8/x150y150g16/i425&Display=hpfr&DefSeekPage=x&SearchBack=ZyActionL&Back=ZyActionS&BackDesc=Results%20page&MaximumPages=1&ZyEntry=1 (accessed on 25 June 2022).
- European Union. Directive 2006/7/EC of the European Parliament and of the Council of 15 February 2006 Concerning the Management of Bathing Water Quality and Repealing Directive 76/160/EEC. Available online: http://data.europa.eu/eli/dir/2006/7/oj/eng (accessed on 25 June 2022).
- World Health Organization. Guidelines on Sanitation and Health. 2018. Available online: https://www.who.int/publications-detail-redirect/9789241514705 (accessed on 25 June 2022).
- World Health Organization. Domestic Water Quantity, Service Level and Health. 2020. Available online: https://www.who.int/publications-detail-redirect/9789240015241 (accessed on 25 June 2022).
- Rodier, J.; Legube, B.; Merlet, N. L’Analyse de l’Eau, 9th ed.; DUNOD: Paris, France, 2009. [Google Scholar]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing. USA. 2017. Available online: chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/https://clsi.org/media/1469/m100s27_sample.pdf (accessed on 10 January 2022).
- Appling, D.; Habteselassie, M.Y.; Radcliffe, D.; Bradshaw, J.K. Preliminary Study on the Effect of Wastewater Storage in Septic Tank on E. coli Concentration in Summer. Water 2013, 5, 1141–1151. [Google Scholar] [CrossRef]
- Camacho, F.P.; Sousa, V.S.; Bergamasco, R.; Ribau Teixeira, M. The use of Moringa oleifera as a natural coagulant in surface water treatment. Chem. Eng. J. 2017, 313, 226–237. [Google Scholar] [CrossRef]
- Delelegn, A.; Sahile, S.; Husen, A. Water purification and antibacterial efficacy of Moringa oleifera Lam. Agric. Food Secur. 2018, 7, 25. [Google Scholar] [CrossRef] [Green Version]
- Fitzpatrick, C.; Fradin, E.; Gregory, J. Temperature effects on flocculation, using different coagulants. Water Sci. Technol. J. Int. Assoc. Water Pollut. Res. 2004, 50, 171–175. [Google Scholar] [CrossRef]
- Summerlin, H.N.; Pola, C.C.; McLamore, E.S.; Gentry, T.; Karthikeyan, R.; Gomes, C.L. Prevalence of Escherichia coli and Antibiotic-Resistant Bacteria During Fresh Produce Production (Romaine Lettuce) Using Municipal Wastewater Effluents. Front. Microbiol. 2021, 12, 660047. [Google Scholar] [CrossRef] [PubMed]
- Mao, D.; Yu, S.; Rysz, M.; Luo, Y.; Yang, F.; Li, F.; Hou, J.; Mu, Q.; Alvarez, P.J.J. Prevalence and proliferation of antibiotic resistance genes in two municipal wastewater treatment plants. Water Res. 2015, 85, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.; Hembach, N.; Schwartz, T. Evaluation of antibiotic resistance dissemination by wastewater treatment plant effluents with different catchment areas in Germany. Sci. Rep. 2020, 10, 8952. [Google Scholar] [CrossRef]
- Grehs, B.W.N.; Linton, M.A.O.; Clasen, B.; de Oliveira Silveira, A.; Carissimi, E. Antibiotic resistance in wastewater treatment plants: Understanding the problem and future perspectives. Arch. Microbiol. 2021, 203, 1009–1020. [Google Scholar] [CrossRef]
- Bréchet, C.; Plantin, J.; Sauget, M.; Thouverez, M.; Talon, D.; Cholley, P.; Guyeux, C.; Hocquet, D.; Bertrand, X. Wastewater Treatment Plants Release Large Amounts of Extended-Spectrum β-Lactamase–Producing Escherichia coli Into the Environment. Clin. Infect. Dis. 2014, 58, 1658–1665. [Google Scholar] [CrossRef]
- Fahrenfeld, N.L.; Ma, Y.; O’Brien, M.; Pruden, A. Reclaimed water as a reservoir of antibiotic resistance genes: Distribution system and irrigation implications. Front. Microbiol. 2013, 4, 130. [Google Scholar] [CrossRef] [Green Version]








| Samples | E. coli | Faecal Coliform | Vibrio cholera | Total Flora | ||||
|---|---|---|---|---|---|---|---|---|
| CFU mL−1 | SD | CFU mL−1 | SD | CFU mL−1 | SD | CFU mL−1 | SD | |
| F0 | 1.97 × 104 | 2.52 × 103 | 2.13 × 104 | 1.59 × 104 | 3.30 × 103 | 4.58 × 102 | 2.63 × 107 | 4.35 × 106 |
| F1 | 5.00 × 104 | 1.00 × 104 | 1.55 × 104 | 1.17 × 103 | 1.35 × 103 | 3.49 × 102 | 1.58 × 108 | 4.05 × 107 |
| F2 | 2.73 × 102 | 3.79× 101 | 2.97 × 102 | 2.52 × 101 | 2.17 × 101 | 4.93 | 2.65 × 106 | 1.37 × 105 |
| F0MOS | 5.47 × 103 | 2.08 × 102 | 3.77 × 103 | 6.66 × 102 | 1.12 × 103 | 5.36 × 102 | 7.00 × 106 | 1.48 × 106 |
| F1MOS | 1.06 × 104 | 7.21 × 102 | 1.11 × 104 | 2.54 × 103 | 5.90 × 101 | 2.21 × 101 | 1.20 × 107 | 3.17 × 106 |
| F2MOS | 1.73 × 102 | 3.51 × 101 | 4.57 × 101 | 9.02 × 100 | 1.40 × 101 | 6.24 | 1.80 × 106 | 3.46 × 105 |
| Samples | AREC | ARFC | ARVC | ARTF | ||||
|---|---|---|---|---|---|---|---|---|
| CFU mL−1 | SD | CFU mL−1 | SD | CFU mL−1 | SD | CFU mL−1 | SD | |
| F0 | 1.49 × 104 | 2.16 × 103 | 1.41 × 104 | 2.32 × 103 | 4.30 × 102 | 1.23 × 102 | 1.92 × 106 | 1.18 × 105 |
| F1 | 1.25 × 104 | 5.03 × 102 | 1.30 × 104 | 8.89 × 103 | 1.47 × 101 | 9.02 | 1.80 × 107 | 2.00 × 105 |
| F2 | 2.03 × 102 | 7.51 × 101 | 1.70 × 102 | 2.65 × 101 | 4.67 | 4.04 | 6.83 × 105 | 1.21 × 105 |
| F0MOS | 1.23 × 103 | 5.77 × 101 | 1.10 × 103 | 1.00 × 102 | 2.20 × 102 | 1.11 × 102 | 1.34 × 106 | 7.21 × 104 |
| F1MOS | 3.23 × 103 | 8.33 × 102 | 2.67 × 102 | 8.02 × 101 | 1.33 × 101 | 5.77 | 1.45 × 106 | 5.84 × 105 |
| F2MOS | 6.00 | 3.46 | 1.03 × 101 | 2.31 | 0.00 | 0.00 | 6.40 × 105 | 1.31 × 105 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sané, N.; Mbengue, M.; Laffite, A.; Stoll, S.; Poté, J.; Le Coustumer, P. Development of a Process for Domestic Wastewater Treatment Using Moringa oleifera for Pathogens and Antibiotic-Resistant Bacteria Inhibition under Tropical Conditions. Water 2022, 14, 2379. https://doi.org/10.3390/w14152379
Sané N, Mbengue M, Laffite A, Stoll S, Poté J, Le Coustumer P. Development of a Process for Domestic Wastewater Treatment Using Moringa oleifera for Pathogens and Antibiotic-Resistant Bacteria Inhibition under Tropical Conditions. Water. 2022; 14(15):2379. https://doi.org/10.3390/w14152379
Chicago/Turabian StyleSané, Nini, Malick Mbengue, Amandine Laffite, Serge Stoll, John Poté, and Philippe Le Coustumer. 2022. "Development of a Process for Domestic Wastewater Treatment Using Moringa oleifera for Pathogens and Antibiotic-Resistant Bacteria Inhibition under Tropical Conditions" Water 14, no. 15: 2379. https://doi.org/10.3390/w14152379
APA StyleSané, N., Mbengue, M., Laffite, A., Stoll, S., Poté, J., & Le Coustumer, P. (2022). Development of a Process for Domestic Wastewater Treatment Using Moringa oleifera for Pathogens and Antibiotic-Resistant Bacteria Inhibition under Tropical Conditions. Water, 14(15), 2379. https://doi.org/10.3390/w14152379