Hydrogeochemistry of Shallow Groundwater and Suitability to Irrigation: The Case of the Karfiguéla Paddy Field in Burkina Faso
Abstract
:1. Introduction
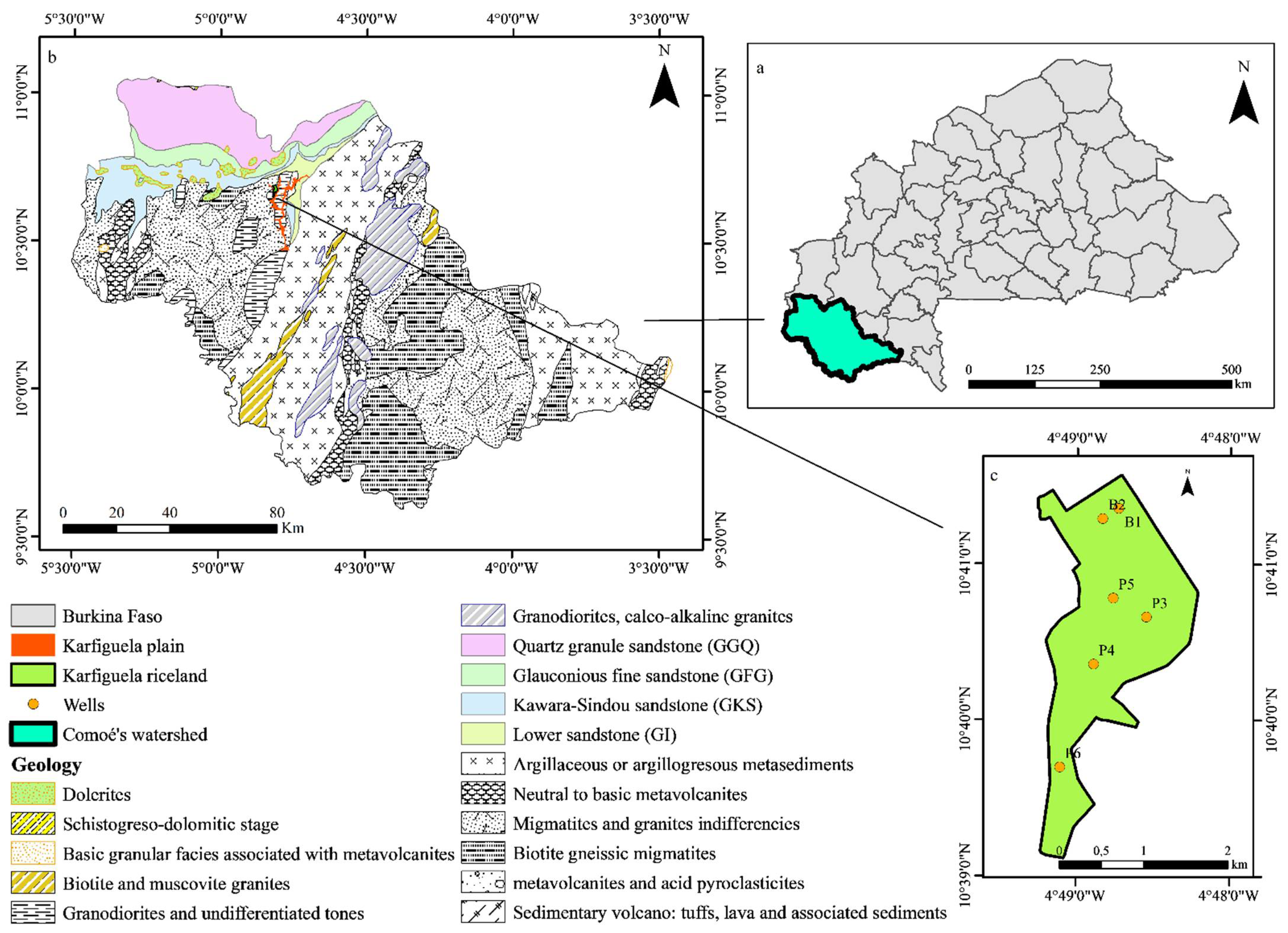
2. Presentation of the Study Area
2.1. Location and Climate
2.2. Hydrological Features
3. Material and Methods
3.1. Sample Collection and Analysis
3.2. Hydrogeochemical Interpretation
3.3. Water Quality for Irrigation
4. Results
4.1. Groundwater Hydrogeochemistry
4.1.1. Physicochemical Parameters and Their Spatial Variability
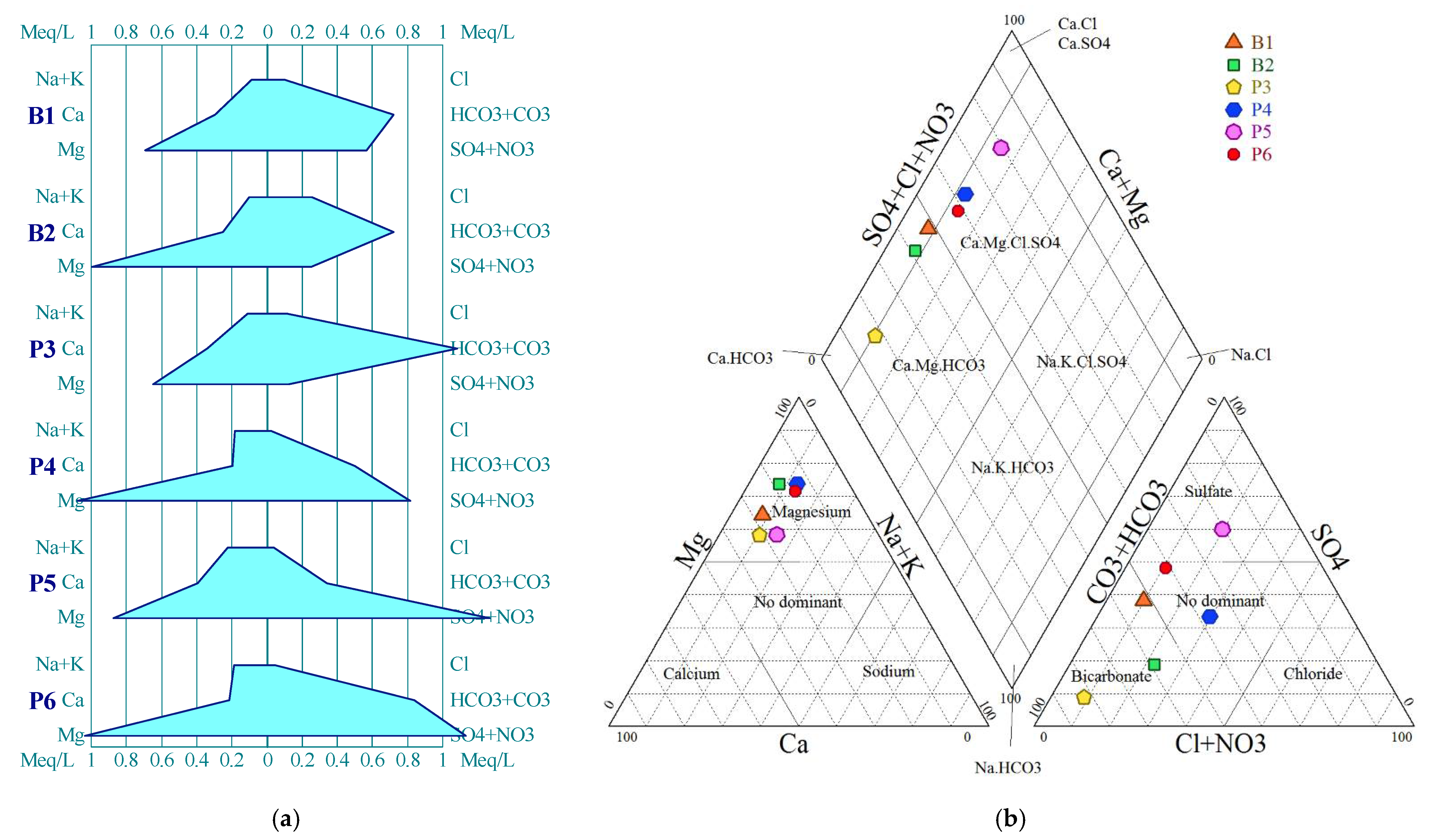
4.1.2. Hydrochemical Facies
4.2. Processes Controlling the Chemistry of the Shallow Aquifer
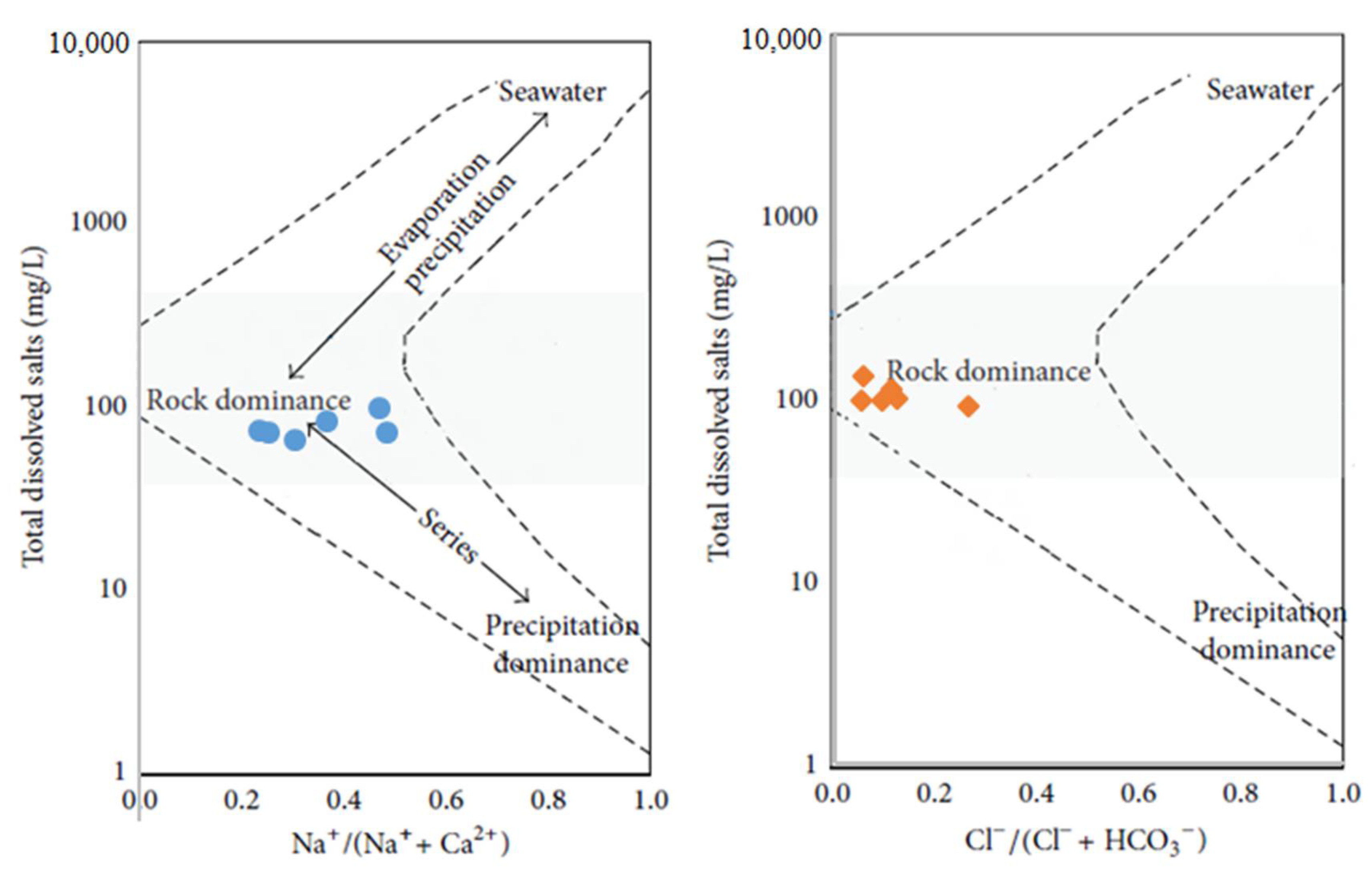
4.2.1. Gibbs Diagram and Multi-Criteria Analysis
4.2.2. Anthropogenic Activities
4.2.3. Cation Exchanges
4.2.4. Water–Rock Interactions
4.2.5. CO2 Dissolution Process
4.3. Shallow Groundwater Suitability for Irrigation
4.3.1. Electrical Conductivity, Sodium Adsorption Ratio, and Sodium Percentage
4.3.2. Chloride and Nitrate (NO3−-N) Hazard
4.3.3. Potential Salinity (PS) and Kelly Ratio (KR)
4.3.4. Residual Sodium Carbonate (RSC)
4.3.5. Magnesium (MH) Hazard
4.3.6. Permeability Index (PI)
4.3.7. Irrigation Coefficient (Ka)
4.3.8. Toxicity and Trace Elements
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Wada, Y.; Van Beek, L.P.; Van Kempen, C.M.; Reckman, J.W.; Vasak, S.; Bierkens, M.F. Global Depletion of Groundwater Resources. Geophys. Res. Lett. 2010, 37. [Google Scholar] [CrossRef] [Green Version]
- Ashraf, B.; AghaKouchak, A.; Alizadeh, A.; Mousavi Baygi, M.; R Moftakhari, H.; Mirchi, A.; Anjileli, H.; Madani, K. Quantifying Anthropogenic Stress on Groundwater Resources. Sci. Rep. 2017, 7, 12910. [Google Scholar] [CrossRef] [Green Version]
- Ashraf, S.; Nazemi, A.; AghaKouchak, A. Anthropogenic Drought Dominates Groundwater Depletion in Iran. Sci. Rep. 2021, 11, 9135. [Google Scholar] [CrossRef] [PubMed]
- Gleeson, T.; Wada, Y. Assessing Regional Groundwater Stress for Nations Using Multiple Data Sources with the Groundwater Footprint. Environ. Res. Lett. 2013, 8, 044010. [Google Scholar] [CrossRef]
- Herbert, C.; Döll, P. Global Assessment of Current and Future Groundwater Stress with a Focus on Transboundary Aquifers. Water Resour. Res. 2019, 55, 4760–4784. [Google Scholar] [CrossRef]
- Richey, A.S.; Thomas, B.F.; Lo, M.-H.; Reager, J.T.; Famiglietti, J.S.; Voss, K.; Swenson, S.; Rodell, M. Quantifying Renewable Groundwater Stress with GRACE. Water Resour. Res. 2015, 51, 5217–5238. [Google Scholar] [CrossRef]
- Ritchie, H.; Roser, M. Water Use and Stress. Our World Data 2017. Available online: https://ourworldindata.org/water-use-stress (accessed on 1 July 2022).
- Thomas, B.F.; Famiglietti, J.S. Identifying Climate-Induced Groundwater Depletion in GRACE Observations. Sci. Rep. 2019, 9, 1–9. [Google Scholar]
- Altchenko, Y.; Villholth, K.G. Mapping Irrigation Potential from Renewable Groundwater in Africa–a Quantitative Hydrological Approach. Hydrol. Earth Syst. Sci. 2015, 19, 1055–1067. [Google Scholar] [CrossRef] [Green Version]
- MacDonald, A.M.; Bonsor, H.C.; Dochartaigh, B.É.Ó.; Taylor, R.G. Quantitative Maps of Groundwater Resources in Africa. Environ. Res. Lett. 2012, 7, 024009. [Google Scholar] [CrossRef]
- Gowing, J.; Walker, D.; Parkin, G.; Forsythe, N.; Haile, A.T.; Ayenew, D.A. Can Shallow Groundwater Sustain Small-Scale Irrigated Agriculture in Sub-Saharan Africa? Evidence from NW Ethiopia. Groundw. Sustain. Dev. 2020, 10, 100290. [Google Scholar] [CrossRef]
- Pavelic, P.; Giordano, M.; Keraita, B.N.; Ramesh, V.; Rao, T. Groundwater Availability and Use in Sub-Saharan Africa: A Review of 15 Countries; International Water Management Institute, 2012; p. 289. Available online: https://www.iwmi.cgiar.org/Publications/Books/PDF/groundwater_availability_and_use_in_sub-saharan_africa_a_review_of_15_countries.pdf (accessed on 1 July 2022).
- Sako, A.; Sawadogo, S.; Nimi, M.; Ouédraogo, M. Hydrogeochemical and Pollution Characterization of a Shallow Glauconitic Sandstone Aquifer in a Peri-Urban Setting of Bobo-Dioulasso, Southwestern Burkina Faso. Environ. Earth Sci. 2020, 79, 1–18. [Google Scholar] [CrossRef]
- Kambou, D. Évaluation Des Performances Techniques de l’irrigation Au Burkina Faso; Université de Liège: Liège, Belgique, 2019. [Google Scholar]
- COWI. Rapport de l’étude de La Stratégie de Suivi et d’évaluation Des Ressources En Eau-Rapport Diagnostic; Le Comité National des Irrigations et du Drainage du Burkina: Ouagadougou, Burkina Faso, 2019. [Google Scholar]
- Daré, W.; Venot, J.-P.; Kaboré, É.; Tapsoba, A.; Traoré, F.; Gérard, F.; Carboni, S.; Idani, D.; Kambiré, H.; Napon, K. Grands Aménagements Hydroagricoles, Inégalités Environnementales et Participation: Le Cas de Bagré Au Burkina Faso. VertigO Rev. Électronique En Sci. L’environnement 2019, 19. [Google Scholar] [CrossRef]
- Dembele, Y.; Yacouba, H.; Keïta, A.; Sally, H. Assessment of Irrigation System Performance in South-western Burkina Faso. Irrig. Drain. 2011, 61, 306–315. [Google Scholar] [CrossRef]
- AEM; AEC; Compaoré, N.F. Rapport de l’étude d’identification, de Localisation et de Caractérisation Physique Des Sources d’eau Dans Les Espaces de Compétence Des Agences de l’eau Du Mouhoun et Des Cascades–Etape 1; Burkina Faso, 2014; p. 88. Available online: http://eaumouhoun.bf/wp-content/uploads/2015/09/Rapport-final-%C3%A9tude-sources-%C3%A9tape-1.pdf (accessed on 1 July 2022).
- Berase. Rapport Etude Diagnostique de l’état Des Lieux Du Périmètre Rizicole de Karfiguéla; Le Comité National des Irrigations et du Drainage du Burkina: Ouagadougou, Burkina Faso, 2018. [Google Scholar]
- Compaoré, N.F.; Dara, A.E.; Koïta, M.; Dao, D.M.; Yonli, H.F. Assessment of the Exploitation Rate and Sustainability of an Alluvial Plain in Southwestern Burkina Faso. J. Nat. Sci. 2017, 5, 16–22. [Google Scholar] [CrossRef] [Green Version]
- Xu, P.; Feng, W.; Qian, H.; Zhang, Q. Hydrogeochemical Characterization and Irrigation Quality Assessment of Shallow Groundwater in the Central-Western Guanzhong Basin, China. Int. J. Environ. Res. Public. Health 2019, 16, 1492. [Google Scholar] [CrossRef] [Green Version]
- Khalid, S. An Assessment of Groundwater Quality for Irrigation and Drinking Purposes around Brick Kilns in Three Districts of Balochistan Province, Pakistan, through Water Quality Index and Multivariate Statistical Approaches. J. Geochem. Explor. 2019, 197, 14–26. [Google Scholar]
- Aher, K.R.; Gaikwad, S.G. Irrigation Groundwater Quality Based on Hydrochemical Analysis of Nandgaon Block, Nashik District in Maharashtra. Int. J. Adv. Geosci. 2017, 5, 7116. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, M.T.; Hasan, M.Y.; Monir, M.U.; Samad, M.A.; Rahman, M.M.; Islam Rifat, M.S.; Islam, M.N.; Khan, A.A.S.; Biswas, P.K.; Jamil, A.H.M.N. Evaluation of Hydrochemical Properties and Groundwater Suitability for Irrigation Uses in Southwestern Zones of Jashore, Bangladesh. Groundw. Sustain. Dev. 2020, 11, 100441. [Google Scholar] [CrossRef]
- Dash, S.; Kalamdhad, A.S. Hydrochemical Dynamics of Water Quality for Irrigation Use and Introducing a New Water Quality Index Incorporating Multivariate Statistics. Environ. Earth Sci. 2021, 80, 1–21. [Google Scholar] [CrossRef]
- Verma, A.; Yadav, B.K.; Singh, N.B. Hydrochemical Monitoring of Groundwater Quality for Drinking and Irrigation Use in Rapti Basin. SN Appl. Sci. 2020, 2, 460. [Google Scholar] [CrossRef] [Green Version]
- Loye, A.S.; Tingueri, R.K.; Zida, Y.; Zoma, L. Monographie de La Région Des Cascades; 2009; p. 180. Available online: https://ireda.ceped.org/inventaire/ressources/bfa-2006-rec-o5_region_cascades.pdf (accessed on 1 July 2022).
- Dembele, Y.; Some, L. Propriétés Hydrodynamiques Des Principaux Types de Sol Du Burkina Faso. In Proceedings of the Niamey, Niger, Workshop, February 1991; IAHS Publ: Niamey, Niger, 1991; Volume 199, pp. 217–227. Available online: https://dokumen.tips/documents/proprietes-hydrodynamiques-des-principaux-types-de-sol-du-burkina-.html?page=1 (accessed on 1 July 2022).
- Hugot, G. A La Recherche Du Gondwana Perdu: Aux Origines Du Monde. 2002, p. 311. Available online: http://www.mgm.fr/UMR/Gondwana.pdf (accessed on 1 July 2022).
- Kouanda, B. Modélisation Intégrée Du Complexe Mouhoun Supérieur-Sourou Dans Le Contexte Des Changements Climatiques; Institut International d’Ingénierie de l’Eau et de l’Environnement: Ouagadougou, Burkina Faso, 2019. [Google Scholar]
- Dakoure, D. Etude Hydrogéologique et Géochimique de La Bordure Sud-Est Du Bassin Sédimentaire de Taoudéni (Burkina Faso-Mali)-Essai de Modélisation. Ph.D. Thesis, Université Pierre et Marie Curie-Paris, Paris, France, 2003. [Google Scholar]
- Sauret, E. Caractérisation Hydrochimique et Qualité Des Eaux Souterraines Du Projet Hydraulique Villageoise 310 Forages, Dans La Boucle Du Mouhoun: Provinces Des Banwa, Des Balés, Du Mouhoun et de La Kossi (Burkina Faso); Mémoire d’Ingénieur, Université Cheikh Anta Diop, Institut des Sciences de la Terre: Dakar, Sénégal, 2005; p. 63. Available online: https://hdl.handle.net/2268/155417 (accessed on 1 July 2022).
- Piper, A.M. A Graphic Procedure in the Geochemical Interpretation of Water-Analyses. Eos Trans. Am. Geophys. Union 1944, 25, 914–928. [Google Scholar] [CrossRef]
- Stiff, H.A., Jr. The Interpretation of Chemical Water Analysis by Means of Patterns. J. Pet. Technol. 1951, 3, 15. [Google Scholar] [CrossRef]
- Sako, A.; Yaro, J.M.; Bamba, O. Impacts of Hydrogeochemical Processes and Anthropogenic Activities on Groundwater Quality in the Upper Precambrian Sedimentary Aquifer of Northwestern Burkina Faso. Appl. Water Sci. 2018, 8, 88. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Wu, J.; Qian, H.; Zhang, Y.; Yang, N.; Jing, L.; Yu, P. Hydrogeochemical Characterization of Groundwater in and around a Wastewater Irrigated Forest in the Southeastern Edge of the Tengger Desert, Northwest China. Expo. Health 2016, 8, 331–348. [Google Scholar] [CrossRef]
- Kumar, M.; Ramanathan, A.L.; Rao, M.S.; Kumar, B. Identification and Evaluation of Hydrogeochemical Processes in the Groundwater Environment of Delhi, India. Environ. Geol. 2006, 50, 1025–1039. [Google Scholar] [CrossRef]
- Yang, Q.; Li, Z.; Ma, H.; Wang, L.; Martín, J.D. Identification of the Hydrogeochemical Processes and Assessment of Groundwater Quality Using Classic Integrated Geochemical Methods in the Southeastern Part of Ordos Basin, China. Environ. Pollut. 2016, 218, 879–888. [Google Scholar] [CrossRef]
- Gibbs, R.J. Mechanisms Controlling World Water Chemistry. Science 1970, 170, 1088–1090. [Google Scholar] [CrossRef]
- Essouli, O.F. Hydrochimie des eaux de surface et souterraines de la partie nord de Brazzaville: Origine et processus de minéralisation. Sci. Appliquées Ing. 2021, 2, 1–15. [Google Scholar]
- Subramani, T.; Rajmohan, N.; Elango, L. Groundwater Geochemistry and Identification of Hydrogeochemical Processes in a Hard Rock Region, Southern India. Environ. Monit. Assess. 2010, 162, 123–137. [Google Scholar] [CrossRef]
- Appelo, C.A.J.; Postma, D. Ion Exchange. In Geochemistry, Groundwater and Pollution; CRC Press: London, UK, 2005; Volume 2, pp. 241–309. [Google Scholar]
- Leclercq, L.; Maquet, B. Deux Nouveaux Indices Diatomique et de Qualité Chimique Des Eaux Courantes. Comparaison Avec Différents Indices Existants. Cah. Biol. Mar. 1987, 28, 303–310. [Google Scholar]
- Orou, R.K.; Soro, G.; Traoré, A.; Fossou, R.M.N.; Soro, N. Aptitude à l’irrigation Des Eaux Souterraines Du Département d’Agboville (Sud-Est de La Côte d’Ivoire). Eur. Sci. J. 2016, 21, 20. Available online: https://eujournal.org/index.php/esj/article/view/7746/7508 (accessed on 1 July 2022).
- Scheiber, L.; Cendón, D.I.; Iverach, C.P.; Hankin, S.I.; Vázquez-Suñé, E.; Kelly, B.F.J. Hydrochemical Apportioning of Irrigation Groundwater Sources in an Alluvial Aquifer. Sci. Total Environ. 2020, 744, 140506. [Google Scholar] [CrossRef]
- Rhoades, J.D. Quality of water for irrigation. Soil Sci. 1972, 113, 277–284. [Google Scholar] [CrossRef]
- Ayers, R.S.; Westcot, D.W. Water Quality for Agriculture; FAO irrigation and drainage paper, 29 Rev.1; FAO Rome: Rome, Italy, 1985; Available online: https://www.fao.org/3/t0234e/t0234e00.htm (accessed on 1 July 2022).
- Senou, I.; Nimi, M.; Sanogo, S.B.; Nacro, H.; Some, A.N. Study of Physicochemical Parameters and Level of Cadmium and Lead Contamination in Irrigation Water in Market Garden Areas in West Burkina Faso. Int. J. Environ. Agric. Biotechnol. 2020, 5, 10. [Google Scholar] [CrossRef]
- Penant, P.; Brouyere, S. Caractérisation Des Sources de Nitrate Dans Les Aquifères Cristallins Du Centre Du Bénin. Mémoire de Master, Université de Liège, Belgique. 2016. Available online: https://matheo.uliege.be/bitstream/2268.2/1650/1/PPENANT_m%C3%A9moire_FINAL.pdf (accessed on 1 July 2022).
- Murray, R.S.; Grant, C.D. The Impact of Irrigation on Soil Structure; National Program for Sustainable Irrigation, Land & Water Australia, 2007; pp. 1–31. Available online: https://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.460.5683&rep=rep1&type=pdf#:~:text=Irrigation%2C%20even%20with%20water%20of,structure%20and%20to%20raise%20the (accessed on 1 July 2022).
- Hillel, D.; Braimoh, A.K.; Vlek, P.L. Soil Degradation under Irrigation. In Land Use and Soil Resources; Springer: Dordrecht, The Netherlands, 2008; pp. 101–119. [Google Scholar] [CrossRef]
- Mohanavelu, A.; Naganna, S.R.; Al-Ansari, N. Irrigation Induced Salinity and Sodicity Hazards on Soil and Groundwater: An Overview of Its Causes, Impacts and Mitigation Strategies. Agriculture 2021, 11, 983. [Google Scholar] [CrossRef]
- Kelley, W.P. Permissible Composition and Concentration of Irrigation Water. Trans. Am. Soc. Civ. Eng. 1941, 106, 849–855. [Google Scholar] [CrossRef]
- Yang, Q.; Li, Z.; Xie, C.; Liang, J.; Ma, H. Risk Assessment of Groundwater Hydrochemistry for Irrigation Suitability in Ordos Basin, China. Nat. Hazards 2018, 101, 309–325. [Google Scholar] [CrossRef]
- Eaton, F.M. Significance of carbonates in irrigation waters. Soil Sci. 1950, 69, 123–134. [Google Scholar] [CrossRef]
- Szabolcs, I.; Darab, K. Radio-Active Technique for Examining the Improving Effect of CaCO3 on Alkali (Szik) Soils. Acta Agron Hung 1964, 13, 93–101. [Google Scholar]
- Raghunath, H.M. Ground Water: Hydrogeology, Ground Water Survey and Pumping Tests, Rural Water Supply and Irrigation Systems, 2nd ed.; Wiley Eastern: New Delhi, India, 1987. [Google Scholar]
- Tahmasebi, P.; Mahmudy-Gharaie, M.H.; Ghassemzadeh, F.; Karouyeh, A.K. Assessment of Groundwater Suitability for Irrigation in a Gold Mine Surrounding Area, NE Iran. Environ. Earth Sci. 2018, 77, 1–12. [Google Scholar] [CrossRef]
- Li, P.; Wu, J.; Qian, H. Assessment of Groundwater Quality for Irrigation Purposes and Identification of Hydrogeochemical Evolution Mechanisms in Pengyang County, China. Environ. Earth Sci. 2013, 69, 2211–2225. [Google Scholar] [CrossRef]
- Zhou, J.L.; Wu, B.; Wang, Y.P.; Guo, X.-J. Distribution and Quality Assessment of Medium Salinity Groundwater in Plain Areas in Tarim Basin, Xinjiang. China Rural Water Hydropower 2009, 9, 32–36. [Google Scholar]
- Huneau, F.; Dakoure, D.; Celle-Jeanton, H.; Vitvar, T.; Ito, M.; Traore, S.; Compaore, N.F.; Jirakova, H.; Le Coustumer, P. Flow Pattern and Residence Time of Groundwater within the South-Eastern Taoudeni Sedimentary Basin (Burkina Faso, Mali). J. Hydrol. 2011, 409, 423–439. [Google Scholar] [CrossRef]
- Dinka, M.O.; Tadesse, K.B. Suitability of Shallow Groundwater for Irrigation: The Case of the Wonji Shoa Sugar Estate (Ethiopia). Irrig. Drain. 2019, 68, 857–866. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, R.H.; Lee, J.; Chang, H.W. Hydrogeochemical Characterization of Major Factors Affecting the Quality of Shallow Groundwater in the Coastal Area at Kimje in South Korea. Environ. Geol. 2003, 44, 478–489. [Google Scholar] [CrossRef]



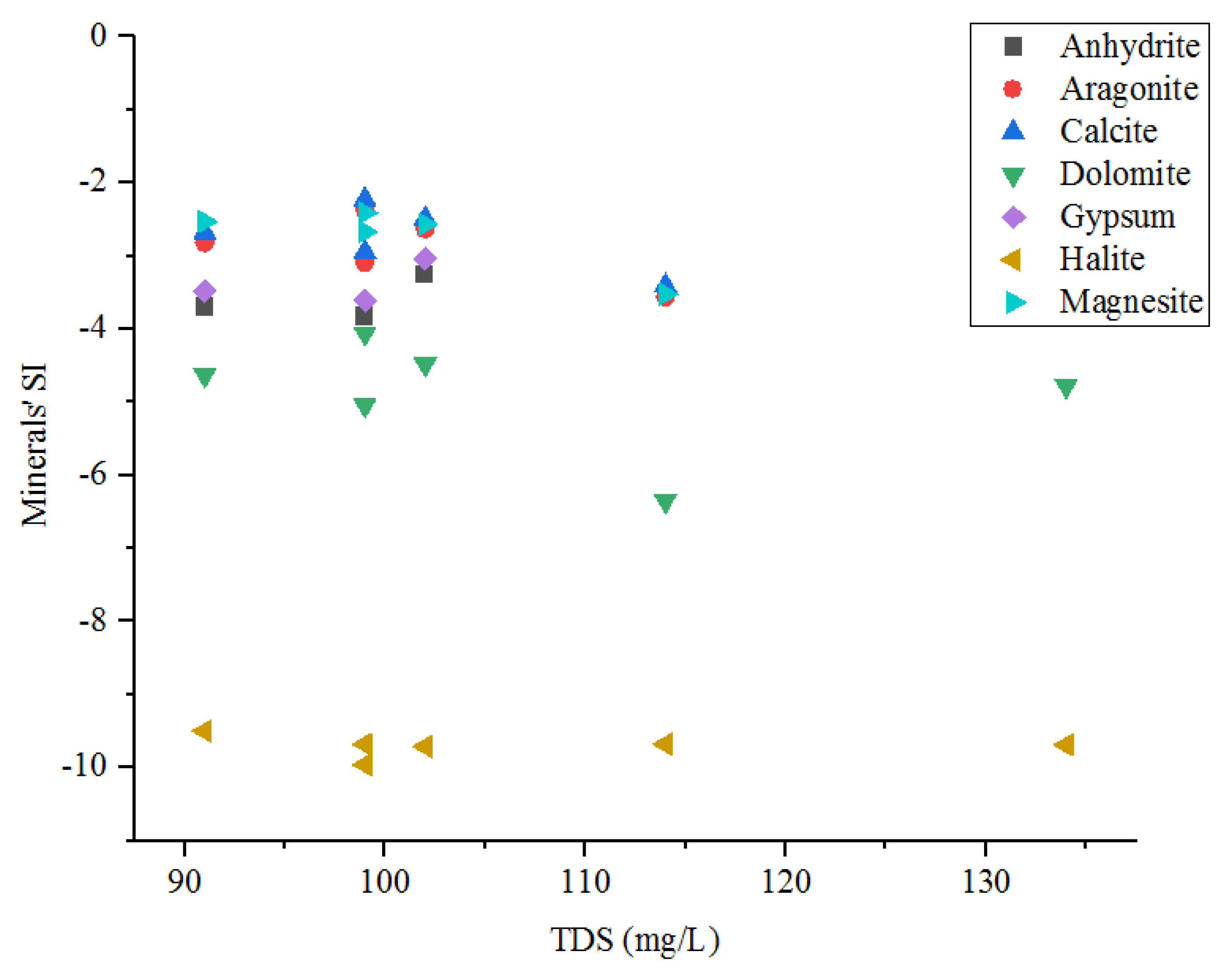



| Anhydrite | CaSO4 ↔ Ca2+ + SO42− |
| Aragonite | CaCO3 + CO2 + H2O ↔ Ca2+ + 2 HCO3− |
| calcite | CaCO3 ↔ Ca2+ + CO32− |
| Dolomite | CaMg(CO3)2 + 2CO2+2H2O ↔ Ca2+ + Mg2+ + 4HCO3− |
| Gypsum | CaSO4:2H2O ↔ Ca2+ + SO42− + 2 H2O |
| halite | NaCl ↔ Na+ + Cl− |
| Magnesite | MgCO3 ↔ Mg2+ + CO32− |
| Parameter | Units | B1 | B2 | P3 | P4 | P5 | P6 | Mean | VC [%] | FAO Standards | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| None | Slight to Moderate | Severe | ||||||||||
| pH | — | 6.5 | 6.3 | 6.5 | 6.3 | 5.7 | 6.2 | 6.2 | 5% | 6.5–8.4 | ||
| EC | μS/cm | 65.1 | 52.1 | 53.2 | 33.7 | 80.0 | 68.1 | 58.7 | 27% | <700 | 700–3000 | 350 |
| TDS | mg·L−1 | 102.0 | 91.0 | 99.0 | 99.0 | 114.0 | 134.0 | 106.5 | 14% | <450 | 450–2000 | 2000 |
| TAC | mg·L−1 | 36.0 | 36.0 | 54.0 | 25.0 | 17.0 | 42.0 | 35.0 | 37% | — | ||
| Ca2+ | mg·L−1 | 6.0 | 5.0 | 7.0 | 4.0 | 8.0 | 4.4 | 5.7 | 27% | — | ||
| Mg2+ | mg·L−1 | 8.5 | 12.2 | 7.9 | 13.1 | 10.7 | 12.6 | 10.8 | 20% | — | ||
| Na+ | mg·L−1 | 1.9 | 1.2 | 1.8 | 3.8 | 4.7 | 4.0 | 2.9 | 50% | 70 | 70–200 | 200 |
| K+ | mg·L−1 | 0.3 | 2.2 | 1.5 | 0.9 | 0.8 | 0.8 | 1.1 | 62% | — | ||
| NH4+ | mg·L−1 | 0.0 | 0.0 | 0.0 | 0.8 | 1.7 | 1.8 | 0.7 | 120% | — | ||
| Fe2+ | mg·L−1 | 0.0 | 0.0 | 0.0 | 1.3 | 4.5 | 3.1 | 1.5 | 130% | — | ||
| Zn2+ | mg·L−1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 112% | <2 | ||
| HCO3− | mg·L−1 | 43.9 | 43.9 | 65.9 | 30.5 | 20.7 | 51.2 | 42.7 | 37% | 90 | 90–500 | 500 |
| CO32− | mg·L−1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | — | — | ||
| Cl− | mg·L−1 | 3.5 | 9.0 | 4.0 | 1.0 | 1.5 | 1.8 | 3.5 | 86% | <140 | 140–355 | 355 |
| NO3− | mg·L−1 | 2.2 | 1.1 | 0.2 | 23.0 | 17.4 | 10.1 | 9.0 | 106% | 5 | 5–30 | 30 |
| NO2− | mg·L−1 | 0.1 | 0.1 | 0.0 | 0.2 | 0.3 | 0.4 | 0.2 | 73% | — | ||
| PO43− | mg·L−1 | 9.9 | 5.7 | 5.2 | 0.5 | 0.7 | 0.1 | 3.7 | 107% | — | ||
| SO42− | mg·L−1 | 25.5 | 11.0 | 5.6 | 21.5 | 47.7 | 46.7 | 26.3 | 67% | — | ||
| Mn2+ | mg·L−1 | 0.00 | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 | 0.0 | 245% | — | ||
| Cd2+ | mg·L−1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.1 | 0.0 | 114% | — | ||
| Pb2+ | mg·L−1 | 0.0 | 0.0 | 0.0 | 0.1 | 0.1 | 0.1 | 0.0 | 120% | — | ||
| Attribute | PC1 | PC2 | PC3 |
|---|---|---|---|
| Na+ | 0.977 | 0.018 | 0.025 |
| Cd2+ | 0.964 | 0.123 | −0.185 |
| Fe2+ | 0.956 | −0.201 | 0.143 |
| Pb2+ | 0.940 | 0.032 | 0.291 |
| SO42− | 0.921 | −0.245 | −0.099 |
| Zn2+ | −0.920 | −0.183 | 0.117 |
| NH4+ | 0.918 | −0.230 | −0.121 |
| NO2−-N | 0.899 | −0.101 | −0.275 |
| pH | −0.814 | 0.233 | −0.502 |
| PO43− | −0.804 | −0.341 | 0.015 |
| NO3−-N | 0.783 | 0.450 | 0.206 |
| TDS | 0.764 | −0.267 | −0.540 |
| Cl− | −0.732 | 0.062 | 0.278 |
| HCO3− | −0.624 | −0.216 | −0.643 |
| K+ | −0.505 | 0.253 | 0.357 |
| Mg2+ | 0.504 | 0.777 | 0.019 |
| Ca2+ | 0.025 | −0.836 | 0.477 |
| EC | 0.439 | −0.817 | 0.066 |
| Mn2+ | −0.499 | −0.354 | −0.171 |
| Eigen value | 11.41 | 2.87 | 1.72 |
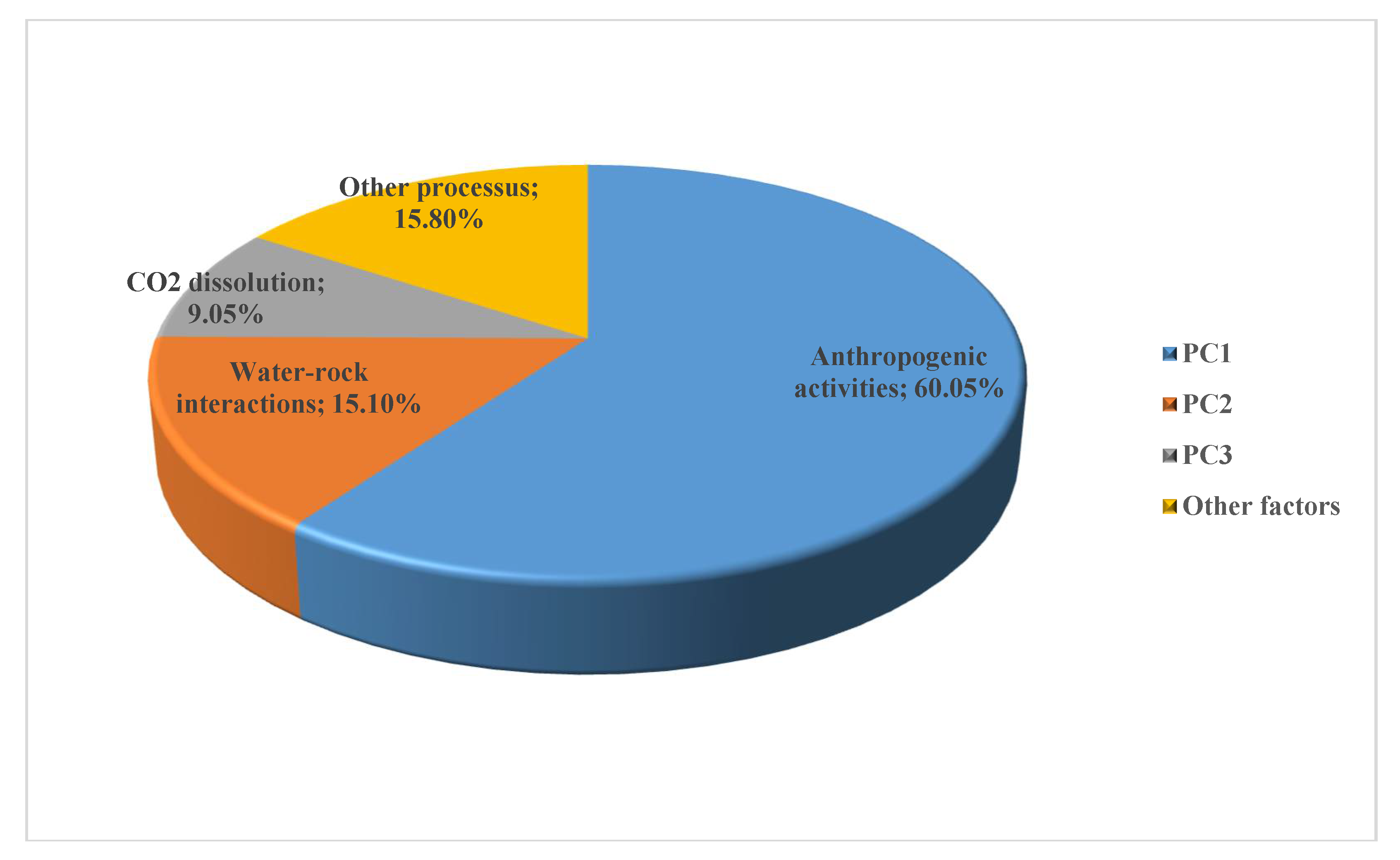
| Var. Expl. | 60.05% (60%) | 15.10% (75%) | 9.05% (84%) |
| B1 | B2 | P3 | P4 | P5 | P6 | ||
|---|---|---|---|---|---|---|---|
| NH4+ | mg·L−1 | 0.0 | 0.0 | 0.0 | 0.8 | 1.7 | 1.790 |
| Index | 5 | 5 | 5 | 4 | 3 | 3 | |
| NO2− | μg/L | 142 | 84 | 27 | 150 | 280 | 370 |
| Index | 2 | 2 | 3 | 1 | 1 | 1 | |
| PO43− | μg/L | 9906 | 5735 | 5214 | 500 | 700 | 50 |
| Index | 1 | 1 | 1 | 2 | 2 | 4 | |
| OPI | 2.7 | 2.7 | 3.0 | 2.3 | 2.0 | 2.7 | |
| Organic pollution | High | High | Moderate | High | High | High | |
| Phasis | IF | Chem. Formula | |||||
|---|---|---|---|---|---|---|---|
| B1 | B2 | P3 | P4 | P5 | P6 | ||
| Anhydrite | −3.26 | −3.7 | −3.83 | — | — | — | CaSO4 |
| Aragonite | −2.63 | −2.82 | −2.37 | −3.09 | −3.56 | −2.93 | CaCO3 |
| calcite | −2.49 | −2.68 | −2.23 | −2.95 | −3.41 | −2.79 | CaCO3 |
| Dolomite | −4.48 | −4.63 | −4.06 | −5.04 | −6.35 | −4.78 | CaMg (CO3)2 |
| Gypsum | −3.04 | −3.48 | −3.61 | — | — | — | CaSO4:2H2O |
| halite | −9.71 | −9.5 | −9.68 | −9.96 | −9.68 | −9.69 | NaCl |
| Magnesite | −2.57 | −2.53 | −2.41 | −2.67 | −3.52 | −2.57 | MgCO3 |
| Units | B1 | B2 | P3 | P4 | P5 | P6 | Average | Standard. Deviation | |
|---|---|---|---|---|---|---|---|---|---|
| EC | [dS/m] | 65.10 | 52.10 | 53.20 | 33.70 | 80.00 | 68.10 | 58.70 | 16.01 |
| SAR | — | 0.12 | 0.07 | 0.11 | 0.20 | 0.26 | 0.22 | 0.16 | 0.07 |
| NO3−-N | [mg/L] | 2.24 | 1.12 | 0.22 | 22.98 | 17.42 | 10.10 | 9.01 | 9.51 |
| %Na | [%] | 8.28% | 7.98% | 10.44% | 12.66% | 15.11% | 13.30% | 11.30% | 2.87% |
| PS | [meq/L] | 0.36 | 0.37 | 0.17 | 0.25 | 0.54 | 0.54 | 0.37 | 0.15 |
| KR | — | 0.08 | 0.04 | 0.08 | 0.13 | 0.16 | 0.14 | 0.10 | 0.04 |
| RSC | [meq/L] | −0.28 | −0.53 | 0.08 | −0.78 | −0.94 | −0.42 | −0.48 | 0.36 |
| MH | [%] | 70.01% | 80.01% | 65.01% | 84.35% | 68.71% | 82.51% | 75.10% | 8.16% |
| IP | [%] | 85.37% | 66.27% | 100.04% | 59.43% | 52.38% | 75.10% | 73.10% | 17.57% |
| Ka | — | 584.57 | 227.33 | 511.50 | 1043.67 | 767.12 | 776.25 | 651.74 | 278.11 |
| Class | Value Range | Water Quality | % Sample | |
|---|---|---|---|---|
| EC [µS/cm] | C1 | <250 | Excellent | 100% |
| C2 | 250–750 | Good | 0% | |
| C3 | 750–2250 | Doubtful | 0% | |
| C4 | >2250 | Unsuitable | 0% | |
| SAR | Low | 0–9 | Use with caution on sodium sensitives crops | 100% |
| Medium | 10–17 | Amendments and leaching need | 0% | |
| High | 18–25 | Unsuitable for continuous use | 0% | |
| Very high | >26 | Unsuitable for use | 0% | |
| NO3−[mg/L] | ≤2 | Excellent | 33.33% | |
| 2–5 | Good | 16.67% | ||
| 5–20 | Permissible | 33.33% | ||
| 20–30 | Doubtful | 16.67% | ||
| >30 | Unsuitable | 0% | ||
| %Na | <20 | Excellent | 100% | |
| 20–40 | Good | 0% | ||
| 40–60 | Permissible | 0% | ||
| 60–80 | Doubtful | 0% | ||
| >80 | Unsuitable | 0% | ||
| PS | <3.0 | Excellent to good | 100% | |
| 3.0–5.0 | Good to injurious | 0% | ||
| >5.0 | Injurious to unsatisfactory | 0% | ||
| KR | <1 | Suitable | 100% | |
| >1 | Unsuitable | 0% | ||
| RSC [meq/L] | <1.25 | Good | 100% | |
| 1.25–2.50 | Doubtful | 0% | ||
| >2.50 | Unsuitable | 0% | ||
| MH | <50 | Suitable | 0% | |
| >50 | Unsuitable | 100% | ||
| PI | Class I | >75 | Suitable | 50% |
| Class II | 25–75 | Marginally suitable | 50% | |
| Class III | <25 | Unsuitable | 0% | |
| Ka | >18 | Excellent | 100% | |
| 6–18 | Permissible | 0% | ||
| 1.2–6 | Doubtful | 0% | ||
| <1.2 | Unsuitable | 0% |
| Units | B1 | B2 | P3 | P4 | P5 | P6 | Recommended Maximum Concentration (R.S. and Westcot, 1985) | |
|---|---|---|---|---|---|---|---|---|
| Cu | mg·L−1 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.2 |
| Ni | mg·L−1 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.2 |
| Cr | mg·L−1 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.1 |
| Mn | mg·L−1 | 0.00 | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 | 0.2 |
| Cd | mg·L−1 | 0.00 | 0.00 | 0.00 | 0.03 | 0.04 | 0.05 | 0.01 |
| Pb | mg·L−1 | 0.00 | 0.00 | 0.00 | 0.07 | 0.12 | 0.06 | 5 |
| Al | mg·L−1 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 5 |
| Zn | mg·L−1 | 0.04 | 0.05 | 0.03 | 0.00 | 0.00 | 0.00 | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serge Gaëtan, S.É.; Marie Michelle, C.H.; Ouindinboudé Jacques, K.; Poulouma Louis, Y.; Idriss, S. Hydrogeochemistry of Shallow Groundwater and Suitability to Irrigation: The Case of the Karfiguéla Paddy Field in Burkina Faso. Water 2022, 14, 2574. https://doi.org/10.3390/w14162574
Serge Gaëtan SÉ, Marie Michelle CH, Ouindinboudé Jacques K, Poulouma Louis Y, Idriss S. Hydrogeochemistry of Shallow Groundwater and Suitability to Irrigation: The Case of the Karfiguéla Paddy Field in Burkina Faso. Water. 2022; 14(16):2574. https://doi.org/10.3390/w14162574
Chicago/Turabian StyleSerge Gaëtan, Sauret Élie, Compaoré Hillary Marie Michelle, Kissou Ouindinboudé Jacques, Yaméogo Poulouma Louis, and Sermé Idriss. 2022. "Hydrogeochemistry of Shallow Groundwater and Suitability to Irrigation: The Case of the Karfiguéla Paddy Field in Burkina Faso" Water 14, no. 16: 2574. https://doi.org/10.3390/w14162574
APA StyleSerge Gaëtan, S. É., Marie Michelle, C. H., Ouindinboudé Jacques, K., Poulouma Louis, Y., & Idriss, S. (2022). Hydrogeochemistry of Shallow Groundwater and Suitability to Irrigation: The Case of the Karfiguéla Paddy Field in Burkina Faso. Water, 14(16), 2574. https://doi.org/10.3390/w14162574






