1. Introduction and Historical Background of the Hula Valley Management
Lake Kinneret is the only natural freshwater lake in Israel. The lake is located below sea level in the northern part of the Syrian-African, a great rift valley. Since 1964, the lake has been the major source of domestic (drinking) water in the country, supplying 200–350 million cubic meters per year (mcm/year, 106 m3 annually). Since early 2010, a major source of domestic water (700 mcm) is desalinization plants. Lake Kinneret is also used for commercial fisheries, recreation and tourism. In the last 20 years, the Kinneret ecosystem structure has undergone significant modifications. The major change was a turnaround in the available nutrients, with limitations from phosphorus to nitrogen. Moreover, the replacement of nitrogen and the outsourcing of ammonium and organic nitrogen to nitrate modified the algal domination of Peridinium and transferring domination to cyanobacteria. The ecological processes of these modifications are evaluated in the present paper with future perspective conclusions.
The Kinneret drainage basin area is 2730 km
2 and is located mostly to the north of the lake, of which the Hula Valley is about 200 km
2. Three major headwater rivers flow from the Hermon mountain region and join into one, the River Jordan. The Jordan River contributes about 63% of the Kinneret water budget. Before 1957, when the Hula Valley was drainage, this land was covered by old Lake Hula and 3500 ha of swampy wetland. Not much information was documented about the chemical composition of the Kinneret headwater, the Hula Valley wetlands and old Lake Hula before the drainage. The Hula swampy wetlands and the old Lake Hula were drained between 1951 and 1957. From 1851 to 1957, about 40 scientific expeditions explored the fauna and the flora of the wetland and temperature, pH, chloride concentration and hardness in old Lake Hula and river waters in the Hula Valley. Nevertheless, information about nutrients in the swamps and the old Lake Hula is not available [
1]. A single documented analysis [
2,
3] reported free and albuminoid ammonia concentrations in the swamps and old Lake Hula, whereas nitrate and nitrite were absent or unmeasurable. The higher concentration of free and albuminoid ammonia in the swampy waters and lower in old Lake Hula were reported. Consequently, the reductive environment of the swamps is confirmed [
3].
The indication of the Hula Valley as a wilderness was defined by the Mongolians (1240 ac) and malaria was introduced during the Crusader period (1099–1291 ac). Enhancement of water surface inundations of the swamps and old Lake Hula resulted in the construction of the Benot Yaakov Bridge by Bivers in 1260. The principal impact on human activity was due to the distribution of the malaria disease. Settling of the Ghawarna nomad tribes in the Hula Valley was carried out between 1840 and 1948, while the nomad population increased from 520 to 31,470 residents. No permanent settlements were constructed and the management policy principle was: “the more dry was the winter more grass for cattle grazing and land for agriculture were available during the spring-summer season”. The Jewish settlements in the Hula Valley were initiated at the end of the 19th century and enhanced in the 1940s. The drainage of old Lake Hula and adjacent swampy wetlands was carried out from 1950 to 1957. The old lake and wetland area was converted into agriculture land and 6900 ha of natural wetland was converted for agricultural cultivation. Consequently, natural fauna and flora of exceptional diversity were demolished. For 40 years, agricultural products (cotton, corn, alfalfa, vegetables, barley and others) were economically produced. As a result of inappropriate management, the soil structure deteriorated, dust storms became frequent, the soil surface subsided by 7–10 cm/year and underground fires occurred frequently. A reclamation project was implemented (1994–2005).
The reclamation project included creating a new shallow Lake Agmon-Hula, (presently with a 0.2 m mean depth and 82 ha surface area) functioning as a collector of flushed nutrients from the peat soil to be removed through the Agmon-Hula system and out of the Kinneret watershed. Independently, human domestic sewage and dairy-farming effluent removal projects were achieved. Collected sewage is transferred into treatment plants and stored in reservoirs for irrigation reuse. From 1970 to 1985, the fish pond area in the Hula Valley was restricted from 1700 to 300 ha. Sewage removal and fish pond restrictions eliminated significant loads of nutrients, mostly organic nitrogen and ammonium, from the external inputs that introduced into Lake Kinneret. As a result of the drainage (1957), the nitrogen form that dominated, contributed to by the Hula Valley, shifted from ammonium and organic nitrogen to nitrate, which was represented by a continuously stable and high concentration. Unlike high and stable concentrations of nitrate, organic nitrogen and ammonium forms significantly declined. The nitrate-flush dynamic resulted from dryness and oxidation of the peat soil after the drainage. The decline of organic nitrogen and ammonium is attributed to sewage removal and diminished aquaculture.
Sewage Removal and Fish Pond Restrictions
As of 2007, 18 reservoirs with a total available volume of 4.1 × 10
6 m
3 were constructed in the Kinneret watershed. Earlier, 12 × 10
6 m
3 of raw sewage used to enter daily into Lake Kinneret [
4,
5,
6]. Between 1996 and 2007, a total capacity of 83 tons/year of TN (concentration of 11–27 ppm) (data on ammonium are not available) was removed into reservoirs, whilst the annual capacity of TN that was removed through the Agmon-Hula system (concentration of 4.6–8.7 ppm) varied between 11–48 ton/year. The removal of the ammonium load (mean concentration of 1.4 ppm) through the Agmon-Hula system during 2000–2005 varied between 5.5 and 3.3 ton/year. Prior to the implementation of the sewage removal project in 1986, an annual load of ammonium in the Jordan discharge was 70.2 tons, whilst from 1999 to 2018 the mean capacity of ammonium in the Jordan was 21 tons, and the mean load of nitrate was 718 tons. Consequently, ammonium removal from the Kinneret mass balance through the sewage treatment, as measured in the Jordan discharge, is more efficient than through the Agmon-Hula system. Nevertheless, heavy loads of nitrates originated in the peat soil and enriched Lake Kinneret’s TN standing stock. Before the drainage, about 293 ton/year of free ammonia was delivered from the swamps into old Lake Hula and 117 ton/year of albuminoid ammonia was forwarded from the old Lake Hula into the Jordan (annual discharge of 650 mcm = 10
6 m
3) [
1,
2,
3].
The total sewage volume that was practically removed was 7.2 mcm out of the optional available 17.1 mcm (42%), of which 1.6 mcm was spilled over and 5.6 mcm (33%) was reused and eliminated from nitrogen consumers in Lake Kinneret [
4,
5,
6,
7,
8,
9,
10,
11,
12]. An average of 19 ppm, as the concentration of TN in the sewage removed into reservoirs, indicates the elimination of 106 tons of TN which mostly is organic and ammonium nitrogenic material. Prior to the Hula drainage, nitrates were almost absent [
1,
2,
3] or negligible within the Jordan loads. Before the drainage, the Jordan River conveyed 2.3 times more ammonium than from 1985 to 1967 and 5.6 times more than from 1999 to 2018. After the drainage, peat soil oxidation was enhanced and nitrates became the major nitrogen form within the Jordan supply to Lake Kinneret. Before sewage removal was accomplished, the flux of organic nitrogen and ammonium continued for the benefit of nitrogen consumers (mostly
Peridinium) in Lake Kinneret. After the achievement of sewage removal and aquaculture restrictions, loads of organic nitrogen and ammonium declined and the
Peridinium bloom diminished.
Until the late 1980s, aquaculture development was intensively implemented in the Hula Valley. The total area of shallow fish ponds had gone up to 1700 ha and were where shoals of cichlids (tilapias) and carps were densely stocked. Tilapias are tropical fishes and temperatures in the Hula Valley in the winter months were too cold for an efficient commercial productive growth rate; therefore, natural, high-quality, warmer surface waters were continuously transferred through the ponds with the aim to warm the water. The result was nutrient (nitrogen, phosphorus, etc.) loading of high-quality water migration downstream into Lake Kinneret. As a result of financial deliberations, the fish pond area was restricted to 350 ha during the 1980s. Both sewage removal and fish pond restrictions significantly affected nutrient loadings from the catchment into Lake Kinneret.
3. Results
The results shown in
Figure 2 indicate an increase in the biomass density of cyanobacteria from the mid-1980s: a 60% increase in the periodically (1969–1985 relative to 1986–2001) averaged cyanobacteria biomass (3.2 and 5.1 g/m
2, respectively) was indicated.
The most common diazotrophic and non-diazotrophic cyanobacteria in Lake Kinneret are as follows:
Heterocystous diazotrophic growth, aerobic:
Aphanizomenon ovalisporum; A. flos-aqua; Anabaena flos-aqua; A. spiroides; A. planctonica
Cylidrospermopsis raciborskii; Chroococcus limneticus; Nostoc sp; Chroococcuc minutus; Planktothrix rubescens.
Anaerobic diazotrophic non-heterocystous:
Plectonema sp.; Oscilatoria limnetica; Lyngby ssp.; Synechococcus sp.
Aerobic diazotrophic heterocystous:
Lingbya limnetica; Oscilatoria, sp.
Non-diazotrophic cyanobacteria:
Spirulina platensis; Merismopedia punctata; Chroococcuc minutus; Planktothrix rubescens
Microcystis aeruginosa; Microcystis flos-aqua; Aphanocapsa spp.
Long-term records of nitrate (NO
3) and ammonium (NH
4) concentrations (ppm) and loads (ton) as annual and monthly means in the waters of River Jordan and the epilimnion of Lake Kinneret were used. The densities of the biomass of cyanobacteria (
Figure 2) and the bloom-forming Pyrrhophyte
Peridinium spp. are presented in
Figure 3,
Figure 4,
Figure 5,
Figure 6,
Figure 7,
Figure 8,
Figure 9,
Figure 10,
Figure 11,
Figure 12,
Figure 13,
Figure 14 and
Figure 15.
The results given in
Figure 3 indicate a decline in the River Jordan discharge attributed to changes in climate conditions.
The results given in
Figure 4 indicate a decline in the ammonium concentrations (ppm) in the Jordan River water between 1968 and 2018.
The results shown in
Figure 5 indicate a positive correlation between the discharge and the concentration of ammonium in the River Jordan water.
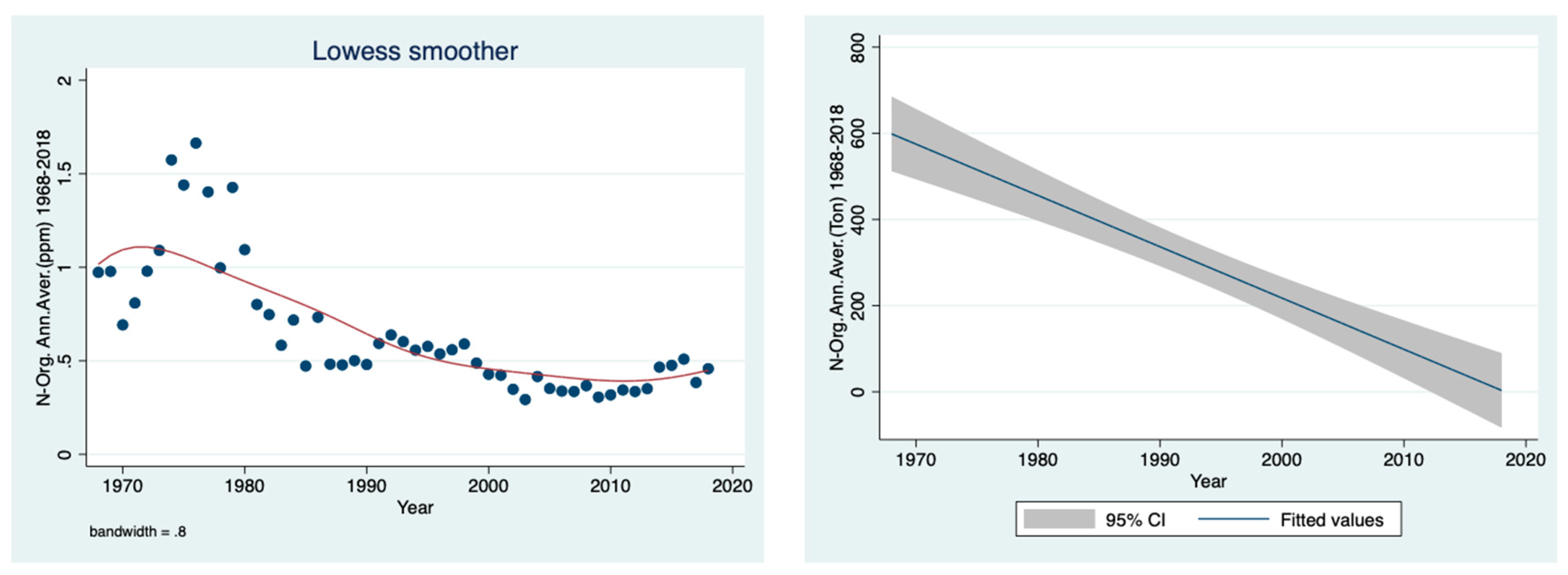
The prominent temporal decline in the organic nitrogen concentration (ppm), shown in
Figure 6, and load (ton) (
Figure 7) in the River Jordan water between 1968 and 2018 is clear.
The prominent temporal decline in the nitrate concentration (ppm), shown in
Figure 8, and load (ton) (
Figure 9) in the River Jordan water from 1968 to 2018 is clear.
The results given in
Figure 10 indicate a significant temporal elevation in the mass (ton) ratio between nitrate and ammonium loads in the water of the River Jordan from 1968 to 2018.
The data evaluation in
Figure 11 and
Figure 12 are the long-term (1970–2018) monthly means of nitrate and ammonium loads (ton) (
Figure 11) and the
Peridinium wet biomass and total nitrogen (
Figure 12) in the epilimnion of Lake Kinneret evaluated as a LOWESS smoother (bandwidth 0.8) trend of changes. The monthly changes in the TN (ppm and ton) and
Peridinium biomass are similar, being high in winter and low in the summer months, whilst the dynamics of ammonium are slightly different, with the decline from winter to spring and an increase from summer to fall, which is probably affected by the thermal structure (stratification–destratification) changes.
The positive relationship between the
Peridinium biomass and the load (ton) of the total nitrogen in the epilimnion is prominently indicated in
Figure 13 based on the full year (1–12) cycle evaluation. Moreover, when computed for the bloom season (January–June) (
Figure 12), similar relations are indicated.
The step forward in the data evaluation is presented in
Figure 15.
Data given in
Figure 15 represent a temporal comprehensive overview of the seasonal tropical pattern of the Lake Kinneret ecosystem: the intensive winter nutrient influx induces a
Peridinium bloom formation, whereas the summer induces a nutrient deficiency; due to the temperature elevation, the eco-physiological activity is enhanced, but total production diminishes.
4. Discussion
The impact of the drainage basin on Lake Kinneret is defined by two major traits: hydrological and chemical. This paper evaluates aspects of the chemistry in which the impact of nitrogen is discussed. The last 100 years of the Anthropocene era were defined in the Kinneret drainage basin by population enhancement, and hydrological and agricultural management modifications: the Hula Valley drainage, domestic and dairy sewage removal, and later, the Hula Reclamation Project implementation and climate condition changes.
Consideration of the nutrient inputs into Lake Kinneret prior to 1968 reveals that they are missing, but later on (
Table 1) they are discussed.
Data given in
Table 1 reflect the historical anthropogenic involvements in the Hula Valley structure, which affected the nitrogen supply from the drainage basin into Lake Kinneret. It is suggested that immediately after the drainage, nitrogen supported as ammonium and organic nitrogen was reduced and continued later.
Figure 3 confirms the reduction in the River Jordan discharge as a result of climate conditions, especially due to a decline in rainfall. Moreover, the temporal discharge decline was accompanied by a decrease in the ammonium concentration in the River Jordan waters (
Figure 4), reflecting the diminishing contribution from the Hula Valley which probably was also affected by the sewage removal and fish pond restrictions. The direct impact of climate conditions (rainfall regime) on the mechanism of nutrient flushing is confirmed in
Figure 5, where the higher the discharge below 700 mcm/y, the higher the ammonium concentration. The impact of sewage removal and fish pond restrictions during the 1980s, which was also partly supplemented by a decline in discharge, is indicated in
Figure 6 (as concentration) and
Figure 7 (as loads in tons) as the prominent decline in organic nitrogen migration from the drainage basin into Lake Kinneret via the River Jordan. After the drainage, the dynamics of nitrate, organic nitrogen and ammonium are dissimilar because the nitrate source is oxidized peat soil and the other two originate mostly from domestic and dairy sewage. This is confirmed in
Figure 8 (ppm) and
Figure 9 (ton), where the trend of change indicates temporal elevation. The dissimilarity between the nitrate and ammonium dynamics is also confirmed in
Figure 10, representing temporal fluctuations of the mass ratio between the two forms of nitrogen (NH
4:NO
3). From 1968 to 1985, the ratio declined (
Figure 10) as a result of the sewage and fish pond contributions, which were later removed (with NH
4 and organic N) (
Figure 10), causing the ratio to increase.
Data shown in
Figure 9,
Figure 10,
Figure 11,
Figure 12 and
Figure 13 indicate relations between nitrogen availability and density of the
Peridinium in the epilimnion of Lake Kinneret on a monthly basis. The dynamics of nitrate in the epilimnion are mostly controlled by algal consumption, epilimnetic nitrification, external inputs and oxidation of ammonium during destratification. Monthly changes are shown in
Figure 11: the winter elevation resulted from external inputs and nitrification and the summer decline resulted from algal consumption, and a lack of external inputs and stable stratification, which causes a reduction in ammonium hypolimnetic penetration. On the other hand (
Figure 9, NH
4), the ammonium monthly changes are different: there was a prominent increase from September to January as a result of the upward transfer of NH
4 from the hypolimnion during destratification and a prominent decline between February and August, mostly due to the oxidation from NO
3 algal consumption. The similarity between the monthly distribution of the
Peridinium biomass (
Figure 10) and NO
3 epilimnetic availability reflects the function of nitrogen in the algal growth rate. Moreover, evaluation of the relationship between the annual (12 months) mean averaged from monthly means and a total load of epilimnetic total nitrogen (of which nitrate is the majority) shows that it is significant (
Figure 13). Considering a similar relationship between the
Peridinium biomass and TN during bloom season (January–June) indicates similar relations. A monthly evaluation of the relationship between
Peridinium biomass distribution and nutrient (N, P) availability is given in
Figure 15. Although this paper discusses nitrogen dynamics, it is highly important to consider, briefly, the impact of phosphorus. P and N sourcing is very different because that of nitrogen is mostly external, whilst that of P is internal and external. In fact, the life cycle of
Peridinium [
12,
13,
14] includes summer dormant cystation in the bottom sediments. During summer cystation, P is absorbed, and later, during winter germination, this P is a significant part of the epilimnetic stock.
The increase in the
Peridinium biomass directly corresponds to TN availability (
Figure 15). The heavy bloom formed by germinated cysts and reproducing
Peridinium cells with a partial supportive external P contribution create P stock as well. Three major conclusions were drawn regarding a combination of suitable conditions that enabled the establishment of cyanophyte algae to be a significant component of the Kinneret phytoplankton community: (1) nitrogen deficiency accompanied by stable (even a slight increase) phosphorus availability [
15,
16]; (2) seasonal increase in phosphorus availability in the epilimnion that resulted in a heavy
Peridinium bloom crush, which was an “injection” of soluble phosphorus from degraded cells into the epilimnion [
17]; (3) decline in epilimnetic sulfate concentration that enhanced molybdenum uptake followed by accelerating nitrogen fixation through nitrogenase in the cells of N
2-fixer cyanobacteria [
18].
The nitrogen demand of Peridinium for the optimal growth rate was widely documented earlier [
12,
13,
14,
15,
18,
19,
20,
21], and the competition among algal components for ammonia uptake was previously studied [Sherr et al. 1982; Cochlan and Harirsron 1991]. The role of nitrogen in the metabolism of mixotrophic dinoflagellates was reviewed by Bellefeuille and Morse [
22,
23], where nitrogen starvation induces metabolic modifications to ensure survival. The importance of particulate and dissolved organic nitrogen for dinoflagellates during initiation and mature bloom was documented by [
24]. The dependence of dinoflagellates and diatom on high N and N:P ratios was documented by [
25,
26]. The sensitivity of freshwater lakes to climate change and nutrient loadings was documented (among others) by [
27,
28,
29,
30,
31,
32,
33].
The maximal concentration of ammonium in the Kinneret epilimnion during the
Peridinium bloom season varies between 1.1–1.7 µM, but it promptly declines later. Ammonium decline is due to algal uptake and microbial nitrification. Considering 3% of the dry weight as nitrogen content in
Peridinium cells, 40% as the DW of wet weight and the commonly documented peak of 150 g(ww)/m
2 [
13,
14], the total nitrogen requirement to compensate for the standing stock is about 30,000 tons for the entire lake (168 km
2 and epilimnion volume of 2000 mcm). Considering the ammonium preferential uptake on nitrate [
22], the standing stock is insufficient. Therefore, the
Peridinium preferential ammonium is a limiting factor, although it is partly replaced by plenty of available nitrates. The high concentration of nitrate in early winter is due to the flux in nitrogen and substantial bacterial nitrifiers originating from the Hula peat soil. The nitrogen sources supplied for the
Peridinium bloom formation are mostly external, although a significant stock of nitrogen is trapped in the bottom sediments [
14]. The prominent decline in the external supply of organic nitrogen via headwater discharges (River Dan, River Jordan) since the early 1980s was documented by [
20,
21]. Sewage removal and fish pond restrictions probably enhanced this trend. A similar decline was also documented in the Golan Heights Meshushim headwater discharge [
20]. During the late 1970s and early 1980s, there was a prominent decline in nitrogen at the expense of the nitrification bacterial process. It was considered [
34] to enhance phytoplankton nitrogen uptake, of which the biomass was prominently increased. Nevertheless, a simultaneous high concentration of ammonium was indicated as well. The option of competition between ammonium and nitrate in the uptake by
Peridinium was not considered. A worldwide, well-known tenet indicates that ammonium inhibits nitrate uptake [
35] and a low rate of nitrate uptake occurs when the ammonium concentration is above 1 µM, as ammonium is preferred as a nitrogen source although growth rates of nitrate continue [
34,
35,
36]. The ammonium supply to the Kinneret epilimnion in early winter originates mostly from the hypolimnion and moves into the epilimnion during destratification, but promptly declines while being oxidized to nitrate. The ammonium decline is attributed to algal uptake and microbial nitrification. During the
Peridinium bloom, the epilimnetic (app volume 2000 mcm; 10
6 m
3) ammonium mean concentration varies between 0.020 and 0.030 ppm (1.1–1.7 µM), which is a total epilimnetic stock of 40–60 tons. The standing stock of intracell nitrogen content within the
Peridinium biomass is 30,000 tons, resulting in ammonium insufficiency, and nitrogen is utilized instead. If the ammonium concentration is higher than 1 µM it is preferred, but if lower, then nitrogen is incorporated. Prior to the sewage removal and aquaculture restrictions, the ammonium supply probably compensated the nitrogen demands of
Peridinium. Later on, when the nitrogen supply originates mostly from nitrate, the
Peridinium decline became realistic. Prior to 1957, ammonium was efficiently supplied from the reductive swampy environment for the beneficial
Peridinium bloom formation. Later on, sewage and aquaculture became major ammonium suppliers, and furthermore, when the major nitrogen source was nitrate, the
Peridinium declined. Nishri and Hamilton [
21] considered a direct relationship between the phytoplankton biomass and nitrates, whilst competition and ammonium preference are not involved. Nishri and Hamilton [
21] did not consider the uptake competition between nitrogen and ammonium by
Peridinium, resulting therefore in an increase in its biomass by NO
3 uptake whilst I related a high biomass during 1970–1980 (
Figure 6) to NH
4 availability.