Small-Scale Variability of Soil Quality in Permafrost Peatland of the Great Hing’an Mountains, Northeast China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Samples Treatment
2.3. Soil Microbial Carbon (Cmic), Basal Respiration (BR), and Metabolic Quotient (qCO2)
2.4. Soil Properties Analyses
2.5. Statistical Date Analyses
3. Results
3.1. Sample Properties Variation
3.2. Microbial Respiration Activities at Small-Scale
3.3. Relationships between Microbial Respiration Activities and Sample Properties
4. Discussion
4.1. Soil Properties at Small Scale in the Permafrost Peatland
4.2. Microbial Respiration Activities at Small Scale in the Permafrost Peatland
4.3. Implications of Variation at Small Scale in Peatland
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gorham, E. Northern peatlands: Role in the carbon cycle and probable responses to climatic warming. Ecol. Appl. 1991, 1, 182–195. [Google Scholar] [CrossRef] [PubMed]
- Tarnocai, C.; Canadell, J.G.; Schuur, E.A.G.; Kuhry, P.; Mazhitova, G.; Zimov, S. Soil organic carbon pools in the northern circumpolar permafrost region. Glob. Biogeochem. Cycles 2009, 23, GB2023. [Google Scholar] [CrossRef]
- Räisänen, J.; Hansson, U.; Ullerstig, A.; Döscher, R.; Graham, L.; Jones, C.; Meier, H.; Samuelsson, P.; Willén, U. European climate in the late twenty-first century: Regional simulations with two driving global models and two forcing scenarios. Clim. Dynam. 2004, 22, 13–31. [Google Scholar] [CrossRef]
- Dorrepaal, E.; Toet, S.; van Logtestijn, R.S.; Swart, E.; van de Weg, M.J.; Callaghan, T.V.; Aerts, R. Carbon respiration from subsurface peat accelerated by climate warming in the subarctic. Nature 2009, 460, 616–619. [Google Scholar] [CrossRef]
- Pengerud, A.; Dignac, M.-F.; Certini, G.; Strand, L.T.; Forte, C.; Rasse, D.P. Soil organic matter molecular composition and state of decomposition in three locations of the European Arctic. Biogeochemistry 2017, 135, 277–292. [Google Scholar] [CrossRef]
- Turetsky, M.R.; Wieder, R.; Vitt, D.; Evans, R.; Scott, K. The disappearance of relict permafrost in boreal north America: Effects on peatland carbon storage and fluxes. Glob. Chang. Biol. 2007, 13, 1922–1934. [Google Scholar] [CrossRef]
- Cresto Aleina, F.; Runkle, B.R.K.; Kleinen, T.; Kutzbach, L.; Schneider, J.; Brovkin, V. Modeling micro-topographic controls on boreal peatland hydrology and methane fluxes. Biogeosciences 2015, 12, 5689–5704. [Google Scholar] [CrossRef] [Green Version]
- Wright, E.L.; Black, C.R.; Turner, B.L.; Sjögersten, S. Environmental controls of temporal and spatial variability in CO2 and CH4 fluxes in a neotropical peatland. Glob. Chang. Biol. 2013, 19, 3775–3789. [Google Scholar] [CrossRef]
- Jenerette, G.D.; Wu, J. Interactions of ecosystem processes with spatial heterogeneity in the puzzle of nitrogen limitation. Oikos 2004, 107, 273–282. [Google Scholar] [CrossRef]
- Evgrafova, A.; de la Haye, T.R.; Haase, I.; Shibistova, O.; Guggenberger, G.; Tananaev, N.; Sauheitl, L.; Spielvogel, S. Small-scale spatial patterns of soil organic carbon and nitrogen stocks in permafrost-affected soils of northern Siberia. Geoderma 2018, 329, 91–107. [Google Scholar] [CrossRef]
- Hobbie, S.E.; Schimel, J.P.; Trumbore, S.E.; Randerson, J.R. Controls over carbon storage in high-latitude soils. Glob. Chang. Biol. 2000, 6, 196–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Limpens, J.; Berendse, F.; Blodau, C.; Canadell, J.; Freeman, C.; Holden, J.; Roulet, N.; Rydin, H.; Schaepman-Strub, G. Peatlands and the carbon cycle: From local processes to global implications—A synthesis. Biogeosciences 2008, 5, 1475–1491. [Google Scholar] [CrossRef] [Green Version]
- Laiho, R. Decomposition in peatlands: Reconciling seemingly contrasting results on the impacts of lowered water levels. Soil Biol. Biochem. 2006, 38, 2011–2024. [Google Scholar] [CrossRef]
- Moore, T.R.; Basiliko, N. Decomposition in boreal peatlands. In Boreal Peatland Ecosystems; Wieder, R.K., Vitt, D.H., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 125–143. [Google Scholar]
- Mathijssen, P.J.H.; Galka, M.; Borken, W.; Knorr, K.H. Plant communities control long term carbon accumulation and biogeochemical gradients in a Patagonian bog. Sci. Total Environ. 2019, 684, 670–681. [Google Scholar] [CrossRef]
- Baldrian, P. Distribution of extracellular enzymes in soils: Spatial heterogeneity and determining factors at various scales. Soil Sci. Soc. Am. J. 2014, 78, 11–18. [Google Scholar] [CrossRef]
- Drollinger, S.; Knorr, K.-H.; Knierzinger, W.; Glatzel, S. Peat decomposition proxies of Alpine bogs along a degradation gradient. Geoderma 2020, 369, 114331. [Google Scholar] [CrossRef]
- Negassa, W.; Baum, C.; Schlichting, A.; Müller, J.; Leinweber, P. Small-scale spatial variability of soil chemical and biochemical properties in a rewetted degraded peatland. Front. Environ. Sci. 2019, 7, 166. [Google Scholar] [CrossRef] [Green Version]
- Ulanowski, T.A.; Branfireun, B.A. Small-scale variability in peatland pore-water biogeochemistry, Hudson Bay Lowland, Canada. Sci. Total Environ. 2013, 454–455, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Holden, J. Peatland hydrology and carbon release: Why small-scale process matters. Philos. Trans. R. Soc. A 2005, 363, 2891–2913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sachs, T.; Giebels, M.; Boike, J.; Kutzbach, L. Environmental controls on CH4 emission from polygonal tundra on the microsite scale in the Lena river delta, Siberia. Glob. Chang. Biol. 2010, 16, 3096–3110. [Google Scholar] [CrossRef]
- Macrae, M.; Devito, K.; Strack, M.; Waddington, J. Effect of water table drawdown on peatland nutrient dynamics: Implications for climate change. Biogeochemistry 2013, 112, 661–676. [Google Scholar] [CrossRef]
- Nungesser, M.K. Modelling microtopography in boreal peatlands: Hummocks and hollows. Ecol. Model. 2003, 165, 175–207. [Google Scholar] [CrossRef]
- Cannone, N.; Guglielmin, M.; Gerdol, R. Relationships between vegetation patterns and periglacial landforms in northwestern Svalbard. Polar Biol. 2004, 27, 562–571. [Google Scholar] [CrossRef] [Green Version]
- Blaško, R.; Holm Bach, L.; Yarwood, S.A.; Trumbore, S.E.; Högberg, P.; Högberg, M.N. Shifts in soil microbial community structure, nitrogen cycling and the concomitant declining N availability in ageing primary boreal forest ecosystems. Soil Biol. Biochem. 2015, 91, 200–211. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, M.W.; Torn, M.S.; Abiven, S.; Dittmar, T.; Guggenberger, G.; Janssens, I.A.; Kleber, M.; Kogel-Knabner, I.; Lehmann, J.; Manning, D.A.; et al. Persistence of soil organic matter as an ecosystem property. Nature 2011, 478, 49–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drzymulska, D. Peat decomposition-shaping factors, significance in environmental studies and methods of determination; a literature review. Geologos 2016, 22, 61–69. [Google Scholar] [CrossRef] [Green Version]
- Romaniuk, R.; Giuffré, L.; Costantini, A.; Bartoloni, N.; Nannipieri, P. A comparison of indexing methods to evaluate quality of soils: The role of soil microbiological properties. Soil Res. 2011, 49, 733–741. [Google Scholar] [CrossRef]
- Jin, H.; Yu, Q.; Lü, L.; Guo, D.; He, R.; Yu, S.; Sun, G.; Li, Y. Degradation of permafrost in the Xing’anling Mountains, Northeastern China. Permafr. Periglac. Processes 2007, 18, 245–258. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Hu, Y.; Lü, J.; Sun, J.; Li, Z.; He, H. Potential carbon mineralization of permafrost peatlands in Great Hing’an Mountains, China. Wetlands 2010, 30, 747–756. [Google Scholar] [CrossRef]
- Zhang, Z. Development and Utilization of Peat Resources; Chinese Jilin Science and Technology Press: Changchun, China, 2000; pp. 185–193. [Google Scholar]
- Anderson, J.; Domsch, K. A physiological method for the quantitative measurement of microbial biomass in soils. Soil Biol. Biochem. 1978, 10, 215–221. [Google Scholar] [CrossRef]
- Susyan, E.A.; Wirth, S.; Ananyeva, N.D.; Stolnikova, E.V. Forest succession on abandoned arable soils in European Russia–Impacts on microbial biomass, fungal-bacterial ratio, and basal CO2 respiration activity. Eur. Soil Biol. 2011, 47, 169–174. [Google Scholar] [CrossRef]
- Leifeld, J.; Gubler, L.; Grünig, A. Organic matter losses from temperate ombrotrophic peatlands: An evaluation of the ash residue method. Plant Soil 2011, 341, 349–361. [Google Scholar] [CrossRef]
- Griffiths, N.A.; Sebestyen, S.D.; Oleheiser, K.C. Variation in peatland porewater chemistry over time and space along a bog to fen gradient. Sci. Total Environ. 2019, 697, 134152. [Google Scholar] [CrossRef] [PubMed]
- Harrell, F. Hmisc: Harrell Miscellaneous. R Package Version 3.17-4. 2016. Available online: http://CRAN.R-project.org/package=Hmisc (accessed on 1 December 2019).
- Eppinga, M.B.; Rietkerk, M.; Wassen, M.J.; De Ruiter, P.C. Linking habitat modification to catastrophic shifts and vegetation patterns in bogs. Plant Ecol. 2009, 200, 53–68. [Google Scholar] [CrossRef] [Green Version]
- Girkin, N.T.; Vane, C.H.; Cooper, H.V.; Moss-Hayes, V.; Craigon, J.; Turner, B.L.; Ostle, N.; Sjögersten, S. Spatial variability of organic matter properties determines methane fluxes in a tropical forested peatland. Biogeochemistry 2018, 142, 231–245. [Google Scholar] [CrossRef]
- Hoyos-Santillan, J.; Lomax, B.H.; Large, D.; Turner, B.L.; Boom, A.; Lopez, O.R.; Sjögersten, S. Getting to the root of the problem: Litter decomposition and peat formation in lowland Neotropical peatlands. Biogeochemistry 2015, 126, 115–129. [Google Scholar] [CrossRef]
- Mao, R.; Zhang, X.; Song, C.; Wang, X.; Finnegan, P.M. Plant functional group controls litter decomposition rate and its temperature sensitivity: An incubation experiment on litters from a boreal peatland in northeast China. Sci. Total Environ. 2018, 626, 678–683. [Google Scholar] [CrossRef]
- Xu, J.; Lin, G.; Liu, B.; Mao, R. Linking leaf nutrient resorption and litter decomposition to plant mycorrhizal associations in boreal peatlands. Plant Soil 2020, 448, 413–424. [Google Scholar] [CrossRef]
- Wang, M.; Larmola, T.; Murphy, M.T.; Moore, T.R.; Bubier, J.L. Stoichiometric response of shrubs and mosses to long-term nutrient (N, P and K) addition in an ombrotrophic peatland. Plant Soil 2016, 400, 403–416. [Google Scholar] [CrossRef]
- Basiliko, N.; Blodau, C.; Roehm, C.; Bengtson, P.; Moore, T.R. Regulation of decomposition and methane dynamics across natural, commercially mined, and restored northern peatlands. Ecosystems 2007, 10, 1148–1165. [Google Scholar] [CrossRef]
- Miao, Y.; Song, C.; Sun, L.; Wang, X.; Meng, H.; Mao, R. Growing season methane emission from a boreal peatland in the continuous permafrost zone of Northeast China: Effects of active layer depth and vegetation. Biogeosciences 2012, 9, 4455–4464. [Google Scholar] [CrossRef] [Green Version]
- Gil-Sotres, F.; Trasar-Cepeda, C.; Leirós, M.C.; Seoane, S. Different approaches to evaluating soil quality using biochemical properties. Soil Biol. Biochem. 2005, 37, 877–887. [Google Scholar] [CrossRef]
- Viers, J.; Prokushkin, A.S.; Pokrovsky, O.S.; Auda, Y.; Kirdyanov, A.V.; Beaulieu, E.; Zouiten, C.; Oliva, P.; Dupré, B. Seasonal and spatial variability of elemental concentrations in boreal forest larch foliage of Central Siberia on continuous permafrost. Biogeochemistry 2012, 113, 435–449. [Google Scholar] [CrossRef]
- Adumitroaei, M.V.; Iancu, G.O.; Rățoi, B.G.; Doru, C.S.; Sandu, C.M. Spatial distribution and geochemistry of major and trace elements from Mohoș peatland, Harghita Mountains, Romania. Holocene 2018, 28, 1936–1947. [Google Scholar] [CrossRef]
- Siewert, M.B. High-resolution digital mapping of soil organic carbon in permafrost terrain using machine learning: A case study in a sub-Arctic peatland environment. Biogeosciences 2018, 15, 1663–1682. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Moore, T.R.; Talbot, J.; Richard, P.J.H. The cascade of C:N:P stoichiometry in an ombrotrophic peatland: From plants to peat. Environ. Res. Lett. 2014, 9, 024003. [Google Scholar] [CrossRef] [Green Version]
- Chiwa, M.; Sheppard, L.J.; Leith, I.D.; Leeson, S.R.; Tang, Y.S.; Neil Cape, J. Long-term interactive effects of N addition with P and K availability on N status of Sphagnum. Environ. Pollut. 2018, 237, 468–472. [Google Scholar] [CrossRef] [Green Version]
- Juutinen, S.; Moore, T.R.; Bubier, J.L.; Arnkil, S.; Humphreys, E.; Marincak, B.; Roy, C.; Larmola, T. Long-term nutrient addition increased CH4 emission from a bog through direct and indirect effects. Sci. Rep. 2018, 8, 3838. [Google Scholar] [CrossRef]
- Limpens, J.; Holmgren, M.; Jacobs, C.M.; Van der Zee, S.E.; Karofeld, E.; Berendse, F. How does tree density affect water loss of peatlands? A mesocosm experiment. PLoS ONE 2014, 9, e91748. [Google Scholar]
- Hájek, M.; Hekera, P.; Hájková, P. Spring fen vegetation and water chemistry in the Western Carpathian flysch zone. Folia Geobot. 2002, 37, 205–224. [Google Scholar] [CrossRef]
- Fisk, M.C.; Ruether, K.F.; Yavitt, J.B. Microbial activity and functional composition among northern peatland ecosystems. Soil Biol. Biochem. 2003, 35, 591–602. [Google Scholar] [CrossRef]
- Brouns, K.; Keuskamp, J.A.; Potkamp, G.; Verhoeven, J.T.A.; Hefting, M.M. Peat origin and land use effects on microbial activity, respiration dynamics and exo-enzyme activities in drained peat soils in the Netherlands. Soil Biol. Biochem. 2016, 95, 144–155. [Google Scholar] [CrossRef]
- Myers, B.; Webster, K.L.; McLaughlin, J.W.; Basiliko, N. Microbial activity across a boreal peatland nutrient gradient: The role of fungi and bacteria. Wetl. Ecol. Manag. 2011, 20, 77–88. [Google Scholar] [CrossRef]
- Preston, M.D.; Smemo, K.A.; McLaughlin, J.W.; Basiliko, N. Peatland microbial communities and decomposition processes in the James Bay Lowlands, Canada. Front. Microbiol. 2012, 3, 70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grodnitskaya, I.D.; Karpenko, L.V.; Knorre, A.A.; Syrtsov, S.N. Microbial activity of peat soils of boggy larch forests and bogs in the permafrost zone of central Evenkia. Eurasian Soil Sci. 2013, 46, 61–73. [Google Scholar] [CrossRef]
- Kraigher, B.; Stres, B.; Hacin, J.; Ausec, L.; Mahne, I.; Vanelsas, J.; Mandicmulec, I. Microbial activity and community structure in two drained fen soils in the Ljubljana Marsh. Soil Biol. Biochem. 2006, 38, 2762–2771. [Google Scholar] [CrossRef]
- Ausec, L.; Kraigher, B.; Mandic-Mulec, I. Differences in the activity and bacterial community structure of drained grassland and forest peat soils. Soil Biol. Biochem. 2009, 41, 1874–1881. [Google Scholar] [CrossRef]
- Säurich, A.; Tiemeyer, B.; Don, A.; Fiedler, S.; Bechtold, M.; Amelung, W.; Freibauer, A. Drained organic soils under agriculture-The more degraded the soil the higher the specific basal respiration. Geoderma 2019, 355, 113911. [Google Scholar] [CrossRef]
- Waddington, J.M.; Roulet, N.T. Carbon balance of a boreal patterned peatland. Glob. Chang. Biol. 2008, 6, 87–97. [Google Scholar] [CrossRef]
- Pelletier, L.; Garneau, M.; Moore, T.R. Variation in CO2 exchange over three summers at microform scale in a boreal bog, Eastmain region, Québec, Canada. J. Geophys. Res. 2011, 116, G03019. [Google Scholar]
- Pullens, J.W.M.; Sottocornola, M.; Kiely, G.; Toscano, P.; Gianelle, D. Carbon fluxes of an alpine peatland in Northern Italy. Agr. For. Meteorol. 2016, 220, 69–82. [Google Scholar] [CrossRef] [Green Version]
- Dieleman, C.M.; Branfireun, B.A.; McLaughlin, J.W.; Lindo, Z. Climate change drives a shift in peatland ecosystem plant community: Implications for ecosystem function and stability. Glob. Chang. Biol. 2015, 21, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Treat, C.C.; Marushchak, M.E.; Voigt, C.; Zhang, Y.; Tan, Z.; Zhuang, Q.; Virtanen, T.A.; Rasanen, A.; Biasi, C.; Hugelius, G.; et al. Tundra landscape heterogeneity, not interannual variability, controls the decadal regional carbon balance in the Western Russian Arctic. Glob. Chang. Biol. 2020, 24, 5188–5204. [Google Scholar] [CrossRef] [PubMed] [Green Version]


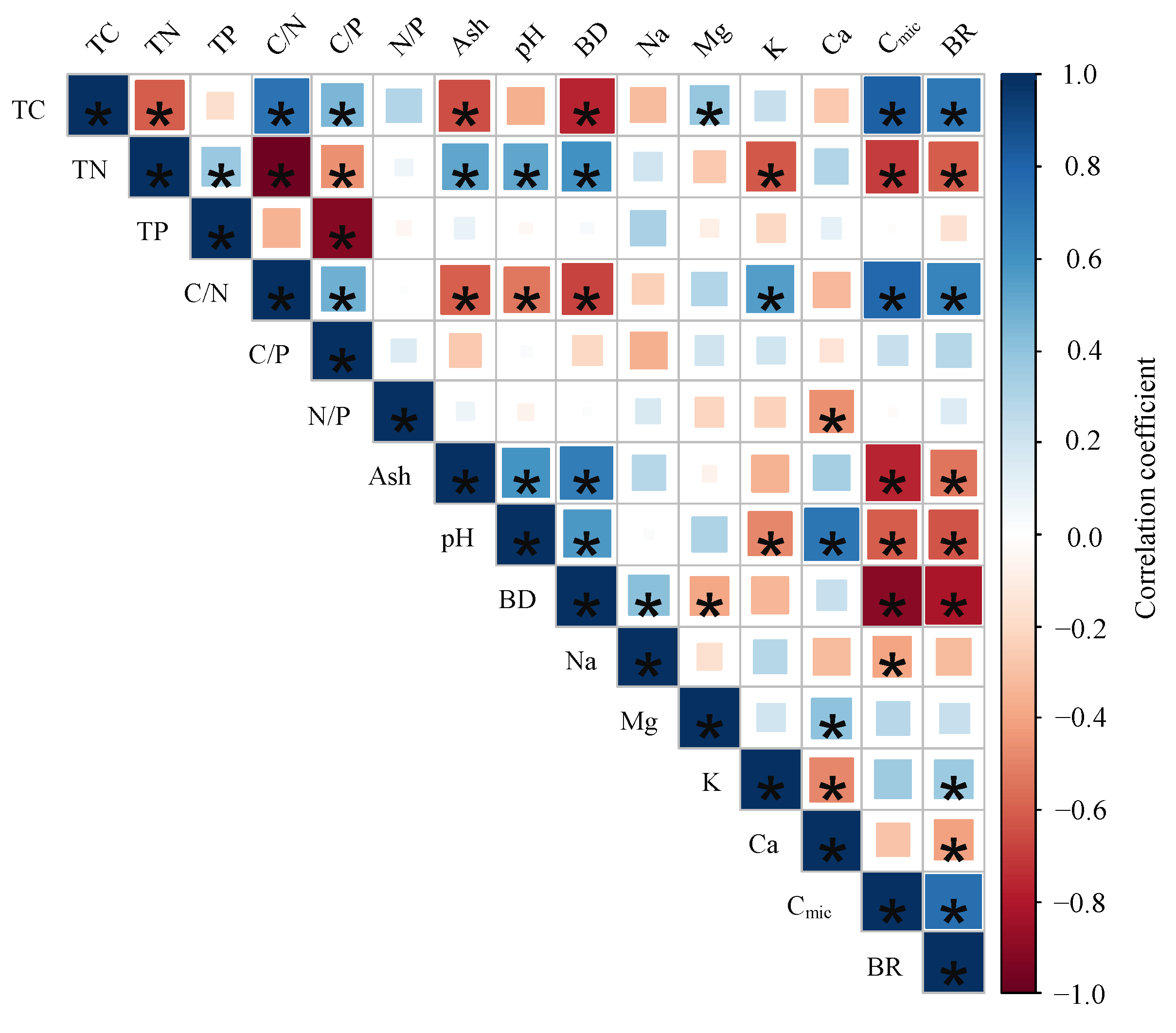
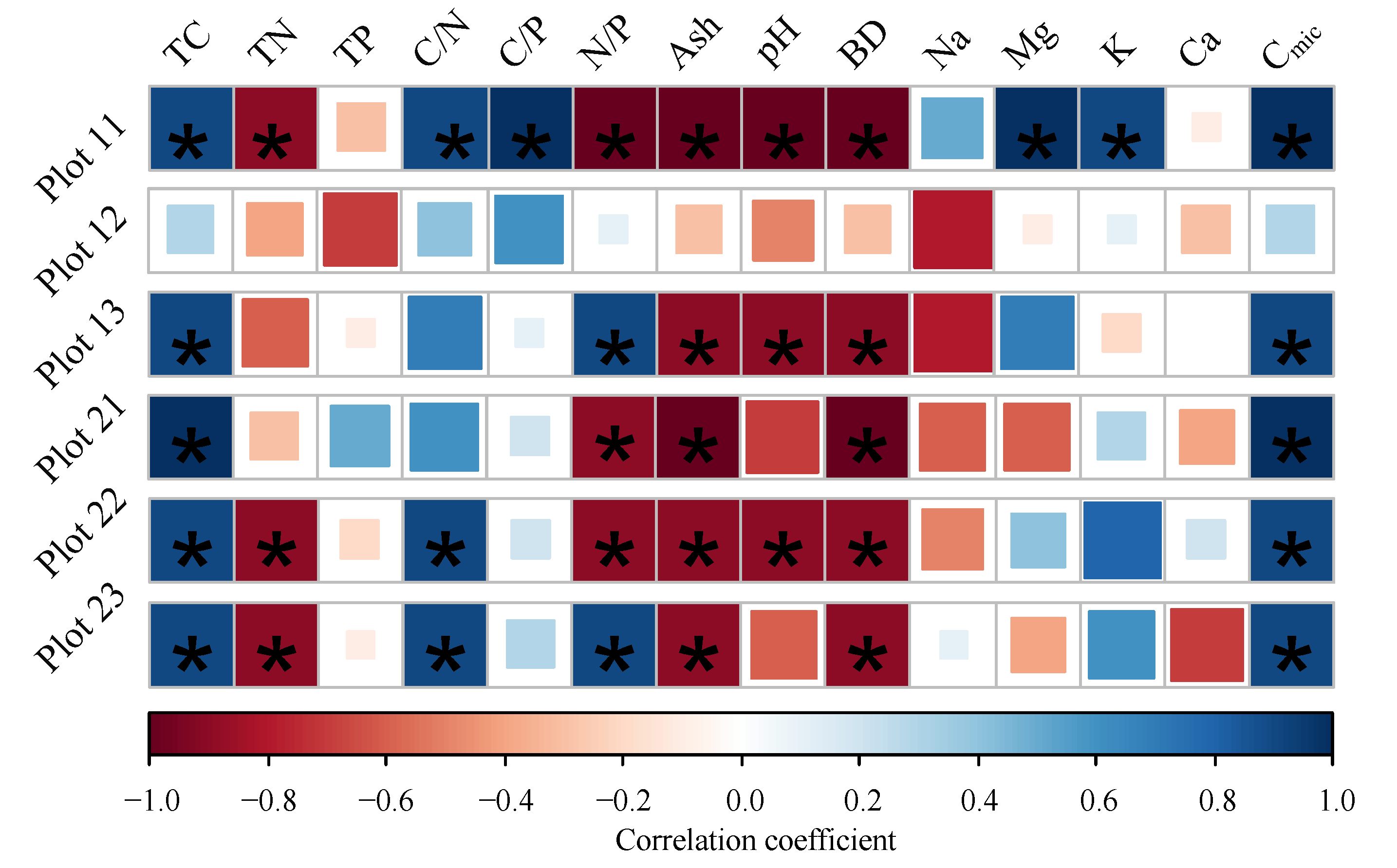
| Parameter | Mean | SD | Min. | Max. | CV% |
|---|---|---|---|---|---|
| TC (g kg−1) | 448.11 | 24.84 | 386.63 | 486.00 | 5.54 |
| TN (g kg−1) | 21.664 | 5.67 | 11.55 | 33.55 | 26.18 |
| TP (g kg−1) | 2.04 | 0.36 | 1.38 | 2.91 | 17.52 |
| C/N | 22.66 | 7.96 | 12.69 | 42.11 | 35.14 |
| C/P | 227.14 | 41.88 | 161.66 | 339.10 | 18.44 |
| N/P | 10.81 | 3.04 | 6.53 | 17.44 | 28.13 |
| Ash (%) | 19.76 | 8.58 | 6.67 | 39.69 | 43.42 |
| pH | 4.92 | 0.39 | 4.30 | 5.77 | 7.91 |
| Bulk density (g cm−3) | 0.15 | 0.05 | 0.08 | 0.26 | 35.66 |
| Na (g kg−1) | 1.11 | 0.64 | 0.50 | 3.02 | 57.50 |
| Mg (g kg−1) | 1.77 | 0.41 | 1.23 | 2.73 | 23.22 |
| K (g kg−1) | 2.36 | 0.67 | 1.36 | 4.10 | 28.36 |
| Ca (g kg−1) | 11.34 | 2.69 | 7.44 | 17.32 | 23.67 |
| Cmic (µg C g−1 soil) | 1816.52 | 1239.38 | 468.61 | 4592.44 | 68.23 |
| Cmic/Corg (%) | 0.40 | 0.26 | 0.11 | 0.95 | 64.70 |
| BR (µg CO2–C g−1 soil h−1) | 8.53 | 7.71 | 1.40 | 26.95 | 90.42 |
| qCO2 (µg CO2–C mg–1 Cmic h−1) | 4.69 | 2.42 | 1.59 | 11.12 | 51.69 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Sun, X.; Sun, L.; Chen, N.; Du, Y. Small-Scale Variability of Soil Quality in Permafrost Peatland of the Great Hing’an Mountains, Northeast China. Water 2022, 14, 2597. https://doi.org/10.3390/w14172597
Wang X, Sun X, Sun L, Chen N, Du Y. Small-Scale Variability of Soil Quality in Permafrost Peatland of the Great Hing’an Mountains, Northeast China. Water. 2022; 14(17):2597. https://doi.org/10.3390/w14172597
Chicago/Turabian StyleWang, Xianwei, Xiaoxin Sun, Li Sun, Ning Chen, and Yu Du. 2022. "Small-Scale Variability of Soil Quality in Permafrost Peatland of the Great Hing’an Mountains, Northeast China" Water 14, no. 17: 2597. https://doi.org/10.3390/w14172597
APA StyleWang, X., Sun, X., Sun, L., Chen, N., & Du, Y. (2022). Small-Scale Variability of Soil Quality in Permafrost Peatland of the Great Hing’an Mountains, Northeast China. Water, 14(17), 2597. https://doi.org/10.3390/w14172597







