Effective Removal of Pb2+ from Aqueous Solution Using Magnetic Mesoporous Silica Prepared by Rubidium-Containing Biotite Leaching Residues and Wastewater
Abstract
:1. Introduction
2. Materials and Methods
2.1. Regents and Materials
2.2. Direct Acid Leaching of Rubidium with Sulfuric Acid
2.3. Pretreatment of Leaching Residues
2.4. Synthesis of Ferric Oxide/Mesoporous SiO2 (FO/MS)
2.5. Batch Experiments of Pb2+ Removal
2.6. Adsorption Kinetics and Isothermal Studies
2.7. Characterizations
3. Results and Discussion
3.1. Behaviors of Rubidium and Iron in Their Leaching Environments
3.1.1. Influence of the Concentration of Sulfuric Acid on Rubidium and Iron Extraction Processes
3.1.2. Influence of the Temperature of the Leaching Process on the Recovery of Rubidium and Iron
3.1.3. Influence of the Amount of Leaching Time Spent on the Iron and Rubidium Extraction
3.1.4. XRD Analysis of Leaching Residue Slags
3.2. Pb2+ Removal Experiments Using the Mesoporous Materials
3.2.1. Effect of Different Adsorbents on the Amount of Lead Removed
3.2.2. Effect of the Initial Pb2+ Concentration on the Amount of Lead Removed
3.2.3. Determination of the Kinetic Model for the Adsorption Process
3.2.4. Fitting of the Adsorption Isotherm Model
3.2.5. Effect of Initial pH Value on Pb2+ Removal Efficiency
3.2.6. Effect of Temperature on Pb2+ Removal Efficiency
3.2.7. Effect of Recycling on Pb2+ Removal Efficiency
3.2.8. Selective Removal of Pb2+ from Multiple Heavy Metals Coexisting Solution
3.3. Characterization and Pb2+ Removal Mechanism
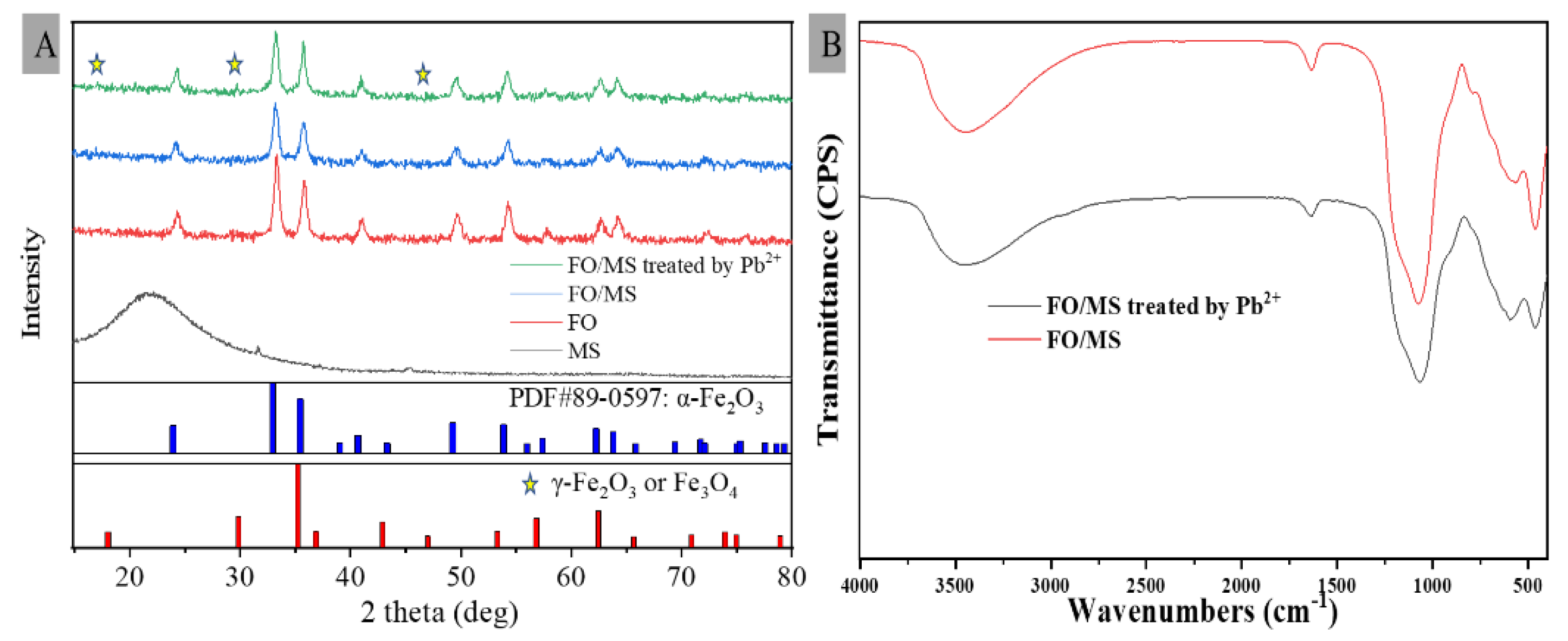
3.3.1. XRD Analysis
3.3.2. FTIR Analysis
3.3.3. Microstructure Analysis
3.3.4. Specific Surface Areas Analysis
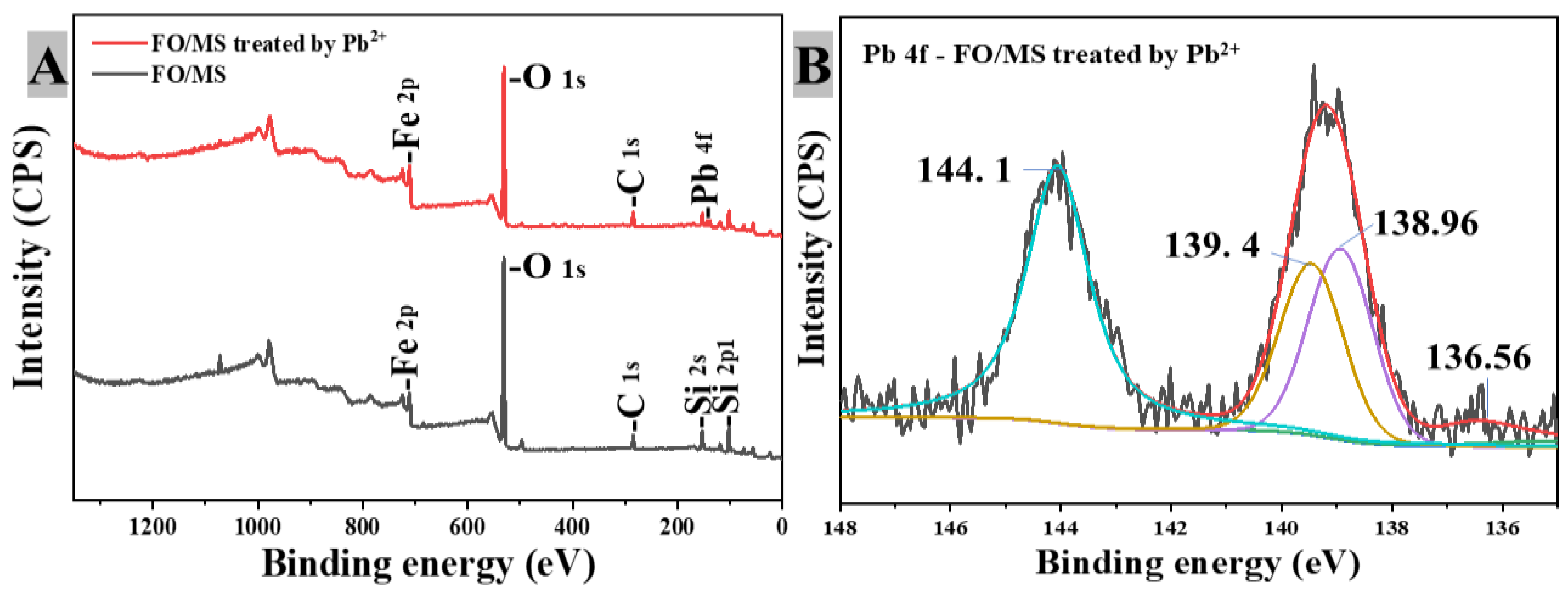
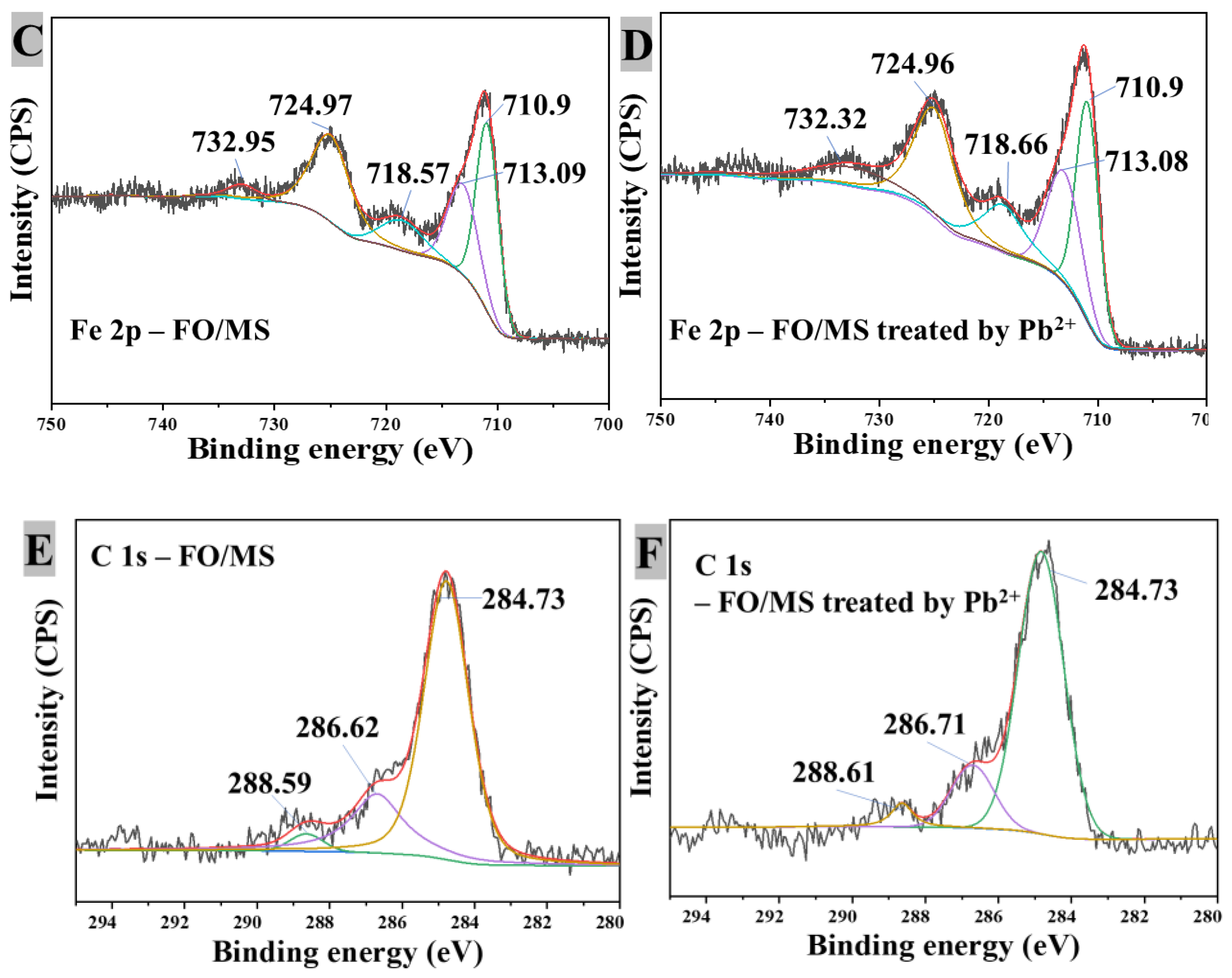
3.3.5. XPS Analysis
3.3.6. Pb2+ Removal Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xing, P.; Wang, C.; Chen, Y.; Ma, B. Rubidium extraction from mineral and brine resources: A review. Hydrometallurgy 2021, 203, 105644. [Google Scholar] [CrossRef]
- Ma, X.; Ma, H.; Yuan, J. Kinetics and Mechanism of Leaching Potassium from Biotite in H2SO4 Solution. ChemistrySelect 2020, 38, 11955–11960. [Google Scholar] [CrossRef]
- Zeng, Q.; Li, S.; Sun, W.; Hu, L.; Zhong, H.; He, Z. Eco-friendly leaching of rubidium from biotite-containing minerals with oxalic acid and effective removal of Hg2+ from aqueous solution using the leaching residues. J. Clean. Prod. 2021, 306, 127167. [Google Scholar] [CrossRef]
- Xing, P.; Wang, C.; Ma, B.; Chen, Y. Removal of Pb(II) from aqueous solution using a new zeolite-type absorbent: Potassium ore leaching residue. J. Environ. Chem. Eng. 2018, 6, 7138–7143. [Google Scholar] [CrossRef]
- Xing, P.; Wang, C.; Ma, B.; Wang, L.; Zhang, W.; Chen, Y. Rubidium and Potassium Extraction from Granitic Rubidium Ore: Process Optimization and Mechanism Study. ACS Sustain. Chem. Eng. 2018, 6, 4922–4930. [Google Scholar] [CrossRef]
- Luo, Z.; Yang, J.; Ma, H.; Liu, M.; Ma, X. Recovery of magnesium and potassium from biotite by sulfuric acid leaching and alkali precipitation with ammonia. Hydrometallurgy 2015, 157, 188–193. [Google Scholar] [CrossRef]
- Li, M.; Huang, F.; Hu, L.; Sun, W.; Li, E.; Xiong, D.; Zhong, H.; He, Z. Efficient activation of peroxymonosulfate by a novel catalyst prepared directly from electrolytic manganese slag for degradation of recalcitrant organic pollutes. Chem. Eng. J. 2020, 401, 126085. [Google Scholar] [CrossRef]
- Song, J.; Huang, G.; Han, D.; Hou, Q.; Gan, L.; Zhang, M. A review of reactive media within permeable reactive barriers for the removal of heavy metal(loid)s in groundwater: Current status and future prospects. J. Clean. Prod. 2021, 319, 128644. [Google Scholar] [CrossRef]
- Zeng, Q.; Huang, L.; Ouyang, D.; Hu, Y.; Zhong, H.; He, Z. Process optimization on the extraction of rubidium from rubidium-bearing biotite. Miner. Eng. 2019, 137, 87–93. [Google Scholar] [CrossRef]
- Lu, C.; Yang, H.; Wang, J.; Tan, Q.; Fu, L. Utilization of iron tailings to prepare high-surface area mesoporous silica materials. Sci. Total Environ. 2020, 736, 139483. [Google Scholar] [CrossRef]
- Meléndez-Ortiz, H.; García-Cerda, L.; Olivares-Maldonado, Y.; Castruita, G.; Mercado-Silva, J.; Perera-Mercado, Y. Preparation of spherical MCM-41 molecular sieve at room temperature: Influence of the synthesis conditions in the structural properties. Ceram. Int. 2012, 38, 6353–6358. [Google Scholar] [CrossRef]
- Al-Ghouti, M.A.; Da’Ana, D.A. Guidelines for the use and interpretation of adsorption isotherm models: A review. J. Hazard. Mater. 2020, 393, 122383. [Google Scholar] [CrossRef]
- Figueira, P.; Lopes, C.; Daniel-Da-Silva, A.; Pereira, E.; Duarte, A.; Trindade, T. Removal of mercury (II) by dithiocarbamate surface functionalized magnetite particles: Application to synthetic and natural spiked waters. Water Res. 2011, 45, 5773–5784. [Google Scholar] [CrossRef]
- Mohmood, I.; Lopes, C.; Lopes, I.; Tavares, D.; Soares, A.; Duarte, A.; Trindade, T.; Ahmad, I.; Pereira, E. Remediation of mercury contaminated saltwater with functionalized silica coated magnetite nanoparticles. Sci. Total Environ. 2016, 557–558, 712–721. [Google Scholar] [CrossRef]
- Elwakeel, K.Z.; Guibal, E. Selective removal of Hg(II) from aqueous solution by functionalized magnetic-macromolecular hybrid material. Chem. Eng. J. 2015, 281, 345–359. [Google Scholar] [CrossRef]
- Gupta, V.K. Removal of lindane and malathion from wastewater using bagasse fly ash—A sugar industry waste. Water Res. 2002, 36, 2483–2490. [Google Scholar] [CrossRef]
- Song, S.; Lopez-Valdivieso, A.; Hernandez-Campos, D.; Peng, C.; Monroy-Fernandez, M.; Razo-Soto, I. Arsenic removal from high-arsenic water by enhanced coagulation with ferric ions and coarse calcite. Water Res. 2006, 40, 364–372. [Google Scholar] [CrossRef]
- Hu, P.; Zhang, Y.; Liu, T.; Huang, J.; Yuan, Y.; Yang, Y. Separation and recovery of iron impurity from a vanadium-bearing stone coal via an oxalic acid leaching-reduction precipitation process. Sep. Purif. Technol. 2017, 180, 99–106. [Google Scholar] [CrossRef]
- Hu, P.; Zhang, Y.; Liu, T.; Huang, J.; Yuan, Y.; Zheng, Q. Highly selective separation of vanadium over iron from stone coal by oxalic acid leaching. J. Ind. Eng. Chem. 2016, 45, 241–247. [Google Scholar] [CrossRef]
- Vernon, J.D.; Bonzongo, J.C.J. Volatilization and sorption of dissolved mercury by metallic iron of different particle sizes: Implications for treatment of mercury contaminated water effluents. J. Hazard. Mater. 2014, 276, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Falyouna, O.; Eljamal, O.; Maamoun, I.; Tahara, A.; Sugihara, Y. Magnetic zeolite synthesis for efficient removal of cesium in a lab-scale continuous treatment system. J. Colloid Interf. Sci. 2020, 571, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, S.; Wen, N.; Wei, D.; Zhang, Y. Highly effective removal of lead and cadmium ions from wastewater by bifunctional magnetic mesoporous silica. Sep. Purif. Technol. 2021, 265, 118341. [Google Scholar] [CrossRef]
- Singh, S.K.; Townsend, T.G.; Mazyck, D.; Boyer, T.H. Equilibrium and intra-particle diffusion of stabilized landfill leachate onto micro- and meso-porous activated carbon. Water Res. 2012, 46, 491–499. [Google Scholar] [CrossRef] [PubMed]
- He, S.Y.; Han, C.Y.; Wang, H.; Liu, C.X.; Shan, Y.; Luo, Y.M. Kinetic Study of Arsenic(V) Absorption with Al2O3 Functionalized SBA-15. Adv. Mater. Res. 2013, 726–731, 2191–2197. [Google Scholar] [CrossRef]
- Ma, W.; Meng, F.; Cheng, Z.; Xin, G.; Duan, S. Synthesis of macroporous silica biomass nanocomposite based on XG/MgSiO3 for the removal of toxic ions. Bioresour. Technol. 2015, 186, 356–359. [Google Scholar] [CrossRef]
- Venkateswarlu, S.; Yoon, M. Core–Shell Ferromagnetic Nanorod Based on Amine Polymer Composite (Fe3O4@DAPF) for Fast Removal of Pb(II) from Aqueous Solutions. ACS Appl. Mater. Interfaces 2015, 7, 25362–25372. [Google Scholar] [CrossRef]
- Ramos-Guivar, J.A.; Taipe, K.; Schettino, M.A., Jr.; Silva, E.; Torres, M.A.M.; Passamani, E.C.; Litterst, F.J. Improved Removal Capacity and Equilibrium Time of Maghemite Nanoparticles Growth in Zeolite Type 5A for Pb(II) Adsorption. Nanomaterials 2020, 10, 1668. [Google Scholar] [CrossRef]
- Chen, W.; Lu, Z.; Xiao, B.; Gu, P.; Yao, W.; Xing, J.; Asiri, A.M.; Alamry, K.A.; Wang, X.; Wang, S. Enhanced removal of lead ions from aqueous solution by iron oxide nanomaterials with cobalt and nickel doping. J. Clean. Prod. 2019, 211, 1250–1258. [Google Scholar] [CrossRef]
- Yin, W.; Dai, D.; Hou, J.; Wang, S.; Wu, X.; Wang, X. Hierarchical porous biochar-based functional materials derived from biowaste for Pb(II) removal. Appl. Surf. Sci. 2018, 465, 297–302. [Google Scholar] [CrossRef]
- Zhang, F.; Tang, X.; Huang, Y.; Keller, A.A.; Lan, J. Competitive removal of Pb2+ and malachite green from water by magnetic phosphate nanocomposites. Water Res. 2018, 150, 442–451. [Google Scholar] [CrossRef]
- Chen, D.; Shen, W.; Wu, S.; Chen, C.; Luo, X.; Guo, L. Ion exchange induced removal of Pb(ii) by MOF-derived magnetic inorganic sorbents. Nanoscale 2016, 8, 7172–7179. [Google Scholar] [CrossRef]
- Ma, Z.; Guan, Y.; Liu, H. Synthesis and characterization of micron-sized monodisperse superparamagnetic polymer particles with amino groups. J. Polym. Sci. Part A Polym. Chem. 2005, 43, 3433–3439. [Google Scholar] [CrossRef]
- Mishra, S.; Verma, N. Surface ion imprinting-mediated carbon nanofiber-grafted highly porous polymeric beads: Synthesis and application towards selective removal of aqueous Pb(II). Chem. Eng. J. 2017, 313, 1142–1151. [Google Scholar] [CrossRef]
- Ahmad, R.; Mirza, A. Facile one pot green synthesis of Chitosan-Iron oxide (CS-Fe2O3) nanocomposite: Removal of Pb(II) and Cd(II) from synthetic and industrial wastewater. J. Clean. Prod. 2018, 186, 342–352. [Google Scholar] [CrossRef]
- Oyetade, O.A.; Skelton, A.A.; Nyamori, V.; Jonnalagadda, S.B.; Martincigh, B. Experimental and DFT studies on the selective adsorption of Pb 2+ and Zn 2+ from aqueous solution by nitrogen-functionalized multiwalled carbon nanotubes. Sep. Purif. Technol. 2017, 188, 174–187. [Google Scholar] [CrossRef]
- Zarime, N.; Zuhairi, W.Y.W.; Krishna, S. Adsorption of Nickel and Zinc by Residual Soils. Am. J. Environ. Sci. 2014, 10, 523–529. [Google Scholar] [CrossRef]
- Yu, C.; Shao, Z.; Hou, H. A functionalized metal–organic framework decorated with O− groups showing excellent performance for lead(ii) removal from aqueous solution. Chem. Sci. 2017, 8, 7611–7619. [Google Scholar] [CrossRef]
- Bérubé, F.; Kaliaguine, S. Calcination and thermal degradation mechanisms of triblock copolymer template in SBA-15 materials. Microporous Mesoporous Mater. 2008, 115, 469–479. [Google Scholar] [CrossRef]
- Boukoussa, B.; Hakiki, A.; Moulai, S.; Chikh, K.; Kherroub, D.E.; Bouhadjar, L.; Guedal, D.; Messaoudi, K.; Mokhtar, F.; Hamacha, R. Adsorption behaviors of cationic and anionic dyes from aqueous solution on nanocomposite polypyrrole/SBA-15. J. Mater. Sci. 2018, 53, 7372–7386. [Google Scholar] [CrossRef]
- Xu, H.; Ni, K.; Li, X.; Fang, G.; Fan, G. Structural transformation of Pd-α-Fe2O3 and Pd-γ-Fe2O3 catalysts and application in the CO oxidation reaction. RSC Adv. 2017, 7, 51403–51410. [Google Scholar] [CrossRef] [Green Version]
- Lu, H.; Wen, C.; Gao, S.; Dong, Y.; Zhang, M.; Li, B.; Hu, W.; Dong, J. Incorporation of nanoscale zero-valent iron particles in monodisperse mesoporous silica nanospheres: Characterization, reactivity, transport in porous media. Colloids Surf. A Physicochem. Eng. Asp. 2018, 553, 28–34. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, S.; Sun, Y.; Cheng, K.; Li, J.; Tsang, D.C. Fabrication and characterization of hydrophilic corn stalk biochar-supported nanoscale zero-valent iron composites for efficient metal removal. Bioresour. Technol. 2018, 265, 490–497. [Google Scholar] [CrossRef]
- Mikhailov, M.N.; Kazansky, V.B.; Kustov, L.M. Intensities of IR stretching bands as a criterion of the strength of Lewis acid sites in ZSM-5 zeolites with bivalent cations. Catal. Lett. 2007, 116, 81–86. [Google Scholar] [CrossRef]
- Zeng, Q.; Huang, Y.; Huang, L.; Hu, L.; Sun, W.; Zhong, H.; He, Z. High adsorption capacity and super selectivity for Pb(Ⅱ) by a novel adsorbent: Nano humboldtine/almandine composite prepared from natural almandine. Chemosphere 2020, 253, 126650. [Google Scholar] [CrossRef]
- Tanaka, S.; Kaneti, Y.V.; Septiani, N.L.W.; Dou, S.X.; Bando, Y.; Hossain, M.S.A.; Yamauchi, Y. A Review on Iron Oxide-Based Nanoarchitectures for Biomedical, Energy Storage, and Environmental Applications. Small Methods 2019, 5, 1800512. [Google Scholar] [CrossRef]
- Jabeen, H.; Kemp, K.; Chandra, V. Synthesis of nano zerovalent iron nanoparticles—Graphene composite for the treatment of lead contaminated water. J. Environ. Manag. 2013, 130, 429–435. [Google Scholar] [CrossRef]
- Deng, S.; Zhang, G.; Liang, S.; Wang, P. Microwave Assisted Preparation of Thio-Functionalized Polyacrylonitrile Fiber for the Selective and Enhanced Adsorption of Mercury and Cadmium from Water. ACS Sustain. Chem. Eng. 2017, 5, 6054–6063. [Google Scholar] [CrossRef]
- Yamashita, T.; Hayes, P. Analysis of XPS spectra of Fe2+ and Fe3+ ions in oxide materials. Appl. Surf. Sci. 2008, 254, 2441–2449. [Google Scholar] [CrossRef]
- Penke, Y.K.; Anantharaman, G.; Ramkumar, J.; Kar, K.K. Aluminum Substituted Cobalt Ferrite (Co−Al−Fe) Nano Adsorbent for Arsenic Adsorption in Aqueous Systems and Detailed Redox Behavior Study with XPS. ACS Appl. Mater. Interfaces 2017, 9, 11587–11598. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Tang, J.; Gai, L.; Gong, Y.; Guan, H.; He, R.; Lyu, H. Different approaches for preparing a novel thiol-functionalized graphene oxide/Fe-Mn and its application for aqueous methylmercury removal. Chem. Eng. J. 2017, 319, 229–239. [Google Scholar] [CrossRef]
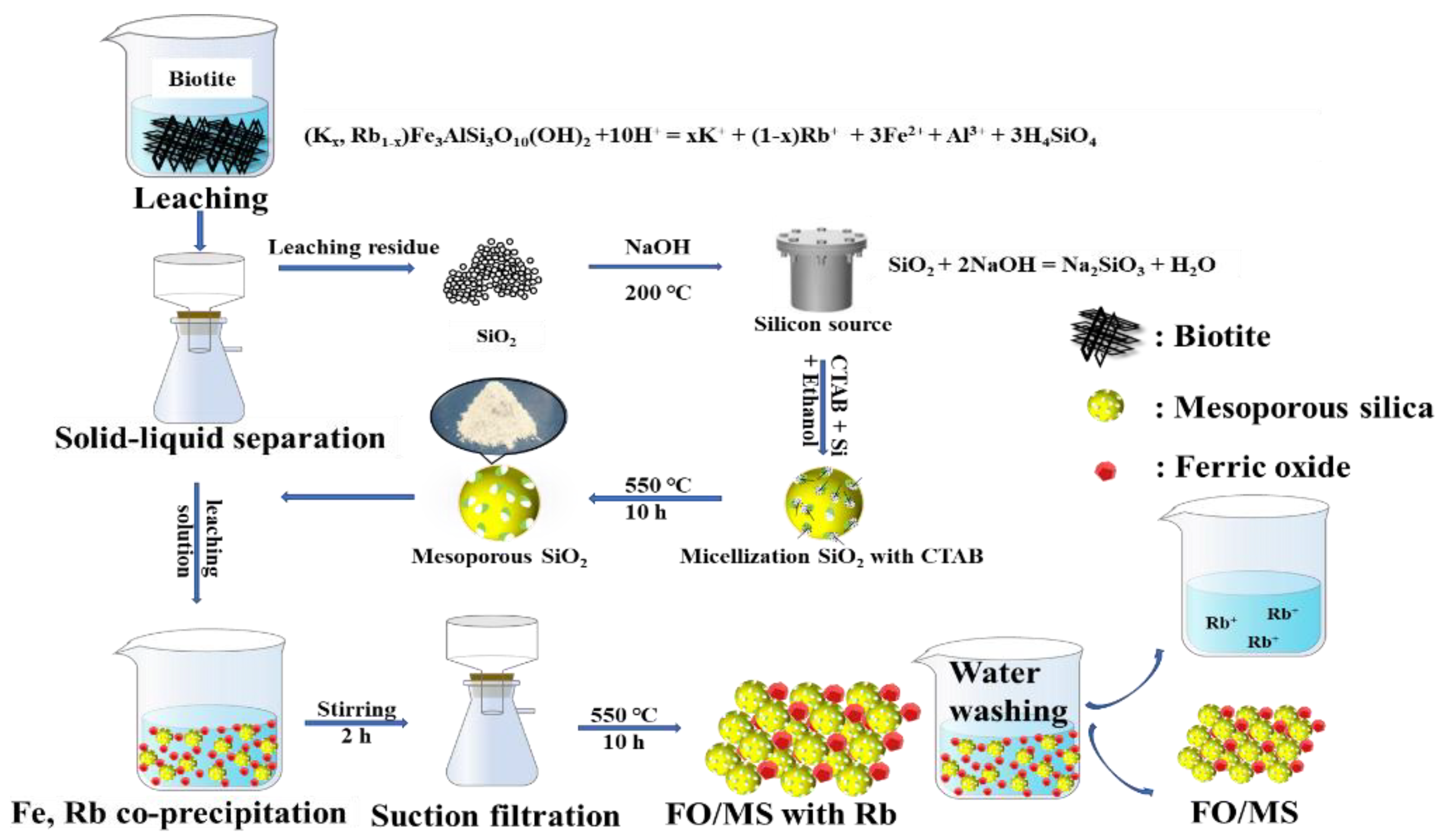




| Elements | O | Fe | Mg | Al | Si | Rb | S | K | Ca | Ti | Others |
| Contents | 26.1 | 4.43 | 0.61 | 6.37 | 36.7 | 0.41 | 0.68 | 4.48 | 0.19 | 0.57 | 19.42 |
| Initial Pb2+ Concentration (mg/L) | 50 | 100 | 150 | 200 | |
|---|---|---|---|---|---|
| Pseudo-first-order model | Qe (mg/g) | 51.5 | 105.76 | 142.87 | 141.46 |
| R2 | 0.94023 | 0.92826 | 0.97492 | 0.99359 | |
| Pseudo-second-order model | Qe (mg/g) | 54.11 | 112.23 | 146.84 | 146.41 |
| R2 | 0.96866 | 0.96405 | 0.99215 | 0.99887 | |
| Intra-particle diffusion model | Qe (mg/g) | 45.81 | 90.91 | 124.56 | 128.46 |
| R2 | 0.96934 | 0.90293 | 0.99662 | 0.99233 | |
| Model | Langmuir | Freundlich | Temkin | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameters | Qm | KL | R2 | n | KF | R2 | AT | BT | R2 |
| Values | 143.47 | 4.74 | 0.999 | 6.82 | 119.94 | 0.666 | 1274 | 13.16 | 0.793 |
| Materials | Qm (mg/g) | Removal Efficiency (%) | Initial Concentration (mg/L) | Equilibrium Time (min) | References |
|---|---|---|---|---|---|
| NPZEO3 | 265 | 99.9 | 50 | 5 | [26] |
| Fe3O4@DC | 83.3 | 99.5 | 80 | 85 | [27] |
| Co-Fe2O3 | 136 | 98.5 | 100 | 120 | [28] |
| Ni-Fe2O3 | 97.5 | 99.5 | 100 | 80 | [28] |
| FBM/700 | 133.61 | 99.9 | 50 | 90 | [29] |
| FSP(Fe3O4) | 202 | 99.5 | 50 | 65 | [30] |
| FeIII-MOF-5 | 345 | 99.7 | 100 | 105 | [31] |
| CoFe2O4 | 51 | 98 | 20 | 30 | [32] |
| FO/MS | 143.47 | 99.7 | 100 | 60 | This work |
| Temperature (°C) | Pseudo Second-Order Model | ||
|---|---|---|---|
| Qe (mg/g) | k2 | R2 | |
| 25 | 150.60 | 0.001184 | 0.99559 |
| 35 | 151.75 | 0.000609 | 0.98861 |
| 45 | 149.93 | 0.000527 | 0.98621 |
| Materials | BET Specific Surface Area (m2/g) | Average Pore Size (nm) |
|---|---|---|
| Fe2O3 | 34.5 | 2 |
| Mesoporous silica | 517.4 | 2 |
| FO/MS | 146.12 | 1 |
| FO/MS treated with Pb2+ | 89.96 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, Y.; Han, Z.; Zeng, Q.; Wang, S.; Sun, W.; Zhong, H.; He, Z. Effective Removal of Pb2+ from Aqueous Solution Using Magnetic Mesoporous Silica Prepared by Rubidium-Containing Biotite Leaching Residues and Wastewater. Water 2022, 14, 2652. https://doi.org/10.3390/w14172652
Liang Y, Han Z, Zeng Q, Wang S, Sun W, Zhong H, He Z. Effective Removal of Pb2+ from Aqueous Solution Using Magnetic Mesoporous Silica Prepared by Rubidium-Containing Biotite Leaching Residues and Wastewater. Water. 2022; 14(17):2652. https://doi.org/10.3390/w14172652
Chicago/Turabian StyleLiang, Yuting, Zhengwei Han, Qiang Zeng, Shouxi Wang, Wei Sun, Hui Zhong, and Zhiguo He. 2022. "Effective Removal of Pb2+ from Aqueous Solution Using Magnetic Mesoporous Silica Prepared by Rubidium-Containing Biotite Leaching Residues and Wastewater" Water 14, no. 17: 2652. https://doi.org/10.3390/w14172652
APA StyleLiang, Y., Han, Z., Zeng, Q., Wang, S., Sun, W., Zhong, H., & He, Z. (2022). Effective Removal of Pb2+ from Aqueous Solution Using Magnetic Mesoporous Silica Prepared by Rubidium-Containing Biotite Leaching Residues and Wastewater. Water, 14(17), 2652. https://doi.org/10.3390/w14172652






