Fabrication of Polyethyleneimine-Modified Nanocellulose/Magnetic Bentonite Composite as a Functional Biosorbent for Efficient Removal of Cu(Ⅱ)
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of TOCN
2.3. Synthesis of TOCN–PEI
2.4. Synthesis of Magnetic Bentonite
2.5. Synthesis of PNMBC
2.6. Characterization
2.7. Batch Adsorption Test
3. Results and Discussion
3.1. Characterization of PNMBC
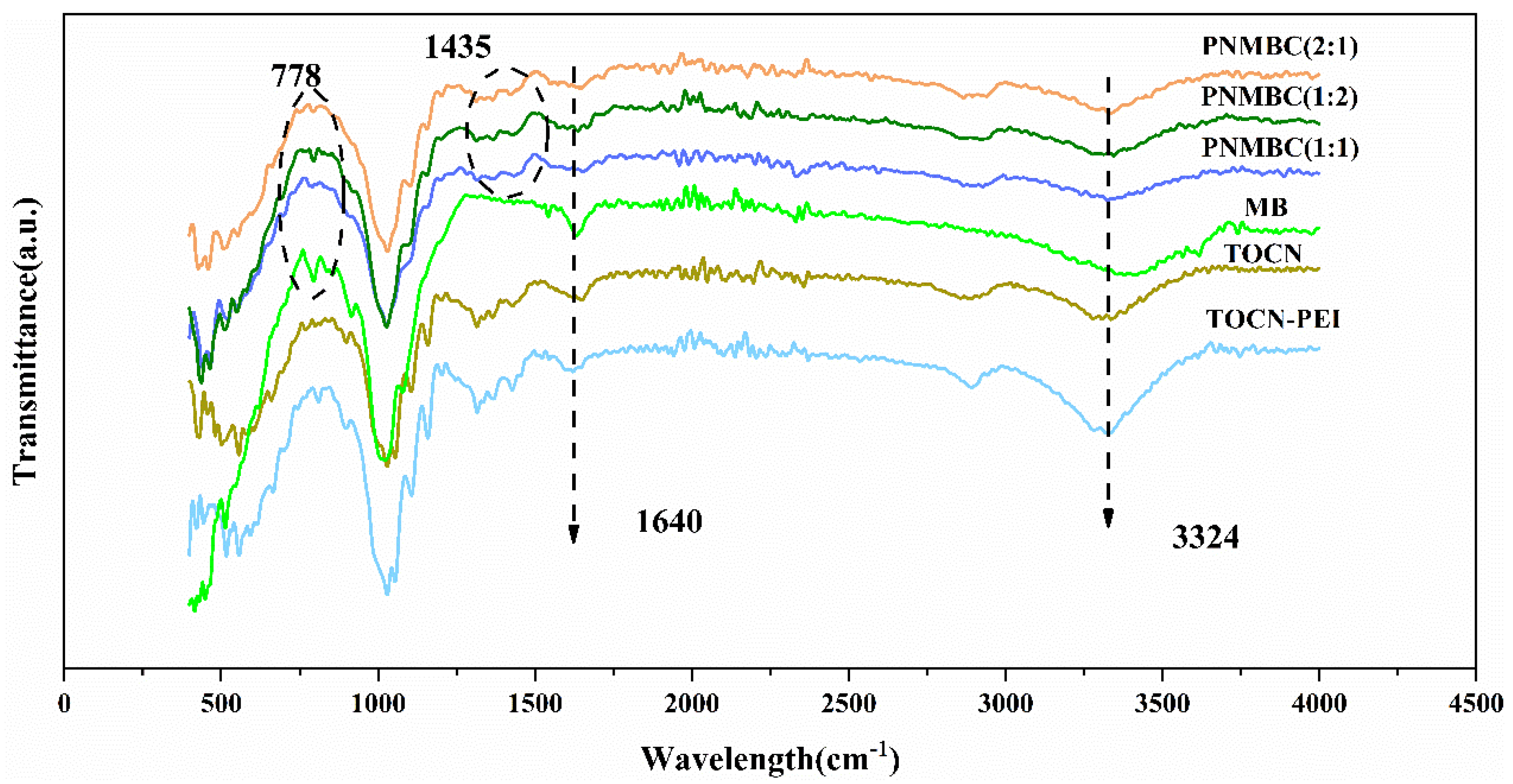
3.1.1. FT-IR Analysis
3.1.2. XRD Analysis
3.1.3. SEM Analysis
3.1.4. TG Analysis
3.1.5. BET Analysis
3.1.6. Zeta Potential
3.2. Effect of Batch Adsorption Performance
3.2.1. Effect of Biosorbent Dosage
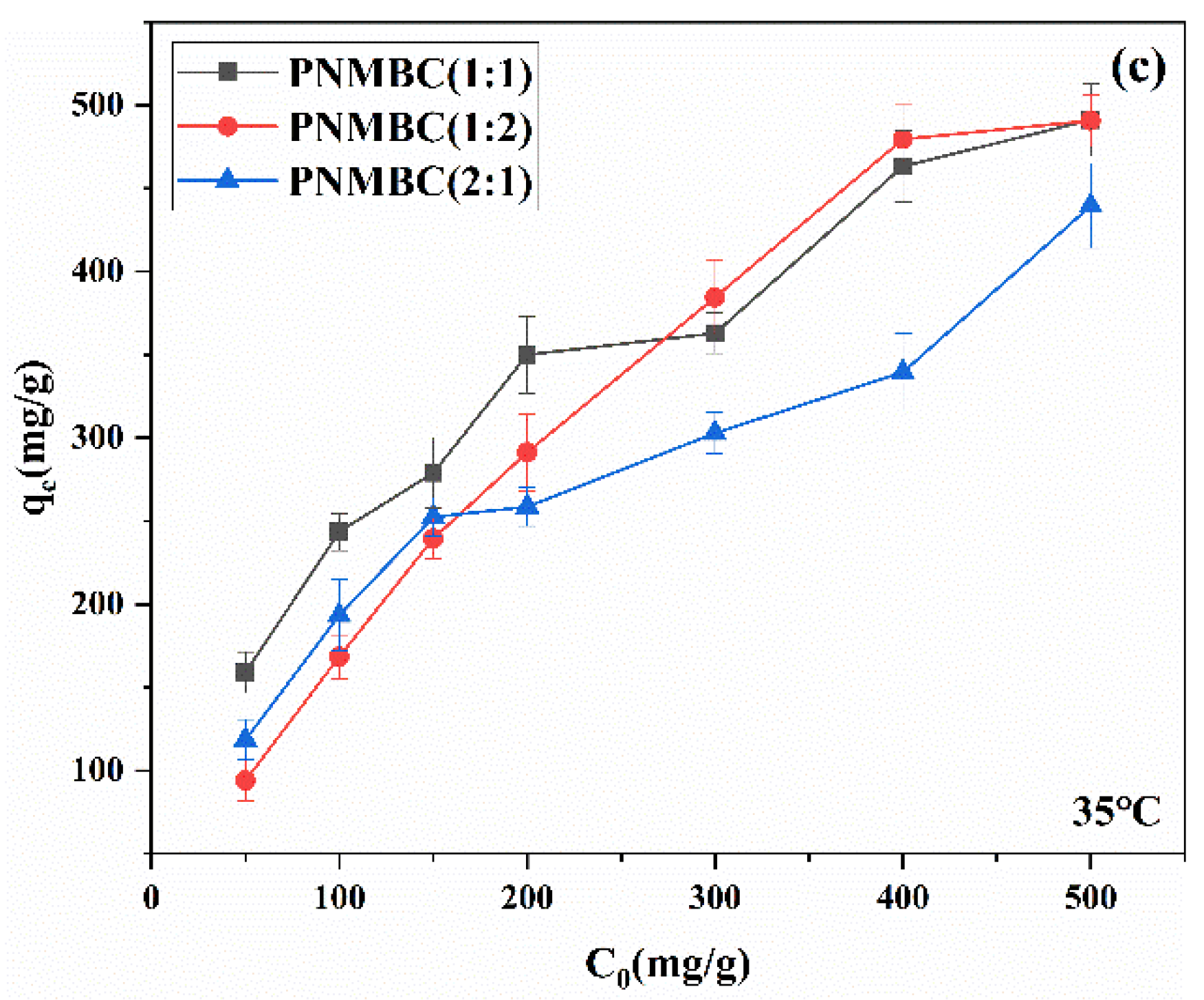
3.2.2. Effect of Initial Concentration of Cu(Ⅱ)
3.2.3. Effect of Adsorption Time
3.2.4. Effect of Temperature
3.3. Adsorption Mechanism
3.4. Reusability of PNMBC
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wong, S.M.; Zulkifli, M.Z.A.; Nordin, D.; Teow, Y.H. Synthesis of cellulose/nano-hydroxyapatite composite hydrogel absorbent for removal of heavy metal ions from palm oil mill effluents. J. Polym. Environ. 2021, 29, 4106–4119. [Google Scholar] [CrossRef]
- Kameda, T.; Suzuki, Y.; Yoshioka, T. Removal of arsenic from an aqueous solution by coprecipitation with manganese oxide. J. Environ. Chem. Eng. 2014, 2, 2045–2049. [Google Scholar] [CrossRef]
- Radchenko, V.; Engle, J.W.; Wilson, J.J.; Maassen, J.R.; Nortier, F.M.; Taylor, W.A.; Birnbaum, E.R.; Hudston, L.A.; John, K.D.; Fassbender, M.E. Application of ion exchange and extraction chromatography to the separation of actinium from proton-irradiated thorium metal for analytical purposes. J. Chromatogr. A 2015, 1380, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Sunil, K.; Karunakaran, G.; Yadav, S.; Padaki, M.; Zadorozhnyy, V.; Pai, R.K. Al-Ti2O6 a mixed metal oxide based composite membrane: A unique membrane for removal of heavy metals. Chem. Eng. J. 2018, 348, 678–684. [Google Scholar] [CrossRef]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef]
- Manfoudhi, N.; Boufi, S. Porous material from cellulose nanofibrils coated with aluminum hydroxyde as an effective adsorbent for fluoride. J. Environ. Chem. Eng. 2020, 8, 103779. [Google Scholar] [CrossRef]
- Manfoudhi, N.; Boufi, S. Nanocellulose as a novel nanostructured adsorbent for environmental remediation: A review. Cellulose 2017, 24, 1171–1197. [Google Scholar] [CrossRef]
- Chen, Q.; Zheng, J.; Wen, L.; Yang, C.; Zhang, L. A multi-functional-group modified cellulose for enhanced heavy metal cadmium adsorption: Performance and quantum chemical mechanism. Chemosphere 2019, 224, 509–518. [Google Scholar] [CrossRef]
- Rani, K.; Gomathi, T.; Vijayalakshmi, K.; Saranya, M.; Sudha, P.N. Banana fiber cellulose nano crystals grafted with butyl acrylate for heavy metal lead (II) removal. Int. J. Biol. Macromol. 2019, 131, 461–472. [Google Scholar]
- Kardam, A.; Raj, K.R.; Srivastava, S.; Srivastava, M.M. Nanocellulose fibers for biosorption of cadmium, nickel, and lead ions from aqueous solution. Clean. Technol. Environ. Policy 2013, 16, 385–393. [Google Scholar] [CrossRef]
- Qu, J.; Tian, X.; Jiang, Z.; Cao, B.; Akindolie, M.S.; Hu, Q.; Feng, C.; Feng, Y.; Meng, X.; Zhang, Y. Multi-component adsorption of Pb(II), Cd(II) and Ni(II) onto microwave-functionalized cellulose: Kinetics, isotherms, thermodynamics, mechanisms and application for electroplating wastewater purification. J. Hazard. Mater. 2020, 387, 121718. [Google Scholar] [CrossRef]
- Zhang, Y.; Yue, X.; Xu, W.; Zhang, H.; Li, F. Amino modification of rice straw-derived biochar for enhancing its cadmium (II) ions adsorption from water. J. Hazard. Mater. 2019, 379, 120783. [Google Scholar] [CrossRef]
- Puangsin, B.; Fujisawa, S.; Kuramae, R.; Saito, T.; Isogai, A. TEMPO-mediated oxidation of hemp bast holocellulose to prepare cellulose nanofibrils dispersed in water. J. Polym. Environ. 2013, 21, 555–563. [Google Scholar] [CrossRef]
- Kuramae, R.; Saito, T.; Isogai, A. TEMPO-oxidized cellulose nanofibrils prepared from various plant holocelluloses. React. Funct. Polym. 2014, 85, 126–133. [Google Scholar] [CrossRef]
- Saito, T.; Isogai, A. TEMPO-mediated oxidation of native cellulose. The effect of oxidation conditions on chemical and crystal structures of the water-insoluble fractions. Biomacromolecules 2004, 5, 1983–1989. [Google Scholar] [CrossRef]
- Khanjani, P.; Kosonen, H.; Ristolainen, M.; Virtanen, P.; Vuorinen, T. Interaction of divalent cations with carboxylate group in TEMPO-oxidized microfibrillated cellulose systems. Cellulose 2019, 26, 4841–4851. [Google Scholar] [CrossRef]
- Cao, L.; Li, Z.; Xiang, S.; Huang, Z.; Ruan, R.; Liu, Y. Preparation and characteristics of bentonite–zeolite adsorbent and its application in swine wastewater. Bioresour. Technol. 2019, 284, 448–455. [Google Scholar] [CrossRef]
- Kostenko, L.; Artiushenko, O.; Kovalchuk, T.; Tomashchuk, I.; Zaitsev, V. Preparation and characterization of organofunctionalized bentonite clay bearing aminophosphonic groups in heavy metal uptake. J. Environ. Chem. Eng. 2019, 7, 103434. [Google Scholar] [CrossRef]
- Ain, Q.U.; Rasheed, U.; Yaseen, M.; Zhang, H.; Tong, Z. Superior dye degradation and adsorption capability of polydopamine modified Fe3O4-pillared bentonite composite. J. Hazard. Mater. 2020, 397, 122758. [Google Scholar] [CrossRef]
- Khandal, D.; Riedl, B.; Tavares, J.R.; Carreau, P.J.; Heuzey, M.-C. Tailoring cellulose nanocrystals rheological behavior in aqueous suspensions through surface functionalization with polyethyleneimine. Phys. Fluids 2019, 31, 021207. [Google Scholar] [CrossRef]
- Song, L.; Liu, F.; Zhu, C.; Li, A. Facile one-step fabrication of carboxymethyl cellulose based hydrogel for highly efficient removal of Cr(VI) under mild acidic condition. Chem. Eng. J. 2019, 369, 641–651. [Google Scholar] [CrossRef]
- Qin, F.; Fang, Z.; Zhou, J.; Sun, C.; Chen, K.; Ding, Z.; Li, G.; Qiu, X. Efficient removal of Cu2+ in water by carboxymethylated cellulose nanofibrils: Performance and mechanism. Biomacromolecules 2019, 20, 4466–4475. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Xiang, Z.; Liu, Q.; Chen, Y.; Lu, F. Polyethyleneimine-bacterial cellulose bioadsorbent for effective removal of copper and lead ions from aqueous solution. Bioresour. Technol. 2017, 244, 844–849. [Google Scholar] [CrossRef] [PubMed]
- Hoang, M.T.; Pham, T.D.; Verheyen, D.; Nguyen, M.K.; Pham, T.T.; Zhu, J.; der Bruggen, B.V. Fabrication of thin film nanocomposite nanofiltration membrane incorporated with cellulose nanocrystals for removal of Cu(II) and Pb(II). Chem. Eng. Sci. 2020, 228, 115998. [Google Scholar] [CrossRef]
- Hong, H.J.; Yu, H.; Park, M.; Jeong, H.S. Recovery of platinum from waste effluent using polyethyleneimine-modified nanocelluloses: Effects of the cellulose source and type. Carbohydr. Polym. 2019, 210, 167–174. [Google Scholar] [CrossRef]
- Saito, T.; Kimura, S.; Nishiyama, Y.; Isogai, A. Cellulose nanofibers prepared by TEMPO-mediated oxidation of native cellulose. Biomacromolecules 2007, 8, 2485–2491. [Google Scholar] [CrossRef]
- Saito, T.; Yanagisawa, M.; Isogai, A. TEMPO-mediated oxidation of native cellulose: SEC–MALLS analysis of water-soluble and insoluble fractions in the oxidized products. Cellulose 2005, 12, 305–315. [Google Scholar] [CrossRef]
- Zhang, N.; Zang, G.-L.; Shi, C.; Yu, H.-Q.; Sheng, G.-P. A novel adsorbent TEMPO-mediated oxidized cellulose nanofibrils modified with PEI: Preparation, characterization, and application for Cu(II) removal. J. Hazard. Mater. 2016, 316, 11–18. [Google Scholar] [CrossRef]
- Palani, S.; Lingam, R.; Srinivasan, G.R. Synthesis and characterisation of carboxymethyl cellulose based bentonite polymer blend. Int. J. Recent Technol. Eng. 2020, 8, 5661–5664. [Google Scholar] [CrossRef]
- Luo, H.; Feng, F.; Yao, F.; Zhu, Y.; Yang, Z.; Wan, Y. Improved removal of toxic metal ions by incorporating graphene oxide into bacterial cellulose. J. Nanosci. Nanotechnol. 2020, 20, 719–730. [Google Scholar] [CrossRef]
- Liu, M.; Liu, Y.; Shen, J.; Zhang, S.; Liu, X.; Chen, X.; Ma, Y.; Ren, S.; Fang, G.; Li, S.; et al. Simultaneous removal of Pb2+, Cu2+ and Cd2+ ions from wastewater using hierarchical porous polyacrylic acid grafted with lignin. J. Hazard. Mater. 2020, 392, 122208. [Google Scholar] [CrossRef]
- dos Reis, G.S.; Sampaio, C.H.; Lima, E.C.; Wilhelm, M. Preparation of novel adsorbents based on combinations of polysiloxanes and sewage sludge to remove pharmaceuticals from aqueous solutions. Colloids Surf. A Physicochem. Eng. Asp. 2016, 497, 304–315. [Google Scholar] [CrossRef]
- Agaba, A.; Cheng, H.; Zhao, J.; Zhang, C.; Tebyetekerwa, M.; Rong, L.; Sui, X.; Wang, B. Precipitated silica agglomerates reinforced with cellulose nanofibrils as adsorbents for heavy metals. RSC Adv. 2018, 8, 33129–33137. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, X.; Lv, X.; Wang, T.; Xue, B. Synthesis of novel lignosulfonate-modified graphene hydrogel for ultrahigh adsorption capacity of Cr(VI) from wastewater. J. Clean. Prod. 2021, 295, 126406. [Google Scholar] [CrossRef]
- Pottathara, Y.B.; Narwade, V.N.; Bogle, K.A.; Kokol, V. TEMPO-oxidized cellulose nanofibrils–graphene oxide composite films with improved dye adsorption properties. Polym. Bull. 2020, 77, 6175–6189. [Google Scholar] [CrossRef]
- Dai, H.; Huang, Y.; Huang, H. Eco-friendly polyvinyl alcohol/carboxymethyl cellulose hydrogels reinforced with graphene oxide and bentonite for enhanced adsorption of methylene blue. Carbohydr. Polym. 2018, 185, 1–11. [Google Scholar] [CrossRef]
- Hokkanen, S.; Doshi, B.; Srivastava, V.; Puro, L.; Koivula, R. Arsenic (III) removal from water by hydroxyapatite-bentonite clay-nanocrystalline cellulose. Environ. Progr. Sustain. Energy 2019, 38, 13147. [Google Scholar] [CrossRef]
- Gopinath, S.; Sugunan, S. Enzymes immobilized on montmorillonite K 10: Effect of adsorption and grafting on the surface properties and the enzyme activity. Appl. Clay Sci. 2007, 35, 67–75. [Google Scholar] [CrossRef]
- Ahmad, R.; Hasan, I. L-cystein modified bentonite-cellulose nanocomposite (cellu/cys-bent) for adsorption of Cu2+, Pb2+, and Cd2+ ions from aqueous solution. Sep. Sci. Technol. 2016, 51, 381–394. [Google Scholar] [CrossRef]
- Liu, D.; Bian, Q.; Li, Y.; Wang, Y.; Xiang, A.; Tian, H. Effect of oxidation degrees of graphene oxide on the structure and properties of poly (vinyl alcohol) composite films. Compos. Sci. Technol. 2016, 129, 146–152. [Google Scholar] [CrossRef]
- Qin, L.; Yan, L.; Chen, J.; Liu, T.; Yu, H.; Du, B. Enhanced removal of Pb2+, Cu2+, and Cd2+ by amino-functionalized magnetite kaolin clay. Ind. Eng. Chem. Res. 2016, 55, 7344–7354. [Google Scholar] [CrossRef]
- Larraza, I.; López-Gónzalez, M.; Corrales, T.; Marcelo, G. Hybrid materials: Magnetite–Polyethylenimine–Montmorillonite, as magnetic adsorbents for Cr(VI) water treatment. J. Colloid Interf. Sci. 2012, 385, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Li, Y.; Wang, S.; Zhou, Y. Synthesis of montmorillonite/Fe3O4-OTAB composite capable of using as anisotropic nanoparticles. Appl. Surf. Sci. 2017, 402, 384–391. [Google Scholar] [CrossRef]
- Chen, X.; Cui, J.; Xu, X.; Sun, B.; Zhang, L.; Dong, W.; Chen, C.; Sun, D. Bacterial cellulose/attapulgite magnetic composites as an efficient adsorbent for heavy metal ions and dye treatment. Carbohydr. Polym. 2019, 229, 115512. [Google Scholar] [CrossRef]
- Lu, B.; Lin, Q.; Yin, Z.; Lin, F.; Chen, X.; Huang, B. Robust and lightweight biofoam based on cellulose nanofibrils for high-efficient methylene blue adsorption. Cellulose 2021, 28, 273–288. [Google Scholar] [CrossRef]
- Yu, X.; Tong, S.; Ge, M.; Zuo, J.; Cao, C.; Song, W. One-step synthesis of magnetic composites of cellulose@iron oxide nanoparticles for arsenic removal. J. Mater. Chem. A 2013, 1, 959–965. [Google Scholar] [CrossRef]
- Jiang, R.; Zhu, H.Y.; Fu, Y.Q.; Zong, E.M.; Jiang, S.T.; Li, J.B.; Zhu, J.Q.; Zhu, Y.Y. Magnetic NiFe2O4/MWCNTs functionalized cellulose bioadsorbent with enhanced adsorption property and rapid separation. Carbohydr. Polym. 2021, 252, 117158. [Google Scholar] [CrossRef]
- Xing, X.; Li, W.; Zhang, J.; Wu, H.; Guan, Y.; Gao, H. TEMPO-oxidized cellulose hydrogel for efficient adsorption of Cu2+ and Pb2+ modified by polyethyleneimine. Cellulose 2021, 28, 7953–7968. [Google Scholar] [CrossRef]
- Chen, Q.; Zheng, J.; Zheng, L.; Dang, Z.; Zhang, L. Classical theory and electron-scale view of exceptional Cd(II) adsorption onto mesoporous cellulose biochar via experimental analysis coupled with DFT calculations. Chem. Eng. J. 2018, 350, 1000–1009. [Google Scholar] [CrossRef]
- Park, S.H.; Shin, S.S.; Park, C.H.; Jeon, S.; Gwon, J.; Lee, S.Y.; Kim, S.J.; Kim, H.J.; Lee, J.H. Poly(acryloyl hydrazide)-grafted cellulose nanocrystal adsorbents with an excellent Cr(VI) adsorption capacity. J. Hazard. Mater. 2020, 394, 122512. [Google Scholar] [CrossRef]
- Mahdavinia, G.R.; Soleymani, M.; Sabzi, M.; Azimi, H.; Atlasi, Z. Novel magnetic polyvinyl alcohol/laponite RD nanocomposite hydrogels for efficient removal of methylene blue. J. Environ. Chem. Eng. 2017, 5, 2617–2630. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, W.; Zhu, L. Mono-/competitive adsorption of cadmium(II) and lead(II) using straw/bentonite-g-poly(acrylic acid-co-acrylamide) resin. Polym. Bull. 2019, 77, 3795–3811. [Google Scholar] [CrossRef]
- Das, S.; Das, P.; Chakraborty, S. Optimization of hazardous crystal violet by chemically treated rice husk: Using central composite response surface methodology. Arch. Environ. Sci. 2012, 6, 57–61. [Google Scholar]
- Chakraborty, S.; Chowdhury, S.; Saha, P.D. Adsorption of crystal violet from aqueous solution onto NaOH-modified rice husk. Carbohydr. Polym. 2011, 86, 1533–1541. [Google Scholar] [CrossRef]
- Chakraborty, S.; Mukherjee, A.; Das, S.; Maddela, N.R.; Iram, S.; Das, P. Study on isotherm, kinetics, and thermodynamics of adsorption of crystal violet dye by calcium oxide modified fly ash. Environ. Eng. Res. 2020, 26, 190372. [Google Scholar] [CrossRef]
- Hokkanen, S.; Bhatnagar, A.; Srivastava, V.; Suorsa, V.; Sillanpää, M. Removal of Cd2+, Ni2+ and PO43− from aqueous solution by hydroxyapatite-bentonite clay-nanocellulose composite. Int. J. Biol. Macromol. 2018, 118, 903–912. [Google Scholar] [CrossRef]
- Liu, S.; Xie, Z.; Zhu, Y.; Zhu, Y.; Jiang, Y.; Wang, Y.; Gao, H. Adsorption characteristics of modified rice straw biochar for Zn and in-situ remediation of Zn contaminated soil. Environ. Technol. Innov. 2021, 22, 101388. [Google Scholar] [CrossRef]
- Adil, H.I.; Thalji, M.R.; Yasin, S.A.; Saeed, I.A.; Assiri, M.A.; Chong, K.F.; Ali, G.A.M. Metal–organic frameworks (MOFs) based nanofiber architectures for the removal of heavy metal ions. RSC Adv. 2022, 12, 1433–1450. [Google Scholar] [CrossRef]
- Shayegan, H.; Ali, G.A.M.; Safarifard, V. Recent progress in the removal of heavy metal ions from water using metal-organic frameworks. ChemistrySelect 2020, 5, 124–146. [Google Scholar] [CrossRef]
- Shayegan, H.; Ali, G.A.M.; Safarifard, V. Amide-functionalized metal–organic framework for high efficiency and fast removal of Pb (II) from aqueous solution. J. Inorg. Organomet. Polym. Mater. 2020, 30, 3170–3178. [Google Scholar] [CrossRef]
- Sadegh, H.; Ali, G.A.M.; Makhlouf, A.S.H.; Chong, K.F.; Alharbi, N.S.; Agarwal, S.; Gupta, V.K. MWCNTs-Fe3O4 nanocomposite for Hg(II) high adsorption efficiency. J. Mol. Liq. 2018, 258, 345–353. [Google Scholar] [CrossRef]














| Adsorbent | Pollutant | Maximum Adsorption Capacity (mg/g) | Reference |
|---|---|---|---|
| CMCNFs | Cu(II) | 115.3 | [22] |
| PEI-BC | Cu(II) | 148 | [23] |
| TOCN–PEI | Cu(II) | 52.32 | [28] |
| BC/GO | Cu(II) | 65 | [30] |
| cellu/cys-bent | Cu(II) | 32.36 | [39] |
| MKC | Cu(II) | 16.5 | [41] |
| Fe3O4/ATP@(BCNs/CS) | Cu(II) | 70.5 | [44] |
| TCP | Cu(II) | 109.89 | [48] |
| PNMBC | Cu(II) | 757.45 | This work |
| Composite | Temperature (°C) | Langmuir Models | Freundlich Models | ||||
|---|---|---|---|---|---|---|---|
| Qmax (mg/g) | KL (L/mg) | R2 | n | KF (mg/g)(L/mg)−1/n | R2 | ||
| 1:1 | 15 | 2790.02 | 0.00104 | 0.09003 | 1.20 | 5.84 | 0.90881 |
| 25 | 847.45 | 0.00593 | 0.91955 | 1.96 | 29.31 | 0.93284 | |
| 35 | 555.55 | 0.0135 | 0.96048 | 2.79 | 57.35 | 0.96510 | |
| 1:2 | 15 | 1306.5 | 0.00304 | 0.64308 | 1.56 | 16.14 | 0.93969 |
| 25 | 588.23 | 0.0110 | 0.93572 | 2.51 | 46.37 | 0.95370 | |
| 35 | 813.01 | 0.00400 | 0.98150 | 1.69 | 15.25 | 0.98320 | |
| 2:1 | 15 | 1288.95 | 0.00204 | 0.47880 | 1.45 | 9.72 | 0.94024 |
| 25 | 704.22 | 0.00600 | 0.82663 | 2.31 | 38.94 | 0.94024 | |
| 35 | 487.80 | 0.00900 | 0.92437 | 2.41 | 33.39 | 0.93280 | |
| Composite | Temperature (°C) | Pseudo-First-Order Adsorption Kinetics | Pseudo-Second-Order Adsorption Kinetics | ||||
|---|---|---|---|---|---|---|---|
| K1 (min−1) | Qe (mg/g) | R2 | K2 (g/(mg·min)) | Qe (mg/g) | R2 | ||
| 1:1 | 15 | 0.102 | 28.20 | 0.55969 | 0.013 | 273.22 | 0.99998 |
| 25 | 0.14 | 16.82 | 0.60238 | 0.026 | 245.70 | 0.99999 | |
| 35 | 0.091 | 25.95 | 0.82591 | 0.015 | 190.11 | 0.99998 | |
| 1:2 | 15 | 0.085 | 11.44 | 0.57244 | 0.033 | 248.76 | 1 |
| 25 | 0.052 | 21.76 | 0.39796 | 0.014 | 238.09 | 0.99985 | |
| 35 | 0.049 | 17.84 | 0.42768 | 0.016 | 234.19 | 0.99989 | |
| 2:1 | 15 | 0.099 | 4.34 | 0.52444 | 0.098 | 211.86 | 1 |
| 25 | 0.065 | 4.51 | 0.31011 | 0.073 | 215.52 | 1 | |
| 35 | 0.098 | 21.06 | 0.86403 | 0.019 | 173.31 | 0.99999 | |
| Composite | ΔG (KJ/mol) | ΔH (KJ/mol) | ΔS (J/mol·K) | ||
|---|---|---|---|---|---|
| 15 °C | 25 °C | 35 °C | |||
| 1:1 | −4.335 | −3.625 | −2.916 | −24.774 | −70.97 |
| 1:2 | −3.777 | −3.762 | −3.748 | −4.195 | −1.451 |
| 2:1 | −3.268 | −2.964 | −2.660 | −12.022 | −30.399 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, X.; Lv, X.; Han, C.; Bai, L.; Wang, T.; Sun, Y. Fabrication of Polyethyleneimine-Modified Nanocellulose/Magnetic Bentonite Composite as a Functional Biosorbent for Efficient Removal of Cu(Ⅱ). Water 2022, 14, 2656. https://doi.org/10.3390/w14172656
Sun X, Lv X, Han C, Bai L, Wang T, Sun Y. Fabrication of Polyethyleneimine-Modified Nanocellulose/Magnetic Bentonite Composite as a Functional Biosorbent for Efficient Removal of Cu(Ⅱ). Water. 2022; 14(17):2656. https://doi.org/10.3390/w14172656
Chicago/Turabian StyleSun, Xiaoyin, Xintian Lv, Caohui Han, Lu Bai, Tingting Wang, and Yongchang Sun. 2022. "Fabrication of Polyethyleneimine-Modified Nanocellulose/Magnetic Bentonite Composite as a Functional Biosorbent for Efficient Removal of Cu(Ⅱ)" Water 14, no. 17: 2656. https://doi.org/10.3390/w14172656
APA StyleSun, X., Lv, X., Han, C., Bai, L., Wang, T., & Sun, Y. (2022). Fabrication of Polyethyleneimine-Modified Nanocellulose/Magnetic Bentonite Composite as a Functional Biosorbent for Efficient Removal of Cu(Ⅱ). Water, 14(17), 2656. https://doi.org/10.3390/w14172656







