Physical, Chemical, and Mineralogical Controls on Retardation of Anatoxin-a Migration by Sorption to Natural Soils with Implications for Groundwater Protection
Abstract
:1. Introduction
2. Materials and Methods
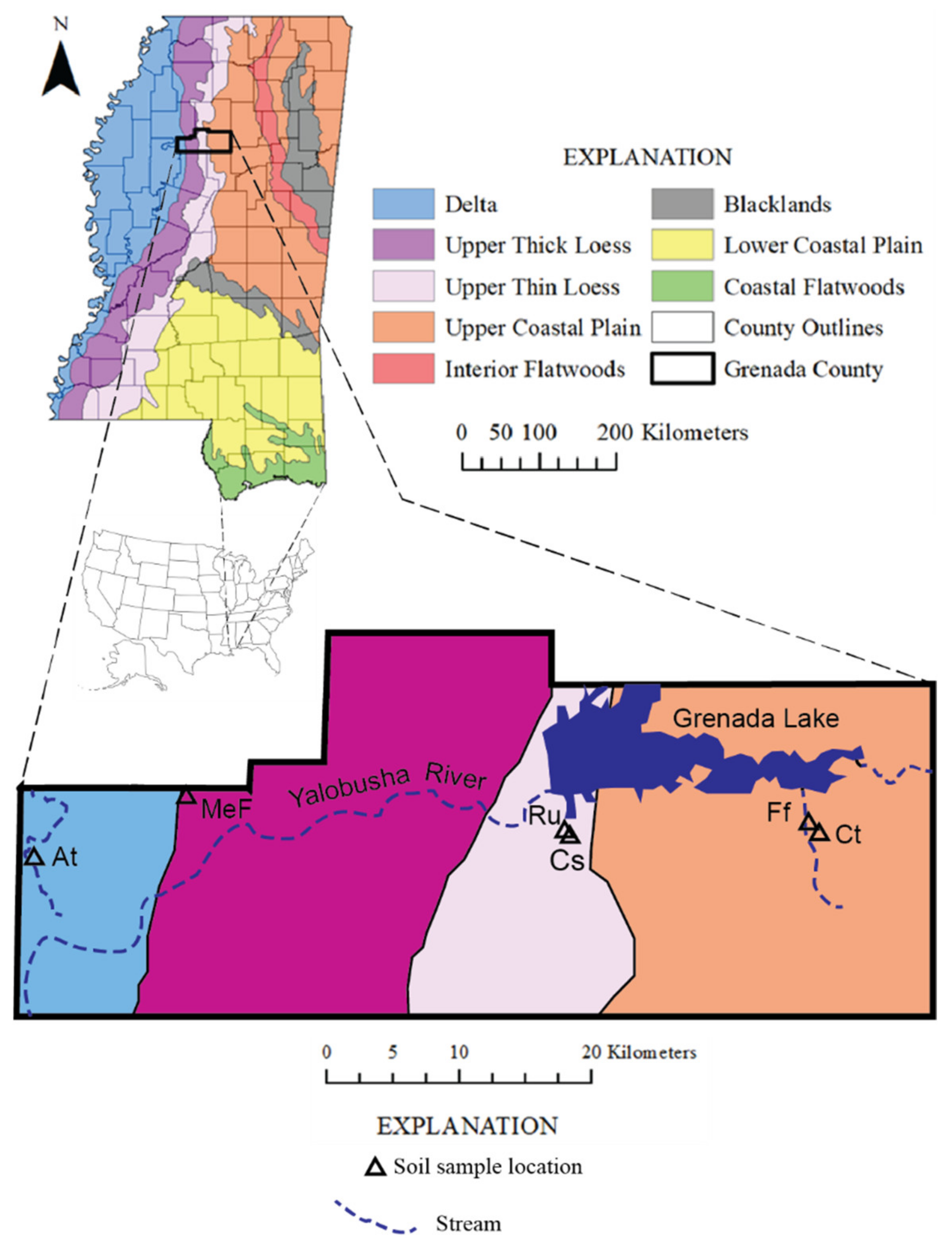
2.1. Study Area
2.2. Soil Characteristics
2.2.1. Pedology
Alligator Association
Memphis Silt Loam Series
Falaya Silt Loam Series
Ruston Series
Cuthbert Series
Channel Sand
2.2.2. Sample Collection
2.2.3. Physical and Extractable Chemical Properties
2.2.4. Elemental Composition and Mineralogy
2.3. Batch Experiments
2.4. Data Analysis
2.4.1. Sorption Isotherms
2.4.2. Retardation Factors
2.4.3. Statistics and Correlation
3. Results
3.1. Soil Properties
3.1.1. Physical and Extractable Chemical Properties
3.1.2. Elemental and Mineralogical Composition
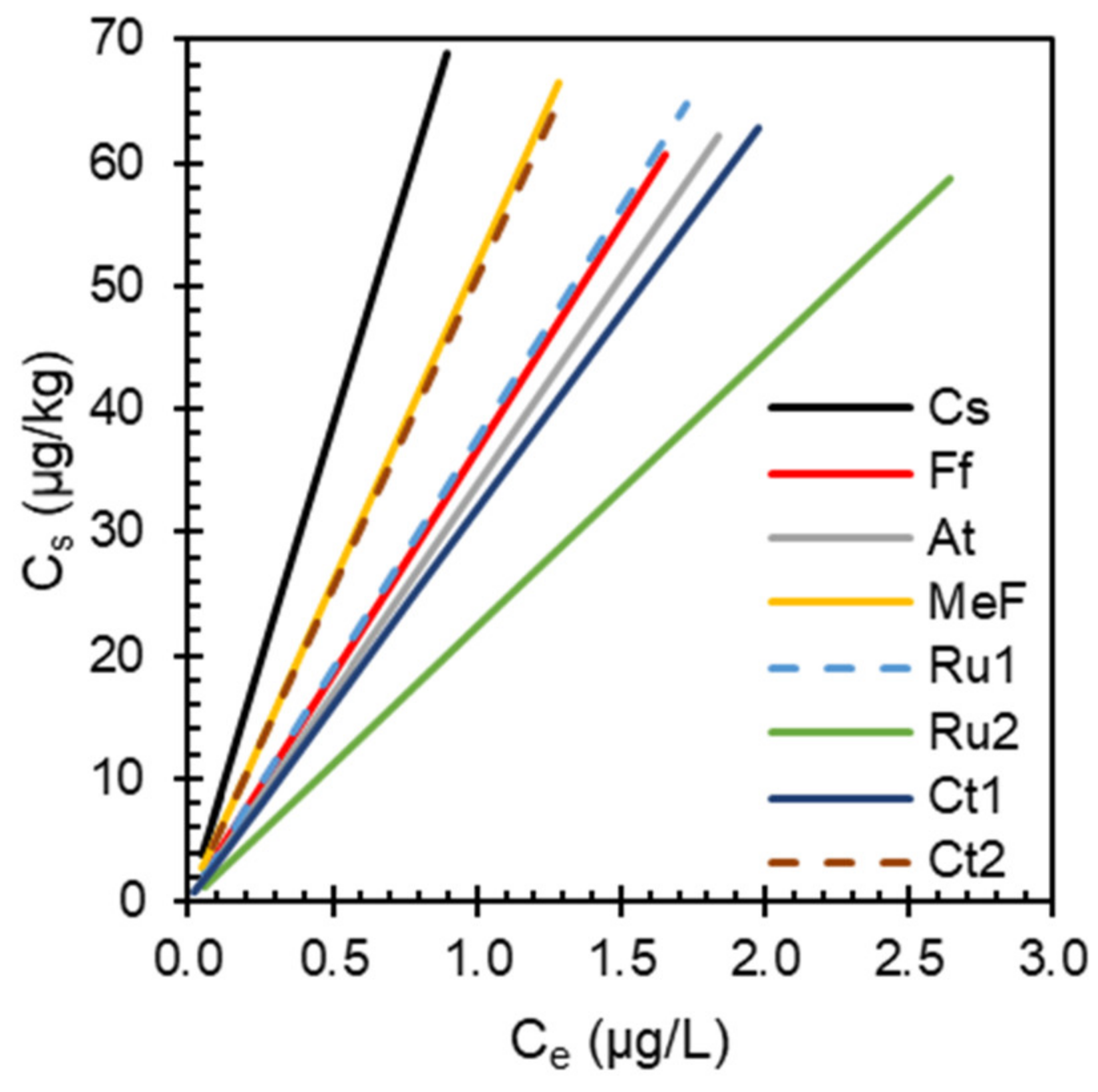
3.2. Sorption Isotherms
3.3. Retardation Factors
3.4. Association between Sorption Affinity and Textural and Mineralogical Characteristics
4. Discussion
4.1. Sorption Isotherm Characteristics
4.2. Influence of Clay Mineralogy on Anatoxin-a Sorption
4.3. Influence of Soil Horizons on Anatoxin-a Migration
4.4. Implications of Anatoxin-a Migration on Groundwater Protection
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Döll, P.; Schmied, H.M.; Schuh, C.; Portmann, F.T.; Eicker, A. Global-scale assessment of groundwater depletion and related groundwater abstractions: Combining hydrological modeling with information from well observations and GRACE satellites. Water Resour. Res. 2014, 50, 5698–5720. [Google Scholar] [CrossRef]
- Stefan, C.; Ansems, N. Web-based global inventory of managed aquifer recharge applications. Sustain. Water Resour. Manag. 2018, 4, 153–162. [Google Scholar] [CrossRef]
- Chorus, I.; Bartram, J. (Eds.) Toxic Cyanobacteria in Water. A Guide to Their Public Health Consequences, Monitoring, and Management; World Health Organization: Geneva, Switzerland, 1999. [Google Scholar]
- Merel, S.; Walker, D.; Chicana, R.; Snyder, S.; Baurès, E.; Thomas, O. State of knowledge and concerns on cyanobacterial blooms and cyanotoxins. Environ. Int. 2013, 59, 303–327. [Google Scholar] [CrossRef] [PubMed]
- Loftin, K.A.; Graham, J.L.; Hilborn, E.D.; Lehmann, S.C.; Meyer, M.T.; Dietze, J.E.; Griffith, C.B. Cyanotoxins in inland lakes of the United States: Occurrence and potential recreational health risks in the EPA National Lakes Assessment 2007. Harmful Algae 2016, 56, 77–90. [Google Scholar] [CrossRef]
- Snow, D.D.; Cassada, D.A.; Larsen, M.L.; Mware, N.A.; Li, X.; D’Alessio, M.; Zhang, Y.; Sallach, J.B. Detection, Occurrence and Fate of Emerging Contaminants in Agricultural Environments. Water Environ. Res. 2017, 89, 897–920. [Google Scholar] [CrossRef] [PubMed]
- Codd, G.; Bell, S.; Kaya, K.; Ward, C.; Beattie, K.; Metcalf, J. Cyanobacterial toxins, exposure routes and human health. Eur. J. Phycol. 1999, 34, 405–415. [Google Scholar] [CrossRef]
- Corbel, S.; Mougin, C.; Bouaïcha, N. Cyanobacterial toxins: Modes of actions, fate in aquatic and soil ecosystems, phytotoxicity and bioaccumulation in agricultural crops. Chemosphere 2014, 96, 1–15. [Google Scholar] [CrossRef]
- Liang, C.; Wang, W.; Wang, Y. Effect of irrigation with microcystins-contaminated water on growth, yield and grain quality of rice (Oryza sativa). Environ. Earth Sci. 2016, 75, 505. [Google Scholar] [CrossRef]
- Lee, S.; Jiang, X.; Manubolu, M.; Riedl, K.; Ludsin, S.A.; Martin, J.F.; Lee, J. Fresh produce and their soils accumulate cyanotoxins from irrigation water: Implications for public health and food security. Food Res. Int. 2017, 102, 234–245. [Google Scholar] [CrossRef]
- Lahti, K.; Vaitomaa, J.; Kivimaki, A.-L.; Sivonen, K. Fate of cyanobacterial hepatotoxins in artificial recharge of groundwater and in bank filtration. In Artificial Recharge of Groundwater; Peters, J.H., Ed.; A.A. Balkema: Nieuwegein, The Netherlands, 1998; pp. 211–216. [Google Scholar]
- O’Reilly, A.M.; Wanielista, M.P.; Loftin, K.A.; Chang, N. Bin Laboratory simulated transport of microcystin-LR and cylindrospermopsin in groundwater under the influence of stormwater ponds: Implications for harvesting of infiltrated stormwater. In Proceedings of the IAHS-AISH Publication, Zurich, Switzerland, 13–18 June 2010; Volume 342. [Google Scholar]
- U.S. Environmental Protection Agency (USEPA). Enhanced Aquifer Recharge of Stormwater in the United States: State of the Science Review; Report # EPA/600/R-21/037F; USEPA: Washington, DC, USA. Available online: http://www.epa.gov/research (accessed on 7 February 2022).
- Trainer, V.L.; Hardy, F.J. Integrative Monitoring of Marine and Freshwater Harmful Algae in Washington State for Public Health Protection. Toxins 2015, 7, 1206–1234. [Google Scholar] [CrossRef]
- Colas, S.; Marie, B.; Lance, E.; Quiblier, C.; Tricoire-Leignel, H.; Mattei, C. Anatoxin-a: Overview on a harmful cyanobacterial neurotoxin from the environmental scale to the molecular target. Environ. Res. 2020, 193, 110590. [Google Scholar] [CrossRef] [PubMed]
- Van der Merwe, D. Freshwater cyanotoxins. In Biomarkers in Toxicology; Academic Press: Cambridge, MA, USA, 2014. [Google Scholar]
- Gorham, P.R. Laboratory Studies on the Toxins Produced by Waterblooms of Blue-Green Algae. Am. J. Public Health Nations Health 1962, 52, 2100–2105. [Google Scholar] [CrossRef] [PubMed]
- Ferrão-Filho, A.D.S.; Kozlowsky-Suzuki, B. Cyanotoxins: Bioaccumulation and effects on aquatic animals. Mar. Drugs 2011, 9, 2729–2772. [Google Scholar] [CrossRef]
- Krienitz, L.; Ballot, A.; Kotut, K.; Wiegand, C.; Pütz, S.; Metcalf, J.S.; Codd, G.A.; Stephan, P. Contribution of hot spring cyanobacteria to the mysterious deaths of Lesser Flamingos at Lake Bogoria, Kenya. FEMS Microbiol. Ecol. 2003, 43, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Rantala-Ylinen, A.; Känä, S.; Wang, H.; Rouhiainen, L.; Wahlsten, M.; Rizzi, E.; Berg, K.; Gugger, M.; Sivonen, K. Anatoxin-a Synthetase Gene Cluster of the Cyanobacterium Anabaena sp. Strain 37 and Molecular Methods to Detect Potential Producers. Appl. Environ. Microbiol. 2011, 77, 7271–7278. [Google Scholar] [CrossRef] [PubMed]
- Walker, H.W. Harmful Algae Blooms in Drinking Water; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Ministry of Health. Guidelines for Drinking-Water Quality Management for New Zealand, 2nd ed.; Ministry of Health: Wellington, New Zealand, 2016; ISBN 9780947491789.
- Cadel-Six, S.; Peyraud-Thomas, C.; Brient, L.; de Marsac, N.T.; Rippka, R.; Méjean, A. Different Genotypes of Anatoxin-Producing Cyanobacteria Coexist in the Tarn River, France. Appl. Environ. Microbiol. 2007, 73, 7605–7614. [Google Scholar] [CrossRef] [PubMed]
- Matlock, S. Toxic algae blamed for elk deaths in northeastern New Mexico. Santa Fe New Mexican, 22 October 2013. [Google Scholar]
- Puschner, B.; Hoff, B.; Tor, E.R. Diagnosis of Anatoxin-a Poisoning in Dogs from North America. J. Vet. Diagn. Investig. 2008, 20, 89–92. [Google Scholar] [CrossRef]
- Biré, R.; Bertin, T.; Dom, I.; Hort, V.; Schmitt, C.; Diogène, J.; Lemée, R.; De Haro, L.; Nicolas, M. First Evidence of the Presence of Anatoxin-A in Sea Figs Associated with Human Food Poisonings in France. Mar. Drugs 2020, 18, 285. [Google Scholar] [CrossRef]
- Ibelings, B.W.; Backer, L.C.; Kardinaal, W.E.A.; Chorus, I. Current approaches to cyanotoxin risk assessment and risk management around the globe. Harmful Algae 2014, 40, 63–74. [Google Scholar] [CrossRef]
- Testai, E. Anatoxin-a and analogues. In Toxic Cyanobacteria in Water; Chorus, I., Welker, M., Eds.; World Health Organization: Geneva, Switzerland; Boca Raton, FL, USA, 2021; pp. 72–93. [Google Scholar]
- Wood, S.A.; Holland, P.T.; MacKenzie, L. Development of solid phase adsorption toxin tracking (SPATT) for monitoring anatoxin-a and homoanatoxin-a in river water. Chemosphere 2011, 82, 888–894. [Google Scholar] [CrossRef]
- Rapala, J.; Lahti, K.; Sivonen, K.; Niemelä, S. Biodegradability and adsorption on lake sediments of cyanobacterial hepatotoxins and anatoxin-a. Lett. Appl. Microbiol. 1994, 19, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Stevens, D.; Krieger, R. Stability studies on the cyanobacterial nicotinic alkaloid snatoxin-A. Toxicon 1991, 29, 167–179. [Google Scholar] [CrossRef]
- Vlad, S.; Anderson, W.B.; Peldszus, S.; Huck, P.M. Removal of the cyanotoxin anatoxin-a by drinking water treatment processes: A review. J. Water Health 2014, 12, 601–617. [Google Scholar] [CrossRef]
- Smith, C.; Sutton, A. The Persistence of Anatoxin-a in Reservoir Water; Report No. FR0427; Foundation for Water Research: Marlow, Buckinghamshire, UK, 1993. [Google Scholar]
- Kaminski, A.; Bober, B.; Lechowski, Z.; Bialczyk, J. Determination of anatoxin-a stability under certain abiotic factors. Harmful Algae 2013, 28, 83–87. [Google Scholar] [CrossRef]
- Zhang, Y.; Husk, B.R.; Duy, S.V.; Dinh, Q.T.; Sanchez, J.S.; Sauvé, S.; Whalen, J.K. Quantitative screening for cyanotoxins in soil and groundwater of agricultural watersheds in Quebec, Canada. Chemosphere 2021, 274, 129781. [Google Scholar] [CrossRef]
- Miller, A.; Russell, C. Food crops irrigated with cyanobacteria-contaminated water: An emerging public health issue in Canada. Environ. Health Rev. 2017, 60, 58–63. [Google Scholar] [CrossRef]
- Mohamed, Z.A.; Al Shehri, A.M. Microcystins in groundwater wells and their accumulation in vegetable plants irrigated with contaminated waters in Saudi Arabia. J. Hazard. Mater. 2009, 172, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Klitzke, S.; Beusch, C.; Fastner, J. Sorption of the cyanobacterial toxins cylindrospermopsin and anatoxin-a to sediments. Water Res. 2011, 45, 1338–1346. [Google Scholar] [CrossRef]
- Bialczyk, J.; Natkański, P.; Kuśtrowski, P.; Czaja-Prokop, U.; Bober, B.; Kaminski, A. Removal of cyanobacterial anatoxin-a from water by natural clay adsorbents. Appl. Clay Sci. 2017, 148, 17–24. [Google Scholar] [CrossRef]
- Aba, R.; Mugani, R.; Hejjaj, A.; de Fraissinette, N.B.; Oudra, B.; Ouazzani, N.; Campos, A.; Vasconcelos, V.; Carvalho, P.; Mandi, L. First Report on Cyanotoxin (MC-LR) Removal from Surface Water by Multi-Soil-Layering (MSL) Eco-Technology: Preliminary Results. Water 2021, 13, 1403. [Google Scholar] [CrossRef]
- Klitzke, S.; Apelt, S.; Weiler, C.; Fastner, J.; Chorus, I. Retention and degradation of the cyanobacterial toxin cylindrospermopsin in sediments—The role of sediment preconditioning and DOM composition. Toxicon 2010, 55, 999–1007. [Google Scholar] [CrossRef] [PubMed]
- United States Dep. Agric. (USDA). Land Resource Regions and Major Land Resource Areas of the United States, the Caribbean, and the Pacific Basin, Handbook 296; USDA: Washington, DC, USA, 2006.
- Thomas, A.E. Soil Survey of Grenada County, Mississippi; US Dep. Agric.: Washington, DC, USA, 1967; pp. 1–76.
- Dash, P.; Silwal, S.; Ikenga, J.O.; Pinckney, J.L.; Arslan, Z.; Lizotte, R.E. Water Quality of Four Major Lakes in Mississippi, USA: Impacts on Human and Aquatic Ecosystem Health. Water 2015, 7, 4999–5030. [Google Scholar] [CrossRef]
- Vanderford, H.B. Soils of Mississippi; Mississippi Agricultural Experiment Station: Starkville, MS, USA, 1962. [Google Scholar]
- NOAA National Centers for Environmental Information, U.S. Climate Normals Quick Access. Available online: https://www.ncei.noaa.gov/access/us-climate-normals/ (accessed on 8 October 2021).
- Natural Resources Conservation Service. Web Soil Survey. Available online: https://websoilsurvey.sc.egov.usda.gov/App/HomePage.htm (accessed on 23 May 2019).
- Natural Resources Conservation Service. Official Soil Series Descriptions. Available online: https://www.nrcs.usda.gov/wps/portal/nrcs/detail/soils/scientists/?cid=nrcs142p2_053587 (accessed on 5 October 2021).
- Brady, N.C.; Weil, R.R. Nature and Properties of Soil, 13th ed.; Prentice-Hall Inc.: Hoboken, NJ, USA, 1998; 960p. [Google Scholar]
- Snowden, J.O.; Priddy, R.R. Loess Investigations in Mississippi: Geology of Mississippi Loess; Bulletin 111; Mississippi Geological, Economic and Topographical Survey: Jackson, MS, USA, 1968; pp. 13–204. [Google Scholar]
- Bruce, R.; Raney, W.A.; Broadfoot, W.M.; Vanderford, H.B.; Lyle, C.Y. Characteristics of Important Mississippi Soils; Mississippi State University, Agricultural Experiment Station: Starkville, MS, USA, 1958. [Google Scholar]
- Skikora, F.J.; Crouse, K.K.; Heckendorn, S.; Huluka, G.; Mitchell, C.C.; Moore, K.P.; Oldham, J.L. Cation Exchange Capacity. In Soil Test Methods From the Southeastern United States; Skikora, F.J., Moore, K.P., Eds.; South. Coop. Ser. Bull. No. 419; Southern Extension and Research Activity Information Exchange Group: Clemson, SC, USA, 2014; pp. 170–179. [Google Scholar]
- Kaplan, D.I.; Bertsch, P.M.; Adriano, D.C.; Miller, W.P. Soil-borne mobile colloids as influenced by water flow and organic carbon. Environ. Sci. Technol. 1993, 27, 1193–1200. [Google Scholar] [CrossRef]
- Harris, W.; White, G.N. X-ray diffraction techniques for soil mineral identification. In Methods of Soil Analysis, Part 5: Mineralogical Methods; Soil Science Society of America (SSSA) Book Series 5; SSSA: Madison, WI, USA, 2015. [Google Scholar]
- Poppe, L.J.; Paskevich, V.F.; Hathaway, J.C.; Blackwood, D.S. A Laboratory Manual for X-ray Powder Diffraction; Open-File Report 2001-41; U.S. Geol. Survey: Woods Hole, MA, USA, 2001; 88p. [CrossRef]
- Miller, M.; Critchley, M.; Hutson, J.; Fallowfield, H. The adsorption of cyanobacterial hepatotoxins from water onto soil during batch experiments. Water Res. 2001, 35, 1461–1468. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency (USEPA). Health Effects Support Document for the Cyanobacterial Toxin Anatoxin-A; USEPA: Washington, DC, USA, 2015.
- Appelo, C.A.J.; Postma, D. Geochemistry, Groundwater, and Pollution; A.A. Balkema Publishers: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Hemond, H.F.; Fechner, E.J. Chemical Fate and Transport in the Environment, 3rd ed.; Academic Press: Cambridge, MA, USA, 2014. [Google Scholar]
- Keenan, W.E.; McNutt, E.J.; Warren, R.B.; Morris, W.M.; Wynn, A.H. Soil Survey of Leflore County, Mississippi; US Dep. Agric.: Washington, DC, USA, 1959; pp. 1–62.
- Longwell, T.J.; Parks, W.L.; Springer, M.E. Moisture Characteristics of Tennessee Soils; University of Tennessee Agricultural Experiment Station: Knoxville, TN, USA, 1963; p. 47. Available online: https://trace.tennessee.edu/utk_agbulletin/303/ (accessed on 8 September 2022).
- Homenauth, O.P.; McBride, M.B. Adsorption of Aniline on Layer Silicate Clays and an Organic Soil. Soil Sci. Soc. Am. J. 1994, 58, 347–354. [Google Scholar] [CrossRef]
- Barton, C.D.; Karathanasis, A.D. Clay Minerals. In Encyclopedia of Soil Science; Lal, R., Ed.; Marcel Dekker: New York, NY, USA, 2002; pp. 187–192. [Google Scholar]
- Starkey, H.C. The Role of Clays in Fixing Lithium; Geological Survey Bulletin 1278-F; US Government Printing Office: Washington, DC, USA, 1982. [CrossRef]
- Kosmulski, M. The pH-dependent surface charging and points of zero charge. J. Colloid Interface Sci. 2010, 353, 1–15. [Google Scholar] [CrossRef]
- White, G.N.; Dixon, J.B. Kaolin-serpentine minerals. In Soil Mineralogy with Environmental Applications; Dixon, J.B., Schulze, D.G., Eds.; Soil Science Society of America (SSSA) Book Series 7; SSSA, Inc.: Madison, WI, USA, 2002; pp. 389–414. [Google Scholar]
- McBride, M.B. Environmental Chemistry of Soils; Oxford University Press, Inc.: New York, NY, USA, 1994. [Google Scholar]
- Kolstad, D.C.; Benson, C.H.; Edil, T.B. Hydraulic Conductivity and Swell of Nonprehydrated Geosynthetic Clay Liners Permeated with Multispecies Inorganic Solutions. J. Geotech. Geoenviron. Eng. 2004, 130, 1236–1249. [Google Scholar] [CrossRef]
- Norrish, K. The swelling of montmorillonite. Discuss. Faraday Soc. 1954, 18, 120–134. [Google Scholar] [CrossRef]
- Newcombe, G. Removal of natural organic material and algal metabolites using activated carbon. In Interface Science and Technology; Newcombe, G., Dixon, D., Eds.; Elsevier: Amsterdam, The Netherlands, 2006; Volume 10, pp. 133–153. [Google Scholar]
- Reid-Soukup, D.A.; Ulery, A.L. Smectites. In Soil Mineralogy with Environmental Applications; Dixon, J.B., Schulze, D.G., Eds.; Soil Science Society of America (SSSA) Book Series 7; SSSA, Inc.: Madison, WI, USA, 2002; pp. 467–499. [Google Scholar]
- Kahut, C.K.; Warren, C.J. Chlorites. In Soil Mineralogy with Environmental Applications; Dixon, J.B., Schulze, D.G., Eds.; Soil Science Society of America (SSSA) Book Series 7; SSSA, Inc.: Madison, WI, USA, 2002; pp. 531–553. [Google Scholar]
- Osman, M.A.; Suter, U.W. Determination of the Cation-Exchange Capacity of Muscovite Mica. J. Colloid Interface Sci. 2000, 224, 112–115. [Google Scholar] [CrossRef]
- Czajkowsky, D.M.; Shao, Z. Inhibition of protein adsorption to muscovite mica by monovalent cations. J. Microsc. 2003, 211, 1–7. [Google Scholar] [CrossRef]
- Bigham, J.M.; Fitzpatrick, R.W.; Schulze, D.G. Iron Oxides. In Soil Mineralogy with Environmental Applications; Dixon, J.B., Schulze, D.G., Eds.; Soil Science Society of America (SSSA) Book Series 7; SSSA, Inc.: Madison, WI, USA, 2002; pp. 323–366. [Google Scholar]
- O’Reilly, A.M.; Wanielista, M.P.; Chang, N.-B.; Xuan, Z.; Harris, W.G. Nutrient removal using biosorption activated media: Preliminary biogeochemical assessment of an innovative stormwater infiltration basin. Sci. Total Environ. 2012, 432, 227–242. [Google Scholar] [CrossRef] [PubMed]
- Payne, E.G.; McCarthy, D.T.; Deletic, A.; Zhang, K. Biotreatment technologies for stormwater harvesting: Critical perspectives. Curr. Opin. Biotechnol. 2019, 57, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Shokri, M.; Kibler, K.M.; Hagglund, C.; Corrado, A.; Wang, D.; Beazley, M.; Wanielista, M. Hydraulic and nutrient removal performance of vegetated filter strips with engineered infiltration media for treatment of roadway runoff. J. Environ. Manag. 2021, 300, 113747. [Google Scholar] [CrossRef]
- Wen, D.; Chang, N.-B.; Wanielista, M.P. Assessing Nutrient Removal in Stormwater Runoff for Urban Farming with Iron filings-based Green Environmental Media. Sci. Rep. 2020, 10, 9379. [Google Scholar] [CrossRef]
- Chang, N.-B.; Wen, D.; Colona, W.; Wanielista, M.P. Comparison of Biological Nutrient Removal via Two Biosorption-Activated Media Between Laboratory-Scale and Field-Scale Linear Ditch for Stormwater and Groundwater Co-treatment. Water Air Soil Pollut. 2019, 230, 151. [Google Scholar] [CrossRef]
- Krasucka, P.; Pan, B.; Ok, Y.S.; Mohan, D.; Sarkar, B.; Oleszczuk, P. Engineered biochar—A sustainable solution for the removal of antibiotics from water. Chem. Eng. J. 2021, 405, 126926. [Google Scholar] [CrossRef]
- Zhu, S.; Xia, M.; Chu, Y.; Khan, M.A.; Lei, W.; Wang, F.; Muhmood, T.; Wang, A. Adsorption and Desorption of Pb(II) on l-Lysine Modified Montmorillonite and the simulation of Interlayer Structure. Appl. Clay Sci. 2018, 169, 40–47. [Google Scholar] [CrossRef]
- Zhu, S.; Chen, Y.; Khan, M.A.; Xu, H.; Wang, F.; Xia, M. In-Depth Study of Heavy Metal Removal by an Etidronic Acid-Functionalized Layered Double Hydroxide. ACS Appl. Mater. Interfaces 2022, 14, 7450–7463. [Google Scholar] [CrossRef]
- Stuckey, J.W.; Livada, J.; Martínez, C.E. Structural charge location dictates speciation and lability of copper in swelling layer silicates. Appl. Clay Sci. 2022, 216, 106332. [Google Scholar] [CrossRef]
- Zhang, J.; Mallants, D.; Brady, P.V. Molecular dynamics study of uranyl adsorption from aqueous solution to smectite. Appl. Clay Sci. 2022, 218, 106361. [Google Scholar] [CrossRef]
- Barrientos-Velázquez, A.L.; Cardona, A.M.; Liu, L.; Phillips, T.; Deng, Y. Influence of layer charge origin and layer charge density of smectites on their aflatoxin adsorption. Appl. Clay Sci. 2016, 132–133, 281–289. [Google Scholar] [CrossRef]
- D’Ascanio, V.; Greco, D.; Menicagli, E.; Santovito, E.; Catucci, L.; Logrieco, A.F.; Avantaggiato, G. The role of geological origin of smectites and of their physico-chemical properties on aflatoxin adsorption. Appl. Clay Sci. 2019, 181, 105209. [Google Scholar] [CrossRef]
- Shen, C.C.; Petit, S.; Li, C.J.; Li, C.S.; Khatoon, N.; Zhou, C.H. Interactions between smectites and polyelectrolytes. Appl. Clay Sci. 2020, 198, 105778. [Google Scholar] [CrossRef]
- Vlad, S.; Peldszus, S.; Anderson, W.B.; Huck, P.M. Anatoxin-a adsorption by virgin and preloaded granular activated carbon. AWWA Water Sci. 2019, 1, e1116. [Google Scholar] [CrossRef] [Green Version]


| Sample | Soil Type | Horizon | Depth | Silt + Clay | pH | SC a | CEC b | Extractable Concentrations (mg/kg) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (cm) | (wt%) | Soil c | Soil + ATX d | (μS/cm) | (cmol+/kg) | Na | Mg | P | K | Ca | Zn | |||
| Ru2 | Ultisol | E | 15 | 24.3 | 4.0 | 5.3 | 40.4 | 5.2 | 16 | 92 | 21 | 86 | 227 | 2.6 |
| Ct1 | Ultisol | A | ~8 | 87.8 | 5.0 | 6.4 | 113.2 | 18.0 | 21 | 699 | 46 | 461 | 4062 | 14.3 |
| At | Vertisol | A | ~30 | 88.4 | 4.3 | 5.5 | 40.0 | 33.2 | 158 | 2191 | 123 | 660 | 5500 | 7.9 |
| Ff | Inceptisol | A | 15 | 83.0 | 4.4 | 5.0 | 48.6 | 10.5 | 48 | 256 | 120 | 195 | 1135 | 6.2 |
| Ru1 | Ultisol | A | 8 | 29.9 | 4.1 | 5.5 | 43.8 | 7.1 | 25 | 138 | 33 | 114 | 444 | 3.0 |
| Ct2 | Ultisol | B | ~15 | 96.7 | 4.4 | 5.6 | 31.2 | 14.5 | 30 | 596 | 40 | 430 | 2432 | 6.8 |
| MeF | Alfisol | A | 15 | 80.1 | 4.0 | 5.5 | 31.5 | 14.2 | 25 | 839 | 98 | 308 | 1010 | 4.2 |
| Cs e | - | - | ~30 | 28.1 | 4.5 | 5.4 | 44.4 | 7.5 | 33 | 293 | 42 | 106 | 1179 | 6.9 |
| Sample | Al | Si | P | S | K | Ca | Ti | Cr | Mn | Fe | Zn | Sr | Zr | SiO2 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ru2 | 29,757 | 543,071 | 4044 | 3239 | 5584 | 3433 | 3268 | 641 | 264 | 36,664 | 40 | 41 | 470 | 1,161,734 |
| Ct1 | 67,519 | 378,292 | 4288 | 2484 | 18,937 | 5931 | 3922 | 81 | 611 | 22,602 | 87 | 98 | 487 | 809,241 |
| At | 86,600 | 279,652 | 4314 | 1099 | 19,187 | 5766 | 3751 | 75 | 537 | 39,178 | 109 | 103 | 217 | 598,230 |
| Ff | 72,471 | 415,679 | 3903 | 2776 | 16,860 | 4150 | 3762 | 68 | 467 | 17,195 | 57 | 90 | 499 | 889,218 |
| Ru1 | 46,026 | 517,550 | 4308 | 2699 | 8330 | 3533 | 3016 | 122 | 259 | 10,765 | 34 | 39 | 427 | 1,107,141 |
| Ct2 | 84,261 | 349,958 | 4764 | 1261 | 19,195 | 4737 | 4158 | 63 | 445 | 32,091 | 87 | 93 | 431 | 748,628 |
| MeF | 72,480 | 378,583 | 4002 | 1787 | 20,579 | 4422 | 3573 | 80 | 678 | 22,771 | 76 | 109 | 506 | 809,863 |
| Cs | 64,389 | 408,612 | 4096 | 2504 | 10,988 | 4475 | 3100 | 129 | 411 | 24,979 | 64 | 61 | 381 | 874,101 |
| Sample | Clay Fraction Mineral Abundance (wt%) | ||||
|---|---|---|---|---|---|
| Chlorite | Kaolinite | Mica | Smectite | Quartz | |
| Ru2 | 0 | 9 | 0 | 0 | 91 |
| Ct1 | 0 | 28 | 35 | 0 | 37 |
| At | 0 | 19 | 30 | 16 | 35 |
| Ff | 0 | 35 | 33 | 0 | 32 |
| Ru1 | 0 | 44 | 0 | 0 | 56 |
| Ct2 | 3 | 25 | 37 | 0 | 35 |
| MeF | 0 | 37 | 0 | 0 | 63 |
| Cs | 0 | 53 | 0 | 13 | 34 |
| Sample | Linear Isotherm | Freundlich Isotherm | Langmuir Isotherm | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kd (L/kg) | RMSE a (μg/kg) | ME b (μg/kg) | r2 c | Kf (L/kg) | n | RMSE (μg/kg) | ME (μg/kg) | r2 | Kl (L/μg) | Cm (μg/kg) | RMSE (μg/kg) | ME (μg/kg) | r2 | |
| Ru2 | 22.31 | 2.60 | −7.12 | 0.99 | 25.56 | 0.83 | 1.19 | −0.25 | 1.00 | 0.15 | 202.6 | 1.27 | −1.85 | 1.00 |
| Ct1 | 31.83 | 3.56 | −10.21 | 0.99 | 35.87 | 0.76 | 0.40 | 0.66 | 1.00 | 0.33 | 152.9 | 0.75 | −1.55 | 1.00 |
| At | 33.83 | 6.31 | −5.40 | 0.92 | 35.41 | 0.89 | 6.10 | −0.78 | 0.92 | 0.12 | 335.1 | 6.12 | −1.65 | 0.92 |
| Ff | 36.73 | 4.11 | 3.01 | 0.97 | 36.73 | 1.00 | 4.11 | 3.01 | 0.97 | 0.01 | 3141.0 | 4.16 | 2.92 | 0.97 |
| Ru1 | 37.54 | 4.48 | −10.93 | 0.98 | 41.16 | 0.75 | 1.89 | 2.19 | 0.99 | 0.46 | 138.4 | 1.28 | 0.66 | 1.00 |
| Ct2 | 50.74 | 4.73 | 2.98 | 0.96 | 50.78 | 0.98 | 4.72 | 4.06 | 0.96 | 0.10 | 580.8 | 4.61 | 5.71 | 0.97 |
| MeF | 51.75 | 3.90 | −5.29 | 0.97 | 52.29 | 0.84 | 2.72 | 3.50 | 0.99 | 0.39 | 191.9 | 1.97 | 4.08 | 1.00 |
| Cs d | 77.15 | 6.08 | −7.92 | 0.94 | 73.16 | 0.82 | 5.06 | 3.24 | 0.96 | 0.68 | 175.4 | 4.57 | 4.06 | 0.96 |
| Sample | |||
|---|---|---|---|
| Ru2 | 1.35 | 0.49 | 62 |
| Ct1 | 1.22 | 0.54 | 73 |
| At | 1.96 | 0.26 | 256 |
| Ff | 1.36 | 0.49 | 104 |
| Ru1 | 1.22 | 0.54 | 86 |
| Ct2 | 1.37 | 0.48 | 145 |
| Mef | 1.36 | 0.49 | 146 |
| Cs | 1.29 | 0.52 | 193 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hobart, J.L.; O’Reilly, A.M.; Gifford, J.N. Physical, Chemical, and Mineralogical Controls on Retardation of Anatoxin-a Migration by Sorption to Natural Soils with Implications for Groundwater Protection. Water 2022, 14, 2869. https://doi.org/10.3390/w14182869
Hobart JL, O’Reilly AM, Gifford JN. Physical, Chemical, and Mineralogical Controls on Retardation of Anatoxin-a Migration by Sorption to Natural Soils with Implications for Groundwater Protection. Water. 2022; 14(18):2869. https://doi.org/10.3390/w14182869
Chicago/Turabian StyleHobart, Justin L., Andrew M. O’Reilly, and Jennifer N. Gifford. 2022. "Physical, Chemical, and Mineralogical Controls on Retardation of Anatoxin-a Migration by Sorption to Natural Soils with Implications for Groundwater Protection" Water 14, no. 18: 2869. https://doi.org/10.3390/w14182869
APA StyleHobart, J. L., O’Reilly, A. M., & Gifford, J. N. (2022). Physical, Chemical, and Mineralogical Controls on Retardation of Anatoxin-a Migration by Sorption to Natural Soils with Implications for Groundwater Protection. Water, 14(18), 2869. https://doi.org/10.3390/w14182869








