Geochemical Composition, Source and Geothermometry of Thermal Water in the Bugok Area, South Korea
Abstract
:1. Introduction
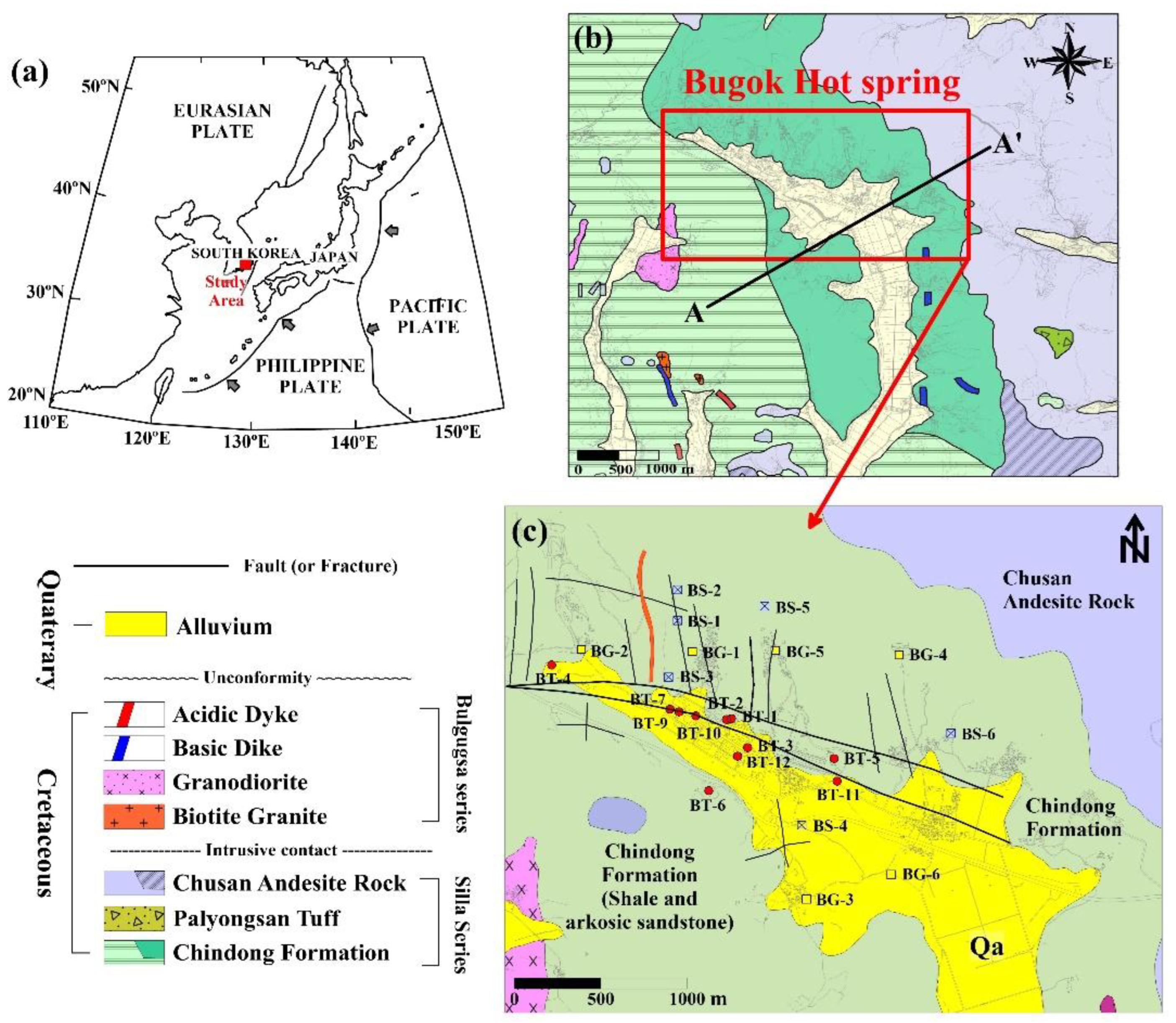
2. Study Site
3. Materials and Methods
3.1. Sample and In-Situ Measurement
3.2. Chemical and Isotopic Analysis
3.3. Noble Gas Analysis
3.4. Geothermometry
4. Results
4.1. Geochemical Composition
4.2. Oygen and Hydrogen Isotope
4.3. Sulfur Isotope
4.4. Helium and Neon Isotopes
4.5. Thermal Reservoir Temperature
4.6. Conceptual Model for the Formation of Geothermal Water
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ministry of the Interior and Safety. The Hot Springs Status of all the Country in 2020; United Nations Office for Disaster Risk Reduction (UNDRR): Brussel, Belgium, 2020; pp. 1–115. [Google Scholar]
- Jeong, C.H.; Lee, B.D.; Yang, J.H.; Nagao, K.; Kim, K.H.; Ahn, S.W.; Lee, Y.C.; Lee, Y.J.; Jang, H.W. Geochemical and isotopic compositions and geothermometry of thermal waters in the Magumsan area, South Korea. Water 2019, 11, 1774. [Google Scholar] [CrossRef]
- Kim, H.C.; Lee, S.; Song, M.Y. Relationship analysis between lithology, geological time and geothermal gradient of South Korea. Econ. Environ. Geol. 2002, 35, 163–170. [Google Scholar]
- Lim, J.U. Resources Survey and Analysis Report of Bugok Hot Spring; Geological Survey of Korea: Daejeon, Korea, 1989.
- Honda, M.; Reynolds, J.H.; Roedder, E.; Epstein, S. Noble gases in diamonds: Occurrences of solar like helium and neon. J. Geophys. Res. 1987, 92, 12507–12521. [Google Scholar] [CrossRef]
- Ozima, M.; Zashu, S. Noble gases in submarine pillow volcanic glasses. Earth Planet. Sci. Lett. 1983, 62, 24–40. [Google Scholar] [CrossRef]
- Sarda, P.; Staudacher, T.; Allegre, C.J. Neon isotopes in submarine basalts. Earth Planet. Sci. Lett. 1988, 91, 73–88. [Google Scholar] [CrossRef]
- Graham, D.W.; Lupton, F.; Albarède, F.; Condomines, M. Extreme temporal homogeneity of helium isotopes at Piton de la Fournaise, Réunion. Nature 1990, 347, 545–548. [Google Scholar] [CrossRef]
- Ballentine, C.J.; Burnard, P.G. Production, release and transport of noble gases in continental crust. Rev. Mineral. Geochem. 2002, 47, 481–538. [Google Scholar] [CrossRef]
- Torgersen, T.; Jenkins, W.J. Helium isotopes in geothermal system: Iceland, The Geysers, Raft River and Steamboat Springs. Geochem. Cosmochim. Acta 1982, 46, 739–748. [Google Scholar] [CrossRef]
- Hulston, J.R.; Lupton, J.E. Helium isotope studies of geothermal fluids in the Taupo Volcanic Zone, New Zealand. J. Volcanol. Geotherm. Res. 1996, 74, 297–321. [Google Scholar] [CrossRef]
- Yokoyama, T.; Nakai, S.I.; Wakita, H. Helium and carbon isotopic compositions of hot spring gases in the Tibetan Plateau. J. Volcanol. Geotherm. Res. 1999, 88, 99–107. [Google Scholar] [CrossRef]
- Graham, D.W. Noble gas isotope geochemistry of mid-ocean ridge and ocean island basalts: Characterization of mantle source reservoirs. Rev. Mineral. Geochem. 2002, 47, 247–317. [Google Scholar] [CrossRef] [Green Version]
- Graham, D.W.; Humphris, S.E.; Jenkins, W.J.; Kurz, M.D. Helium isotope geochemistry of some volcanic rocks from Saint Helena. Earth Planet. Sci. Lett. 1992, 110, 121–131. [Google Scholar] [CrossRef]
- Class, C.; Goldstein, S.L.; Stute, M.; Kurz, M.D.; Scholsser, P. Grand Comore Island: A well-constrained “low 3He/4He” mantle plume. Earth Planet. Sci. Lett. 2005, 233, 391–409. [Google Scholar] [CrossRef]
- Füri, E.; Hilton, D.R.; Murton, B.J.; Hemond, C.; Dyment, J.; Day, J.M.D. Helium isotope variations between Réunion Island and the Central Indian Ridge (17°–21°S): New evidence for ridge-hotspot interaction. J. Geophys. Res. 2011, 116, B02207. [Google Scholar] [CrossRef]
- Jeong, C.H.; Hur, H.S.; Nagao, K.; Kim, K.H. Hydrochemical and isotopic characteristics, and origin of noble gas for low-temperature hot spring waters in the Honam area. Econ. Envion. Geol. 2007, 40, 635–649. [Google Scholar]
- Jeong, C.H.; Nagao, K.; Kim, K.H.; Choi, H.K.; Sumino, H.; Park, J.; Park, C.H.; Lee, J.I.; Hur, S.D. Hydrochemistry and noble gas origin of various hot spring waters from the eastern area in South Korea. J. Soil Groundw. Environ. 2008, 13, 1–12. [Google Scholar]
- Jeong, C.H.; Koh, Y.K.; Shin, S.H.; Nagao, K.; Kim, K.H.; Kim, G.Y. Hydrochemistry and noble gas origin of hot spring waters of Icheon and Pocheon area in Korea. J. Eng. Geol. 2009, 19, 529–541. [Google Scholar]
- Park, J.; Jeong, C.H.; Nagao, K.; Yang, J.H.; Sumino, H.; Kim, K.H.; Kim, M.S.; Lee, J.I.; Park, C.H.; Koh, Y.K.; et al. Hydrochemistry and noble gas geochemistry of geothermal water in Chungcheong province, South Korea. Geochem. J. 2016, 50, 89–103. [Google Scholar] [CrossRef]
- Jeong, C.H.; Yoo, S.W.; Kim, K.H.; Nagao, K. Hydrochemistry and origin of noble gases and CO2 Gas within carbonated mineral waters in the Kyeongbuk-Kangwon province, Korea. J. Eng. Geol. 2011, 21, 65–77. [Google Scholar] [CrossRef]
- Jeong, C.H.; Lee, Y.C.; Lee, Y.J.; Choi, H.Y.; Koh, G.W.; Moon, D.C.; Jung, C.Y.; Jo, S.B. Origin and hydrochemical characteristics of natural carbonated water at Seoqwipo, Jeju, Island. J. Eng. Geol. 2016, 26, 515–529. [Google Scholar] [CrossRef]
- Fournier, R.O.; Truesdell, A.H. An empirical Na-K-Ca geothermometer for natural waters. Geochim. Cosmochim. Acta 1973, 37, 1255–1275. [Google Scholar] [CrossRef]
- Truesdell, A.H. Summary of section III: Geochemical techniques in exploration. In Proceedings of the Second United Nations Symposium on the Development and Use of Geothermal Resources, San Francisco, CA, USA, 20–29 May 1975. [Google Scholar]
- Truesdell, A.H.; Fournier, R.O. Procedure for estimating the temperature of a hot-water component in a mixed water by using a plot of dissolved silica versus enthalpy. J. Res. Geol. Surv. 1977, 5, 49–52. [Google Scholar]
- Tonani, F.B. Some remarks on the application of geothermal techniques in geothermal exploration. In Advances in European Geothermal Research; Strub, A.S., Ungemach, P., Eds.; Springer: Dordrecht, The Netherlands, 1980; pp. 428–443. [Google Scholar]
- Fournier, R.O. Application of Water Geochemistry to Geothermal Exploration and Reservoir Engineering. In Geothermal System: Principles and Case Histories; John Willey & Sons: Now York, NY, USA, 1981. [Google Scholar]
- Arnórsson, S. Chemical equilibria in icelandic geothermal systems-implications for chemical geothermometry investigations. Geothermics 1983, 12, 119–128. [Google Scholar] [CrossRef]
- Nieva, D.; Nieva, R. Developments in geothermal energy in Mexico-Part 12. A cationic composition geothermometer for prospection of geothermal resources. Heat Recovery CHP 1987, 7, 243–258. [Google Scholar] [CrossRef]
- Giggenbach, W.F. Geothermal solute equilibria. Derivation of Na-K-Mg-Ca geoindicators. Geochim. Cosmochim. Acta 1988, 52, 2749–2765. [Google Scholar] [CrossRef]
- Kim, N.J.; Lee, H.K. The Geological Map of Yongsan Sheet (1:50,000); Korea Institute of Geoscience and Mineral Resources: Daejeon, Korea, 1964; pp. 1–31. [Google Scholar]
- Paik, I.S.; Kim, H.J.; Lee, J.D.; Kim, I.S.; Kim, J.S.; Moon, B.C. Comparative sedimentology for the lacustrine deposits of the upper Gyeonsang supergroup in the Southeastern Gyeongsang basin Korea. J. Korean Earth Sci. Soc. 2000, 21, 423–436. [Google Scholar]
- Koh, Y.K.; Yun, S.T.; Lim, C.S.; Bae, D.S.; Park, S.S. Geochemical evolution and deep environment of the geothermal waters in the Bogok area: Reconsideration on the origin of sulfate-type geothermal water. Econ. Environ. Geol. 2001, 37, 328–343. [Google Scholar]
- Kim, S.G. Investigation of geothermal sites in Korea. Eco. Environ. Geol. 1985, 18, 167–175. [Google Scholar]
- Aka, F.T.; Kusakabe, M.; Nagao, K.; Tanyileke, G. Noble gas isotopic compositions and water/gas chemistry of soda springs from the islands of Bioko, Saão Tomé and Annobon, along with Cameroon Volcanic Line, West Africa. Appl. Geochem. 2001, 16, 323–338. [Google Scholar] [CrossRef]
- Fournier, R.O.; White, D.E.; Truesdell, A.H. Geochemical Indicators of Subsurface Temperature-Part 1, Basic Assumptions; USGS: Reston, VA, USA, 1974.
- Parkjurst, D.L.; Appelo, C.A.J. Description of Input and Examples for PHREEQC Version 3—A Computer Program for Speciation, Batch-Reaction, One-Dimensional Transport, and Inverse Geochemical Calculations; USGS: Reston, VA, USA, 2013.
- Ongay, E. Hydrogeological and Hydrochemical Investigation of Balikesir-Pamukcu Geothermal Area and Its Vicinity. Master’s Thesis, Dokuz Eylul University, Izmir, Turkey, 2004. [Google Scholar]
- Yuce, G.; Taskiran, L. Isotope and Chemical Composition of Thermal Fluids at Tekman geothermal area (Eastern Turkey). Geochem. J. 2013, 47, 423–435. [Google Scholar] [CrossRef]
- Jeong, C.H.; Kim, C.S.; Kim, T.K.; Kim, S.J. Reaction path modeling on geochemical evolution of groundwater and formation of secondary minerals in water-gneiss reaction system. J. Mineral. Soc. Korea 1997, 10, 33–44. [Google Scholar]
- Craig, H. Isotopic variations in meteoric water. Science 1961, 133, 1702–1703. [Google Scholar] [CrossRef] [PubMed]
- Koh, Y.K.; Bae, D.S.; Kim, C.S.; Kim, K.S.; Chung, H.J.; Kim, S.Y. Consideration of the groundwater recharge based on environmental isotopic characteristics of the small basin in the Yeosu area. J. Soil Groundw. Environ. 2001, 6, 93–106. [Google Scholar]
- Geyh, M. Groundwater: Saturated and unsaturated zone. Environmental Isotopes in the Hydrological Cycle Principles and Applications, Technical Document in Hydrology; UNESCO: Paris, France, 2000; Volume 4, p. 196. [Google Scholar]
- D’amore, F.; Panichi, C. Geochemistry in geothermal exploration. Energy Res. 1985, 9, 277–298. [Google Scholar] [CrossRef]
- Yoo, B.C.; You, B.W. Geopung copper deposit in Ogcheon, Chungcheongbuk-do: Mineralogy, fluid inclusion and stable isotope studies. Eco.Environ. Geol. 2011, 44, 193–201. [Google Scholar] [CrossRef]
- Allegre, C.J. Isotope Geololgy; Cambridge University Press: Cambridge, UK, 2008; p. 534. [Google Scholar]
- Yoo, B.C. Genesis of the Ogcheon gold-silver deposite in Republic of Korea: Ore minerals, fluid inclusion and stable isotope studies. Eco. Environ. Geol. 2013, 46, 153–163. [Google Scholar] [CrossRef]
- Yu, H.M.; Shin, D.B. Mineralization and genetic environments of the central and main orebodies in the Manjang deposit, Goesan. Mineral. Soc. Korea 2018, 31, 87–101. [Google Scholar] [CrossRef]
- Lim, J.U.; Chung, S.Y. Geothermal Resources Survey in the Bugok Area (Changnyeong-Masan Area); Korea Institute of Geoscience and Mineral Resources: Daejeon, Korea, 1980. [Google Scholar]
- Sasaki, A. Variation in sulfur isotope composition of oceanic sulfate. Int. Geol. Congr. Sect. 1971, 1, 342–345. [Google Scholar]
- Zak, I.; Sakai, H.; Kaplan, R. Factors controlling the 18O/16O and 34S/32S isotopic ratios of ocean sulfates and interstitial sulfates from modern deep sea sediments. In Isotope Marine Chemistry; Goldberg, E.D., Horibe, Y., Saruhaki, K., Eds.; Geochemical Research Association: Tokyo, Japan, 1980; pp. 339–373. [Google Scholar]
- Berner, R.A. Sulphate Reduction, Organic Matter Decomposition and Pyrite Formation. R. Soc. 1985, 315, 25–38. [Google Scholar]
- Ohmoto, H.; Lasaga, A.C. Kinetics of Reactions between aqueous Sulfates and Sulfides in Hydrothermal System. Geochim. Cosmochim. Acta 1982, 46, 1727–1745. [Google Scholar] [CrossRef]
- Li, Z.; Li, J.; Cui, H.; Wang, D.; Gong, S.; Li, J.; Zhang, Y.; Ma, C.; Wang, Y. Geochemistry Characteristics of Hydrogen Sulphide-Bearing Gas Pools in Sichuan Basin. Energy Explor. Exploit. 2014, 32, 691–708. [Google Scholar] [CrossRef]
- Robert, R.S. Sulfur Isotope Geochemistry of Sulfide Minerals; USGS: Reston, VA, USA, 2006.
- Mamyrin, B.A.; Tolstikhin, I.N. Helium Isotopes in Nature, 1st ed.; Elsevier: Amsterdam, The Netherlands, 1984; p. 274. [Google Scholar]
- Ozima, M.; Podosek, F.A. Noble Gases in the Earth. In Noble Gas Geochemistry, 2nd ed.; Cambridge University Press: Cambridge, UK, 2002; pp. 217–249. [Google Scholar]
- Polyak, B.G.; Tolstikhin, I.N. Isotopic composition of the Earth’s helium and the motive forces of tectogenesis. Chem. Geol. 1985, 52, 9–33. [Google Scholar] [CrossRef]
- Italiano, F.G.; Martinelli, G.; Nuccio, P.M. Anomalies of mantle-derived helium during the 1997-1998 seismic swarm of Umbria-Marche, Italy. Geophys. Res. Lett. 2001, 28, 839–842. [Google Scholar] [CrossRef]
- Klemperer, S.L.; Mack Kennedy, B.; Satry, S.R.; Makovsky, Y.; Harinarayana, T.; Leech, M.L. Mantle fluids in the Karakoram fault: Helium isotope evidence. Earth Planet. Sci. Lett. 2013, 366, 59–70. [Google Scholar] [CrossRef]
- Bräuer, K.; Geissler, W.H.; Kämpf, H.; Niedermannn, S.; Rman, N. Helium and carbon isotope signatures of gas exhalations in the westernmost part of the Pannonian Basin (SE Austria/Ne Slovenia): Evidence for active lithospheric mantle degassing. Chem. Geol. 2016, 422, 60–70. [Google Scholar] [CrossRef]
- Jeong, C.H.; Park, J.; Nagao, K.; Sumino, H.; Kim, K.H.; Hur, S.D.; Lee, J.I.; Koh, Y.K.; Park, C.H. Hydrochemistry and origin of noble gases of hot spring water in Korea: Daejeon-Chungcheong area. In Proceedings of the Annual Meeting(Autumn Season) of KoSSGE, Jeonju University, Jeonju, Korea, 9–10 September 2004. [Google Scholar]
- Jeong, C.H.; Nagao, K.; Kim, K.H.; Sumino, H.; Park, J.S.; Lee, J.I.; Hur, S.D.; Ahn, S.W.; Choi, H.K.; Hur, H.S.; et al. Geochemical evolution, heat source and noble gas of hot spring, geothermal water and geological environment. In Proceedings of the 22th Joint Symposium of KSEEG and KSG, Daejeon University, Daejeon, Korea, 30 May 2006. [Google Scholar]
- Park, S.S. Hydrogeochemical Studies on the Origin and Geochemical Environments of Thermal Groundwaters in Bugok and Magumsan Area, Southern Korea. Master’s Thesis, Korea University, Seoul, South Korea, 2005. [Google Scholar]
- Kotarba, M.J.; Nagao, K. Composition and origin of natural gases accumulated in the Polish and Ukrainian parts of the Carpathian region: Gaseous hydrocarbons, noble gases, carbon dioxide and nitrogen. Chem. Geol. 2008, 255, 426–438. [Google Scholar] [CrossRef]
- Li, N.; Zhou, H.; Wen, Z.; Jakada, H. Formation mechanism and mixing behavior of Nanyang thermal spring, Xingshan County of Hubei Province, central China. Hydrogeol. J. 2019, 27, 2933–2953. [Google Scholar] [CrossRef]
- Han, S.J. A Study on the Hydrogeochemical Characteristics of Thermal and Groundwaters, in Dongrea-gu, Pusan. Master’s Thesis, Pusan National University, Pusan, Korea, 1999. [Google Scholar]
- Lajwe, G. Comparison, Characterization, and Interpretation of Geothermal Fluid Geochemistry in the Sedimentary Environments of Kibiro, Panyimur, and Öxarfjördur. In Proceedings of the Geothermal Training Programme, Tokyo, Japan; 2013. [Google Scholar]
- Wang, J.; Jin, M.; Jia, B.; Kang, F. Hydrochemical characteristics and geothermometry applications of thermal groundwater in northern Jinan, Shandong, China. Geothermics 2015, 57, 185–195. [Google Scholar] [CrossRef]
- Reed, M.; Spycher, N. Calculation of pH and Mineral Equilibria in Hydrothermal Waters with Application to Geothermometry and Studies of Boiling and Dilution. Geochim. Cosmochim. Acta 1984, 48, 1479–1492. [Google Scholar] [CrossRef]
- Pang, Z.H.; Reed, M. Theoretical Chemical Thermometry on Geothermal Waters: Problems and Methods. Geochim. Cosmochim. Acta 1998, 62, 1083–1091. [Google Scholar] [CrossRef]










| Sample ID | Temp. (°C) | pH | Eh (mV) | EC (μS/cm) | DO | Na+ | K+ | Ca2+ | Mg2+ | Sr2+ | Fe |
| BT-1 | 75.6 | 7.81 | −250 | 568 | 1.50 | 93.2 | 3.58 | 12.8 | 0.12 | 0.49 | 0.01 |
| BT-2 | 74.0 | 7.97 | −290 | 535 | 0.00 | 88.7 | 3.43 | 7.69 | 0.08 | 0.33 | 0.02 |
| BT-3 | 63.4 | 7.90 | −240 | 590 | 1.10 | 96.7 | 3.81 | 13.3 | 0.38 | 0.49 | 0.03 |
| BT-4 | 58.8 | 8.49 | −440 | 465 | 0.50 | 78.7 | 2.92 | 4.09 | 0.02 | 0.15 | 0.01 |
| BT-5 | 25.7 | 7.71 | −28 | 475 | 0.70 | 74.2 | 2.13 | 19.7 | 1.93 | 0.69 | 0.02 |
| BT-6 | 52.6 | 8.50 | −65 | 378 | 3.40 | 66.7 | 2.00 | 3.38 | 0.03 | 0.12 | 0.02 |
| BT-7 | 78.7 | 8.37 | −450 | 506 | 2.60 | 83.7 | 2.94 | 4.13 | 0.01 | 0.16 | 0.01 |
| BT-9 | 78.7 | 8.41 | −490 | 494 | 0.30 | 89.5 | 3.04 | 5.14 | 0.03 | 0.19 | 0.01 |
| BT-10 | 76.3 | 8.67 | −390 | 506 | 2.10 | 91.1 | 3.02 | 4.49 | 0.06 | 0.18 | <0.01 |
| BT-11 | 26.7 | 8.71 | −130 | 392 | 3.00 | 65.2 | 0.81 | 9.74 | 1.07 | 0.16 | 0.01 |
| BT-12 | 65.8 | 7.81 | −170 | 495 | 0.40 | 75.6 | 2.69 | 21.2 | 0.22 | 0.31 | 0.01 |
| Sample ID | Mn | SiO2 | HCO3− | F− | Cl− | SO42− | *E (%) | δ18O (‰) | δ2H (‰) | δ34S (‰) | Group |
| BT-1 | 1.98 | 55.4 | 148 | 0.73 | 7.65 | 110 | −1.77 | −8.70 | −59.0 | 7.60 | Group Ⅰ |
| BT-2 | 1.87 | 53.5 | 115 | 0.68 | 7.36 | 85.3 | 5.25 | −8.90 | −59.0 | 9.10 | Group Ⅰ |
| BT-3 | 3.80 | 47.9 | 209 | 0.51 | 5.91 | 72.6 | −1.18 | −8.60 | −57.0 | 4.60 | Group Ⅱ |
| BT-4 | 0.24 | 61.4 | 125 | 0.93 | 4.20 | 81.9 | −2.86 | −9.10 | −61.0 | 14.0 | Group Ⅱ |
| BT-5 | 2.59 | 21.2 | 192 | 0.36 | 11.0 | 50.3 | −1.03 | −7.50 | −51.0 | −3.00 | Group Ⅲ |
| BT-6 | 0.60 | 46.0 | 129 | 0.49 | 8.40 | 51.2 | −4.87 | −8.60 | −57.0 | 4.60 | Group Ⅱ |
| BT-7 | 0.26 | 63.7 | 118 | 1.26 | 4.64 | 79.2 | −1.60 | −9.00 | −61.0 | 15.0 | Group Ⅰ |
| BT-9 | 0.21 | 62.9 | 118 | 0.95 | 4.86 | 93.2 | 2.18 | −9.00 | −61.0 | 13.0 | Group Ⅰ |
| BT-10 | 0.23 | 70.6 | 121 | 0.99 | 5.68 | 93.1 | 2.55 | −9.10 | −61.0 | 13.0 | Group Ⅰ |
| BT-11 | 1.89 | 19.0 | 122 | 0.59 | 15.6 | 36.4 | 3.07 | −7.40 | −51.0 | 1.00 | Group Ⅲ |
| BT-12 | 1.73 | 49.8 | 176 | 0.57 | 11.9 | 78.5 | −2.90 | −8.50 | −58.0 | 9.90 | Group Ⅱ |
| Sample ID | Temp. (°C) | pH | Eh (mV) | EC (μS/cm) | DO | Na+ | K+ | Ca2+ | Mg2+ | Sr2+ |
| BG-1 | 23.3 | 7.53 | 220 | 359 | 4.70 | 10.7 | 1.00 | 50.5 | 14.2 | 0.69 |
| BG-2 | 22.7 | 7.13 | 240 | 53.0 | 7.40 | 3.08 | 0.69 | 6.37 | 0.80 | 0.03 |
| BG-3 | 22.8 | 8.10 | 160 | 267 | 7.60 | 9.54 | 3.38 | 38.8 | 4.92 | 0.21 |
| BG-4 | 23.4 | 7.70 | 180 | 331 | 4.80 | 14.4 | 0.88 | 44.7 | 9.77 | 0.57 |
| BG-5 | 18.1 | 7.22 | 160 | 365 | 3.10 | 17.3 | 4.29 | 45.2 | 11.1 | 0.39 |
| BG-6 | 18.2 | 7.47 | 3 | 406 | 7.70 | 19.7 | 1.93 | 50.7 | 7.45 | 0.39 |
| BS-1 | 20.2 | 8.13 | 240 | 425 | 7.60 | 11.1 | 1.09 | 67.3 | 15.1 | 0.56 |
| BS-2 | 20.1 | 7.92 | 240 | 372 | 6.00 | 8.84 | 1.24 | 62.1 | 12.7 | 0.44 |
| BS-3 | 23.3 | 6.72 | 250 | 296 | 6.30 | 7.12 | 1.27 | 41.3 | 9.28 | 0.29 |
| BS-4 | 25.3 | 7.54 | 140 | 425 | 3.70 | 10.8 | 1.54 | 53.3 | 5.06 | 0.38 |
| BS-5 | 21.3 | 7.61 | 210 | 235 | 6.00 | 8.16 | 0.73 | 32.4 | 8.00 | 0.24 |
| BS-6 | 20.9 | 7.86 | 230 | 101 | 6.80 | 5.96 | 0.84 | 10.3 | 2.81 | 32.0 |
| Sample ID | Fe | Mn | SiO2 | HCO3− | F− | Cl− | SO42− | *E (%) | δ18O (‰) | δ2H (‰) |
| BG-1 | <0.01 | <0.01 | 17.7 | 253 | N.D | 1.98 | 4.15 | −1.57 | −8.20 | −57.0 |
| BG-2 | 0.01 | 0.01 | 8.90 | 27.5 | 0.04 | 1.93 | 1.60 | −2.79 | −7.80 | −53.0 |
| BG-3 | 0.00 | <0.01 | 17.6 | 153 | N.D | 5.38 | 4.15 | −0.86 | −7.80 | −55.0 |
| BG-4 | 0.00 | <0.01 | 22.7 | 190 | N.D | 6.04 | 15.5 | 0.03 | −7.80 | −56.0 |
| BG-5 | <0.01 | <0.01 | 18.6 | 250 | N.D | 9.29 | 3.42 | −6.71 | −8.20 | −56.0 |
| BG-6 | 3.02 | <0.01 | 19.7 | 165 | 0.19 | 11.8 | 20.9 | 6.25 | −7.30 | −50.0 |
| BS-1 | <0.01 | <0.01 | 14.1 | 229 | N.D | 1.64 | 38.2 | 5.39 | −7.70 | −54.0 |
| BS-2 | 0.01 | 0.01 | 15.6 | 302 | N.D | 2.73 | 3.75 | −6.08 | −7.70 | −53.0 |
| BS-3 | 0.21 | 0.21 | 12.2 | 159 | N.D | 6.72 | 37.5 | −6.02 | - | - |
| BS-4 | 0.12 | 0.12 | 16.9 | 169 | 0.13 | 4.11 | 16.0 | 4.13 | −7.80 | −52.0 |
| BS-5 | 0.03 | 0.03 | 14.4 | 129 | 0.04 | 1.70 | 10.0 | 4.85 | −7.90 | −53.0 |
| BS-6 | 0.07 | 0.07 | 14.4 | 54.9 | N.D | 2.39 | 4.75 | −3.61 | - | - |
| Sample ID. | 4He | 3He | ||||
|---|---|---|---|---|---|---|
| Air % | Mantle % | Crust % | Air % | Mantle % | Crust % | |
| BT-1 | 0.90 | 2.90 | 96.2 | 3.50 | 95.2 | 1.30 |
| BT-2 | 1.07 | 2.50 | 96.4 | 4.60 | 93.9 | 1.50 |
| BT-3 | 1.34 | 2.60 | 96.0 | 5.50 | 93.1 | 1.40 |
| BT-4 | 0.57 | 2.70 | 96.7 | 2.40 | 96.2 | 1.40 |
| BT-5 | 21.0 | 0.40 | 78.6 | 84.3 | 14.6 | 1.10 |
| BT-7 | 0.70 | 2.80 | 96.5 | 2.90 | 95.7 | 1.40 |
| BT-9 | 0.49 | 2.70 | 96.8 | 2.00 | 96.6 | 1.40 |
| BT-11 | 8.54 | 2.20 | 89.5 | 33.3 | 65.4 | 1.20 |
| BT-12 | 0.70 | 2.80 | 96.5 | 2.70 | 96.0 | 1.40 |
| Sample ID. | Discharge Temp. (°C) | Na−K−Mg Reservoir (°C) | Water−Mineral Equilibria Model (°C) | |||||
|---|---|---|---|---|---|---|---|---|
| Na−K (1) | K−Mg (2) | Calcedony | Quartz | Ca−Montmorillonite | K−Feldspar | Muscovite | ||
| BT-1 | 75.6 | 166 | 95 | (74) | 105 | (70) | (58.0) | 108 |
| BT-2 | 74.0 | 166 | 100 | (73) | 101 | (67) | (57) | 105 |
| BT-4 | 58.8 | 164 | 115 | 74 | 101 | (58) | (57) | 94 |
| BT-6 | 52.6 | 152 | 98 | 62 | 90 | 54 | (49) | 90 |
| BT-7 | 78.7 | 161 | 126 | (77) | 104 | (61) | (58) | 97 |
| BT-9 | 78.7 | 159 | 110 | (76) | 103 | (60) | (59) | 96 |
| BT-10 | 76.3 | 157 | 100 | 77 | 103 | (56) | (54) | 91 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, C.; Lee, Y.; Lee, Y.; Ahn, S.; Nagao, K. Geochemical Composition, Source and Geothermometry of Thermal Water in the Bugok Area, South Korea. Water 2022, 14, 3008. https://doi.org/10.3390/w14193008
Jeong C, Lee Y, Lee Y, Ahn S, Nagao K. Geochemical Composition, Source and Geothermometry of Thermal Water in the Bugok Area, South Korea. Water. 2022; 14(19):3008. https://doi.org/10.3390/w14193008
Chicago/Turabian StyleJeong, Chanho, Yujin Lee, Yongcheon Lee, Sangwon Ahn, and Keisuke Nagao. 2022. "Geochemical Composition, Source and Geothermometry of Thermal Water in the Bugok Area, South Korea" Water 14, no. 19: 3008. https://doi.org/10.3390/w14193008
APA StyleJeong, C., Lee, Y., Lee, Y., Ahn, S., & Nagao, K. (2022). Geochemical Composition, Source and Geothermometry of Thermal Water in the Bugok Area, South Korea. Water, 14(19), 3008. https://doi.org/10.3390/w14193008






