What Inspiring Elements from Natural Services of Water Quality Regulation Could Be Applied to Water Management?
Abstract
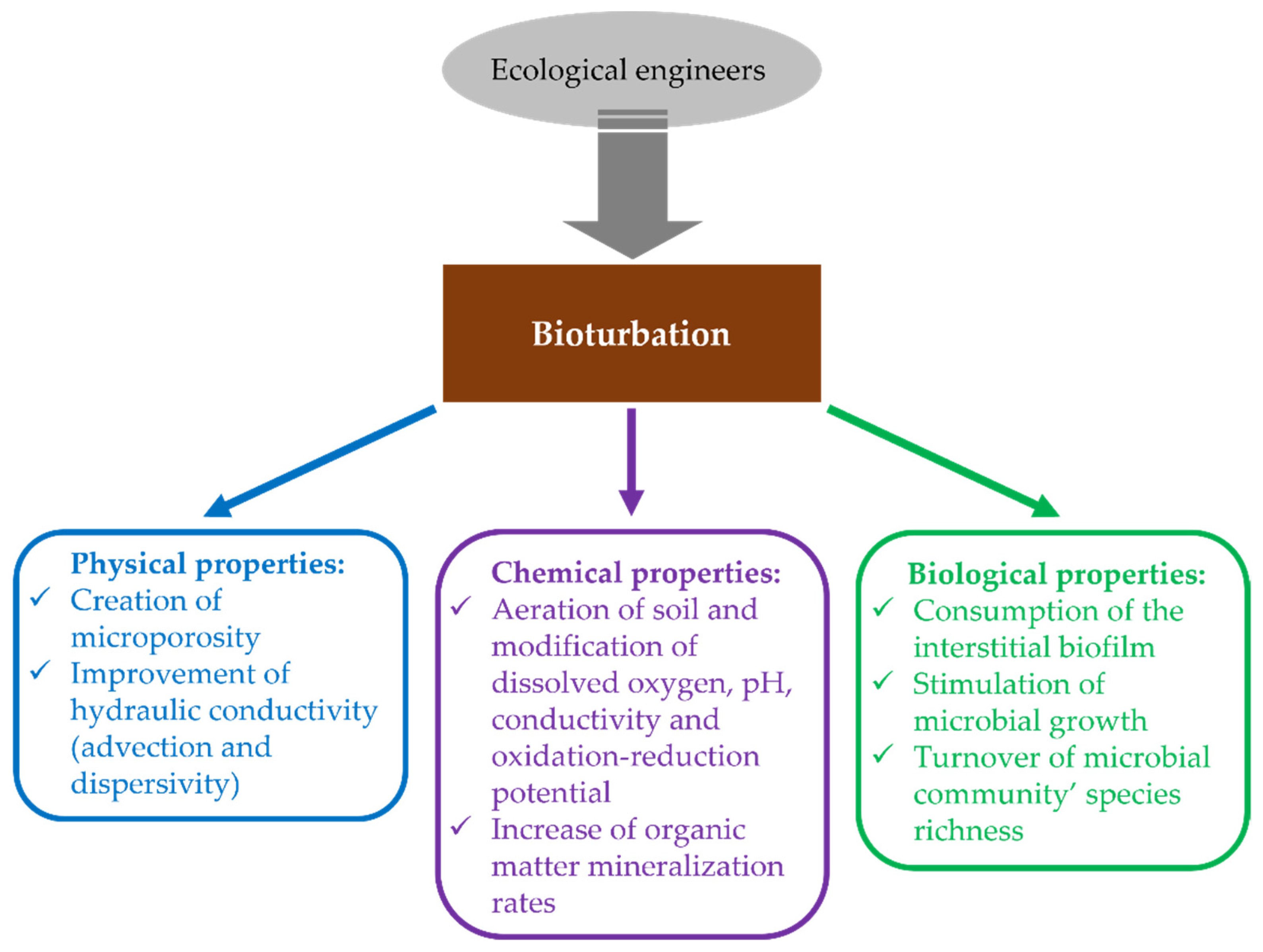
:1. Introduction
2. Biodiversity Influence on Physical Properties of the Environment
2.1. The Mix of Sediments and Soils by Bioturbation and Pollution Pumping
2.2. The Influence of Biogenic Structures on the Porosity of the Substrate
2.3. The Influence of Biofilm Consumption on Sediment Permeability
3. Biodiversity Influence on Chemical Properties of the Environment
4. Bioturbation Influence on Organic Matter Degradation and Oxygen Saving
5. Biodiversity Influence on Biological Properties of the Environment
6. Biodiversity Influence on Sewage Quantity Reduction
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lecerf, A.; Usseglio-Polatera, P.; Charcosset, J.Y.; Lambrigot, D.; Bracht, B.; Chauvet, E. Assessment of functional integrity of eutrophic streams using litter breakdown and benthic macroinvertebrates. Arch. Hydrobiol. 2006, 165, 105–126. [Google Scholar] [CrossRef]
- Naeem, S. Advancing realism in biodiversity research. Trends. Ecol. Evol. 2008, 23, 414–416. [Google Scholar] [CrossRef]
- Martín-López, B.; Church, A.; Başak Dessane, E.; Berry, P.; Chenu, C.; Christie, M.; Gerino, M.; Keune, H.; Oteros-Rozas, E.; Paillard, S.; et al. Chapter 2: Nature’s contributions to people and quality of life. In The IPBES Regional Assessment Report on Biodiversity and Ecosystem Services for Europe and Central Asia; Rounsevell, M., Fischer, M., Torre-Marin Rando, A., Mader, A., Eds.; Secretariat of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services: Bonn, Germany, 2018; Chapter 2; pp. 57–185. [Google Scholar] [CrossRef]
- Kukwa, D.T.; Afolabi, F.O.; Tetteh, E.K.; Anekwe, I.M.S.; Chetty, M. Bioremediation of hazardous wastes. In Hazardous Waste Management; Jeyakumar, R.B., Sankarapandian, K., Ravi, Y.K., Eds.; IntechOpen: London, UK, 2022; Chapter 2; pp. 1–23. [Google Scholar] [CrossRef]
- Bengtsson, J. Which species? What kind of diversity? Which ecosystem function? Some problems in studies of relations between biodiversity and ecosystem function. Appl. Soil Ecol. 1998, 10, 191–199. [Google Scholar] [CrossRef]
- Mathieu, J.; Grimaldi, M.; Jouquet, P.; Rouland, C.; Lavelle, P.; Desjardins, T.; Rossi, J.-P. Spatial patterns of grasses influence soil macrofauna biodiversity in Amazonian pastures. Soil Biol. Biochem. 2009, 41, 586–593. [Google Scholar] [CrossRef]
- Cardinale, B.; Duffy, J.; Gonzalez, A. Biodiversity loss and its impact on humanity. Nature 2012, 486, 59–67. [Google Scholar] [CrossRef]
- Jouquet, P.; Janeau, J.-L.; Pisano, A.; Tran Sy, H.; Orange, D.; Luu, T.N.M.; Valentin, C. Influence of earthworms and termites on runoff and erosion in a tropical steep slope fallow in Vietnam: A rainfall simulation experiment. Appl. Soil Ecol. 2012, 61, 161–168. [Google Scholar] [CrossRef]
- Mace, G.M.; Norris, K.; Fitter, A.H. Biodiversity and ecosystem services: A multilayered relationship. Trends Ecol. Evol. 2012, 27, 19–26. [Google Scholar] [CrossRef]
- Maes, J.; Egoh, B.; Willemen, L.; Liquete, C.; Vihervaara, P.; Schägner, J.P.; Grizzetti, B.; Drakou, E.G.; La Notte, A.; Zulian, G.; et al. Mapping ecosystem services for policy support and decision making in the European Union. Ecosyst. Serv. 2012, 1, 31–39. [Google Scholar] [CrossRef]
- Quijas, S.; Jackson, L.E.; Maass, M.; Schmid, B.; Raffaelli, D.; Balvanera, P. Plant diversity and generation of ecosystem services at the landscape scale: Expert knowledge assessment. J. Appl. Ecol. 2012, 49, 929–940. [Google Scholar] [CrossRef]
- Santos-Martín, F.; Martín-López, B.; García-Llorente, M.; Aguado, M.; Benayas, J.; Montes, C. Unraveling the relationships between ecosystems and human wellbeing in Spain. PLoS ONE 2013, 8, e73249. [Google Scholar] [CrossRef]
- Harrison, P.A.; Berry, P.M.; Simpson, G.; Haslett, J.R.; Blicharska, M.; Bucur, M.; Dunford, R.; Egoh, B.; Garcia-Llorente, M.; Geamănă, N.; et al. Linkages between biodiversity attributes and ecosystem services: A systematic review. Ecosyst. Serv. 2014, 9, 191–203. [Google Scholar] [CrossRef]
- Bennett, E.M.; Cramer, W.; Begossi, A.; Cundill, G.; Díaz, S.; Egoh, B.N.; Geijzendorffer, I.R.; Krug, C.B.; Lavorel, S.; Lazos, E.; et al. Linking biodiversity, ecosystem services, and human well-being: Three challenges for designing research for sustainability. Curr. Opin. Environ. Sustain. 2015, 14, 76–85. [Google Scholar] [CrossRef]
- Díaz, S.; Settele, J.; Brondízio, E.S.; Ngo, H.T.; Guèze, M.; Agard, J.; Arneth, A.; Balvanera, P.; Brauman, K.A.; Butchart, S.H.M.; et al. Summary for Policymakers of the Global Assessment Report on Biodiversity and Ecosystem Services; IPBES secretariat: Bonn, Germany, 2019. [Google Scholar] [CrossRef]
- Díaz, S.; Demissew, S.; Joly, C.; Lonsdale, W.M.; Larigauderie, A. A Rosetta stone for nature’s benefits to people. PLoS Biol. 2015, 13, e1002040. [Google Scholar] [CrossRef] [PubMed]
- Blackwell, M.S.A.; Pilgrim, E.S. Ecosystem services delivered by small-scale wetlands. Hydrol. Sci. J. 2011, 56, 1467–1484. [Google Scholar] [CrossRef]
- Grizzetti, B.; Passy, P.; Billen, G.; Bouraoui, F.; Garnier, J.; Lassaletta, L. The role of water nitrogen retention in integrated nutrient management: Assessment in a large basin using different modelling approaches. Environ. Res. Lett. 2015, 10, 065008. [Google Scholar] [CrossRef]
- Geijzendorffer, I.R.; Galewski, T.; Guelmami, A.; Perennou, C.; Popoff, N.; Grillas, P. Mediterranean wetlands: A gradient from natural resilience to a fragile social-ecosystem. In Atlas of Ecosystem Services; Schröter, M., Bonn, A., Klotz, S., Seppelt, R., Baessler, C., Eds.; Springer: Cham, Switzerland, 2019; Chapter 57; pp. 83–89. [Google Scholar] [CrossRef]
- Cakir, R.; Raimonet, M.; Sauvage, S.; Walcker, R.; Gerino, M.; Sánchez-Pérez, J.M. Assessment of water quality regulation functions in Southwestern Europe watersheds. Water 2021, 13, 2980. [Google Scholar] [CrossRef]
- Cakir, R.; Sauvage, S.; Gerino, M.; Volk, M.; Sánchez-Pérez, J.M. Assessment of ecological function indicators related to nitrate under multiple human stressors in a large watershed. Ecol. Indic. 2020, 111, 106016. [Google Scholar] [CrossRef]
- Tapia, J.; Bielsa, J.; Martínez, Y.; Sauvage, S.; Cakir, R.; Raimonet, M.; Gerino, M.; Sánchez-Pérez, J.M. Economic valuation of the natural service of nitrate regulation provided by rivers including dilution effects: Application to a semiarid region, the Ebro basin (Spain). Ecol. Indic. 2020, 117, 106608. [Google Scholar] [CrossRef]
- Boulton, A.J.; Findlay, S.; Marmonier, P.; Stanley, E.H.; Valett, H.M. The functional significance of the hyporheic zone in streams and rivers. Ann. Rev. Ecol. Syst. 1998, 29, 59–81. [Google Scholar] [CrossRef]
- Datry, T.; Larned, S.T. River flow controls ecological processes and invertebrate assemblages in subsurface flowpaths of an ephemeral river reach. Can. J. Fish. Aquat. Sci. 2008, 65, 1532–1544. [Google Scholar] [CrossRef]
- Prior, H.; Johnes, P.J. Regulation of surface water quality in a Cretaceous Chalk catchment, UK: An assessment of the relative importance of instream and wetland processes. Sci. Total Environ. 2002, 282–283, 159–174. [Google Scholar] [CrossRef]
- Pinay, G.; Décamps, H.; Chauvet, E.; Fustec, E. Functions of ecotones in fluvial systems. In The Ecology and Management of Aquatic-Terrestrial Ecotones, Man and the Biosphere Series; Naiman, R.J., Décamps, H., Eds.; Parthenon Press Publ.: Carnforth, UK, 1990; Chapter 8; Volume 4, pp. 141–169. [Google Scholar]
- Sauvage, S.; Sánchez-Pérez, J.M.; Vervier, P.; Naiman, R.-J.; Alexandre, H.; Bernard-Jannin, L.; Boulêtreau, S.; Delmotte, S.; Julien, F.; Peyrard, D.; et al. Modelling the role of riverbed compartments in the regulation of water quality as an ecological service. Ecol. Eng. 2018, 118, 19–30. [Google Scholar] [CrossRef]
- Balvanera, P.; Pfisterer, A.B.; Buchmann, N.; He, J.S.; Nakashizuka, T.; Raffaeli, D.; Schmid, B. Quantifying the evidence for biodiversity effects on ecosystem functioning and services. Ecol. Lett. 2006, 9, 1146–1156. [Google Scholar] [CrossRef]
- Jouquet, P.; Bernard-Reversat, F.; Bottinelli, N.; Orange, D.; Rouland-Lefèvre, C.; Tran Duc, T.; Podwojewski, P. Influence of changes in land use and earthworm activities on carbon and nitrogen dynamics in a steepland ecosystem in Northern Vietnam. Biol. Fertil. Soils 2007, 44, 69–77. [Google Scholar] [CrossRef]
- Yao, J.; Colas, F.; Solimini, A.G.; Battin, T.J.; Gafny, S.; Morais, M.; Puig, M.A.; Martí, E.; Pusch, M.T.; Voreadou, C.; et al. Macroinvertebrate community traits and nitrate removal in stream sediments. Freshw. Biol. 2017, 62, 929–944. [Google Scholar] [CrossRef]
- Yao, J.; Sánchez-Pérez, J.M.; Sauvage, S.; Teissier, S.; Attard, E.; Lauga, B.; Duran, R.; Julien, F.; Bernard-Jannin, L.; Ramburn, H.; et al. Biodiversity and ecosystem purification service in an alluvial wetland. Ecol. Eng. 2017, 103, 359–371. [Google Scholar] [CrossRef]
- Le, H.T.; Ho, C.T.; Trinh, Q.H.; Trinh, D.A.; Luu, M.T.N.; Tran, H.S.; Orange, D.; Janeau, J.-L.; Merroune, A.; Rochelle-Newall, E.; et al. Responses of aquatic bacteria to terrestrial runoff: Effects on community structure and key taxonomic groups. Front. Microbiol. 2016, 7, 889. [Google Scholar] [CrossRef]
- Liu, Y.; Dedieu, K.; Sánchez-Pérez, J.M.; Montuelle, B.; Buffan-Dubau, E.; Julien, F.; Azémar, F.; Sauvage, S.; Marmonier, P.; Yao, J.; et al. Role of biodiversity in the biogeochemical processes at the water-sediment interface of macroporous river bed: An experimental approach. Ecol. Eng. 2017, 103, 385–393. [Google Scholar] [CrossRef]
- Nas, B.; Ateş, H.; Dolu, T.; Yel, E.; Argun, M.E.; Koyuncu, S.; Kara, M.; Dinç, S. Evaluation of occurrence, fate and removal of priority phthalate esters (PAEs) in wastewater and sewage sludge by advanced biological treatment, waste stabilization pond and constructed wetland. Chemosphere 2022, 295, 133864. [Google Scholar] [CrossRef]
- Venditti, S.; Brunhoferova, H.; Hansen, J. Behaviour of 27 selected emerging contaminants in vertical flow constructed wetlands as post-treatment for municipal wastewater. Sci. Total Environ. 2022, 819, 153234. [Google Scholar] [CrossRef]
- Rhoads, D.C. Organism-sediment relations on the muddy sea floor. Oceanogr. Mar. Biol. Annu. Rev. 1974, 12, 263–300. [Google Scholar]
- Aller, J.Y.; Aller, R.C. Evidence for localized enhancement of biological activity associated with tube and burrow structures in deep-sea sediments at the Hebble Site, Western North Atlantic. Deep-Sea Res. Oceanogr. A 1986, 33, 755–790. [Google Scholar] [CrossRef]
- Gerino, M.; Aller, R.C.; Lee, C.; Cochran, J.K.; Aller, J.Y.; Green, M.A.; Hirschberg, D. Comparison of different tracers and methods used to quantify bioturbation during a spring bloom: 234-thorium, luminophores and chlorophylla. Estuar. Coast. Shelf Sci. 1998, 46, 531–547. [Google Scholar] [CrossRef]
- Kristensen, E.; Penha-Lopes, G.P.; Delefosse, M.; Valdemarsen, T.; Organo Quintana, C.; Banta, G.T. What is bioturbation? Need for a precise definition for fauna in aquatic science. Mar. Ecol. Prog. Ser. 2012, 446, 285–302. [Google Scholar] [CrossRef]
- Hoang, T.K.; Probst, A.; Orange, D.; Gilbert, F.; Elger, A.; Kallerhoff, J.; Laurent, F.; Bassil, S.; Duong, T.T.; Gerino, M. Bioturbation effects on bioaccumulation of cadmium in the wetland plant Typha latifolia: A nature-based experiment. Sci. Total Environ. 2018, 618, 1284–1297. [Google Scholar] [CrossRef]
- Aller, R.C.; Cochran, J.K. The critical role of bioturbation for particle dynamics, priming potential, and organic C remineralization in marine sediments: Local and basin scales. Front. Earth Sci. 2019, 7, 157. [Google Scholar] [CrossRef]
- Jones, C.G.; Lawton, J.H.; Shachak, M. Organisms as ecosystem engineers. Oikos 1994, 69, 373–386. [Google Scholar] [CrossRef]
- Adams, G.O.; Tawari-Fufeyin, P.; Okoro, S.E.; Ehinomen, I. Bioremediation, biostimulation and bioaugmentation: A review. Int. J. Environ. Bioremediation Biodegrad. 2015, 3, 28–39. [Google Scholar] [CrossRef]
- Gerino, M.; Laffont-Schwob, I. La remédiation naturelle et l’autoépuration des milieux aquatiques. In L’eau à Découvert; Euzen, A., Jeandel, C., Mosseri, R., Eds.; CNRS Éditions: Paris, France, 2015; pp. 234–235. Available online: https://books.openedition.org/editionscnrs/10014?lang=fr (accessed on 1 December 2021).
- Orange, D. Towards a sustainable green world? A better use of OM. Significances Bioeng. Biosci. Opin. Pap. 2020, 4. [Google Scholar] [CrossRef]
- Yu, Z.; Lu, C.; Cai, J.; Yu, D.; Mahe, G.; Mishra, A.; Cudennec, C.; Van Lanen, H.A.J.; Orange, D.; Amani, A. Preface: Hydrological processes and water security in a changing world. Proc. Int. Assoc. Hydrol. Sci. 2020, 383, 3–4. [Google Scholar] [CrossRef]
- Eggermont, H.; Balian, E.; Azevedo, J.M.N.; Beumer, V.; Brodin, T.; Claudet, J.; Fady, B.; Grube, M.; Keune, H.; Lamarque, P.; et al. Nature-based solutions: New influence for environmental management and research in Europe. Gaia 2015, 24, 243–248. [Google Scholar] [CrossRef]
- Clark, W.C.; Harley, A.G. Sustainability science: Toward a synthesis. Annu. Rev. Environ. Resour. 2020, 45, 331–386. [Google Scholar] [CrossRef]
- Gerino, M.; Stora, G.; François-Carcaillet, F.; Gilbert, J.-C.; Poggiale, F.; Mermillod-Blondin, F.; Desrosiers, G.; Vervier, P. Macro-invertebrate functional groups in freshwater and marine sediments: A common mechanistic classification. Vie Milieu 2003, 53, 221–231. Available online: https://hal.sorbonne-universite.fr/hal-03205285 (accessed on 1 December 2021).
- Meysman, F.J.R.; Middelburg, J.J.; Heip, C.H.R. Bioturbation: A fresh look at Darwin’s last idea. Trends Ecol. Evol. 2006, 21, 688–695. [Google Scholar] [CrossRef]
- Mermillod-Blondin, F.; Rosenberg, R. Ecosystem engineering: The impact of bioturbation on biogeochemical processes in marine and freshwater benthic habitats. Aquat. Sci. 2006, 68, 434–442. [Google Scholar] [CrossRef]
- Delmotte, S.; Gerino, M.; Thebault, J.M.; Meysman, F.J.R. Modeling effects of patchiness and biological variability on transport rates within bioturbated sediments. J. Mar. Res. 2008, 66, 191–218. [Google Scholar] [CrossRef]
- Meysman, F.J.R.; Boudreau, B.P.; Middelburg, J.J. When and why does bioturbation lead to diffusive mixing? J. Mar. Res. 2010, 68, 881–920. [Google Scholar] [CrossRef]
- Gardner, L.R.; Sharma, P.; Moore, W.S. A regeneration model for the effect of bioturbation by fiddler crabs on 210Pb profiles in salt marsh sediments. J. Environ. Radioact. 1987, 5, 25–36. [Google Scholar] [CrossRef]
- Wang, X.; Matisoff, G. Solute transport in sediments by a large freshwater oligochaete, Branchiura sowerbyi. Environ. Sci. Technol. 1997, 31, 1926–1933. [Google Scholar] [CrossRef]
- Officer, C.B.; Lynch, D.R. Bioturbation, sedimentation and sediment-water exchanges. Estuar. Coast. Shelf Sci. 1989, 28, 1–12. [Google Scholar] [CrossRef]
- Clough, L.M.; Lopez, G.R. Potential carbon sources for the head-down deposit-feeding polychaete Heteromastus Filiformis. J. Mar. Res. 1993, 51, 595–616. [Google Scholar] [CrossRef]
- Gerino, M.; Stora, G.; Durbec, J.-P. Quantitative estimation of biodiffusive and bioadvective sediment mixing: In situ experimental approach. Oceanol. Acta 1994, 17, 547–554. Available online: https://archimer.ifremer.fr/doc/00099/21010/18636.pdf (accessed on 20 December 2021).
- Matisoff, G.; Fisher, J.B.; Matis, S. Effects of benthic macroinvertebrates on the exchange of solutes between sediments and freshwater. Hydrobiologia 1985, 122, 19–33. [Google Scholar] [CrossRef]
- Rice, D.L. Early diagenesis in bioadvective sediments: Relationships between the diagenesis of beryllium-7, sediment reworking rates, and the abundance of conveyor-belt deposit-feeders. J. Mar. Res. 1986, 44, 149–184. [Google Scholar] [CrossRef]
- Robbins, J.A. A model for particle-selective transport of tracers in sediments with conveyor belt deposit feeders. J. Geophys. Res., C 1986, 91, 8542–8558. [Google Scholar] [CrossRef]
- Granberg, M.E.; Selck, H. Effects of sediment organic matter quality on bioaccumulation, degradation, and distribution of pyrene in two macrofaunal species and their surrounding sediment. Mar. Environ. Res. 2007, 64, 313–335. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.; Cochran, J.K. Radium and radium-daughter nuclides in carbonates: A brief overview of strategies for determining chronologies. J. Environ. Radioact. 2010, 101, 530–537. [Google Scholar] [CrossRef]
- Cochran, J.K.; Aller, R.C.; Aller, J.Y.; Hirschberg, D.J.; Mackin, J.E. Long Island Sound Study: Sediment Geochemistry and Biology; US EPA Final Report, Contract CE 002870026; Marine Sciences Research Center, Stony Brook Univ.: New York, NY, USA, 1991. [Google Scholar]
- Cochran, J.K.; Hirschberg, D.; Amiel, D. Particle Mixing and Sediment Accumulation Rates of Peconic Estuary Sediments: A Sediment Accretion Study in Support of the Peconic Estuary Program; Final Report of Project #0014400498181563; Marine Sciences Research Center, Stony Brook Univ.: New York, NY, USA, 2000. [Google Scholar]
- Teal, L.R.; Parker, E.R.; Solan, M. Coupling bioturbation activity to metal (Fe and Mn) profiles in situ. Biogeosciences 2013, 10, 2365–2378. [Google Scholar] [CrossRef]
- Ciutat, A.; Anschutz, P.; Gerino, M.; Boudou, A. Effects of bioturbation on cadmium transfer and distribution into freshwater sediments. Environ. Toxicol. Chem. 2005, 24, 1048–1058. [Google Scholar] [CrossRef] [Green Version]
- Anschutz, P.; Ciutat, A.; Lecroart, P.; Gerino, M.; Boudou, A. Effects of tubificid worm bioturbation on freshwater sediment biogeochemistry. Aquat. Geochem. 2012, 18, 475–497. [Google Scholar] [CrossRef]
- Iwamoto, T.; Nasu, M. Current bioremediation practice and perspective. J. Biosci. Bioeng. 2001, 92, 1–8. [Google Scholar] [CrossRef]
- Kumar, A.; Bisht, B.S.; Joshi, V.D.; Dhewa, T. Review on bioremediation of polluted environment: A management tool. Int. J. Environ. Sci. 2011, 1, 1079–1093. Available online: https://doczz.net/doc/7561258/review-on-bioremediation-of-polluted-environment--a-manag (accessed on 7 September 2022).
- Hoang, T.K. Ecological Engineering for the Bioremediation of Aquatic Systems: Effects of the Combined Bioturbation and Phytoremediation on Cadmium and Atrazine Removal. Doctoral Thesis, Paul Sabatier University, Toulouse, France, 16 November 2018. Available online: https://tel.archives-ouvertes.fr/tel-02193335/document (accessed on 9 September 2022).
- Vidali, M. Bioremediation. An overview. Pure Appl. Chem. 2001, 73, 1163–1172. [Google Scholar] [CrossRef]
- Stoodley, P.; Dodds, I.; De Beer, D.; Lappin Scott, H.; Boyle, J.D. Flowing biofilms as a transport mechanism for biomass through porous media under laminar and turbulent conditions in a laboratory reactor system. Biofouling 2005, 21, 161–168. [Google Scholar] [CrossRef]
- Shafahi, M.; Vafai, K. Biofilm affected characteristics of porous structures. Int. J. Heat Mass Transf. 2009, 52, 574–581. [Google Scholar] [CrossRef]
- Davit, Y.; Iltis, G.; Debenest, G.; Veran-Tissoires, S.; Wildenschild, D.; Gerino, M.; Quintard, M. Imaging biofilm in porous media using X-ray computed microtomography. J. Microsc. 2011, 242, 15–25. [Google Scholar] [CrossRef]
- Karrabi, S.M.; Séchet, P.; Morra, C.; Cartellier, A.H.; Geindreau, C.; Martins, J.M.F. Investigation of hydrodynamics/biomass growth coupling in a pilot scale granular bioreactor at low pore Reynolds number. Chem. Eng. Sci. 2011, 66, 1765–1782. [Google Scholar] [CrossRef]
- Hua, G.F.; Zhu, W.; Shen, J.Q.; Zhang, Y.H.; Zeng, Y.T. The role of biofilm in clogging process in vertical flow constructed wetland. Appl. Eng. Agric. 2013, 29, 61–66. [Google Scholar] [CrossRef]
- Rolland du Roscoat, S.; Ivankovic, T.; Lenoir, N.; Dekic, S.; Martins, J.M.F.; Geindreau, C. First visualisation of bacterial biofilms in 3D porous media with neutron microtomography without contrast agent. J. Microsc. 2021, 13063. [Google Scholar] [CrossRef]
- Taylor, S.W.; Jaffé, P.R. Biofilm growth and the related changes in the physical properties of a porous medium. 1. Experimental investigation. Water Resour. Res. 1990, 26, 2153–2159. [Google Scholar] [CrossRef]
- Holm, J. Effects of Biomass Growth on the Hydrodynamic Properties of Groundwater Aquifers. Ph.D. Thesis, Department of Hydrodynamics and Water Resources, Technical University of Denmark, Lyngby, Denmark, April 2000. [Google Scholar]
- Bielefeldt, A.R.; Illangasekare, T.; Uttecht, M.; LaPlante, R. Biodegradation of propylene glycol and associated hydrodynamic effects in sand. Water Res. 2002, 36, 1707–1714. [Google Scholar] [CrossRef]
- Bielefeldt, A.R.; McEachern, C.; Illangasekare, T. Hydrodynamic changes in sand due to biogrowth on naphthalene and decane. J. Environ. Eng. 2002, 128, 51–59. [Google Scholar] [CrossRef]
- Cheik, S.; Bottinelli, N.; Minh, T.T.; Doan, T.T.; Jouquet, P. Quantification of three dimensional characteristics of macrofauna macropores and their effects on soil hydraulic conductivity in Northern Vietnam. Front. Environ. Sci. 2019, 7, 31. [Google Scholar] [CrossRef]
- Bastardie, F.; Capowiez, Y.; de Dreuzy, J.-R.; Cluzeau, D. X-ray tomographic and hydraulic characterization of burrowing by three earthworm species in repacked soil cores. Appl. Soil Ecol. 2003, 24, 3–16. [Google Scholar] [CrossRef]
- Capowiez, Y.; Bottinelli, N.; Sammartino, S.; Michel, E.; Jouquet, P. Morphological and functional characterisation of the burrow systems of six earthworm species (Lumbricidae). Biol. Fertil. Soils 2015, 51, 869–877. [Google Scholar] [CrossRef]
- Thullner, M.; Zeyer, J.; Kinzelbach, W. Influence of microbial growth on hydraulic properties of pore networks. Transp. Porous Media 2002, 49, 99–122. [Google Scholar] [CrossRef]
- Seifert, D.; Engesgaard, P. Use of tracer tests to investigate changes in flow and transport properties due to bioclogging of porous media. J. Contam. Hydrol. 2007, 93, 58–71. [Google Scholar] [CrossRef]
- Bottinelli, N.; Hedde, M.; Jouquet, P.; Capowiez, Y. An explicit definition of earthworm ecological categories-Marcel Bouché’s triangle revisited. Geoderma 2000, 372, 114361. [Google Scholar] [CrossRef]
- Capowiez, Y.; Sammartino, S.; Keller, T.; Bottinelli, N. Decreased burrowing activity of endogeic earthworms and effects on water infiltration in response to an increase in soil bulk density. Pedobiologia 2021, 85–86, 150728. [Google Scholar] [CrossRef]
- Gilibert, O.; Gerino, M.; Costa, D.-T.; Sauvage, S.; Julien, F.; Capowiez, Y.; Orange, D. Density effect of Eisenia sp. epigeic earthworms on the hydraulic conductivity of sand filters for wastewater treatment. Water 2022, 14, 1048. [Google Scholar] [CrossRef]
- Ye, J.; Xu, Z.; Chen, H.; Wang, L.; Benoit, G. Reduction of clog matter in constructed wetlands by metabolism of Eisenia foetida: Process and modeling. Environ. Pollut. 2018, 238, 803–811. [Google Scholar] [CrossRef] [PubMed]
- Tompkins, D.; Bumbac, C.; Clifford, E.; Dussaussois, J.-B.; Hannon, L.; Salvadó, V.; Schellenberg, T. EU horizon 2020 research for a sustainable future: INNOQUA-A nature-based sanitation solution. Water 2019, 11, 2461. [Google Scholar] [CrossRef]
- Biles, C.L.; Paterson, D.M.; Ford, R.B.; Solan, M.; Raffaelli, D.G. Bioturbation, ecosystem functioning and community structure. Hydrol. Earth Syst. Sci. 2002, 6, 999–1005. [Google Scholar] [CrossRef]
- Gilbert, F.; Aller, R.C.; Hulth, S. The influence of macrofaunal burrow spacing and diffusive scaling on sedimentary nitrification and denitrification: An experimental simulation and model approach. J. Mar. Res. 2003, 61, 101–125. [Google Scholar] [CrossRef]
- Mermillod-Blondin, F.; Gaudet, J.P.; Gerino, M.; Desrosiers, G.; Creuzé des Châtelliers, M. Influence of macroinvertebrates on physico-chemical and microbial processes in hyporheic sediments. Hydrol. Process. 2003, 17, 779–794. [Google Scholar] [CrossRef]
- Chowdhury, A.; Pradhan, S.; Saha, M.; Sanyal, N. Impact of pesticides on soil microbiological parameters and possible bioremediation strategies. Indian J. Microbiol. 2008, 48, 114–127. [Google Scholar] [CrossRef]
- Leveque, T.; Capowiez, Y.; Schreck, E.; Xiong, T.; Foucault, Y.; Dumat, C. Earthworm bioturbation influences the phytoavailability of metals released by particles in cultivated soils. Environ. Pollut. 2014, 191, 199–206. [Google Scholar] [CrossRef]
- Farenhorst, A.; Topp, E.; Bowman, B.T.; Tomlin, A.D. Earthworms and the dissipation and distribution of atrazine in the soil profile. Soil Biol. Biochem. 2000, 32, 23–33. [Google Scholar] [CrossRef]
- Kersanté, A.; Martin-Laurent, F.; Soulas, G.; Binet, F. Interactions of earthworms with atrazine-degrading bacteria in an agricultural soil. FEMS Microbiol. Ecol. 2006, 57, 192–205. [Google Scholar] [CrossRef]
- Monard, C.; Martin-Laurent, F.; Vecchiato, C.; Francez, A.-J.; Vandenkoornhuyse, P.; Binet, F. Combined effect of bioaugmentation and bioturbation on atrazine degradation in soil. Soil Biol. Biochem. 2008, 40, 2253–2259. [Google Scholar] [CrossRef]
- Marmonier, P.; Archambaud, G.; Belaidi, N.; Bougon, N.; Breil, P.; Chauvet, E.; Claret, C.; Cornut, J.; Datry, T.; Dole-Olivier, M.-J.; et al. The role of organisms in hyporheic processes: Gaps in current knowledge, needs for future research and applications. Ann. Limnol.-Int. J. Limnol. 2012, 48, 253–266. [Google Scholar] [CrossRef]
- Triska, F.J.; Kennedy, V.C.; Avanzino, R.J.; Zellweger, G.W.; Bencala, K.E. Retention and transport of nutrients in a third-order stream: Channel processes. Ecology 1989, 70, 1877–1892. [Google Scholar] [CrossRef]
- Lewandowski, J.; Putschew, A.; Schwesig, D.; Neumann, C.; Radke, M. Fate of organic micropollutants in the hyporheic zone of a eutrophic lowland stream: Results of a preliminary field study. Sci. Total Environ. 2011, 409, 1824–1835. [Google Scholar] [CrossRef]
- Destrieux, D. Résidus de Médicaments d’un Cours d’eau Urbain: Constitution d’une Base de données Pour la Gestion des risques Ecotoxicologiques. Ph.D. Thesis, Paul Sabatier University, Toulouse, France, 28 March 2018. [Google Scholar]
- Schittko, S.; Putschew, A.; Jekel, M. Bank filtration: A suitable process for the removal of iodinated X-ray contrast media? Water Sci. Technol. 2004, 50, 261–268. [Google Scholar] [CrossRef]
- Kundle, U.; Radke, M. Biodegradation of acidic pharmaceuticals in bed sediments: Insight from a laboratory experiment. Environ. Sci. Technol. 2008, 42, 7273–7279. [Google Scholar] [CrossRef]
- Schultz, M.; Löffler, D.; Wagner, M.; Ternes, T. Transformation of the X-ray contrast medium iopromide in soil and biological wastewater treatment. Environ. Sci. Technol. 2008, 42, 7207–7217. [Google Scholar] [CrossRef]
- Radke, M.; Lauwigi, C.; Heinkele, G.; Mürdter, T.E.; Letzel, M. Fate of the antibiotic sulfamethoxazole and its two major human metabolites in a water sediment test. Environ. Sci. Technol. 2009, 43, 3135–3141. [Google Scholar] [CrossRef]
- Wang, J.-M.; Patterson, B.; Bodour, A.; Maier, R.M.; Brusseau, M.L. Biodegradation during contaminant transport in porous media: 7. Impact of multiple-degrader community dynamics. Environ. Toxicol. Chem. 2005, 24, 2806–2811. [Google Scholar] [CrossRef]
- Aller, R.C. Diagenetic processes near the sediment-water interface of Long Island Sound. I. Decomposition and nutrient element geochemistry (S,N,P). Adv. Geophys. 1980, 22, 237–350. [Google Scholar] [CrossRef]
- Aller, R.C. The effects of macrobenthos on the chemical properties of marine sediments and overlying water. In Animal-Sediment Relations; Topics in Geobiology; McCall, P.L., Tevez, M.J.S., Eds.; Springer: Boston, MA, USA, 1982; Volume 100, pp. 53–102. [Google Scholar] [CrossRef]
- Kunihiro, T.; Miyazaki, T.; Uramoto, Y.; Kinoshita, K.; Inoue, A.; Tamaki, S.; Hama, D.; Tsutsumi, H.; Ohwada, K. The succession of microbial community in the organic rich fish-farm sediment during bioremediation by introducing artificially mass-cultured colonies of a small polychaete, Capitella sp. I. Mar. Pollut. Bull. 2008, 57, 68–77. [Google Scholar] [CrossRef]
- Wada, M.; Zhang, D.; Do, H.-K.; Nishimura, M.; Tsutsumi, H.; Kogure, K. Co-inoculation of Capitella sp. I with its synergetic bacteria enhances degradation of organic matter in organically enriched sediment below fish farms. Mar. Pollut. Bull. 2008, 57, 86–93. [Google Scholar] [CrossRef]
- Guinasso, N.L., Jr.; Schink, D.R. Quantitative estimates of biological mixing rates in abyssal sediments. J. Geophys. Res. 1975, 80, 3032–3043. [Google Scholar] [CrossRef]
- Waugh, S.; Aller, R.C. N2 production and fixation in deep-tier burrows of Squilla empusa in muddy sediments of Great Peconic Bay. J. Sea Res. 2017, 129, 36–41. [Google Scholar] [CrossRef]
- Pischedda, L.; Poggiale, J.C.; Cuny, P.; Gilbert, F. Imaging oxygen distribution in marine sediments. The importance of bioturbation and sediment heterogeneity. Acta Biotheor. 2008, 56, 123–135. [Google Scholar] [CrossRef]
- Loreau, M.; Naeem, S.; Inchausti, P. Biodiversity and ecosystem functioning: Current knowledge and future challenges. Science 2011, 294, 804–808. [Google Scholar] [CrossRef]
- Nichols, D.S. Capacity of natural wetlands to remove nutrients from wastewater. J. Water Pollut. Control Fed. 1983, 55, 495–505. Available online: https://www.jstor.org/stable/25041910 (accessed on 9 September 2022).
- Verhoeven, J.T.A.; Meuleman, A.F.M. Wetlands for wastewater treatment: Opportunities and limitations. Ecol. Eng. 1999, 12, 5–12. [Google Scholar] [CrossRef]
- Jahangir, M.M.R.; Richards, K.G.; Healy, M.G.; Gill, L.; Müller, C.; Johnston, P.G.; Fenton, O. Carbon and nitrogen dynamics and greenhouse gas emissions in constructed wetlands treating wastewater: A review. Hydrol. Earth Syst. Sci. 2016, 20, 109–123. [Google Scholar] [CrossRef]
- Khursheed, A.; Kazmi, A.A. Retrospective of ecological approaches to excess sludge reduction. Water Res. 2011, 45, 4287–4310. [Google Scholar] [CrossRef]
- Guo, W.Q.; Yang, S.S.; Xiang, W.S.; Wang, X.J.; Ren, N.Q. Minimization of excess sludge production by in-situ activated sludge treatment processes-A comprehensive review. Biotechnol. Adv. 2013, 31, 1386–1396. [Google Scholar] [CrossRef]
- Liang, P.; Huang, X.; Qian, Y. Excess sludge reduction in activated sludge process through predation of Aeolosoma hemprichi. Biochem. Eng. J. 2006, 28, 117–122. [Google Scholar] [CrossRef]
- Guo, X.-S.; Liu, J.-X.; Wei, Y.-S.; Li, L. Sludge reduction with Tubificidae and the impact on the performance of the wastewater treatment process. J. Environ. Sci. 2007, 19, 257–263. [Google Scholar] [CrossRef]
- Buys, B.R.; Klapwijk, A.; Elissen, H.; Rulkens, W.H. Development of a test method to assess the sludge reduction potential of aquatic organisms in activated sludge. Bioresour. Technol. 2008, 99, 8360–8366. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, Y.; Guo, X.; Liu, J. Sludge reduction potential of the activated sludge process by integrating an oligochaete reactor. J. Hazard. Mater. 2009, 163, 87–91. [Google Scholar] [CrossRef]
- Tamis, J.; van Schouwenburg, G.; Kleerebezem, R.; van Loosdrecht, M.C. A full scale worm reactor for efficient sludge reduction by predation in a wastewater treatment plant. Water Res. 2011, 45, 5916–5924. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, Z.; Wu, Z.; Han, X. Sludge reduction and process performance in a submerged membrane bioreactor with aquatic worms. Chem. Eng. J. 2011, 172, 929–935. [Google Scholar] [CrossRef]
- Tian, Y.; Li, Z.; Chen, L.; Lu, Y. Role of extracellular polymeric substances (EPSs) in membrane fouling of membrane bioreactor coupled with worm reactor. Bioresour. Technol. 2012, 123, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Li, Z.; Lu, Y. Changes in characteristics of soluble microbial products and extracellular polymeric substances in membrane bioreactor coupled with worm reactor: Relation to membrane fouling. Bioresour. Technol. 2012, 122, 62–69. [Google Scholar] [CrossRef]
- Li, Z.; Tian, Y.; Ding, Y.; Wang, H.; Chen, L. Contribution of extracellular polymeric substances (EPS) and their subfractions to the sludge aggregation in membrane bioreactor coupled with worm reactor. Bioresour. Technol. 2013, 144, 328–336. [Google Scholar] [CrossRef]
- Zhang, X.; Tian, Y.; Wang, Q.; Chen, L.; Wang, X. Heavy metal distribution and speciation during sludge reduction using aquatic worms. Bioresour. Technol. 2012, 126, 41–47. [Google Scholar] [CrossRef]
- Hendrickx, T.L.G.; Temmink, H.; Elissen, H.J.; Buisman, C.J.N. Aquatic worms eat sludge: Mass balances and processing of worm faeces. J. Hazard. Mater. 2010, 177, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Bulte, E.; Hector, A.; Larigauderie, A. ecoSERVICES: Assessing the Impacts of Biodiversity Changes on Ecosystem Functioning and Services; DIVERSITAS Report No. 3; DIVERSITAS Secretariat: Paris, France, 2005; 40p. [Google Scholar]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gerino, M.; Orange, D.; Sánchez-Pérez, J.M.; Buffan-Dubau, E.; Canovas, S.; Monfort, B.; Albasi, C.; Sauvage, S. What Inspiring Elements from Natural Services of Water Quality Regulation Could Be Applied to Water Management? Water 2022, 14, 3030. https://doi.org/10.3390/w14193030
Gerino M, Orange D, Sánchez-Pérez JM, Buffan-Dubau E, Canovas S, Monfort B, Albasi C, Sauvage S. What Inspiring Elements from Natural Services of Water Quality Regulation Could Be Applied to Water Management? Water. 2022; 14(19):3030. https://doi.org/10.3390/w14193030
Chicago/Turabian StyleGerino, Magali, Didier Orange, José Miguel Sánchez-Pérez, Evelyne Buffan-Dubau, Sophie Canovas, Bertrand Monfort, Claire Albasi, and Sabine Sauvage. 2022. "What Inspiring Elements from Natural Services of Water Quality Regulation Could Be Applied to Water Management?" Water 14, no. 19: 3030. https://doi.org/10.3390/w14193030
APA StyleGerino, M., Orange, D., Sánchez-Pérez, J. M., Buffan-Dubau, E., Canovas, S., Monfort, B., Albasi, C., & Sauvage, S. (2022). What Inspiring Elements from Natural Services of Water Quality Regulation Could Be Applied to Water Management? Water, 14(19), 3030. https://doi.org/10.3390/w14193030










