First Evidence of Microplastic Contamination in Antarctic Fish (Actinopterygii, Perciformes)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Field Sampling and Data Collection
2.2. Digestion of Samples and MP Analysis
2.3. Quality Assurance/Quality Control (QA/QC)
2.4. Risk Assessment of MPs on Human Health and Organisms
2.5. Statistical Analysis
3. Results
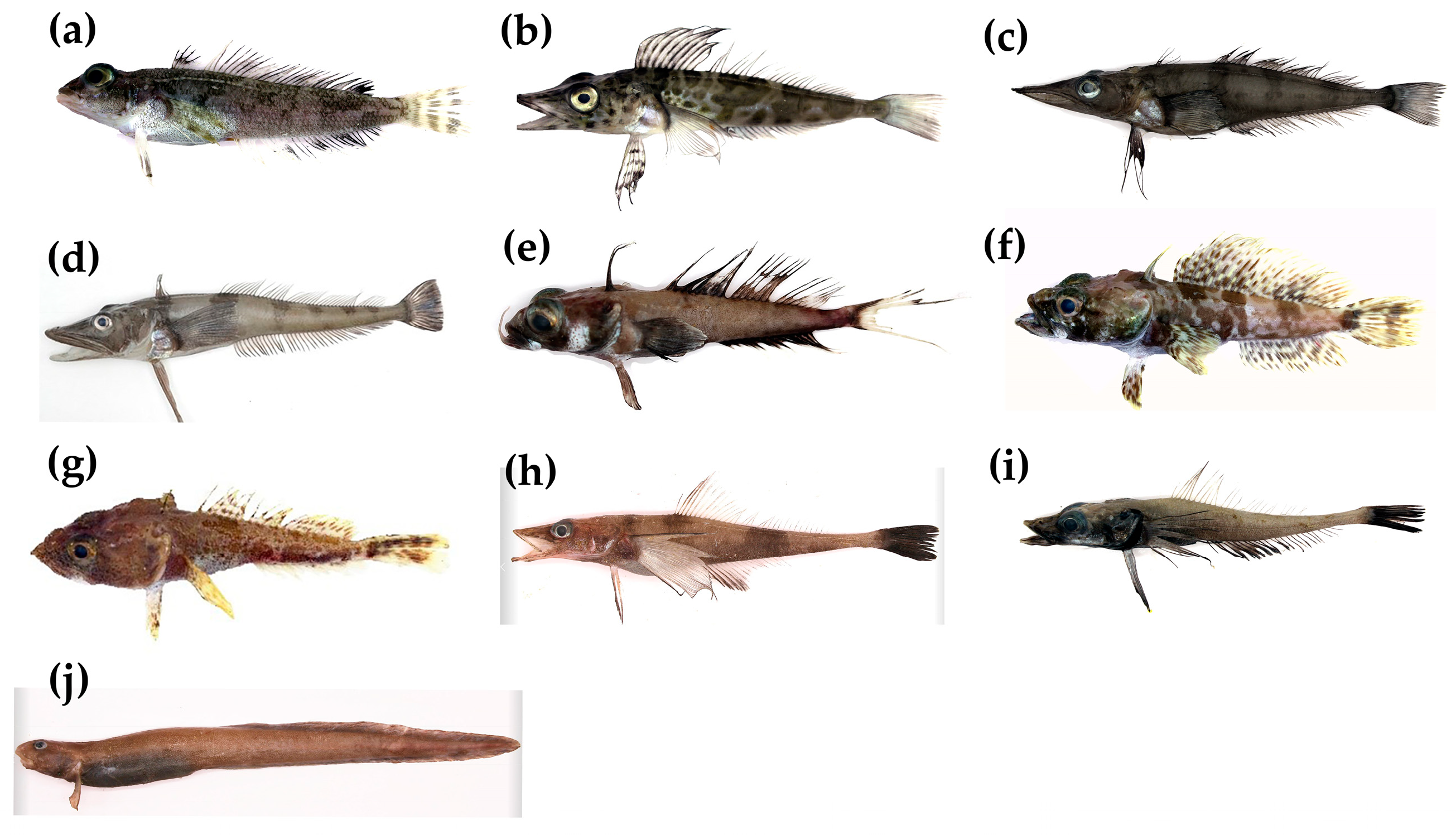
3.1. Biological Characteristics of Collected Fish Samples
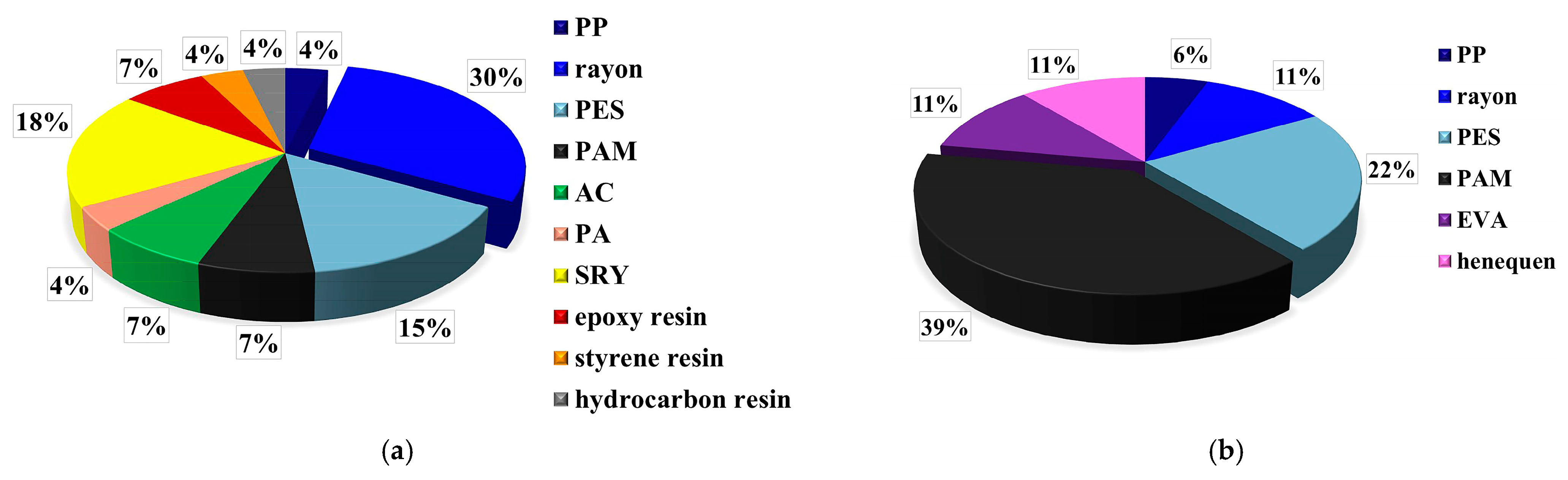
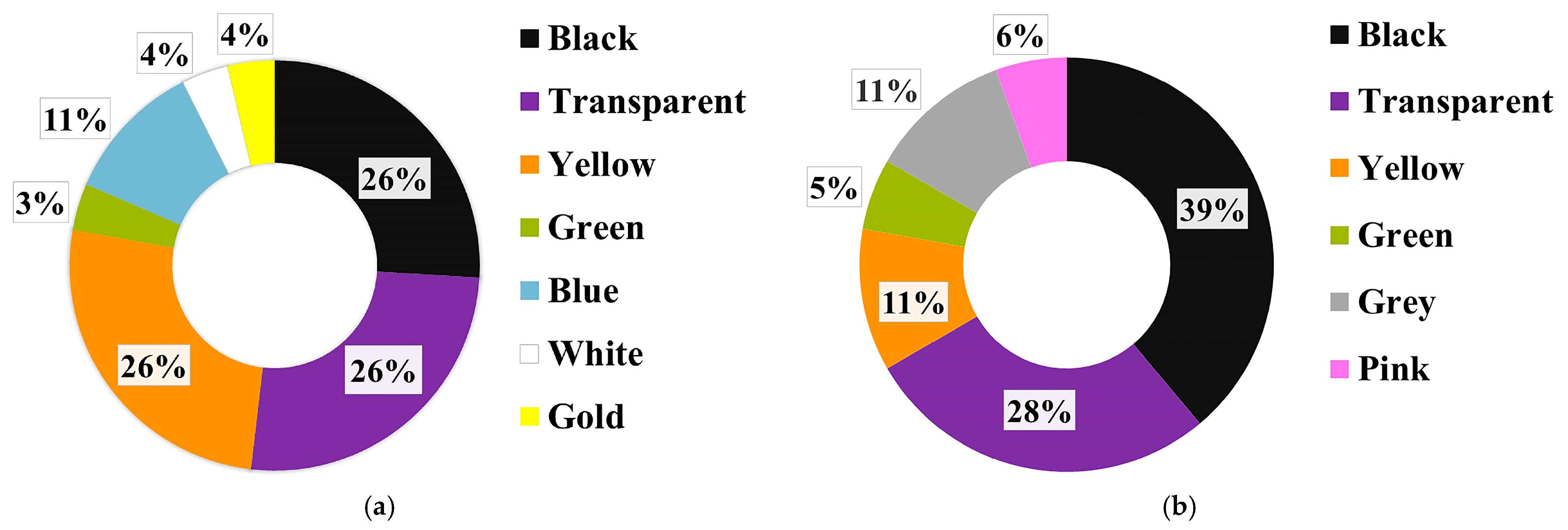
3.2. Comparison of MP Contamination in Order Perciformes between Two Sea Areas
3.3. Comparison of MP Contamination among Different Fish Families of Order Perciformes in the AS
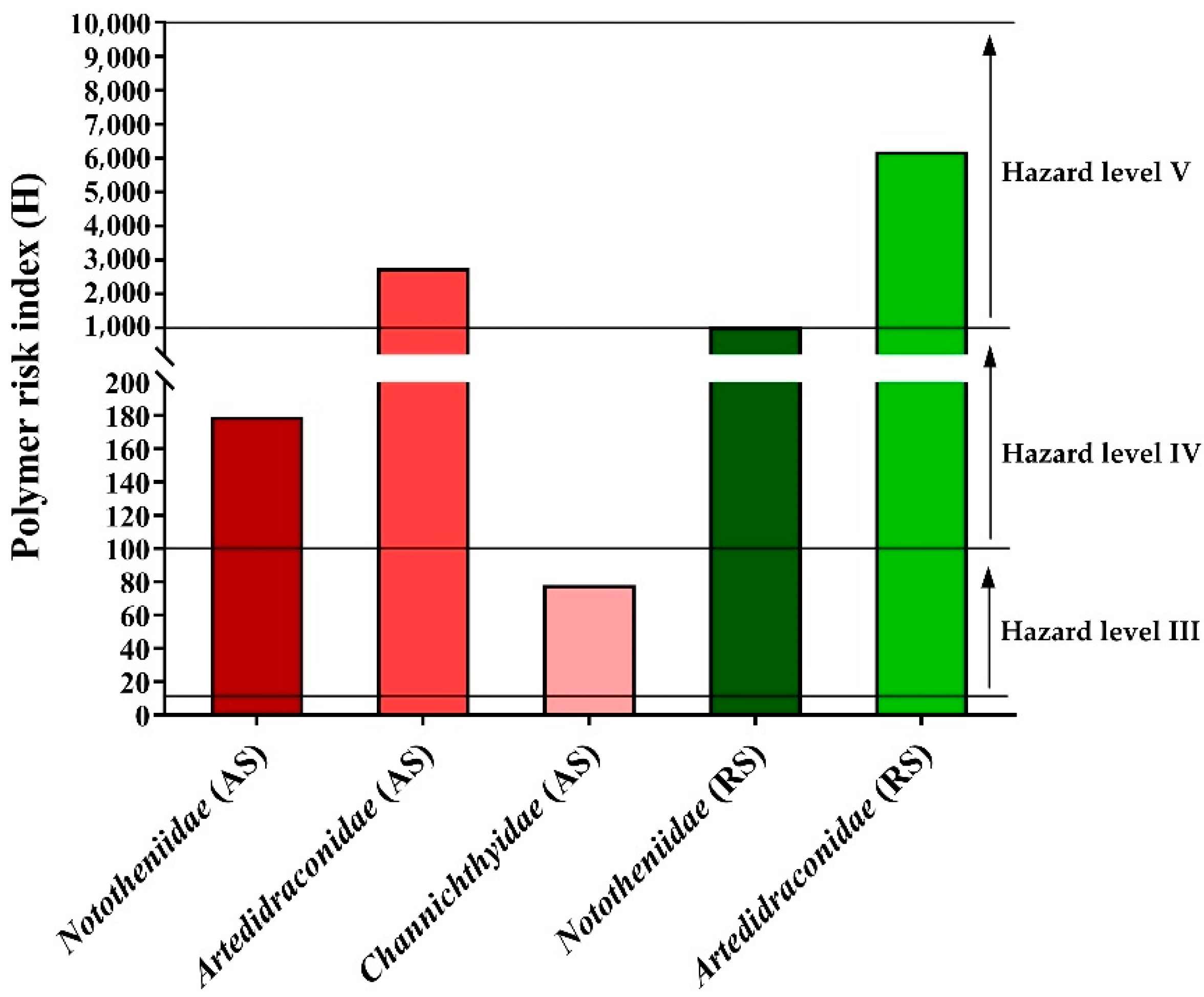
3.4. Environmental and Health Hazard Risk Assessment of MPs
4. Discussion
4.1. Comparison of MP Contamination in Fish between Antarctica and Other Regions Worldwide
4.2. Comparison of MP Contamination in Order Perciformes and Other Antarctic Species
4.3. Comparison of MP Contamination in Fish between AS and RS
4.4. Comparison of MP Contamination among Different Families of Fish
4.5. Hazard Risk Assessment of MPs in the Fish Species
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ding, J.; Li, J.; Sun, C.; Jiang, F.; Ju, P.; Qu, L.; Zheng, Y.; He, C. Detection of microplastics in local marine organisms using a multi-technology system. Anal. Methods. 2019, 11, 78–87. [Google Scholar] [CrossRef]
- Ford, H.V.; Jones, N.H.; Davies, A.J.; Godley, B.J.; Jambeck, J.R.; Napper, I.E.; Suckling, C.C.; Williams, G.J.; Woodall, L.C.; Koldewey, H.J. The fundamental links between climate change and marine plastic pollution. Sci. Total Environ. 2022, 806, 150392. [Google Scholar] [CrossRef] [PubMed]
- Suaria, G.; Perold, V.; Lee, J.R.; Lebouard, F.; Aliani, S.; Ryan, P.G. Floating macro- and microplastics around the Southern Ocean: Results from the Antarctic Circumnavigation Expedition. Environ. Int. 2020, 136, 105494. [Google Scholar] [CrossRef]
- Barnes, D.K.A.; Morley, S.A.; Bell, J.; Brewin, P.; Brigden, K.; Collins, M.; Glass, T.; Goodall-Copestake, W.P.; Henry, L.; Laptikhovsky, V.; et al. Marine plastics threaten giant Atlantic Marine Protected Areas. Curr. Biol. 2018, 28, R1137–R1138. [Google Scholar] [CrossRef]
- Pereira, J.M.; Rodríguez, Y.; Blasco-Monleon, S.; Porter, A.; Lewis, C.; Pham, C.K. Microplastic in the stomachs of open-ocean and deep-sea fishes of the North-East Atlantic. Environ. Pollut. 2020, 265, 115060. [Google Scholar] [CrossRef]
- Collard, F.; Ask, A. Plastic ingestion by Arctic fauna: A review. Sci. Total Environ. 2021, 786, 147462. [Google Scholar] [CrossRef]
- Caruso, G.; Bergami, E.; Singh, N.; Corsi, I. Plastic occurrence, sources, and impacts in Antarctic environment and biota. Water Biol. Secur. 2022, 1, 100034. [Google Scholar] [CrossRef]
- Kershaw, P.J. Marine Plastic Debris and Microplastics Global Lessons and Research to Inspire Action and Guide Policy Change, 1st ed.; United Nations Environment Programme: Nairobi, Kenya, 2016; pp. 26–34. [Google Scholar]
- Andrady, A.L. Microplastics in the marine environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef]
- Cannon, S.M.E.; Lavers, J.L.; Figueiredo, B. Plastic ingestion by fish in the Southern Hemisphere: A baseline study and review of methods. Mar. Pollut. Bull. 2016, 107, 286–291. [Google Scholar] [CrossRef]
- Law, K.L.; Thompson, R.C. Microplastics in the seas. Science 2014, 345, 144–145. [Google Scholar] [CrossRef]
- Waller, C.L.; Griffiths, H.J.; Waluda, C.M.; Thorpe, S.E.; Loaiza, I.; Moreno, B.; Pacherres, C.O.; Hughes, K.A. Microplastics in the Antarctic marine system: An emerging area of research. Sci. Total Environ. 2017, 598, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Kuklinski, P.; Wicikowski, L.; Koper, M.; Grala, T.; Leniec-Koper, H.; Barasiński, M.; Talar, M.; Kamiński, I.; Kibart, R.; Małecki, W. Offshore surface waters of Antarctica are free of microplastics, as revealed by a circum-Antarctic study. Mar. Pollut. Bull. 2019, 149, 110573. [Google Scholar] [CrossRef] [PubMed]
- González-Pleiter, M.; Edo, C.; Velázquez, D.; Casero-Chamorro, M.C.; Leganés, F.; Quesada, A.; Fernández-Piñas, F.; Rosal, R. First detection of microplastics in the freshwater of an Antarctic Specially Protected Area. Mar. Pollut. Bull. 2020, 161, 111811. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Garin, O.; García-Cuevas, I.; Drago, M.; Rita, D.; Parga, M.; Gazo, M.; Cardona, L. No evidence of microplastics in Antarctic fur seal scats from a hotspot of human activity in Western Antarctica. Sci. Total Environ. 2020, 737, 140210. [Google Scholar] [CrossRef] [PubMed]
- Pakhomova, S.; Berezina, A.; Lusher, A.L.; Zhdanov, I.; Silvestrova, K.; Zavialov, P.; van Bavel, B.; Yakushev, E. Microplastic variability in subsurface water from the Arctic to Antarctica. Environ. Pollut. 2022, 298, 118808. [Google Scholar] [CrossRef]
- Cincinelli, A.; Scopetani, C.; Chelazzi, D.; Lombardini, E.; Martellini, T.; Katsoyiannis, A.; Fossi, M.C.; Corsolini, S. Microplastic in the surface waters of the Ross Sea (Antarctica): Occurrence, distribution and characterization by FTIR. Chemosphere 2017, 175, 391–400. [Google Scholar] [CrossRef]
- Cunningham, E.M.; Ehlers, S.M.; Dick, J.T.A.; Sigwart, J.D.; Linse, K.; Dick, J.J.; Kiriakoulakis, K. High Abundances of Microplastic Pollution in Deep-Sea Sediments: Evidence from Antarctica and the Southern Ocean. Environ. Sci. Technol. 2020, 54, 13661–13671. [Google Scholar] [CrossRef]
- Jones-williams, K.; Galloway, T.; Cole, M.; Stowasser, G.; Waluda, C.; Manno, C. Close encounters-microplastic availability to pelagic amphipods in sub- antarctic and antarctic surface waters. Environ. Int. J. 2020, 140, 105792. [Google Scholar] [CrossRef]
- Cossi, P.F.; Ojeda, M.; Chiesa, I.L.; Rimondino, G.N.; Fraysse, C.; Calcagno, J.; Pérez, A.F. First evidence of microplastics in the Marine Protected Area Namuncurá at Burdwood Bank, Argentina: A study on Henricia obesa and Odontaster penicillatus (Echinodermata: Asteroidea). Polar Biol. 2021, 44, 2277–2287. [Google Scholar] [CrossRef]
- Fragão, J.; Bessa, F.; Otero, V.; Barbosa, A.; Sobral, P.; Waluda, C.M.; Guímaro, H.R.; Xavier, J.C. Microplastics and other anthropogenic particles in Antarctica: Using penguins as biological samplers. Sci. Total Environ. 2021, 788, 147698. [Google Scholar] [CrossRef]
- Auman, H.J.; Woehler, E.J.; Riddle, M.J.; Burton, H. First evidence of ingestion of plastic debris by seabirds at sub-Antarctic Heard Island. Mar. Ornithol. 2004, 32, 105–106. [Google Scholar]
- Eriksson, C.; Burton, H. Origins and biological accumulation of small plastic particles in fur seals from Macquarie Island. Ambio 2003, 32, 380–384. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.K.; Singh, J.; Mishra, P.P. Microplastics in polar regions: An early warning to the world’s pristine ecosystem. Sci. Total Environ. 2021, 784, 147149. [Google Scholar] [CrossRef] [PubMed]
- Fraser, C.I.; Morrison, A.K.; Hogg, A.M.C.; Macaya, E.C.; van Sebille, E.; Ryan, P.G.; Padovan, A.; Jack, C.; Valdivia, N.; Waters, J.M. Antarctica’s ecological isolation will be broken by storm-driven dispersal and warming. Nat. Clim. Change 2018, 8, 704–708. [Google Scholar] [CrossRef]
- Barrera-Oro, E. The role of fish in the Antarctic marine food web: Differences between inshore and offshore waters in the southern Scotia Arc and west Antarctic Peninsula. Antarct. Sci. 2002, 14, 293–309. [Google Scholar] [CrossRef]
- Rossi, L.; Caputi, S.S.; Calizza, E.; Careddu, G.; Oliverio, M.; Schiaparelli, S.; Costantini, M.L. Antarctic food web architecture under varying dynamics of sea ice cover. Sci. Rep. 2019, 9, 12454. [Google Scholar] [CrossRef]
- Liu, G.; Zhu, Z.; Yang, Y.; Sun, Y.; Yu, F.; Ma, J. Sorption behavior and mechanism of hydrophilic organic chemicals to virgin and aged microplastics in freshwater and seawater. Environ. Pollut. 2019, 246, 26–33. [Google Scholar] [CrossRef]
- Massos, A.; Turner, A. Cadmium, lead and bromine in beached microplastics. Environ. Pollut. 2017, 227, 139–145. [Google Scholar] [CrossRef]
- Teuten, E.L.; Saquing, J.M.; Knappe, D.R.U.; Barlaz, M.A.; Jonsson, S.; Björn, A.; Rowland, S.J.; Thompson, R.C.; Galloway, T.S.; Yamashita, R.; et al. Transport and release of chemicals from plastics to the environment and to wildlife. Phil. Trans. R. Soc. B 2009, 364, 2027–2045. [Google Scholar] [CrossRef]
- Lithner, D.; Larsson, Å.; Dave, G. Environmental and health hazard ranking and assessment of plastic polymers based on chemical composition. Sci. Total Environ. 2011, 409, 3309–3324. [Google Scholar] [CrossRef]
- Xu, P.; Peng, G.; Su, L.; Gao, Y.; Gao, L.; Li, D. Microplastic risk assessment in surface waters: A case study in the Changjiang Estuary, China. Mar. Pollut. Bull. 2018, 133, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Munari, C.; Infantini, V.; Scoponi, M.; Rastelli, E.; Corinaldesi, C.; Mistri, M. Microplastics in the sediments of Terra Nova Bay (Ross Sea, Antarctica). Mar. Pollut. Bull. 2017, 122, 161–165. [Google Scholar] [CrossRef]
- Grover-Johnson, O. Findings from the Continuous Plankton Recorder in the Ross Sea and the East Antarctic Regions. Supervised Project Report ANTA604, New Zealand, 2017/2018. Findings from the Continuous Plankton Recorder in the Ross Sea and the East Antarctic Regions. Available online: https://ir.canterbury.ac.nz/bitstream/handle/10092/15837/Microplastics%20in%20the%20Southern%20Ocean.pdf?sequence=1&isAllowed=y (accessed on 30 April 2022).
- Feng, J.; Li, D.; Zhang, J.; Zhao, L. Variations in and environmental controls of primary productivity in the Amundsen Sea. Biogeosciences 2021, 296, 1–44. [Google Scholar] [CrossRef]
- Barnes, D.K.A.; Walters, A.; Gonçalves, L. Macroplastics at sea around Antarctica. Mar. Environ. Res. 2010, 70, 250–252. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Zheng, R.; Zhang, Y.; Hong, F.; Mu, J.; Chen, M.; Song, P.; Lin, L.; Lin, H.; Le, F.; et al. Microplastic contamination in benthic organisms from the Arctic and sub-Arctic regions. Chemosphere 2018, 209, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.C.; Fang, C.; Zheng, R.; Hong, F.; Jiang, Y.; Zhang, M.; Li, Y.; Hamid, F.S.; Bo, J.; Lin, L.S. Microplastic pollution in wild commercial nekton from the South China Sea and Indian Ocean, and its implication to human health. Mar. Environ. Res. 2021, 167, 105295. [Google Scholar] [CrossRef] [PubMed]
- Dehaut, A.; Cassone, A.L.; Frère, L.; Hermabessiere, L.; Himber, C.; Rinnert, E.; Rivière, G.; Lambert, C.; Soudant, P.; Huvet, A.; et al. Microplastics in seafood: Benchmark protocol for their extraction and characterization. Environ. Pollut. 2016, 215, 223–233. [Google Scholar] [CrossRef]
- Karami, A.; Golieskardi, A.; Choo, C.K.; Romano, N.; Ho, Y.B.; Salamatinia, B. A high-performance protocol for extraction of microplastics in fish. Sci. Total Environ. 2017, 578, 485–494. [Google Scholar] [CrossRef]
- Zhang, X.; Yan, B.; Wang, X. Selection and optimization of a protocol for extraction of microplastics from Mactra veneriformis. Sci. Total Environ. 2020, 746, 141250. [Google Scholar] [CrossRef]
- Fang, C.; Zhang, Y.; Zheng, R.; Hong, F.; Zhang, M.; Zhang, R.; Mou, J.; Mu, J.; Lin, L.; Bo, J. Spatio-temporal variation of microplastic pollution in the sediment from the Chukchi Sea over five years. Sci. Total Environ. 2021, 806, 150530. [Google Scholar] [CrossRef]
- Hubold, G.; Ekau, W. Feeding Patterns of Post-Larval and Juvenile Notothenioids in the Southern Weddell Sea (Antarctica). Polar Biol. 1990, 10, 255–260. [Google Scholar] [CrossRef]
- Iwami, T.; Numanami, H.; Naito, Y. Behavior of three species of the family Artedidraconidae (Pisces, Notothenioidei), with ref-erence to feeding. Polar Biol. 1996, 9, 225–230. [Google Scholar]
- Lombarte, A.; Olaso, I.; Bozzano, A. Ecomorphological trends in the Artedidraconidae (Pisces: Perciformes: Notothenioidei) of the Weddell Sea. Antarct. Sci. 2003, 15, 211–218. [Google Scholar] [CrossRef]
- Mesa, M.L.; Eastman, J.T.; Licandro, P. Feeding habits of Bathydraco marri (Pisces, Notothenioidei, Bathydraconidae) from the Ross Sea, Antarctica. Polar Biol. 2007, 30, 541–547. [Google Scholar] [CrossRef]
- Fanta, E.; Rios, F.S.A.; Meyer, A.A.; Grötzner, S.R.; Zaleski, T. Chemical and visual sensory systems in feeding behaviour of the Antarctic fish Ophthalmolycus amberensis (Zoarcidae). Antarct. Rec. 2001, 45, 27–42. [Google Scholar]
- Morgana, S.; Ghigliotti, L.; Estévez-Calvar, N.; Stifanese, R.; Wieckzorek, A.; Doyle, T.; Christiansen, J.S.; Faimali, M.; Garaventa, F. Microplastics in the Arctic: A case study with sub-surface water and fish samples off Northeast Greenland. Environ. Pollut. 2018, 242, 1078–1086. [Google Scholar] [CrossRef] [PubMed]
- Lusher, A.L.; O’Donnell, C.; Officer, R.; O’Connor, I. Microplastic interactions with North Atlantic mesopelagic fish. ICES J. Mar. Sci. Marine Sci. 2016, 73, 1214–1225. [Google Scholar] [CrossRef]
- Pinho, I.; Amezcua, F.; Rivera, J.M.; Green-Ruiz, C.; de Jesus Piñón-Colin, T.; Wakida, F. First report of plastic contamination in batoids: Plastic ingestion by Haller’s Round Ray (Urobatis halleri) in the Gulf of California. Environ. Res. 2022, 211, 113077. [Google Scholar] [CrossRef]
- Güven, O.; Gökdağ, K.; Jovanović, B.; Kıdeyş, A.E. Microplastic litter composition of the Turkish territorial waters of the Mediterranean Sea, and its occurrence in the gastrointestinal tract of fish. Environ. Pollut. 2017, 223, 286–294. [Google Scholar] [CrossRef]
- Dantas, N.C.F.M.; Duarte, O.S.; Ferreira, W.C.; Ayala, A.P.; Rezende, C.F.; Feitosa, C.V. Plastic intake does not depend on fish eating habits: Identification of microplastics in the stomach contents of fish on an urban beach in Brazil. Mar. Pollut. Bull. 2020, 153, 110959. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, H.; Chen, B.; Sun, X.; Qu, K.; Xia, B. Microplastic ingestion in deep-sea fish from the South China Sea. Sci. Total Environ. 2019, 677, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Wootton, N.; Ferreira, M.; Reis-Santos, P.; Gillanders, B.M. A comparison of microplastic in fish from Australia and Fiji. Front. Mar. Sci. 2021, 8, 690991. [Google Scholar] [CrossRef]
- Karbalaei, S.; Golieskardi, A.; Hamzah, H.B.; Abdulwahid, S.; Hanachi, P.; Walker, T.R.; Karami, A. Abundance and characteristics of microplastics in commercial marine fish from Malaysia. Mar. Pollut. Bull. 2019, 148, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Daniel, D.B.; Ashraf, P.M.; Thomas, S.N. Microplastics in the edible and inedible tissues of pelagic fishes sold for human consumption in Kerala, India. Environ. Pollut. 2020, 266, 115365. [Google Scholar] [CrossRef] [PubMed]
- Saha, M.; Naik, A.; Desai, A.; Nanajkar, M.; Rathore, C.; Kumar, M.; Gupta, P. Microplastics in seafood as an emerging threat to marine environment: A case study in Goa, west coast of India. Chemosphere 2021, 270, 129359. [Google Scholar] [CrossRef]
- Absher, T.M.; Ferreira, S.L.; Kern, Y.; Ferreira, A.L.; Christo, S.W.; Ando, R.A. Incidence and identification of microfibers in ocean waters in Admiralty Bay, Antarctica. Environ. Sci. Pollut. Res. 2019, 26, 292–298. [Google Scholar] [CrossRef]
- Sfriso, A.A.; Tomio, Y.; Rosso, B.; Gambaro, A.; Sfriso, A.; Corami, F.; Rastelli, E.; Corinaldesi, C.; Mistri, M.; Munari, C. Microplastic accumulation in benthic invertebrates in Terra Nova Bay (Ross Sea, Antarctica). Environ. Int. 2020, 137, 105587. [Google Scholar] [CrossRef]
- Gao, C.; Cao, Z.; Yan, C.; Zhu, G. Traits and distribution of microplastics in stomach and intestinal tract of Pleuragramma antarcticum around the South Shetland Islands. J. Fish. 2021. (In Chinese) [Google Scholar] [CrossRef]
- Le Guen, C.; Suaria, G.; Sherley, R.B.; Ryan, P.G.; Aliani, S.; Boehme, L.; Brierley, A.S. Microplastic study reveals the presence of natural and synthetic fibres in the diet of King Penguins (Aptenodytes patagonicus) foraging from South Georgia. Environ. Int. 2020, 134, 105303. [Google Scholar] [CrossRef]
- Bessa, F.; Ratcliffe, N.; Otero, V.; Sobral, P.; Marques, J.C.; Waluda, C.M.; Trathan, P.N.; Xavier, J.C. Microplastics in gentoo penguins from the Antarctic region. Sci. Rep. 2019, 9, 14191. [Google Scholar] [CrossRef]
- van Franeker, J.A.; Bell, P.J. Plastic ingestion by petrels breeding in Antarctica. Mar. Pollut. Bull. 1988, 19, 672–674. [Google Scholar] [CrossRef]
- Aronson, R.B.; Thatje, S.; Mcclintock, J.B.; Hughes, K.A. Anthropogenic impacts on marine ecosystems in Antarctica. Ann. N. Y. Acad. Sci. 2011, 1223, 82–107. [Google Scholar] [CrossRef] [PubMed]
- Tin, T.; Fleming, Z.L.; Hughes, K.A.; Ainley, D.G.; Convey, P.; Moreno, C.A.; Pfeiffer, S.; Scott, J.; Snape, I. Impacts of local human activities on the Antarctic environment. Antarct. Sci. 2009, 21, 3–33. [Google Scholar] [CrossRef] [Green Version]
- Council of Managers of National Antarctic Programs. Antarctic Station Catalogue; COMNAP: Christchurch, New Zealand, 2017; pp. 86–146. [Google Scholar]
- Wright, S.L.; Thompson, R.C.; Galloway, T.S. The physical impacts of microplastics on marine organisms: A review. Environ. Pollut. 2013, 178, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Maximenko, N.; Hafner, J.; Niiler, P. Pathways of marine debris derived from trajectories of Lagrangian drifters. Mar. Pollut. Bull. 2012, 65, 51–62. [Google Scholar] [CrossRef]
- Jiang, Y.; Yang, F.; Zhao, Y.; Wang, J. Greenland Sea Gyre increases microplastic pollution in the surface waters of the Nordic Seas. Sci. Total Environ. 2020, 712, 136484. [Google Scholar] [CrossRef]
- Stark, J.S.; Corbett, P.A.; Dunshea, G.; Johnstone, G.; King, C.; Mondon, J.A.; Power, M.L.; Samuel, A.; Snape, I.; Riddle, M. The environmental impact of sewage and wastewater outfalls in Antarctica: An example from Davis station, East Antarctica. Water Res. 2016, 105, 602–614. [Google Scholar] [CrossRef]
- Gröndahl, F.; Sidenmark, J.; Thomsen, A. Survey of waste water disposal practices at Antarctic research stations. Polar Res. 2009, 28, 298–306. [Google Scholar] [CrossRef]
- Szopińska, M.; Luczkiewicz, A.; Jankowska, K.; Fudala-Ksiazek, S.; Potapowicz, J.; Kalinowska, A.; Bialik, R.J.; Chmiel, S.; Polkowska, Ż. First evaluation of wastewater discharge influence on marine water contamination in the vicinity of Arctowski Station (Maritime Antarctica). Sci. Total Environ. 2021, 789, 147912. [Google Scholar] [CrossRef]
- Lusher, A.L.; Burke, A.; O’Connor, I.; Officer, R. Microplastic pollution in the Northeast Atlantic Ocean: Validated and opportunistic sampling. Mar. Pollut. Bull. 2014, 88, 325–333. [Google Scholar] [CrossRef]
- Sun, J.; Prabhu, A.; Aroney, S.; Rinke, C. Insights into plastic biodegradation: Community composition and functional capabilities of the superworm (Zophobas morio) microbiome in styrofoam feeding trials. Microb Genom. 2022, 8, 000842. [Google Scholar] [CrossRef] [PubMed]
- Yezek, L.P.; Duval, J.F.L.; Van Leeuwen, H.P. Electrokinetics of diffuse soft interfaces. III. Interpretation of data on the polyacrylamide/water interface. Langmuir 2005, 21, 6220–6227. [Google Scholar] [CrossRef] [PubMed]
- Alomar, C.; Sureda, A.; Capó, X.; Guijarro, B.; Tejada, S.; Deudero, S. Microplastic ingestion by Mullus surmuletus Linnaeus, 1758 fish and its potential for causing oxidative stress. Environ. Res. 2017, 159, 135–142. [Google Scholar] [CrossRef] [PubMed]
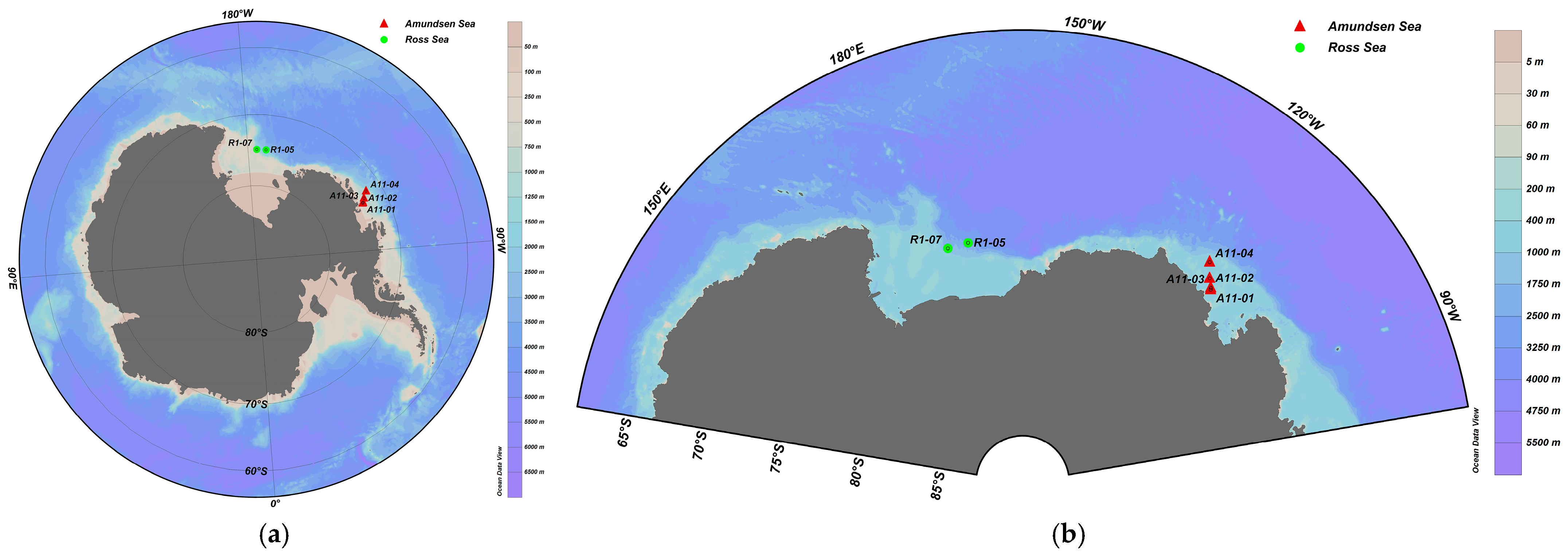

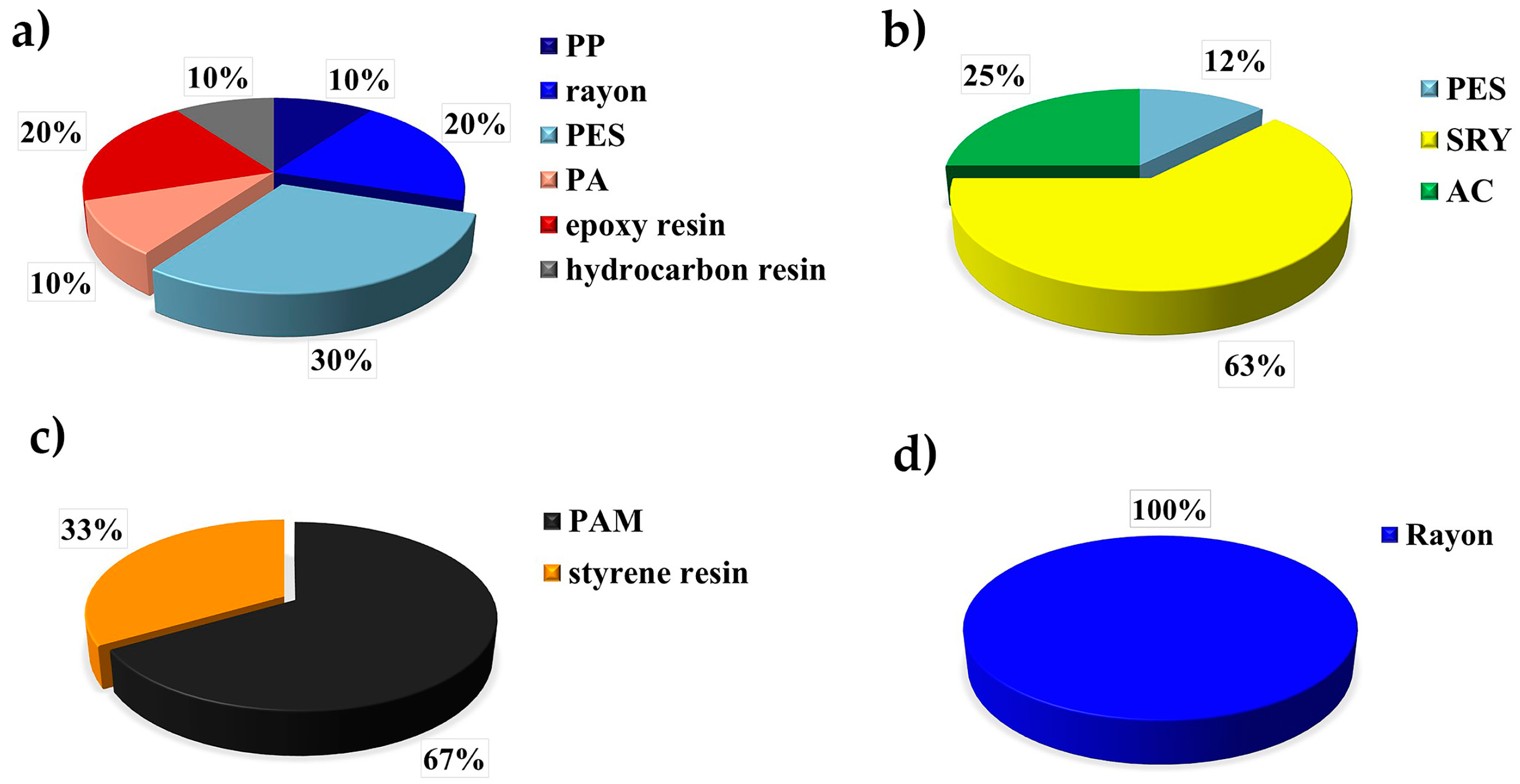
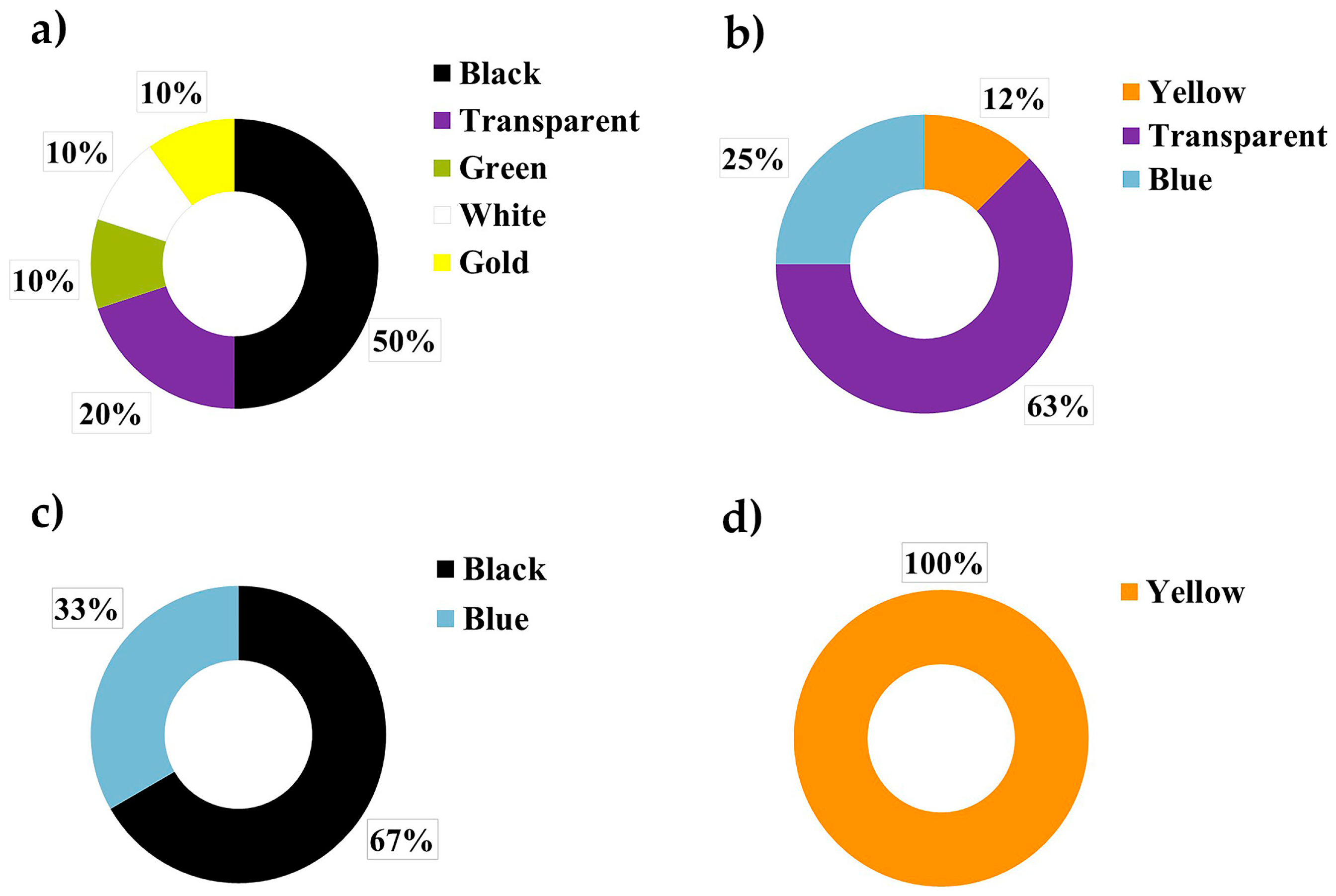
| Site | Depth/m | Salinity | Temperature/°C |
|---|---|---|---|
| A11–01 | 622 | 31.71 | −1.800 |
| A11−02 | 659 | 32.25 | −1.800 |
| A11−03 | 609 | 31.71 | −1.800 |
| A11−04 | 502 | 30.62 | −1.800 |
| R1−05 | 332 | 31.38 | −0.203 |
| R1−07 | 285 | 31.71 | 0.204 |
| Family | Genus, Species | Distribution a | Depth Range a /m | Number of Samples/ Individue | Life Stage | Feeding Methods | Average Length/cm (Average ± SD) |
|---|---|---|---|---|---|---|---|
| Nototheniidae | Trematomus, Trematomus scotti | Southern Ocean: South Shetland, South Orkney islands, RS, Breid Bay, Weddell Sea, and Antarctic Peninsula | 20–793 | 22 | 8 Juveniles, 14 Adults | Carnivore a | 12.22 ± 1.50 |
| Channichthyidae | Pagetopsis, Pagetopsis maculatus | Southern Ocean: circum-Antarctic on the continental shelf | 200–800 | 1 | Adult | Carnivore [43] | 15.80 b |
| Dacodraco, Dacodraco hunteri | Southern Ocean: probably circum-Antarctic on the continental shelf | 300–800 | 1 | Adult | Carnivore [43] | 22.50 b | |
| Chionodraco, Chionodraco myersi | Southern Ocean: circum-Antarctic on the continental shelf | 200–800 | 2 | Adults | Carnivore a | 24.05 ± 3.61 | |
| Artedidraconidae | Dolloidraco, Dolloidraco longedorsalis | Southern Ocean: Weddell Sea, Graham Land, Queen Mary Land, South Victoria Land | 203–1145 | 3 | Adults | Carnivore a | 10.93 ± 0.90 |
| Histiodraco, Histiodraco velifer | Southern Ocean: East Antarctica (South Victoria Land, MacRobertson Land, RS, Weddell Sea) | 210–667 | 1 | Adult | Carnivore [44] | 16.50 b | |
| Pogonophryne, Pogonophryne albipinna | Southern Ocean: RS | 1565–1674 | 1 | Adult | Carnivore [45] | 8.50 b | |
| Bathydraconidae | Vomeridens, Vomeridens infuscipinnis | Southern Ocean: Weddell and RSs, South Orkney Islands and Antarctic Peninsula | 500–813 | 1 | Adult | Carnivore [46] | 21.50 b |
| Akarotaxis, Akarotaxis nudiceps | Southern Ocean: Antarctic continental shelf and west of Adelaide Island at the Antarctic Peninsula | 371–915 | 2 | Adults | Carnivore [46] | 12.75 ± 0.21 | |
| Zoarcidae | Ophthalmolycus, Ophthalmolycus amberensis | Southern Ocean: circum-Antarctic | 140–826 | 2 | 1 Juvenile, 1 Adult | Omnivore [47] | 25.35 ± 5.16 |
| Location | Fish | Sampling Organs | MP Abundance | Main Compositions of MPs | Reference |
|---|---|---|---|---|---|
| Amundsen Sea | demersal fish | gastrointestinal tract | 1.227 items individual−1, 1.914 items g−1 | Rayon, SRY, PES, PAM, AC, epoxy resin | this study |
| Ross Sea | demersal fish | gastrointestinal tract | 1.286 items individual−1, 1.951 items g−1 | PAM, PES, rayon, EVA, henequen | this study |
| Arctic | demersal fish | digestive tract | 1.1 ± 0.3 items individual−1 | PES, acrylic, PA, PE, EVA | [48] |
| North Atlantic | mesopelagic fish | digestive tract | 0.13 items individual−1 | - | [49] |
| the Gulf of California | benthic fish | gastrointestinal tract | 2.72 items individual−1 | PAM, PA, PET, PE, PP, polyacrylic | [50] |
| Turkish territorial waters of the Mediterranean Sea | pelagic fish, demersal fish | gastrointestinal tract | 1.36 items individual−1 | PS, PA, polyamide resin | [51] |
| Fortaleza coastal zone, Brazil | pelagic fish | stomach | 1.53 items individual−1 | PES | [52] |
| South China Sea | deep-sea fish | intestine | 1.77 ± 0.73 items individual−1 | cellophane, PA, PET, PARA, PAE | [53] |
| Australia and Fiji | reef-associated, demersal and benthopelagic | gastrointestinal tract | 1.58 ± 0.23 items individual−1, 0.86 ± 0.14 items individual−1 | polyolefin | [54] |
| Seri Kembangan, Malaysia | reef-associated, pelagic-neritic, benthopelagic and demersal fish | viscera and gills | 0.39 items individual−1 | PE, PP, PET | [55] |
| Kerala, India | pelagic fish | muscle and skin; gill and viscera | 0.53 ± 0.77 items individual−1; 0.07 ± 0.26 items individual−1 | PE, PP, PS, polyurethane | [56] |
| Goa, west coast of India | benthic fish | gastrointestinal tract | 1.4 ± 0.3–7.8 ± 4.4 items individual−1 | PAM, polyacetylene, EVOH, PA, PVC | [57] |
| South China Sea; Indian Ocean | pelagic and benthic nektons | digestive tract | 0.39 ± 0.12 items individual−1; 1.00 ± 0.16 items individual−1 | PES, PA | [38] |
| Location | Species | Sampling | MP Abundance | Main Types of MPs | Reference |
|---|---|---|---|---|---|
| Admiralty Bay, Antarctica | zooplankton | - | 2.40 ± 4.57 items 100 m−3 | polyethyleneglycols, PU, PET, PA | [58] |
| Terra Nova Bay (Ross Sea, Antarctica) | invertebrate species | - | 0.7 items mg−1 | PA, PE | [59] |
| Namuncurá at Burdwood Bank, Southwest Atlantic Ocean | sea stars | soft tissue | 2.84 ± 3.56 items g−1 wet weight | semi-synthetic cellulose | [20] |
| South Shetland Islands | fish | gastrointestinal tract | 0.36 ± 0.51 items individual−1 | PET, PP, PA, PAN | [60] |
| Banzare Banks of Southern Ocean | fish | gastrointestinal tract | 0.04 items individual−1 | acrylic resin | [10] |
| Antarctic Peninsula and Scotia Sea region | penguins | scat | 0.1 items scat−1 | PE, PES | [21] |
| South Georgia | penguins | scat | 0.13 items scat−1 | semi-synthetic (viscose, rayon), PET, acrylic, PP | [61] |
| Bird Island, South Georgia and Signy Station, South Orkney Islands | penguins | scat | 0.23 ± 0.53 items scat−1 | PES, rayon | [62] |
| sub-Antarctic Heard Island | sea birds | digestive systems | 0.11 items individual−1 | - | [22] |
| Ardery Island, near the Australian station Casey | sea birds | stomach | 1.12 items individual−1 | - | [63] |
| North Head Peninsula, Macquarie Island | fur seals | scat | 1.13 items scat−1 | PE, PP | [23] |
| Deception Island, South Shetland Islands | fur seals | scat | 0 items scat−1 | - | [15] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Liu, S.; Bo, J.; Zheng, R.; Hong, F.; Gao, F.; Miao, X.; Li, H.; Fang, C. First Evidence of Microplastic Contamination in Antarctic Fish (Actinopterygii, Perciformes). Water 2022, 14, 3070. https://doi.org/10.3390/w14193070
Zhang M, Liu S, Bo J, Zheng R, Hong F, Gao F, Miao X, Li H, Fang C. First Evidence of Microplastic Contamination in Antarctic Fish (Actinopterygii, Perciformes). Water. 2022; 14(19):3070. https://doi.org/10.3390/w14193070
Chicago/Turabian StyleZhang, Min, Shigang Liu, Jun Bo, Ronghui Zheng, Fukun Hong, Fulong Gao, Xing Miao, Hai Li, and Chao Fang. 2022. "First Evidence of Microplastic Contamination in Antarctic Fish (Actinopterygii, Perciformes)" Water 14, no. 19: 3070. https://doi.org/10.3390/w14193070
APA StyleZhang, M., Liu, S., Bo, J., Zheng, R., Hong, F., Gao, F., Miao, X., Li, H., & Fang, C. (2022). First Evidence of Microplastic Contamination in Antarctic Fish (Actinopterygii, Perciformes). Water, 14(19), 3070. https://doi.org/10.3390/w14193070







