Climate and Land Use Driven Ecosystem Homogenization in the Prairie Pothole Region
Abstract
:1. Introduction
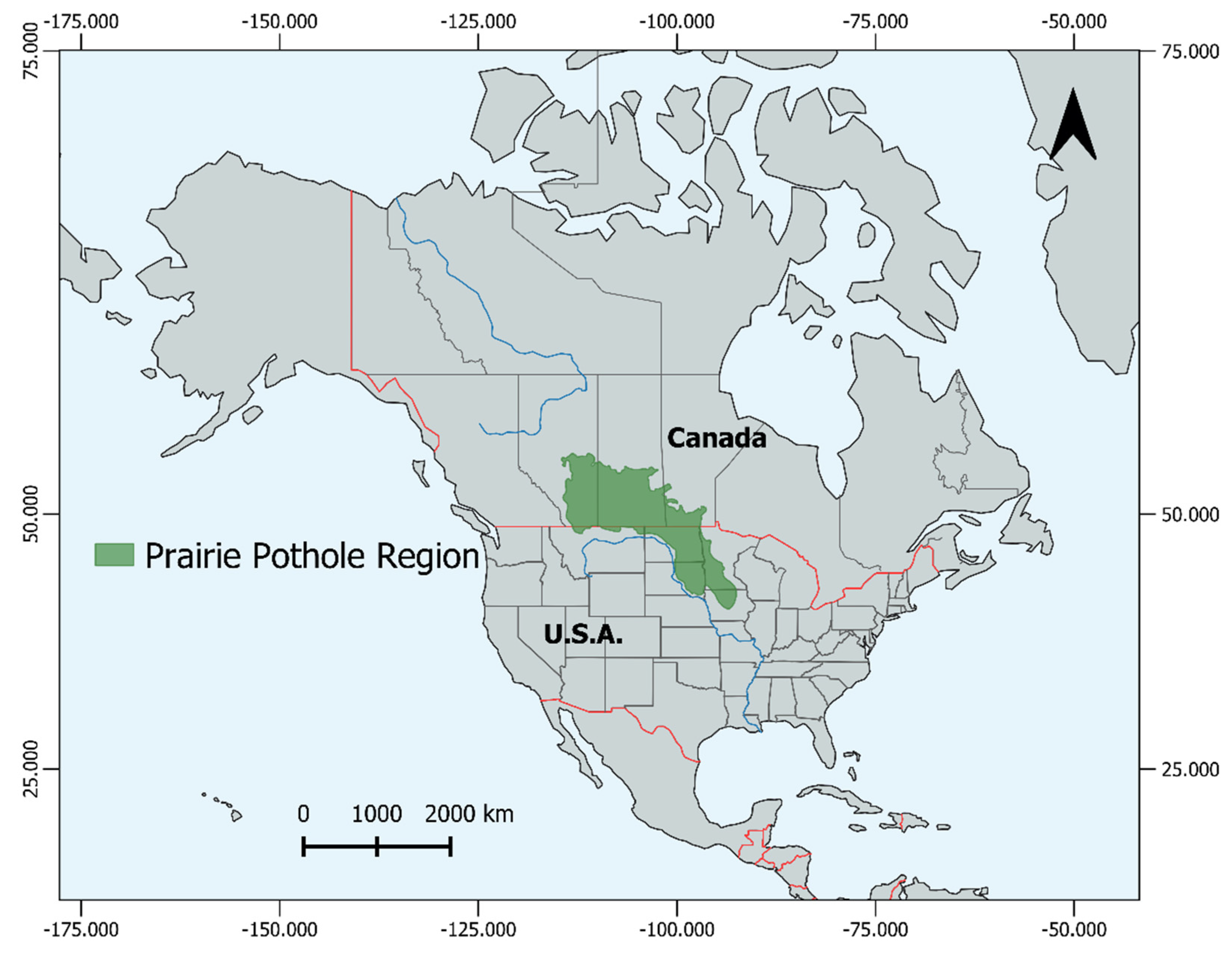
Prairie Pothole Wetland Ecosystem Variability and Function
2. Ecosystem Homogenization
2.1. Conceptual Model for Ecosystem Homogenization
2.2. Mechanisms for Ecosystem Homogenization in the PPR
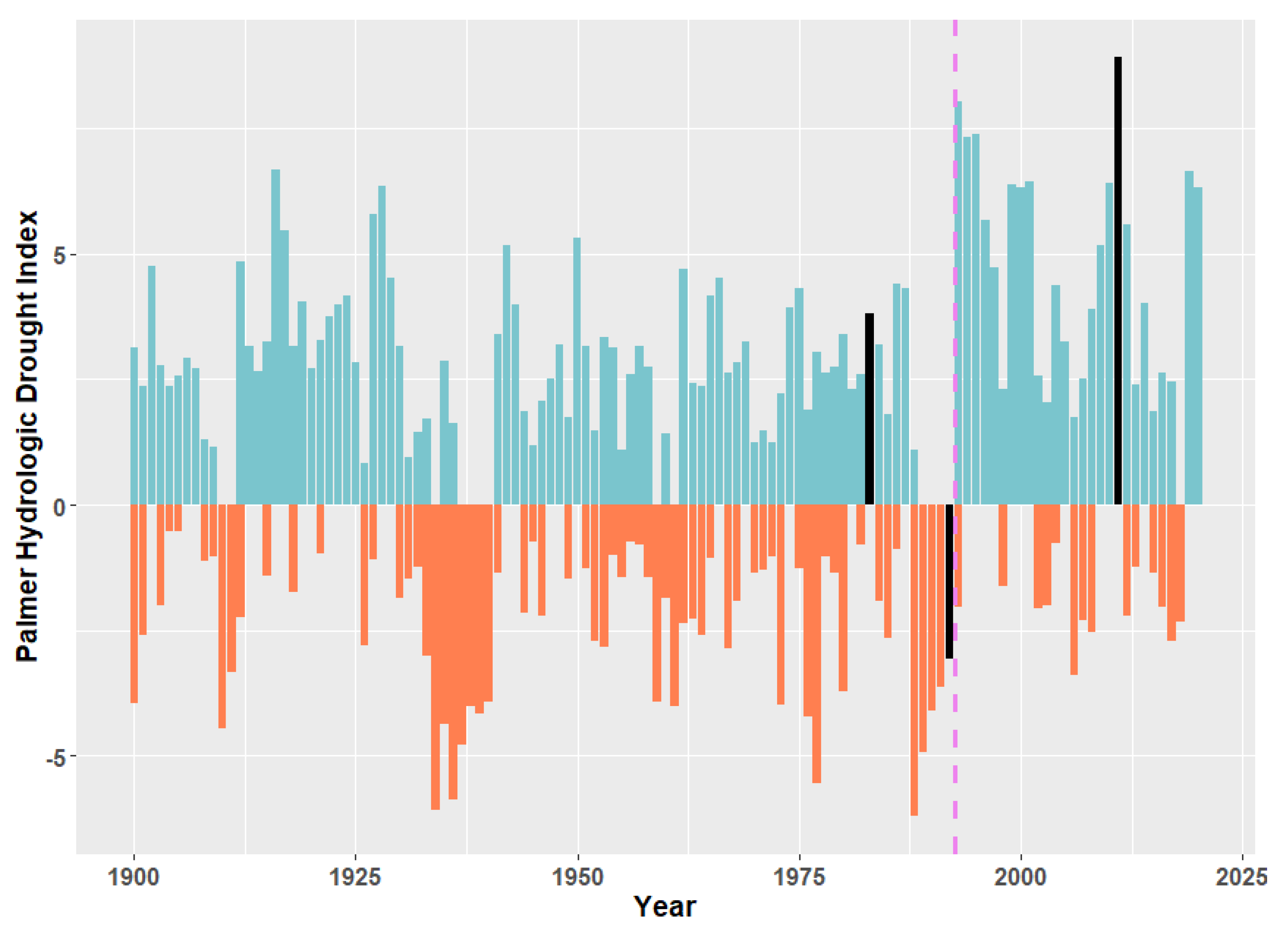
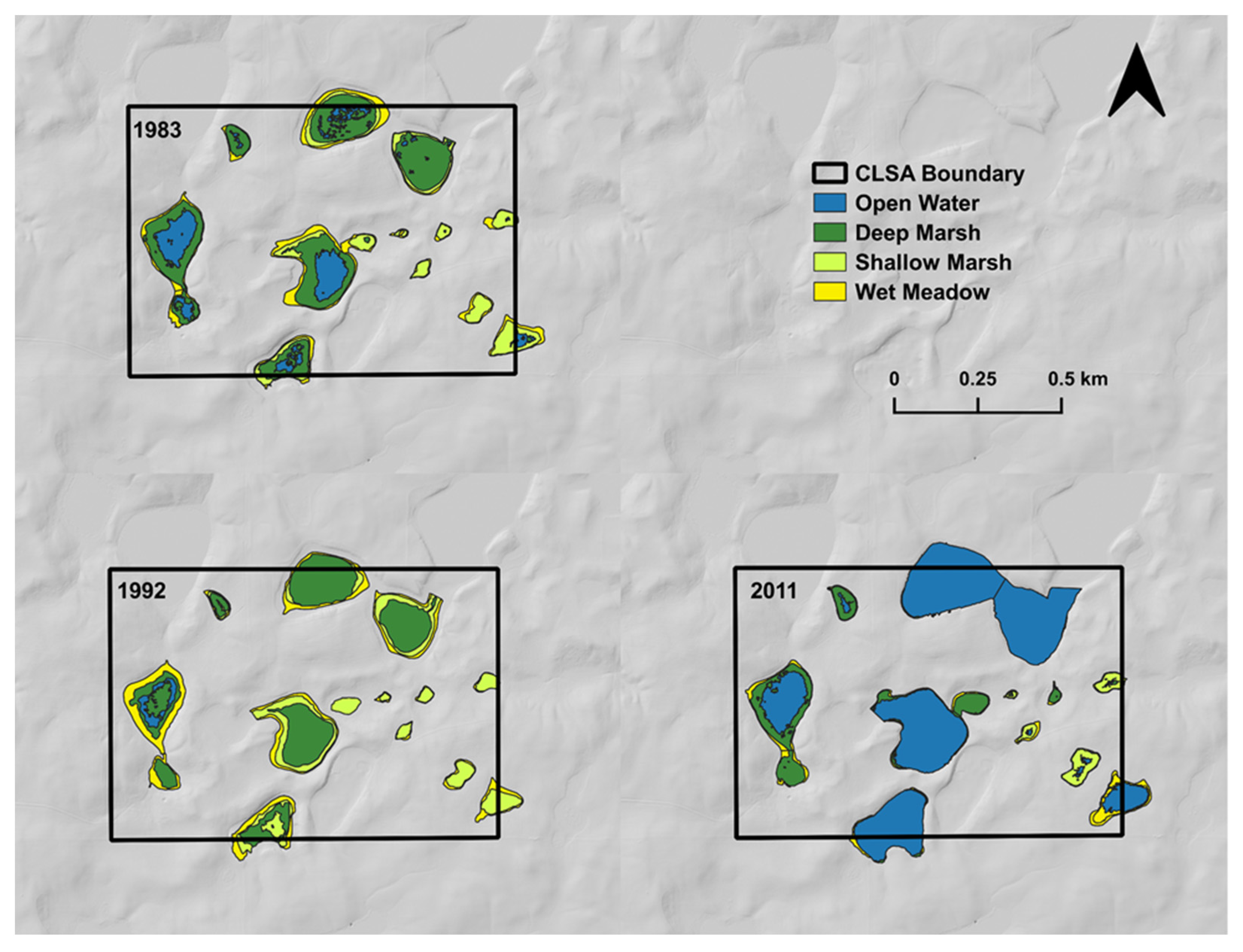
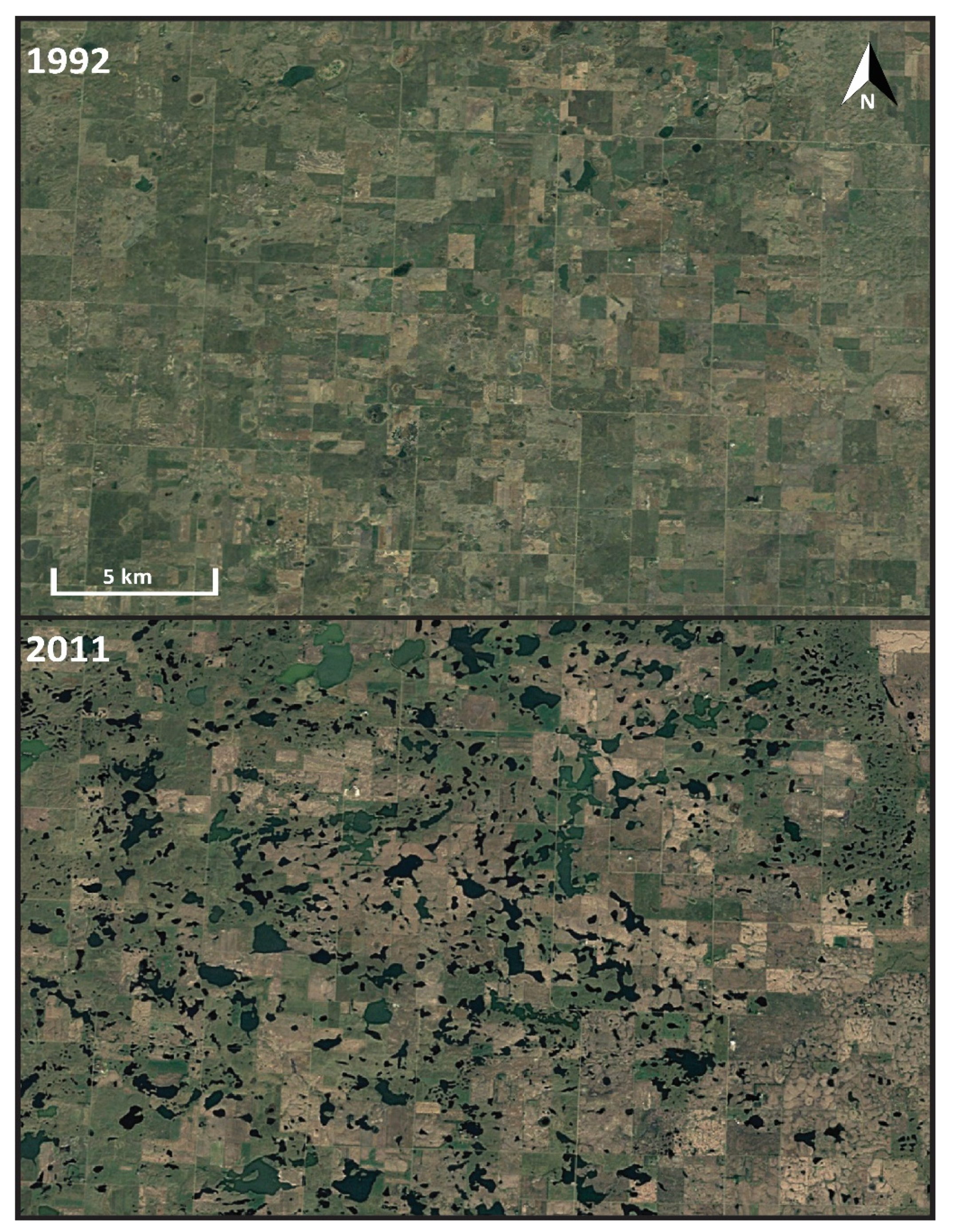
3. Evidence for Ecosystem Homogenization
4. Consequences of Ecosystem Homogenization
5. Potential Impacts of Continued Climate Change
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dudgeon, D.; Arthington, A.H.; Gessner, M.O.; Kawabata, Z.-I.; Knowler, D.J.; Lévêque, C.; Naiman, R.J.; Prieur-Richard, A.-H.; Soto, D.; Stiassny, M.L.J.; et al. Freshwater Biodiversity: Importance, Threats, Status and Conservation Challenges. Biol. Rev. 2006, 81, 163. [Google Scholar] [CrossRef] [PubMed]
- Reid, A.J.; Carlson, A.K.; Creed, I.F.; Eliason, E.J.; Gell, P.A.; Johnson, P.T.; Kidd, K.A.; MacCormack, T.J.; Olden, J.D.; Ormerod, S.J. Emerging Threats and Persistent Conservation Challenges for Freshwater Biodiversity. Biol. Rev. 2019, 94, 849–873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahel, F.J. Homogenization of Freshwater Faunas. Annu. Rev. Ecol. Syst. 2002, 33, 291–315. [Google Scholar] [CrossRef] [Green Version]
- Poff, N.L.; Olden, J.D.; Merritt, D.M.; Pepin, D.M. Homogenization of Regional River Dynamics by Dams and Global Biodiversity Implications. Proc. Natl. Acad. Sci. USA 2007, 104, 5732–5737. [Google Scholar] [CrossRef] [Green Version]
- Bedford, B.L. Cumulative Effects on Wetland Landscapes: Links to Wetland Restoration in the United States and Southern Canada. Wetlands 1999, 19, 775–788. [Google Scholar] [CrossRef]
- Zedler, J.B.; Kercher, S. Wetland Resources: Status, Trends, Ecosystem Services, and Restorability. Annu. Rev. Environ. Resour. 2005, 30, 39–74. [Google Scholar] [CrossRef] [Green Version]
- Otte, A.; Simmering, D.; Wolters, V. Biodiversity at the Landscape Level: Recent Concepts and Perspectives for Multifunctional Land Use. Landsc. Ecol. 2007, 22, 639–642. [Google Scholar] [CrossRef]
- Mouillot, D.; Villéger, S.; Scherer-Lorenzen, M.; Mason, N.W.H. Functional Structure of Biological Communities Predicts Ecosystem Multifunctionality. PLoS ONE 2011, 6, e17476. [Google Scholar] [CrossRef] [Green Version]
- Houghton, D.C. The Effects of Landscape-Level Disturbance on the Composition of Minnesota Caddisfly (Insecta: Trichoptera) Trophic Functional Groups: Evidence for Ecosystem Homogenization. Environ. Monit. Assess. 2007, 135, 253–264. [Google Scholar] [CrossRef]
- Moyle, P.B.; Mount, J.F. Homogenous Rivers, Homogenous Faunas. Proc. Natl. Acad. Sci. USA 2007, 104, 5711–5712. [Google Scholar] [CrossRef]
- Rahel, F.J. Biogeographic Barriers, Connectivity and Homogenization of Freshwater Faunas: It’s a Small World after All. Freshw. Biol. 2007, 52, 696–710. [Google Scholar] [CrossRef]
- Zeni, J.O.; Casatti, L. The Influence of Habitat Homogenization on the Trophic Structure of Fish Fauna in Tropical Streams. Hydrobiologia 2014, 726, 259–270. [Google Scholar] [CrossRef]
- Creed, I.F.; Lane, C.R.; Serran, J.N.; Alexander, L.C.; Basu, N.B.; Calhoun, A.J.K.; Christensen, J.R.; Cohen, M.J.; Craft, C.; D’Amico, E.; et al. Enhancing Protection for Vulnerable Waters. Nat. Geosci. 2017, 10, 809–815. [Google Scholar] [CrossRef] [PubMed]
- Finlayson, M.; Cruz, R.D.; Davidson, N.; Alder, J.; Cork, S.; de Groot, R.S.; Lévêque, C.; Milton, G.R.; Peterson, G.; Pritchard, D. Millennium Ecosystem Assessment: Ecosystems and Human Well-Being: Wetlands and Water Synthesis; Island Press: Washington DC, USA, 2005. [Google Scholar]
- Cohen, M.J.; Creed, I.F.; Alexander, L.; Basu, N.B.; Calhoun, A.J.K.; Craft, C.; D’Amico, E.; DeKeyser, E.; Fowler, L.; Golden, H.E.; et al. Do Geographically Isolated Wetlands Influence Landscape Functions? Proc. Natl. Acad. Sci. USA 2016, 113, 1978–1986. [Google Scholar] [CrossRef] [Green Version]
- Gleason, R.A.; Laubhan, M.K.; Euliss, N.H., Jr.; Tangen, B.A.; Kermes, K.E. Ecosystem Services Derived from Wetland Conservation Practices in the United States Prairie Pothole Region with an Emphasis on the US Department of Agriculture Conservation Reserve and Wetlands Reserve Programs; US Geological Survey: Reston, VA, USA, 2008.
- Frayer, W.E. Status and Trends of Wetlands and Deepwater Habitats in the Conterminous United States, 1950’s to 1970’s. Colorado State University: Fort Collins, CO, USA, 1983; p. 32. [Google Scholar]
- Tiner, R.W. Wetlands of the United States: Current Status and Recent Trends; US Fish & Wildlife Service, National Wetlands Inventory: Washington DC, USA, 1984.
- Brinson, M.M.; Malvárez, A.I. Temperate Freshwater Wetlands: Types, Status, and Threats. Environ. Conserv. 2002, 29, 115–133. [Google Scholar] [CrossRef]
- Stenert, C.; Pires, M.M.; Epele, L.B.; Grech, M.G.; Maltchik, L.; McLean, K.I.; Mushet, D.M.; Batzer, D.P. Climate-versus Geographic-Dependent Patterns in the Spatial Distribution of Macroinvertebrate Assemblages in New World Depressional Wetlands. Glob. Chang. Biol. 2020, 26, 6895–6903. [Google Scholar] [CrossRef]
- Price, E.P.F.; Spyreas, G.; Matthews, J.W. Biotic homogenization of regional wetland plant communities within short time-scales in the presence of an aggressive invader. J. Ecol. 2018, 106, 1180–1190. [Google Scholar] [CrossRef]
- Richardson, J.L.; Arndt, J.L.; Freeland, J. Wetland Soils of the Prairie Potholes. Adv. Agron. 1994, 52, 121–171. [Google Scholar]
- Euliss, N.H.; Mushet, D.M.; Newton, W.E.; Otto, C.R.; Nelson, R.D.; LaBaugh, J.W.; Scherff, E.J.; Rosenberry, D.O. Placing Prairie Pothole Wetlands along Spatial and Temporal Continua to Improve Integration of Wetland Function in Ecological Investigations. J. Hydrol. 2014, 513, 490–503. [Google Scholar] [CrossRef]
- Bam, E.K.; Ireson, A.M.; van der Kamp, G.; Hendry, J.M. Ephemeral Ponds: Are They the Dominant Source of Depression-Focused Groundwater Recharge? Water Resour. Res. 2020, 56, e2019WR026640. [Google Scholar] [CrossRef] [Green Version]
- Anteau, M.J.; Wiltermuth, M.T.; van der Burg, M.P.; Pearse, A.T. Prerequisites for Understanding Climate-Change Impacts on Northern Prairie Wetlands. Wetlands 2016, 36, 299–307. [Google Scholar] [CrossRef]
- Winter, T.C. A Conceptual Framework for Assessing Cumulative Impacts on the Hydrology of Nontidal Wetlands. Environ. Manag. 1988, 12, 605–620. [Google Scholar] [CrossRef]
- Euliss, N.H.; LaBaugh, J.W.; Fredrickson, L.H.; Mushet, D.M.; Laubhan, M.K.; Swanson, G.A.; Winter, T.C.; Rosenberry, D.O.; Nelson, R.D. The Wetland Continuum: A Conceptual Framework for Interpreting Biological Studies. Wetlands 2004, 24, 448–458. [Google Scholar] [CrossRef] [Green Version]
- Smith, A.G.; Stoudt, J.H.; Gollop, J.B. Prairie Potholes and Marshes. In Waterfowl Tomorrow; U.S. Fish and Wildlife Service: Washington, DC, USA, 1964; pp. 39–50. [Google Scholar]
- Van der Valk, A.G. The Prairie Potholes of North America; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Winter, T.C.; Rosenberry, D.O. Hydrology of Prairie Pothole Wetlands during Drought and Deluge: A 17-Year Study of the Cottonwood Lake Wetland Complex in North Dakota in the Perspective of Longer Term Measured and Proxy Hydrological Records. Clim. Chang. 1998, 40, 189–209. [Google Scholar] [CrossRef]
- Hayashi, M.; van der Kamp, G.; Rosenberry, D.O. Hydrology of Prairie Wetlands: Understanding the Integrated Surface-Water and Groundwater Processes. Wetlands 2016, 36, 237–254. [Google Scholar] [CrossRef]
- LaBaugh, J.; Winter, T.C.; Adomaitis, V.; Swanson, G.A. Hydrology and Chemistry of Selected Prairie Wetlands in the Cottonwood Lake Area, Stutsman County, North Dakota, 1979-82; US Geological Survey Prof. Paper; USGS Publications Warehouse: Reston, VA, USA, 1987; p. 1431.
- Cowardin, L.M. Classification of Wetlands & Deepwater Habitats of the U. S.; FWS/OBS-79/31; U.S. Fish and Wildlife Service: Washington, DC, USA, 1979.
- Swanson, G.A. Chemical Characteristics of Prairie Lakes in South-Central North Dakota: Their Potential for Influencing Use by Fish and Wildlife; US Department of the Interior, Fish and Wildlife Service: Washington, DC, USA, 1988.
- Gleason, R.A.; Euliss, N.H.; Tangen, B.A.; Laubhan, M.K.; Browne, B.A. USDA Conservation Program and Practice Effects on Wetland Ecosystem Services in the Prairie Pothole Region. Ecol. Appl. 2011, 21. [Google Scholar] [CrossRef]
- Nahlik, A.M.; Fennessy, M.S. Carbon Storage in US Wetlands. Nat. Commun. 2016, 7, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Crumpton, W.G.; Isenhart, T.M.; Mitchell, P.D. Nitrate and Organic N Analyses with Second-Derivative Spectroscopy. Limnol. Oceanogr. 1992, 37, 907–913. [Google Scholar] [CrossRef] [Green Version]
- van der Kamp, G.; Hayashi, M. The groundwater recharge function of small wetlands in the semi-arid Northern Prairies. Great Plains Res. 1998, 8, 39–56. [Google Scholar]
- Euliss, N.H., Jr.; Gleason, R.A.; Olness, A.; McDougal, R.L.; Murkin, H.R.; Robarts, R.D.; Bourbonniere, R.A.; Warner, B.G. North American Prairie Wetlands Are Important Nonforested Land-Based Carbon Storage Sites. Sci. Total Environ. 2006, 361, 179–188. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.; Young, C.; Feng, M.; Heidemann, K.; Cushing, M.; Mushet, D.M.; Liu, S. Demonstration of a Conceptual Model for Using LiDAR to Improve the Estimation of Floodwater Mitigation Potential of Prairie Pothole Region Wetlands. J. Hydrol. 2011, 405, 417–426. [Google Scholar] [CrossRef] [Green Version]
- Euliss, N.H., Jr.; Wrubleski, D.A.; Mushet, D.M. Wetlands of the Prairie Pothole Region: Invertebrate Species Composition, Ecology, and Management; USGS Publications Warehouse: Southeast Jamestown, ND, USA, 1999.
- Johnson, R.R.; Oslund, F.T.; Hertel, D.R. The Past, Present, and Future of Prairie Potholes in the United States. J. Soil Water Conserv. 2008, 63, 84A–87A. [Google Scholar] [CrossRef]
- Batt, B.D. The Ecology and Management of Breeding Waterfowl; University of Minnesota Press: Minneapolis, MN, USA, 1987; ISBN 978-1-4529-0016-2. [Google Scholar]
- Sorenson, L.G.; Goldberg, R.; Root, T.L.; Anderson, M.G. Potential Effects of Global Warming on Waterfowl Populations Breeding in the Northern Great Plains. Clim. Chang. 1998, 40, 343–369. [Google Scholar] [CrossRef]
- van der Kamp, G.; Hayashi, M.; Gallen, D. Comparing the Hydrology of Grassed and Cultivated Catchments in the Semi-Arid Canadian Prairies. Hydrol. Process. 2003, 17, 559–575. [Google Scholar] [CrossRef]
- Renton, D.A.; Mushet, D.M.; DeKeyser, E.S. Climate Change and Prairie Pothole Wetlands: Mitigating Water-Level and Hydroperiod Effects through Upland Management; US Department of the Interior: Washington, DC, USA, 2015; p. 32.
- Thiere, G.; Milenkovski, S.; Lindgren, P.-E.; Sahlén, G.; Berglund, O.; Weisner, S.E. Wetland Creation in Agricultural Landscapes: Biodiversity Benefits on Local and Regional Scales. Biol. Conserv. 2009, 142, 964–973. [Google Scholar] [CrossRef]
- Shook, K.R.; Pomeroy, J.W. Memory Effects of Depressional Storage in Northern Prairie Hydrology. Hydrol. Process. 2011, 25, 3890–3898. [Google Scholar] [CrossRef]
- McIntyre, N.E.; Wright, C.K.; Swain, S.; Hayhoe, K.; Liu, G.; Schwartz, F.W.; Henebry, G.M. Climate forcing of wetland landscape connectivity in the Great Plains. Front. Ecol. Environ. 2014, 12, 59–64. [Google Scholar] [CrossRef]
- Martin, A.R.; Soupir, M.L.; Kaleita, A.L. Seasonal and Intra-Event Nutrient Levels in Farmed Prairie Potholes of the Des Moines Lobe. Trans. ASABE 2019, 62, 1607–1617. [Google Scholar] [CrossRef]
- Gibbs, J.P. Wetland Loss and Biodiversity Conservation. Conserv. Biol. 2000, 14, 314–317. [Google Scholar] [CrossRef] [Green Version]
- McKenna, O.P.; Mushet, D.M.; Scherff, E.J.; McLean, K.I.; Mills, C.T. The Pothole Hydrology-Linked Systems Simulator (PHyLiSS)—Development and Application of a Systems Model for Prairie-Pothole Wetlands, 2018-1165; Geological Survey (USGS): Reston, VA, USA, 2018; p. 34.
- Elliott, L.H.; Igl, L.D.; Johnson, D.H. The Relative Importance of Wetland Area versus Habitat Heterogeneity for Promoting Species Richness and Abundance of Wetland Birds in the Prairie Pothole Region, USA. Condor 2020, 122, duz060. [Google Scholar] [CrossRef] [Green Version]
- McLean, K.I.; Mushet, D.M.; Sweetman, J.N.; Anteau, M.J.; Wiltermuth, M.T. Invertebrate Communities of Prairie-Pothole Wetlands in the Age of the Aquatic Homogenocene. Hydrobiologia 2019, 1–21. [Google Scholar] [CrossRef]
- Mushet, D.M.; McKenna, O.P.; LaBaugh, J.W.; Euliss, N.H.; Rosenberry, D.O. Accommodating State Shifts within the Conceptual Framework of the Wetland Continuum. Wetlands 2018, 38, 647–651. [Google Scholar] [CrossRef]
- McKenna, O.P.; Mushet, D.M.; Rosenberry, D.O.; LaBaugh, J.W. Evidence for a Climate-Induced Ecohydrological State Shift in Wetland Ecosystems of the Southern Prairie Pothole Region. Clim. Chang. 2017, 145, 273–287. [Google Scholar] [CrossRef]
- McKenna, O.P.; Kucia, S.R.; Mushet, D.M.; Anteau, M.J.; Wiltermuth, M.T. Synergistic Interaction of Climate and Land-Use Drivers Alter the Function of North American, Prairie-Pothole Wetlands. Sustainability 2019, 11, 6581. [Google Scholar] [CrossRef] [Green Version]
- Coote, D.R.; Gregorich, L.J. The Health of Our Water: Toward Sustainable Agriculture in Canada; Agriculture and Agri-Food Canada: Ottawa, CA, Canada, 2000; p. 19. [Google Scholar]
- Anteau, M.J. Do Interactions of Land Use and Climate Affect Productivity of Waterbirds and Prairie-Pothole Wetlands? Wetlands 2012, 32, 1–9. [Google Scholar] [CrossRef]
- Calhoun, A.J.; Mushet, D.M.; Bell, K.P.; Boix, D.; Fitzsimons, J.A.; Isselin-Nondedeu, F. Temporary Wetlands: Challenges and Solutions to Conserving a ‘Disappearing’Ecosystem. Biol. Conserv. 2017, 211, 3–11. [Google Scholar] [CrossRef]
- Rains, M.C.; Leibowitz, S.G.; Cohen, M.J.; Creed, I.F.; Golden, H.E.; Jawitz, J.W.; Kalla, P.; Lane, C.R.; Lang, M.W.; McLaughlin, D.L. Geographically Isolated Wetlands Are Part of the Hydrological Landscape. Hydrol. Process. 2016, 30, 153–160. [Google Scholar] [CrossRef]
- Van Meter, K.J.; Basu, N.B. Signatures of Human Impact: Size Distributions and Spatial Organization of Wetlands in the Prairie Pothole Landscape. Ecol. Appl. 2015, 25, 451–465. [Google Scholar] [CrossRef] [Green Version]
- Serran, J.N.; Creed, I.F. New Mapping Techniques to Estimate the Preferential Loss of Small Wetlands on Prairie Landscapes. Hydrol. Process. 2016, 30, 396–409. [Google Scholar] [CrossRef]
- Dahl, T.E. Wetlands Losses in the United States, 1780’s to 1980’s. Report to the Congress; National Wetlands Inventory: St. Petersburg, FL, USA), 1990.
- Dahl, T.E. Status and Trends of Prairie Wetlands in the United States 1997 to 2009; US Fish and Wildlife Service: Washington, DC, USA, 2014.
- Johnston, C.A. Wetland Losses Due to Row Crop Expansion in the Dakota Prairie Pothole Region. Wetlands 2013, 33, 175–182. [Google Scholar] [CrossRef]
- Jenkins, D.G.; Grissom, S.; Miller, K. Consequences of Prairie Wetland Drainage for Crustacean Biodiversity and Metapopulations. Conserv. Biol. 2003, 17, 158–167. [Google Scholar] [CrossRef]
- Vanderhoof, M.K.; Christensen, J.R.; Alexander, L.C. Patterns and Drivers for Wetland Connections in the Prairie Pothole Region, United States. Wetl. Ecol. Manag. 2017, 25, 275–297. [Google Scholar] [CrossRef] [PubMed]
- McCauley, L.A.; Anteau, M.J.; van der Burg, M.P.; Wiltermuth, M.T. Land Use and Wetland Drainage Affect Water Levels and Dynamics of Remaining Wetlands. Ecosphere 2015, 6, 1–22. [Google Scholar] [CrossRef]
- Mac, M.J.; Opler, P.A.; Haecker, C.E.; Doran, P.D. Status and Trends of the Nation’s Biological Resources, Volume 2; U.S. Department of the Interior, U.S. Geological Survey: Reston, VA, USA, 1998.
- DeKeyser, E.S.; Meehan, M.; Clambey, G.; Krabbenhoft, K. Cool Season Invasive Grasses in Northern Great Plains Natural Areas. Nat. Areas J. 2013, 33, 81–90. [Google Scholar] [CrossRef]
- Toledo, D.; Sanderson, M.; Spaeth, K.; Hendrickson, J.; Printz, J. Extent of Kentucky Bluegrass and Its Effect on Native Plant Species Diversity and Ecosystem Services in the Northern Great Plains of the United States. Invasive Plant Sci. Manag. 2014, 7, 543–552. [Google Scholar] [CrossRef]
- Dixon, C.; Vacek, S.; Grant, T. Evolving Management Paradigms on U.S. Fish and Wildlife Service Lands in the Prairie Pothole Region. Rangelands 2019, 41, 36–43. [Google Scholar] [CrossRef]
- Jones, S. Assessment of Prairie Pothole Conditions and Plant Community Composition on FWS Fee-Title Lands. Master’s Thesis, North Dakota State University, Fargo, ND, USA, 2021. [Google Scholar]
- Gleason, R.A.; Euliss, N.H., Jr. Sedimentation of Prairie Wetlands. Gt. Plains Res. 1998, 97–112. [Google Scholar]
- Main, A.R.; Headley, J.V.; Peru, K.M.; Michel, N.L.; Cessna, A.J.; Morrissey, C.A. Widespread Use and Frequent Detection of Neonicotinoid Insecticides in Wetlands of Canada’s Prairie Pothole Region. PLoS ONE 2014, 9, e92821. [Google Scholar] [CrossRef]
- Williams, N.; Sweetman, J. Distribution and Concentration of Neonicotinoid Insecticides on Waterfowl Production Areas in West Central Minnesota. Wetlands 2019, 39, 311–319. [Google Scholar] [CrossRef]
- Euliss, N.H., Jr.; Mushet, D.M. Impacts of Water Development on Aquatic Macroinvertebrates, Amphibians, and Plants in Wetlands of a Semi-Arid Landscape. Aquat. Ecosyst. Health Manag. 2004, 7, 73–84. [Google Scholar] [CrossRef] [Green Version]
- McIntyre, N.E.; Liu, G.; Gorzo, J.; Wright, C.K.; Guntenspergen, G.R.; Schwartz, F. Simulating the Effects of Climate Variability on Waterbodies and Wetland-Dependent Birds in the Prairie Pothole Region. Ecosphere 2019, 10, e02711. [Google Scholar] [CrossRef] [Green Version]
- Mushet, D.M.; Neau, J.L.; Euliss, N.H., Jr. Modeling Effects of Conservation Grassland Losses on Amphibian Habitat. Biol. Conserv. 2014, 174, 93–100. [Google Scholar] [CrossRef]
- Pearse, A.T.; Anteau, M.J.; Post van der Burg, M.; Sherfy, M.H.; Buhl, T.K.; Shaffer, T.L. Reassessing Perennial Cover as a Driver of Duck Nest Survival in the Prairie Pothole Region. J. Wildl. Manag. 2022, 86, e22227. [Google Scholar] [CrossRef]
- Ballard, T.; Seager, R.; Smerdon, J.E.; Cook, B.I.; Ray, A.J.; Rajagopalan, B.; Kushnir, Y.; Nakamura, J.; Henderson, N. Hydroclimate Variability and Change in the Prairie Pothole Region, the “Duck Factory” of North America. Earth Interact. 2014, 18, 1–28. [Google Scholar] [CrossRef]
- Mushet, D.M.; Goldhaber, M.B.; Mills, C.T.; McLean, K.I.; Aparicio, V.M.; McCleskey, R.B.; Holloway, J.M.; Stockwell, C.A. Chemical and Biotic Characteristics of Prairie Lakes and Large Wetlands in South-Central North Dakota—Effects of a Changing Climate; U.S. Department of the Interior, U.S. Geological Survey: Reston, VA, USA, 2015.
- Johnston, C.A.; McIntyre, N.E. Effects of Cropland Encroachment on Prairie Pothole Wetlands: Numbers, Density, Size, Shape, and Structural Connectivity. Landsc. Ecol. 2019, 34, 827–841. [Google Scholar] [CrossRef]
- Kahara, S.N.; Mockler, R.M.; Higgins, K.F.; Chipps, S.R.; Johnson, R.R. Spatiotemporal Patterns of Wetland Occurrence in the Prairie Pothole Region of Eastern South Dakota. Wetlands 2009, 29, 678–689. [Google Scholar] [CrossRef]
- Wiltermuth, M.T. Influences of Climate Variability and Landscape Modifications on Water Dynamics, Community Structure, and Amphipod Populations in Large Prairie Wetlands: Implications for Waterbird Conservation. Ph.D. Thesis, North Dakota State University, Fargo, ND, USA, 2014. [Google Scholar]
- Liu, G.; Schwartz, F.W. Climate-Driven Variability in Lake and Wetland Distribution across the Prairie Pothole Region: From Modern Observations to Long-Term Reconstructions with Space-for-Time Substitution. Water Resour. Res. 2012, 48. [Google Scholar] [CrossRef]
- Cressey, R.L.; Austin, J.E.; Stafford, J.D. Three Responses of Wetland Conditions to Climatic Extremes in the Prairie Pothole Region. Wetlands 2016, 36, 357–370. [Google Scholar] [CrossRef]
- Millett, B.; Johnson, W.C.; Guntenspergen, G. Climate Trends of the North American Prairie Pothole Region 1906–2000. Clim. Chang. 2009, 93, 243–267. [Google Scholar] [CrossRef]
- Krapu, C.; Kumar, M.; Borsuk, M. Identifying Wetland Consolidation Using Remote Sensing in the North Dakota Prairie Pothole Region. Water Resour. Res. 2018, 54, 7478–7494. [Google Scholar] [CrossRef]
- Mushet, D.M.; McKenna, O.P.; McLean, K.I. Alternative Stable States in Inherently Unstable Systems. Ecol. Evol. 2020, 10, 843–850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McLean, K.I.; Mushet, D.M.; Sweetman, J.N. Temporal Coherence Patterns of Prairie Pothole Wetlands Indicate the Importance of Landscape Linkages and Wetland Heterogeneity in Maintaining Biodiversity. Front. Ecol. Evol. 2022, 801. [Google Scholar] [CrossRef]
- Yansa, C.H. Holocone Paleovegetation and Paleohydrology of a Prairie Pothold in Southern Saskatchewan, Canada. J. Paleolimnol. 1998, 19, 429–441. [Google Scholar] [CrossRef]
- Hu, K.; Mushet, D.M.; Sweetman, J.N. Multiproxy Paleolimnological Records Provide Evidence for a Shift to a New Ecosystem State in the Northern Great Plains, USA. Limnol. Oceanogr. 2022. [Google Scholar] [CrossRef]
- Cressey, R. Changes in Wetland Conditions and Wetland Plant Communities in the Prairie Pothole Region after 50 Years; South Dakota State University: Brookings, SD, USA, 2016. [Google Scholar]
- LaBaugh, J.W.; Rosenberry, D.O.; Mushet, D.M.; Neff, B.P.; Nelson, R.D.; Euliss, N.H., Jr. Long-Term Changes in Pond Permanence, Size, and Salinity in Prairie Pothole Region Wetlands: The Role of Groundwater-Pond Interaction. J. Hydrol. Reg. Stud. 2018, 17, 1–23. [Google Scholar] [CrossRef]
- McCauley, L.A.; Anteau, M.J.; van der Burg, M.P. Consolidation Drainage and Climate Change May Reduce Piping Plover Habitat in the Great Plains. J. Fish Wildl. Manag. 2015, 7, 4–13. [Google Scholar] [CrossRef] [Green Version]
- Niemuth, N.D.; Wangler, B.; Reynolds, R.E. Spatial and Temporal Variation in Wet Area of Wetlands in the Prairie Pothole Region of North Dakota and South Dakota. Wetlands 2010, 30, 1053–1064. [Google Scholar] [CrossRef]
- Vanderhoof, M.K.; Alexander, L.C. The Role of Lake Expansion in Altering the Wetland Landscape of the Prairie Pothole Region, United States. Wetlands 2016, 36, 309–321. [Google Scholar] [CrossRef] [Green Version]
- Vanderhoof, M.K.; Alexander, L.C.; Todd, M.J. Temporal and Spatial Patterns of Wetland Extent Influence Variability of Surface Water Connectivity in the Prairie Pothole Region, United States. Landsc. Ecol. 2016, 31, 805–824. [Google Scholar] [CrossRef] [Green Version]
- Mushet, D.M.; Euliss, N.H., Jr.; Rosenberry, D.O.; LaBaugh, J.W.; Bansal, S.; Levy, Z.F.; McKenna, O.P.; McLean, K.I.; Mills, C.T.; Neff, B.P.; et al. Lessons Learned from Wetlands Research at the Cottonwood Lake Study Area, Stutsman County, North Dakota, 1967–2021; U.S. Department of the Interior, U.S. Geological Survey: Reston, VA, USA, 2022.
- Shaw, D.A.; Vanderkamp, G.; Conly, F.M.; Pietroniro, A.; Martz, L. The Fill–Spill Hydrology of Prairie Wetland Complexes during Drought and Deluge. Hydrol. Process. 2012, 26, 3147–3156. [Google Scholar] [CrossRef]
- Leibowitz, S.G.; Mushet, D.M.; Newton, W.E. Intermittent Surface Water Connectivity: Fill and Spill vs. Fill and Merge Dynamics. Wetlands 2016, 2, 323–342. [Google Scholar] [CrossRef]
- Wiltermuth, M.T.; Anteau, M.J. Is Consolidation Drainage an Indirect Mechanism for Increased Abundance of Cattail in Northern Prairie Wetlands? Wetl. Ecol. Manag. 2016, 24, 533–544. [Google Scholar] [CrossRef]
- McLean, K.I.; Mushet, D.M.; Renton, D.A.; Stockwell, C.A. Aquatic-Macroinvertebrate Communities of Prairie-Pothole Wetlands and Lakes Under a Changed Climate. Wetlands 2016, 36, 423–435. [Google Scholar] [CrossRef]
- McLean, K.I.; Mushet, D.M.; Stockwell, C.A. From “Duck Factory” to “Fish Factory”: Climate Induced Changes in Vertebrate Communities of Prairie Pothole Wetlands and Small Lakes. Wetlands 2016, 36, 407–421. [Google Scholar] [CrossRef]
- Nachshon, U.; Ireson, A.; van der Kamp, G.; Davies, S.R.; Wheater, H.S. Impacts of Climate Variability on Wetland Salinization in the North American Prairies. Hydrol. Earth Syst. Sci. 2014, 18, 1251–1263. [Google Scholar] [CrossRef] [Green Version]
- Maurer, K.M.; Stewart, T.W.; Lorenz, F.O. Direct and Indirect Effects of Fish on Invertebrates and Tiger Salamanders in Prairie Pothole Wetlands. Wetlands 2014, 34, 735–745. [Google Scholar] [CrossRef]
- Lockwood, J.L.; McKinney, M.L. Biotic Homogenization; Springer: Boston, MA, USA, 2001; pp. 1–17. [Google Scholar]
- Olden, J.D. Biotic Homogenization: A New Research Agenda for Conservation Biogeography. J. Biogeogr. 2006, 33, 2027–2039. [Google Scholar] [CrossRef]
- Petsch, D.K. Causes and Consequences of Biotic Homogenization in Freshwater Ecosystems. Int. Rev. Hydrobiol. 2016, 101, 113–122. [Google Scholar] [CrossRef]
- Price, E.P.; Spyreas, G.; Matthews, J.W. Biotic Homogenization of Wetland Vegetation in the Conterminous United States Driven by Phalaris Arundinacea and Anthropogenic Disturbance. Landsc. Ecol. 2020, 1–14. [Google Scholar] [CrossRef]
- Aronson, M.F.J.; Galatowitsch, S. Long-Term Vegetation Development of Restored Prairie Pothole Wetlands. Wetlands 2008, 28, 883–895. [Google Scholar] [CrossRef]
- Stewart, R.E.; Kantrud, H.A. Classification of Natural Ponds and Lakes in the Glaciated Prairie Region; Resource Publication 92; Bureau of Sport Fisheries and Wildlife, U.S. Fish and Wildlife Service: Washington, DC, USA, 1971; p. 57.
- Kantrud, H.A.; Millar, J.B.; van der Valk, A.G. Vegetation of wetlands of the prairie pothole region. North. Prairie Wetl. 1989, 132–187. [Google Scholar]
- van der Valk, A.; Mushet, D.M. Interannual Water-Level Fluctuations and the Vegetation of Prairie Potholes: Potential Impacts of Climate Change. Wetlands 2016, 36, 397–406. [Google Scholar] [CrossRef]
- Glooschenko, W.A.; Tarnocai, C.; Zoltai, S.; Glooschenko, V. Wetlands of Canada and Greenland BT—Wetlands of the World: Inventory, Ecology and Management Volume I: Africa, Australia, Canada and Greenland, Mediterranean, Mexico, Papua New Guinea, South Asia, Tropical South America, United States; Whigham, D.F., Dykyjová, D., Hejný, S., Eds.; Springer: Dordrecht, The Netherlands, 1993; pp. 415–514. ISBN 978-94-015-8212-4. [Google Scholar]
- Bansal, S.; Lishawa, S.C.; Newman, S.; Tangen, B.A.; Wilcox, D.; Albert, D.; Windham-Myers, L. Typha (Cattail) Invasion in North American Wetlands: Biology, Regional Problems, Impacts, Ecosystem Services, and Management. Wetlands 2019, 39, 645–684. [Google Scholar] [CrossRef] [Green Version]
- McLean, K.I.; Mushet, D.M.; Newton, W.E.; Sweetman, J.N. Long-Term Multidecadal Data from a Prairie-Pothole Wetland Complex Reveal Controls on Aquatic-Macroinvertebrate Communities. Ecol. Indic. 2021, 126, 107678. [Google Scholar] [CrossRef]
- Gleason, J.E.; Bortolotti, J.Y.; Rooney, R.C. Wetland Microhabitats Support Distinct Communities of Aquatic Macroinvertebrates. J. Freshw. Ecol. 2018, 33, 73–82. [Google Scholar] [CrossRef] [Green Version]
- Batzer, D.P. The Seemingly Intractable Ecological Responses of Invertebrates in North American Wetlands: A Review. Wetlands 2013, 33, 1–15. [Google Scholar] [CrossRef]
- King, J.L. Loss of diversity as a consequence of habitat destruction in California vernal pools. In Ecology, Conservation, and Management of Vernal Pool Ecosystems; California Native Plant Society: Sacramento, CA, USA,, 1998; pp. 119–123. [Google Scholar]
- Belk, D. Global status and trends in ephemeral pool invertebrate conservation: Implications for Californian fairy shrimp. In Ecology, Conservation, and Management of Vernal Pool Ecosystems; Witham, C.W., Bauder, E.T., Belk, D., Ferren, W.R., Jr., Ornduff, R., Eds.; California Native Plant Society: Sacramento, CA, USA, 1998; pp. 147–150. [Google Scholar]
- Anteau, M.J.; Afton, A.D. Amphipod Densities and Indices of Wetland Quality across the Upper-Midwest, USA. Wetlands 2008, 28, 184–196. [Google Scholar] [CrossRef]
- Hanson, M.A.; Zimmer, K.D.; Butler, M.G.; Tangen, B.A.; Herwig, B.R.; Euliss, N.H. Biotic Interactions as Determinants of Ecosystem Structure in Prairie Wetlands: An Example Using Fish. Wetlands 2005, 25, 764–775. [Google Scholar] [CrossRef]
- Janke, A.K.; Anteau, M.J.; Stafford, J.D. Prairie Wetlands Confer Consistent Migrant Refueling Conditions across a Gradient of Agricultural Land Use Intensities. Biol. Conserv. 2019, 229, 99–112. [Google Scholar] [CrossRef] [Green Version]
- Zimmer, K.D.; Hanson, M.A.; Butler, M.G. Factors Influencing Invertebrate Communities in Prairie Wetlands: A Multivariate Approach. Can. J. Fish. Aquat. Sci. 2000, 57, 76–85. [Google Scholar] [CrossRef]
- Zimmer, K.D.; Hanson, M.A.; Butler, M.G. Effects of Fathead Minnow Colonization and Removal on a Prairie Wetland Ecosystem. Ecosystems 2001, 4, 346–357. [Google Scholar] [CrossRef]
- Peterka, J.J. Fishes in Northern Prairie Wetlands. In Northern Prairie Wetlands; van der Valk, A.G., Ed.; Iowa State University Press: Ames, IA, USA, 1989; pp. 302–315. [Google Scholar]
- Sundberg, M.D.; Baldwin, R.C.; Stewart, T.W.; Weber, M.J. Linkages between Land Use, Invasive Fishes, and Prairie Pothole Wetland Condition. Wetlands 2016, 36, 1097–1107. [Google Scholar] [CrossRef]
- Hanson, M.A.; Riggs, M.R. Potential Effects of Fish Predation on Wetland Invertebrates: A Comparison of Wetlands with and without Fathead Minnows. Wetlands 1995, 15, 167–175. [Google Scholar] [CrossRef]
- Cox Jr, R.R.; Hanson, M.A.; Roy, C.C.; Euliss Jr, N.H.; Johnson, D.H.; Butler, M.G. Mallard Duckling Growth and Survival in Relation to Aquatic Invertebrates. J. Wildl. Manag. 1998, 124–133. [Google Scholar] [CrossRef]
- Pires, M.M.; Sahlén, G.; Périco, E. Agricultural Land Use Affects the Heterogeneity of Odonata Communities in the Brazilian Pampa. J. Insect Conserv. 2021, 26, 503–514. [Google Scholar] [CrossRef]
- Gleason, J.E.; Rooney, R.C. Pond Permanence Is a Key Determinant of Aquatic Macroinvertebrate Community Structure in Wetlands. Freshw. Biol. 2018, 63, 264–277. [Google Scholar] [CrossRef]
- Daniel, J.; Gleason, J.E.; Cottenie, K.; Rooney, R.C. Stochastic and deterministic processes drive wetland community assembly across a gradient of environmental filtering. Oikos 2019, 128, 1158–1169. [Google Scholar] [CrossRef]
- Niemuth, N.D.; Solberg, J.W. Response of Waterbirds to Number of Wetlands in the Prairie Pothole Region of North Dakota, USA. Waterbirds 2003, 26, 233–238. [Google Scholar] [CrossRef]
- U.S. Fish and Wildlife Service. Waterfowl Population Status; U.S. Department of the Interior: Washington, DC, USA, 2019; p. 78.
- Orr, J.T.; Duquette, C.A.; Hovick, T.J.; Geaumont, B.A.; Harms, T.M. Secretive Marsh Bird Densities and Habitat Associations in the Prairie Pothole Region. Wetlands 2020, 40, 1529–1538. [Google Scholar] [CrossRef]
- Hill, N. Marshbird Response to Invasive Cattail Control Using Grazing, Mowing, and Herbicide, Application in the Prairie Pothole Region of Minnesota. Available online: Https://www.Fws.Gov/migratorybirdspdfsurveys--DataWebless20Migratory20Game20BirdsMarsh20Bird20pdf20filesMarshbirdResponseToInvasiveCattailControlInMN.Pdf (accessed on 20 September 2022).
- Ameli, A.A.; Creed, I.F. Groundwaters at Risk: Wetland Loss Changes Sources, Lengthens Pathways, and Decelerates Rejuvenation of Groundwater Resources. JAWRA J. Am. Water Resour. Assoc. 2019, 55, 294–306. [Google Scholar] [CrossRef]
- Zhang, B.; Schwartz, F.W.; Liu, G. Systematics in the Size Structure of Prairie Pothole Lakes through Drought and Deluge. Water Resour. Res. 2009, 45. [Google Scholar] [CrossRef]
- Liu, G.; Schwartz, F.W.; Wright, C.K.; McIntyre, N.E. Characterizing the Climate-Driven Collapses and Expansions of Wetland Habitats with a Fully Integrated Surface–Subsurface Hydrologic Model. Wetlands 2016, 36, 287–297. [Google Scholar] [CrossRef]
- Muhammad, A.; Evenson, G.R.; Stadnyk, T.A.; Boluwade, A.; Jha, S.K.; Coulibaly, P. Assessing the Importance of Potholes in the Canadian Prairie Region under Future Climate Change Scenarios. Water 2018, 10, 1657. [Google Scholar] [CrossRef] [Green Version]
- Hawkins, E.; Sutton, R. The Potential to Narrow Uncertainty in Projections of Regional Precipitation Change. Clim. Dyn. 2011, 37, 407–418. [Google Scholar] [CrossRef]
- Sofaer, H.R.; Skagen, S.K.; Barsugli, J.J.; Rashford, B.S.; Reese, G.C.; Hoeting, J.A.; Wood, A.W.; Noon, B.R. Projected Wetland Densities under Climate Change: Habitat Loss but Little Geographic Shift in Conservation Strategy. Ecol. Appl. Publ. Ecol. Soc. Am. 2016, 26, 1677–1692. [Google Scholar] [CrossRef] [PubMed]
- Steen, V.; Skagen, S.K.; Noon, B.R. Preparing for an Uncertain Future: Migrating Shorebird Response to Past Climatic Fluctuations in the Prairie Potholes. Ecosphere 2018, 9, e02095. [Google Scholar] [CrossRef]
- McKenna, O.P.; Mushet, D.M.; Kucia, S.R.; McCulloch-Huseby, E.C. Limited Shifts in the Distribution of Migratory Bird Breeding Habitat Density in Response to Future Changes in Climate. Ecol. Appl. 2021, 31, e02428. [Google Scholar] [CrossRef]
- LaBaugh, J.W.; Mushet, D.M.; Rosenberry, D.O.; Euliss, N.H.; Goldhaber, M.B.; Mills, C.T.; Nelson, R.D. Changes in Pond Water Levels and Surface Extent Due to Climate Variability Alter Solute Sources to Closed-Basin Prairie-Pothole Wetland Ponds, 1979 to 2012. Wetlands 2016, 36, 343–355. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McLean, K.; Mushet, D.; Sweetman, J. Climate and Land Use Driven Ecosystem Homogenization in the Prairie Pothole Region. Water 2022, 14, 3106. https://doi.org/10.3390/w14193106
McLean K, Mushet D, Sweetman J. Climate and Land Use Driven Ecosystem Homogenization in the Prairie Pothole Region. Water. 2022; 14(19):3106. https://doi.org/10.3390/w14193106
Chicago/Turabian StyleMcLean, Kyle, David Mushet, and Jon Sweetman. 2022. "Climate and Land Use Driven Ecosystem Homogenization in the Prairie Pothole Region" Water 14, no. 19: 3106. https://doi.org/10.3390/w14193106
APA StyleMcLean, K., Mushet, D., & Sweetman, J. (2022). Climate and Land Use Driven Ecosystem Homogenization in the Prairie Pothole Region. Water, 14(19), 3106. https://doi.org/10.3390/w14193106




