Performance of Micropollutant Removal during Wet-Weather Conditions in Advanced Treatment Stages on a Full-Scale WWTP
Abstract
:1. Introduction
1.1. Background and Political Relevance
1.2. State of the Art: OMP Removal under Wet-Weather Conditions
1.3. Impact of Rain Events and Research Gap
- (i)
- Hydraulic retention times (HRT) in the different treatment stages are shortened due to a higher inflow, which can cause a decrease in biological degradation and lower contact time in additional treatment steps as well, affecting sorption processes.
- (ii)
- Wet-weather conditions may change the concentration of OMPs at the inflow of advanced treatment stages, which has an impact on sorption capacity.
- (iii)
- The wastewater matrix changes, for example, due to fluctuations in DOC concentration or the occurrence of rainwater-related high OMP concentrations.
- (iv)
- The boundary conditions for sorption processes change, including variation in temperature, electrical conductivity, and buffer capacity.
1.4. Objectives and Hypotheses
- (1)
- Describe the temporal variations of OMP concentrations and loads at the inflow and outflow of advanced treatment stages with PAC and GAC during wet weather.
- (2)
- Determine the daily OMP release into the receiving water body during wet-weather conditions compared with dry-weather conditions.
- (3)
- Assess variations in OMP removal performance by advanced treatment with PAC and GAC due to wet-weather flows.
- (4)
- Improve the understanding of wet-weather flow dynamics on OMP removal by activated carbon processes.
2. Materials and Methods
2.1. Framework and Study Design
2.2. Monitoring and Sampling Procedure
2.3. Analytical Methods
2.4. Calculations of Phase Distribution, Mass Load, and Removal
3. Results
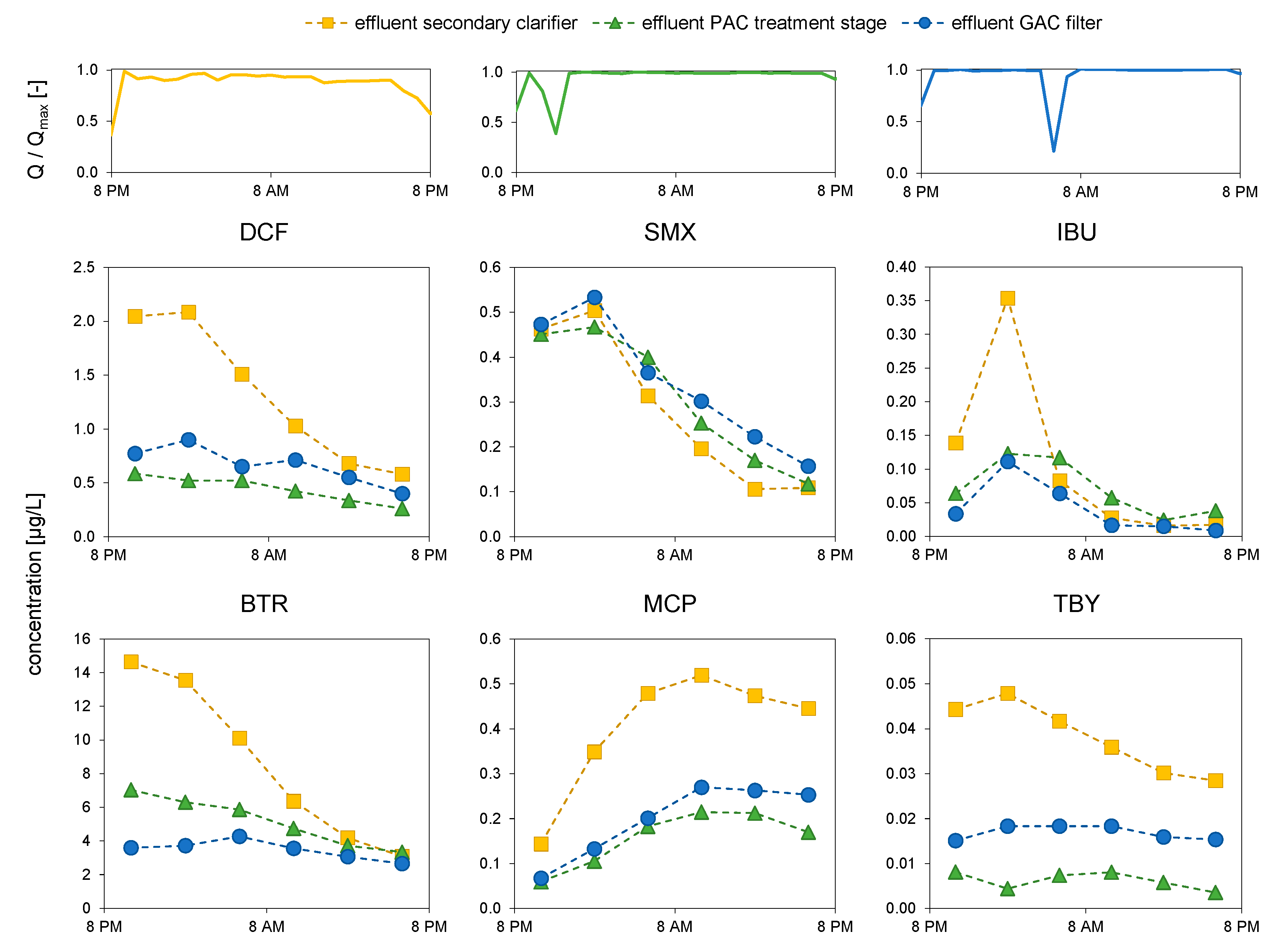
3.1. Flow and Treatment Conditions
3.2. Phase Distribution at the Effluent of the Secondary Clarifier
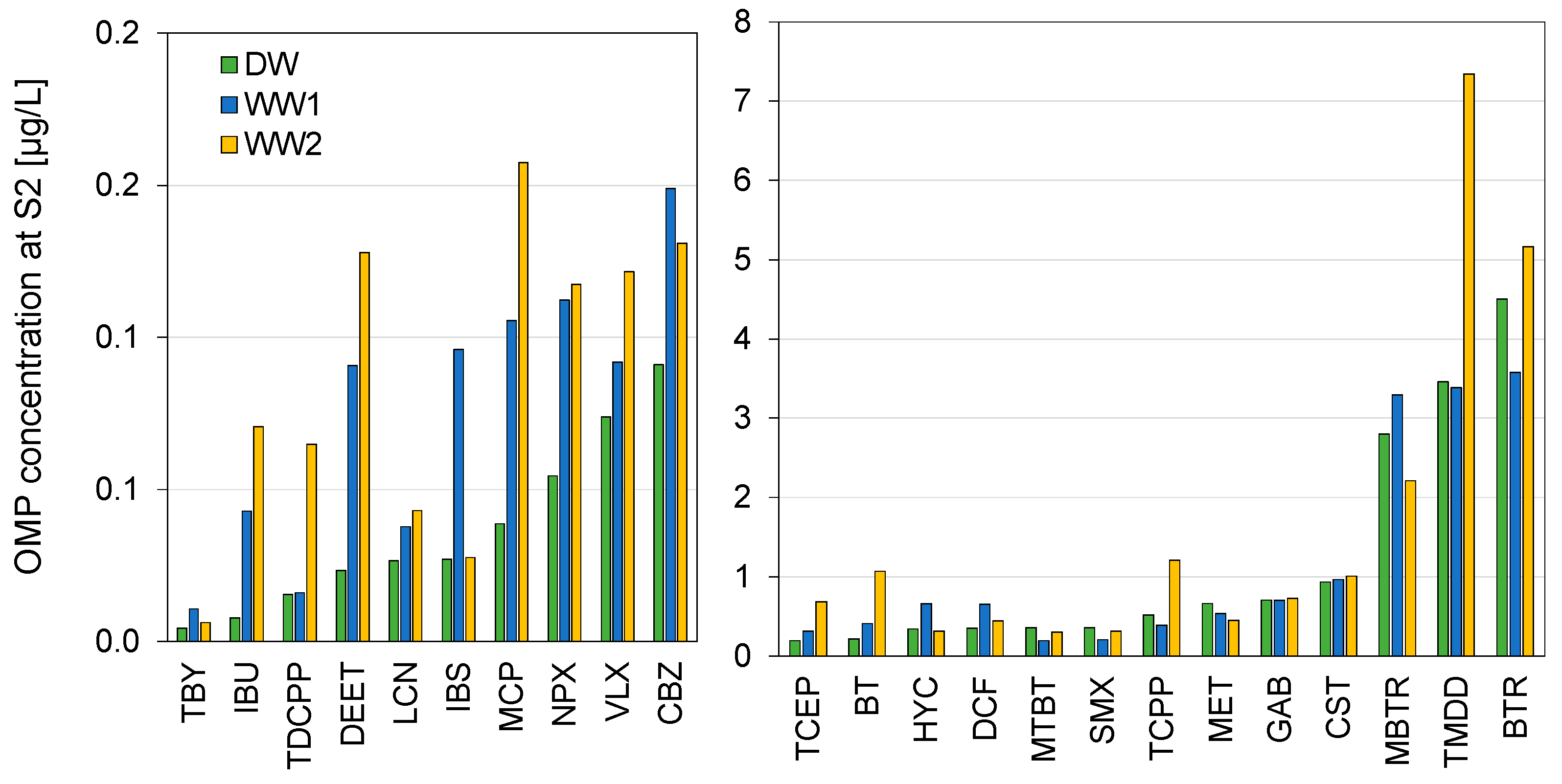
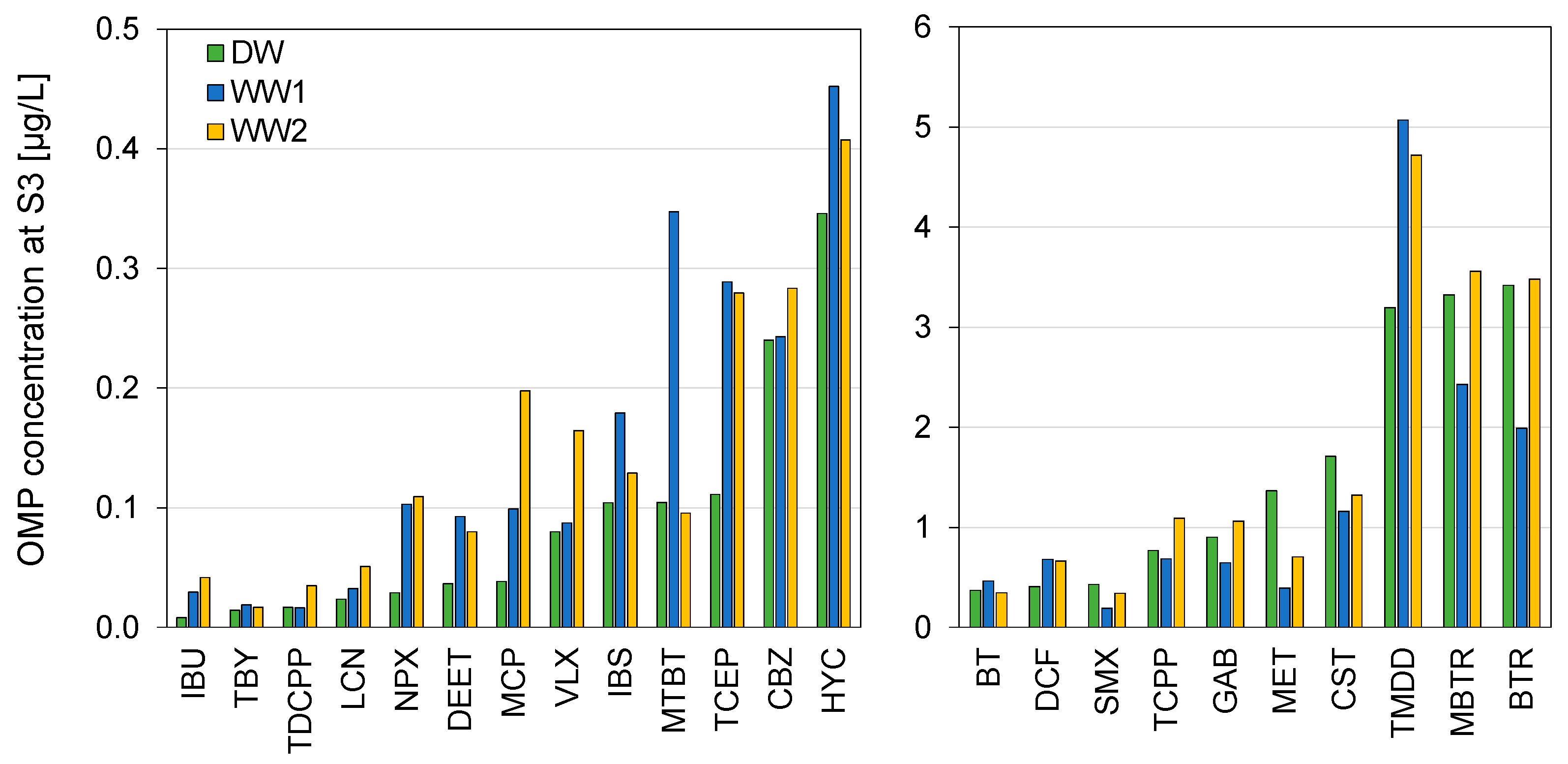
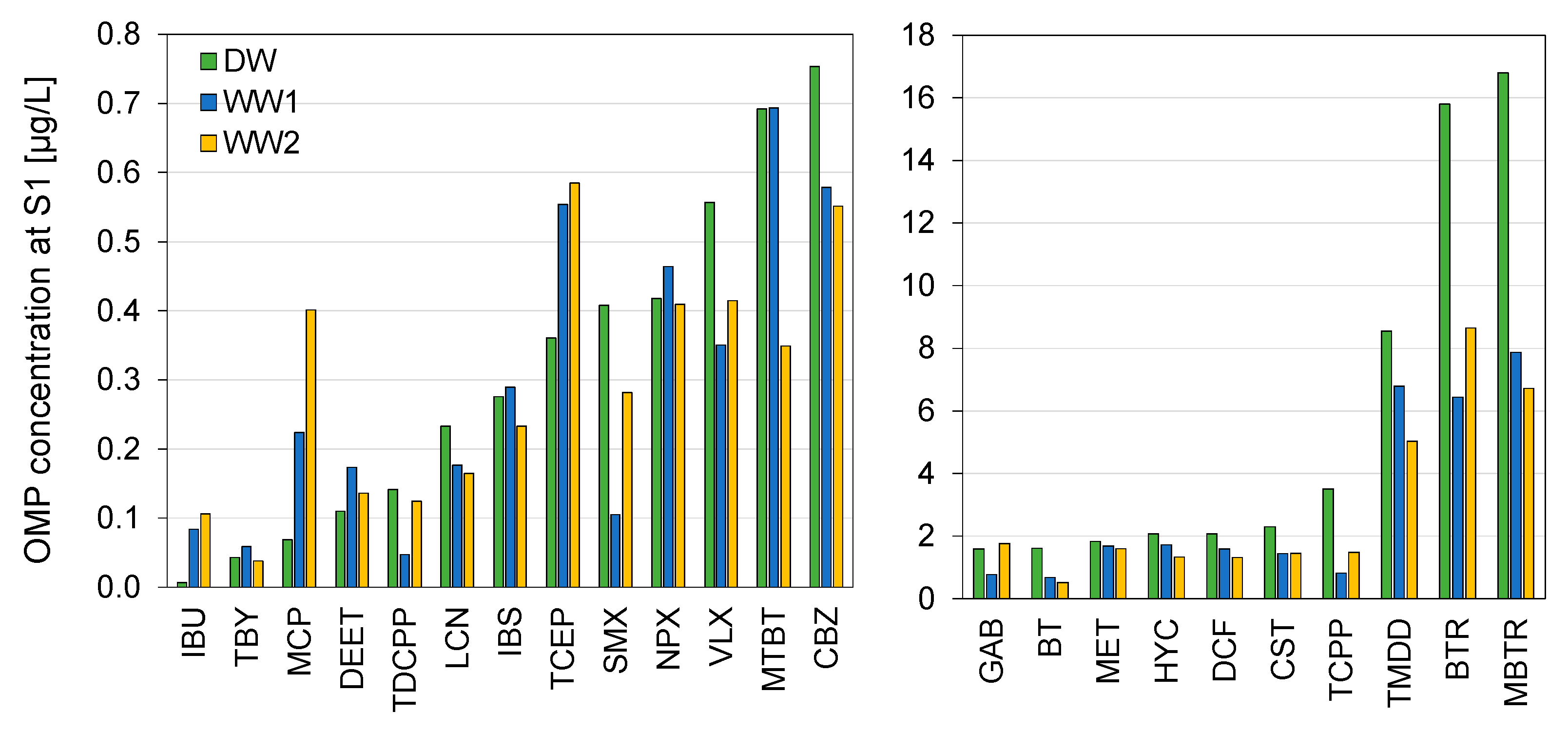
3.3. Concentrations of Micropollutants
3.4. Concentration Dynamics
3.5. Mass Loads of Micropollutants
3.6. Removal of Micropollutants
4. Discussion
4.1. Main Findings
4.1.1. OMP Concentrations
4.1.2. OMP Mass Loads
4.1.3. Removal Efficiency
4.2. Limitations and Critical Remarks
4.3. Future Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| No. | Group | Substance | Abbreviation | CAS |
|---|---|---|---|---|
| 1 | Pharmaceutical | Candesartan | CST | 139481-59-7 |
| 2 | Carbamazepine | CBZ | 298-46-4 | |
| 3 | Diclofenac | DCF | 15307-86-5 | |
| 4 | Gabapentin | GBN | 60142-96-3 | |
| 5 | Hydrochlorothiazide | HYC | 58-93-5 | |
| 6 | Ibuprofen | IBF | 15687-27-1 | |
| 7 | Irbesartan | IBS | 138402-11-6 | |
| 8 | Lidocaine | LCN | 137-58-6 | |
| 9 | Metoprolol | MET | 51384-51-1 | |
| 10 | Naproxen | NPX | 22204-53-1 | |
| 11 | Sulfamethoxazole | SMX | 723-46-6 | |
| 12 | Venlafaxine | VLX | 93413-69-5 | |
| 13 | Food | Caffeine | CAF | 58-08-2 |
| 14 | Corrosion inhibitor | 1H-benzotriazole | BTR | 95-14-7 |
| 15 | Tolyltriazole | MBTR | 29385-43-1 | |
| 16 | Industrial chemical | 4-nonylphenol | 4NP | 104-40-5 |
| 17 | Benzothiazole | BT | 95-16-9 | |
| 18 | 2-Methylthiobenzothiazole | MTBT | 615-22-5 | |
| 19 | 2,4,7,9-Tetramethyl-5-decyn-4,7-diol | TMDD | 126-86-3 | |
| 20 | Flame retardant | Tris(2-chlorethyl)phosphate | TCEP | 115-96-8 |
| 21 | Tris(2-chlorisopropyl)phosphate | TCPP | 13674-84-5 | |
| 22 | Tris(1,3-dichlorisopropyl)phosphate | TDCPP | 13674-87-8 | |
| 23 | Insect repellent | Icaridin | ICN | 119515-38-7 |
| 24 | Diethyltoluamide | DEET | 134-62-3 | |
| 25 | Herbicide | Mecoprop | MCP | 93-65-2 |
| 26 | Terbutryn | TBY | 886-50-0 |

References
- Brunsch, A.F. Mass flow analysis for anthropogenic micropollutants as performed in the Swist river catchment. Water Pollut. 2014, 182, 87–98. [Google Scholar] [CrossRef] [Green Version]
- Eggen, R.I.L.; Hollender, J.; Joss, A.; Schärer, M.; Stamm, C. Reducing the discharge of micropollutants in the aquatic environment: The benefits of upgrading wastewater treatment plants. Environ. Sci. Technol. 2014, 48, 7683–7689. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Guo, W.; Ngo, H.H.; Nghiem, L.D.; Hai, F.I.; Zhang, J.; Liang, S.; Wang, X.C. A review on the occurrence of micropollutants in the aquatic environment and their fate and removal during wastewater treatment. Sci. Total Environ. 2014, 473–474, 619–641. [Google Scholar] [CrossRef] [PubMed]
- European Union. Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 Establishing a Framework for Community Action in the Field of Water Policy. 2000. Available online: https://eur-lex.europa.eu/legal-content/en/ALL/?uri=CELEX%3A32000L0060 (accessed on 3 October 2022).
- United Nations. The 2030 Agenda and the Sustainable Development Goals: An opportunity for Latin America and the Caribbean; United Nations ECLAC: Santiago, Chile, 2018; ISBN 978-92-1-058644-3.
- Stockhaus, H.; Stein, U.; Meinecke, L.F.; Gerstetter, C.; Bodle, R.; Vidaurre, R.; Holmes, A.; Bueb, B.; Hundegger, S. Potenziale anderer Rechtsbereiche zum Erreichen der Ziele der Wasserrahmenrichtlinie: TEXTE 72/2021. In Ressortforschungsplan des Bundesministerium für Umwelt, Naturschutz und nukleare Sicherheit; Forschungskennzahl 3718242100; FB000404; Umweltbundesamt: Dessau-Roßlau, Germany, 2021. [Google Scholar]
- Pistocchi, A.; Alygizakis, N.A.; Brack, W.; Boxall, A.; Cousins, I.T.; Drewes, J.E.; Finckh, S.; Gallé, T.; Launay, M.A.; McLachlan, M.S.; et al. European scale assessment of the potential of ozonation and activated carbon treatment to reduce micropollutant emissions with wastewater. Sci. Total Environ. 2022, 848, 157124. [Google Scholar] [CrossRef] [PubMed]
- Barjenbruch, M.; Sens, K.; Schüler, R.; Stapf, M. Entfernung von Spurenstoffen–Handreichung für Betreiber von Kläranlagen. Available online: https://www.dwa-no.de/files/_media/content/PDFs/LV_Nord-Ost/Internationale_Projekte/2020_Entfernung%20von%20Spurenstoffen%20-%20Handreichung%20f%C3%BCr%20KA-Betreiber.pdf (accessed on 3 October 2022).
- Kompetenzzentrum Spurenstoffe Baden-Württemberg. Kläranlagenausbau in Baden-Württemberg–Aktueller Stand. Available online: https://koms-bw.de/klaeranlagen/uebersichtskarte/ (accessed on 3 October 2022).
- Ministerium für Umwelt, Klima und Energiewirtschaft Baden-Württemberg. Arbeitspapier Spurenstoffelimination auf Arbeitspapier Spurenstoffelimination auf Kommunalen Kläranlagen in Baden-Württemberg. Available online: https://um.baden-wuerttemberg.de/fileadmin/redaktion/m-um/intern/Dateien/Dokumente/3_Umwelt/Wasser/210614-Arbeitspapier-Spurenstoffelimination-kommunale-Klaeranlagen-barrierefrei.pdf (accessed on 3 October 2022).
- ARA Ausbau. Platform for Process Engineering of Micropollutants: Status Quo. Available online: https://micropoll.ch/ara-ausbau/ (accessed on 3 October 2022).
- Pistocchi, A.; Andersen, H.R.; Bertanza, G.; Brander, A.; Choubert, J.M.; Cimbritz, M.; Drewes, J.E.; Koehler, C.; Krampe, J.; Launay, M.; et al. Treatment of micropollutants in wastewater: Balancing effectiveness, costs and implications. Sci. Total Environ. 2022, 850, 157593. [Google Scholar] [CrossRef] [PubMed]
- Fundneider, T.; Acevedo Alonso, V.; Abbt-Braun, G.; Wick, A.; Albrecht, D.; Lackner, S. Empty bed contact time: The key for micropollutant removal in activated carbon filters. Water Res. 2020, 191, 116765. [Google Scholar] [CrossRef] [PubMed]
- Margot, J.; Kienle, C.; Magnet, A.; Weil, M.; Rossi, L.; de Alencastro, L.F.; Abegglen, C.; Thonney, D.; Chèvre, N.; Schärer, M.; et al. Treatment of micropollutants in municipal wastewater: Ozone or powdered activated carbon? Sci. Total Environ. 2013, 461–462, 480–498. [Google Scholar] [CrossRef]
- Benstoem, F.; Nahrstedt, A.; Boehler, M.; Knopp, G.; Montag, D.; Siegrist, H.; Pinnekamp, J. Performance of granular activated carbon to remove micropollutants from municipal wastewater—A meta-analysis of pilot- and large-scale studies. Chemosphere 2017, 185, 105–118. [Google Scholar] [CrossRef]
- Mailler, R.; Gasperi, J.; Coquet, Y.; Deshayes, S.; Zedek, S.; Cren-Olivé, C.; Cartiser, N.; Eudes, V.; Bressy, A.; Caupos, E.; et al. Study of a large scale powdered activated carbon pilot: Removals of a wide range of emerging and priority micropollutants from wastewater treatment plant effluents. Water Res. 2015, 72, 315–330. [Google Scholar] [CrossRef] [Green Version]
- Mailler, R.; Gasperi, J.; Coquet, Y.; Buleté, A.; Vulliet, E.; Deshayes, S.; Zedek, S.; Mirande-Bret, C.; Eudes, V.; Bressy, A.; et al. Removal of a wide range of emerging pollutants from wastewater treatment plant discharges by micro-grain activated carbon in fluidized bed as tertiary treatment at large pilot scale. Sci. Total Environ. 2016, 542, 983–996. [Google Scholar] [CrossRef]
- Zietzschmann, F.; Stützer, C.; Jekel, M. Granular activated carbon adsorption of organic micro-pollutants in drinking water and treated wastewater—Aligning breakthrough curves and capacities. Water Res. 2016, 92, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Guillossou, R.; Le Roux, J.; Mailler, R.; Vulliet, E.; Morlay, C.; Nauleau, F.; Gasperi, J.; Rocher, V. Organic micropollutants in a large wastewater treatment plant: What are the benefits of an advanced treatment by activated carbon adsorption in comparison to conventional treatment? Chemosphere 2019, 218, 1050–1060. [Google Scholar] [CrossRef] [PubMed]
- Schachtler, M.; Otto, J.; Thomann, M. Spurenstoffelimination bei Regenwetter: Wie bei Regenwetter eine Konstante Abbauleistung mit Ozon Erreicht Werden Kann. Aqua Gas N°2 2020, 20–25. Available online: https://micropoll.ch/Mediathek/spurenstoffelimination-bei-regenwetter/ (accessed on 3 October 2022).
- Launay, M.A.; Dittmer, U.; Steinmetz, H. Organic micropollutants discharged by combined sewer overflows–Characterisation of pollutant sources and stormwater-related processes. Water Res. 2016, 104, 82–92. [Google Scholar] [CrossRef]
- Gasperi, J.; Zgheib, S.; Cladière, M.; Rocher, V.; Moilleron, R.; Chebbo, G. Priority pollutants in urban stormwater: Part 2—Case of combined sewers. Water Res. 2012, 46, 6693–6703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dittmer, U.; Bachmann-Machnik, A.; Launay, M.A. Impact of Combined Sewer Systems on the Quality of Urban Streams: Frequency and Duration of Elevated Micropollutant Concentrations. Water 2020, 12, 850. [Google Scholar] [CrossRef] [Green Version]
- Stadt Mannheim. Präsentation Stadtententwässerung Mannheim. Available online: https://www.mannheim.de/sites/default/files/2017-11/EBS-Pr%C3%A4sentation_3.pdf (accessed on 3 October 2022).
- Metzger, S.; Alt, K.; Benstoem, F.; Biebersdorf, N.; Boehler, M.; Bornemann, C.; Fundneider, T.; Hauff, R.; Jedele, K.; Jekel, M.; et al. Spurenstoffentfernung auf Kommunalen Kläranlagen–Teil 2: Einsatz von Aktivkohle–Verfahrensgrundsätze und Bemessung; Deutsche Vereinigung für Wasserwirtschaft, Abwasser und Abfall e.V. (DWA): Hennef, Germany, 2021. [Google Scholar]
- Kompetenzzentrum Spurenstoffe Baden-Württemberg. Handlungsempfehlung für die Vergleichskontrolle und den Betrieb von Verfahrenstechniken zur Gezielten Spurenstoffelimination; Kompetenzzentrum Spurenstoffe Baden-Württemberg: Stuttgart, Germany, 2018. [Google Scholar]
- de Ridder, D.J.; MCConville, M.; Verliefde, A.R.D.; van der Aa, L.T.J.; Heijman, S.G.J.; Verberk, J.Q.J.C.; Rietveld, L.C.; van Dijk, J.C. Development of a predictive model to determine micropollutant removal using granular activated carbon. Drink. Water Eng. Sci. 2009, 2, 57–62. [Google Scholar] [CrossRef] [Green Version]
- Rößler, A.; Metzger, S. Application of SAC254 measurement for the assessment of micropollutant removal in the adsorptive treatment stage of a municipal wastewater treatment plant. Water Pract. Technol. 2016, 11, 503–515. [Google Scholar] [CrossRef] [Green Version]
- Guillossou, R.; Le Roux, J.; Mailler, R.; Morlay, C.; Vulliet, E.; Nauleau, F.; Rocher, V.; Gasperi, J. Influence of the properties of 7 micro-grain activated carbons on organic micropollutants removal from wastewater effluent. Chemosphere 2020, 243, 125306. [Google Scholar] [CrossRef]
- Loos, R.; Carvalho, R.; António, D.C.; Comero, S.; Locoro, G.; Tavazzi, S.; Paracchini, B.; Ghiani, M.; Lettieri, T.; Blaha, L.; et al. EU-wide monitoring survey on emerging polar organic contaminants in wastewater treatment plant effluents. Water Res. 2013, 47, 6475–6487. [Google Scholar] [CrossRef]
- Vetter, W.; Lorenz, J. Determination of benzotriazoles in dishwasher tabs from Germany and estimation of the discharge into German waters. Environ. Sci. Pollut. Res. Int. 2013, 20, 4435–4440. [Google Scholar] [CrossRef]
- Launay, M.A. Organic Micropollutants in Urban Wastewater Systems during Dry and Wet Weather-Occurence, Spatio-Temporal Distribution and Emissions to Surface Waters. Doctoral Thesis, Universität Stuttgart, Stuttgart, Germany, 2017. [Google Scholar]
- Jover, E.; Matamoros, V.; Bayona, J.M. Characterization of benzothiazoles, benzotriazoles and benzosulfonamides in aqueous matrixes by solid-phase extraction followed by comprehensive two-dimensional gas chromatography coupled to time-of-flight mass spectrometry. J. Chromatogr. A 2009, 1216, 4013–4019. [Google Scholar] [CrossRef] [PubMed]
- Warner, W.; Licha, T.; Nödler, K. Qualitative and quantitative use of micropollutants as source and process indicators. A review. Sci. Total Environ. 2019, 686, 75–89. [Google Scholar] [CrossRef] [PubMed]
- Luft, A.; Wagner, M.; Ternes, T.A. Transformation of biocides irgarol and terbutryn in the biological wastewater treatment. Environ. Sci. Technol. 2014, 48, 244–254. [Google Scholar] [CrossRef]
- Joss, A.; Zabczynski, S.; Göbel, A.; Hoffmann, B.; Löffler, D.; McArdell, C.S.; Ternes, T.A.; Thomsen, A.; Siegrist, H. Biological degradation of pharmaceuticals in municipal wastewater treatment: Proposing a classification scheme. Water Res. 2006, 40, 1686–1696. [Google Scholar] [CrossRef]
- Yu, J.T.; Bouwer, E.J.; Coelhan, M. Occurrence and biodegradability studies of selected pharmaceuticals and personal care products in sewage effluent. Agric. Water Manag. 2006, 86, 72–80. [Google Scholar] [CrossRef]
- Bollmann, U.E.; Tang, C.; Eriksson, E.; Jönsson, K.; Vollertsen, J.; Bester, K. Biocides in urban wastewater treatment plant influent at dry and wet weather: Concentrations, mass flows and possible sources. Water Res. 2014, 60, 64–74. [Google Scholar] [CrossRef]
- Burkhardt, M.; Kupper, T.; Hean, S.; Haag, R.; Schmid, P.; Kohler, M.; Boller, M. Biocides used in building materials and their leaching behavior to sewer systems. Water Sci. Technol. 2007, 56, 63–67. [Google Scholar] [CrossRef]
- Singer, H.; Jaus, S.; Hanke, I.; Lück, A.; Hollender, J.; Alder, A.C. Determination of biocides and pesticides by on-line solid phase extraction coupled with mass spectrometry and their behaviour in wastewater and surface water. Environ. Pollut. 2010, 158, 3054–3064. [Google Scholar] [CrossRef]
- Reif, D.; Saracevic, E.; Šabić Runjavec, M.; Haslinger, J.; Schaar, H.; Kreuzinger, N. Desorption of Organic Micropollutants from Loaded Granular Activated Carbon. Water 2020, 12, 2754. [Google Scholar] [CrossRef]
- Wunderlin, P.; Rensch, D.; Abegglen, C.; Dominguez, D.; Egli, C.; LeGoaziou, Y.; Schachtler, M.; Pfund, D.; Urfer, D.; Thonney, D.; et al. Konzepte zur Überwachung der Reinigungsleistung von weitergehenden Verfahren zur Spurenstoffelimination. Available online: https://micropoll.ch/wp-content/uploads/2020/06/2017_VSA_F_Konzepte-zur-%C3%9Cberwachung-der-_d.pdf (accessed on 3 October 2022).



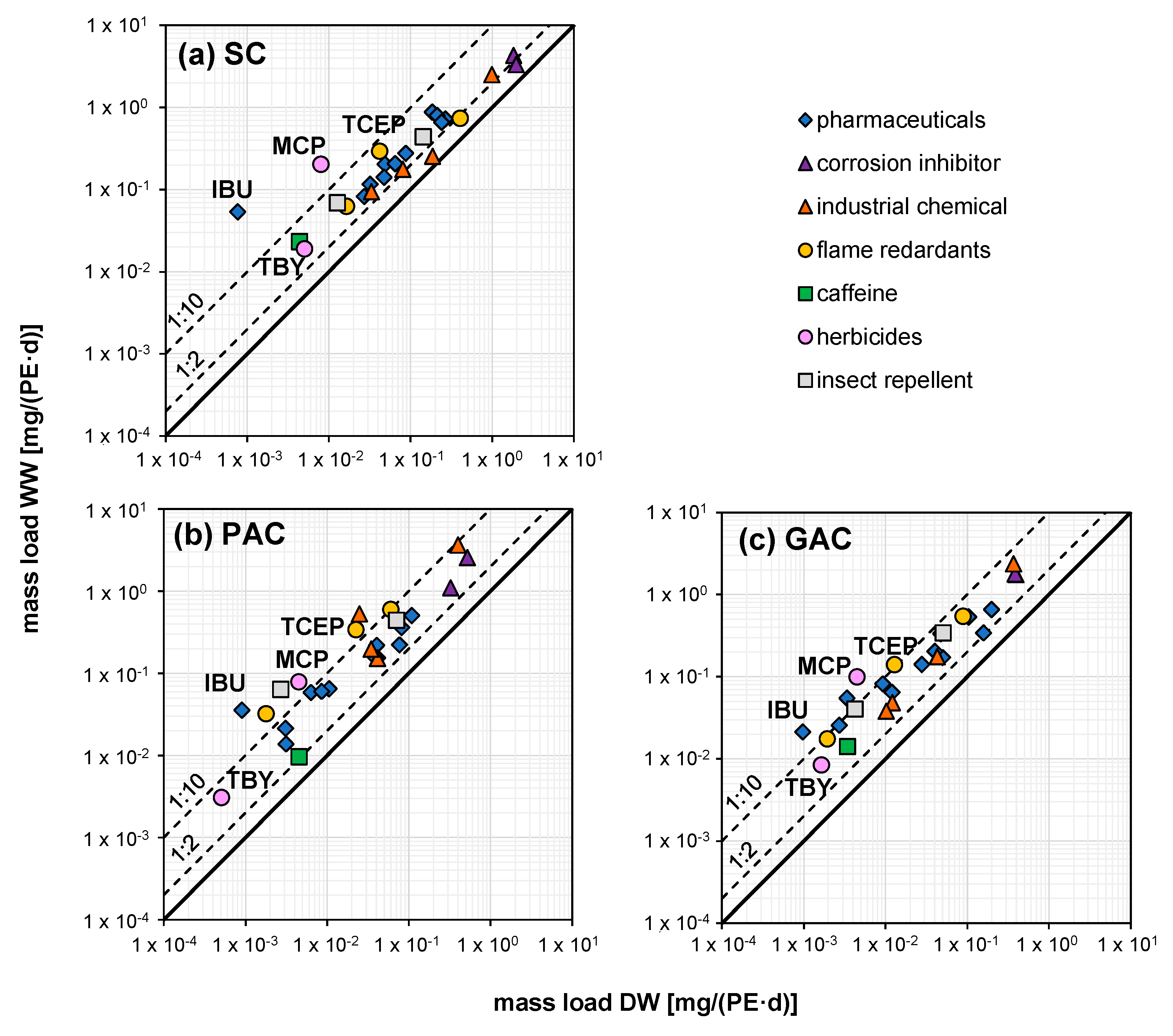

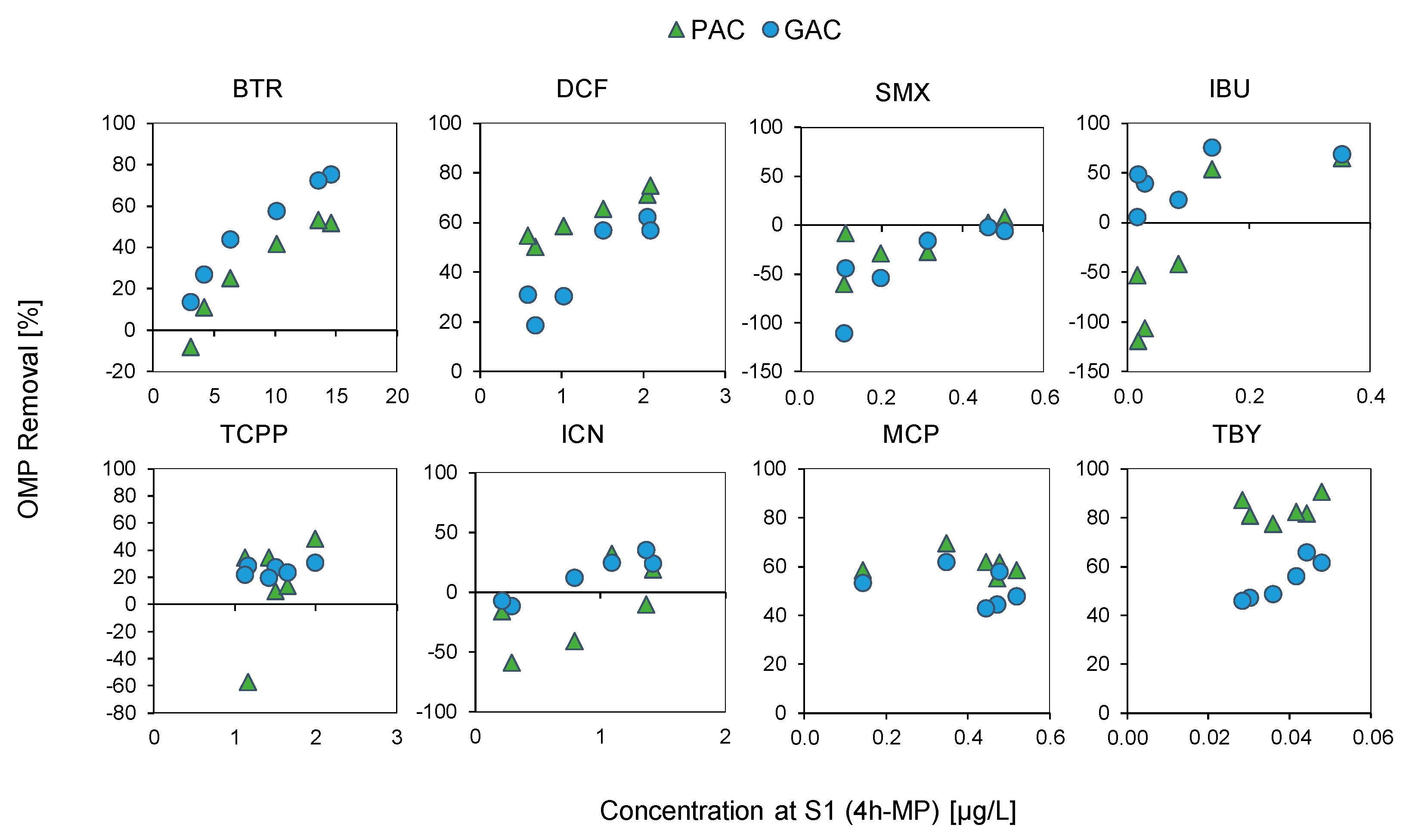
| Sample Number | Unit | DW | WW1 | WW2_1 | WW2_2 | WW2_3 | WW2_4 | WW2_5 | WW2_6 |
|---|---|---|---|---|---|---|---|---|---|
| date in 2019 | 5/6 September | 11 June | 7 September | 8 September | 8 September | 8 September | 8 September | 8 September | |
| time | 0:00–23:59 | 0:00–23:59 | 20:00–23:59 | 0:00–3:59 | 4:00–7:59 | 8:00–11:59 | 12:00–15:59 | 16:00–19:59 | |
| effluent secondary clarifier S1 | |||||||||
| pH | - | - | 7.7 | 8.0 | 7.9 | 7.8 | 7.7 | 7.6 | 7.8 |
| el. conductivity | µS/cm | - | 1150 | 1580 | 1440 | 733 | 516 | 518 | 419 |
| turbidity | NTU | - | - | 2 | 1 | 2 | 2 | 2 | 3 |
| DOC | mg/L | 8.33 | 8.80 | 9.13 | 8.98 | 7.73 | 6.16 | 5.20 | 4.92 |
| SAC254 | 1/m | 21.9 | 18.9 | 24.2 | 26.5 | 23.0 | 18.7 | 14.8 | 13.6 |
| effluent PAC S2 | |||||||||
| pH | - | - | 7.9 | 8.3 | 8.2 | 8.1 | 8.0 | 7.9 | 8.1 |
| el. conductivity | µS/cm | - | 1220 | 1510 | 1490 | 1270 | 888 | 645 | 469 |
| turbidity | NTU | - | - | 2 | 1 | 2 | 2 | 2 | 1 |
| DOC | mg/L | 6.36 | 7.15 | 7.43 | 7.36 | 6.89 | 5.80 | 4.43 | 4.21 |
| SAC254 | 1/m | 14.5 | 14.9 | 16.7 | 18.0 | 17.6 | 14.3 | 11.2 | 9.10 |
| effluent GAC S3 | |||||||||
| pH | - | - | 8.1 | 8.4 | 8.4 | 8.3 | 8.2 | 8.1 | 8.0 |
| el. conductivity | µS/cm | - | 1150 | 1580 | 1540 | - | 800 | 576 | 437 |
| turbidity | NTU | - | - | 1 | 7 | - | 1 | <1 | <1 |
| DOC | mg/L | 7.17 | 6.96 | 7.87 | 8.18 | 5.27 | 6.64 | 5.32 | 4.42 |
| SAC254 | 1/m | 17.5 | 15.8 | 19.3 | 21.3 | 18.3 | 17.0 | 14.1 | 12.4 |
| PAC 1,3 | GAC 2,3 | |||
|---|---|---|---|---|
| DW | WW | DW | WW | |
| IBU | −18 | 40 | −28 | 44 |
| TBY | 90 | 83 | 67 | 62 |
| MCP | 44 | 57 | 44 | 54 |
| DEET | 79 | 27 | 67 | 44 |
| TDCPP | 89 | 57 | 88 | 69 |
| LCN | 89 | 77 | 90 | 76 |
| IBS | 90 | 74 | 62 | 51 |
| TCEP | 46 | 13 | 69 | 50 |
| SMX | 13 | −52 | −6 | −52 |
| NPX | 87 | 74 | 93 | 76 |
| VLX | 87 | 73 | 86 | 68 |
| MTBT | 49 | 43 | 85 | 62 |
| CBZ | 88 | 75 | 68 | 54 |
| GAB | 56 | 34 | 43 | 28 |
| BT | 87 | −35 | 77 | 32 |
| MET | 64 | 70 | 25 | 67 |
| HYC | 84 | 69 | 83 | 72 |
| DCF | 83 | 56 | 80 | 36 |
| CST | 59 | 32 | 25 | 14 |
| TCPP | 85 | 36 | 78 | 21 |
| TMDD | 59 | 2 | 63 | 16 |
| BTR | 72 | 42 | 78 | 65 |
| MBTR | 83 | 63 | 80 | 58 |
| pharmaceuticals (12) | 65 | 52 | 52 | 44 |
| corrosion inhibitors (2) | 78 | 52 | 79 | 61 |
| industrial chemicals (3) | 65 | 3 | 75 | 36 |
| flame retardants (3) | 73 | 35 | 78 | 47 |
| herbicides (2) | 67 | 70 | 56 | 58 |
| insect repellent (1) | 79 | 27 | 67 | 44 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neef, J.; Leverenz, D.; Launay, M.A. Performance of Micropollutant Removal during Wet-Weather Conditions in Advanced Treatment Stages on a Full-Scale WWTP. Water 2022, 14, 3281. https://doi.org/10.3390/w14203281
Neef J, Leverenz D, Launay MA. Performance of Micropollutant Removal during Wet-Weather Conditions in Advanced Treatment Stages on a Full-Scale WWTP. Water. 2022; 14(20):3281. https://doi.org/10.3390/w14203281
Chicago/Turabian StyleNeef, Johanna, Dominik Leverenz, and Marie Alexandra Launay. 2022. "Performance of Micropollutant Removal during Wet-Weather Conditions in Advanced Treatment Stages on a Full-Scale WWTP" Water 14, no. 20: 3281. https://doi.org/10.3390/w14203281
APA StyleNeef, J., Leverenz, D., & Launay, M. A. (2022). Performance of Micropollutant Removal during Wet-Weather Conditions in Advanced Treatment Stages on a Full-Scale WWTP. Water, 14(20), 3281. https://doi.org/10.3390/w14203281






