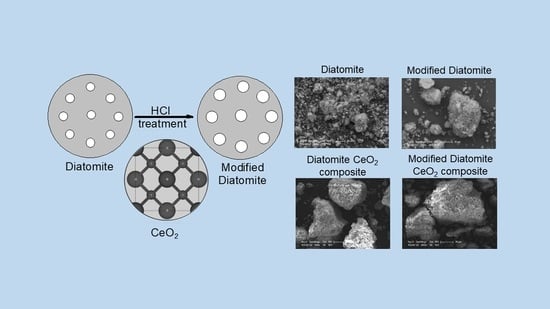
A Novel Low-Cost Photocatalyst: Preparation, Characterization, and Photocatalytic Properties of CeO2-Diatomite Composites
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Modified Diatomite
2.3. Preparation of CeO2-Diatomite Composites
2.4. Characterization Techniques
2.5. Assessment of Initial Adsorptive Surface Interactions
2.6. Photocatalytic Activity Assessment
3. Results and Discussion
3.1. Characterization of the Diatomites and CeO2-Diatomite Composites
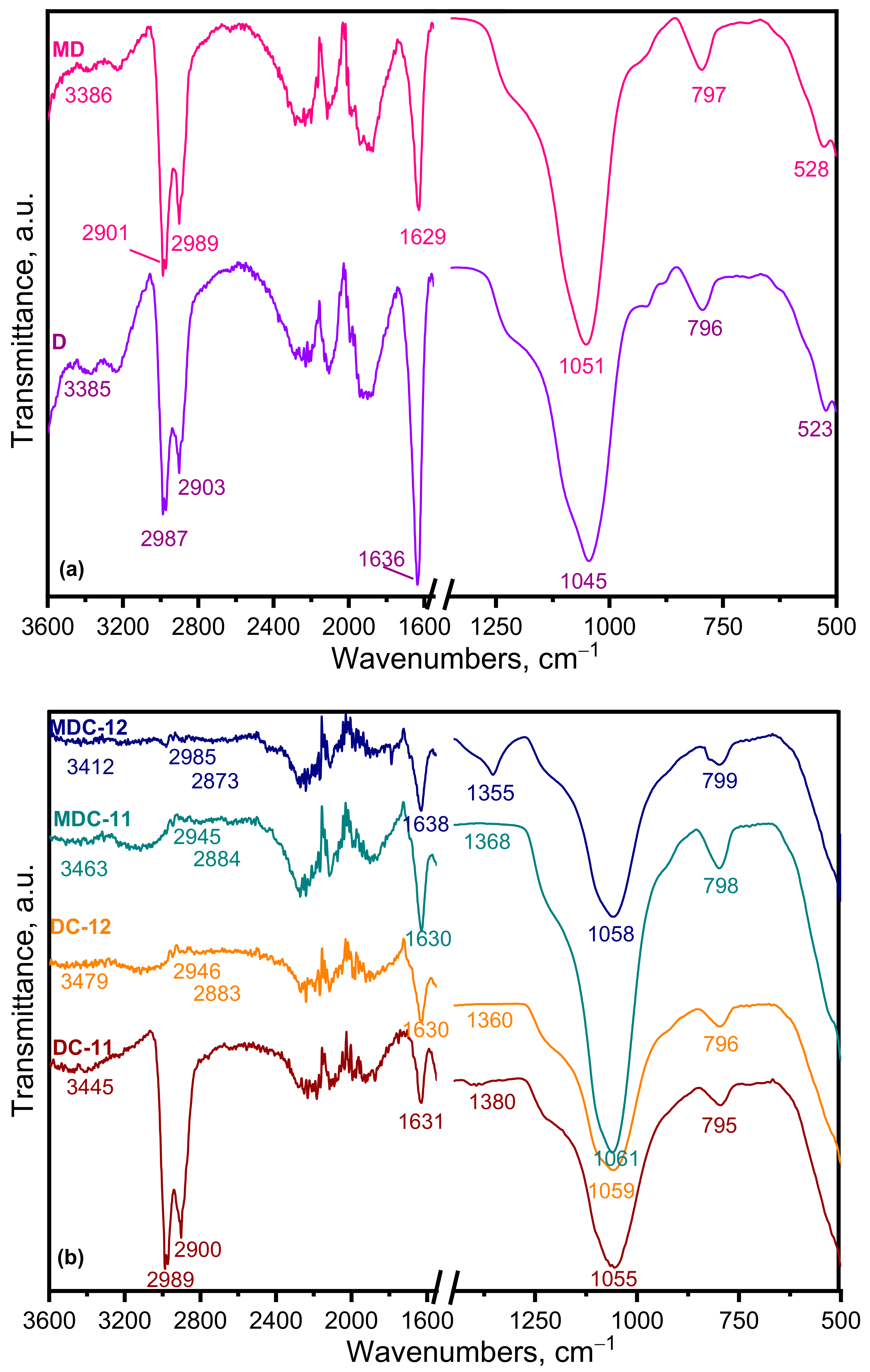
3.1.1. FTIR Analysis
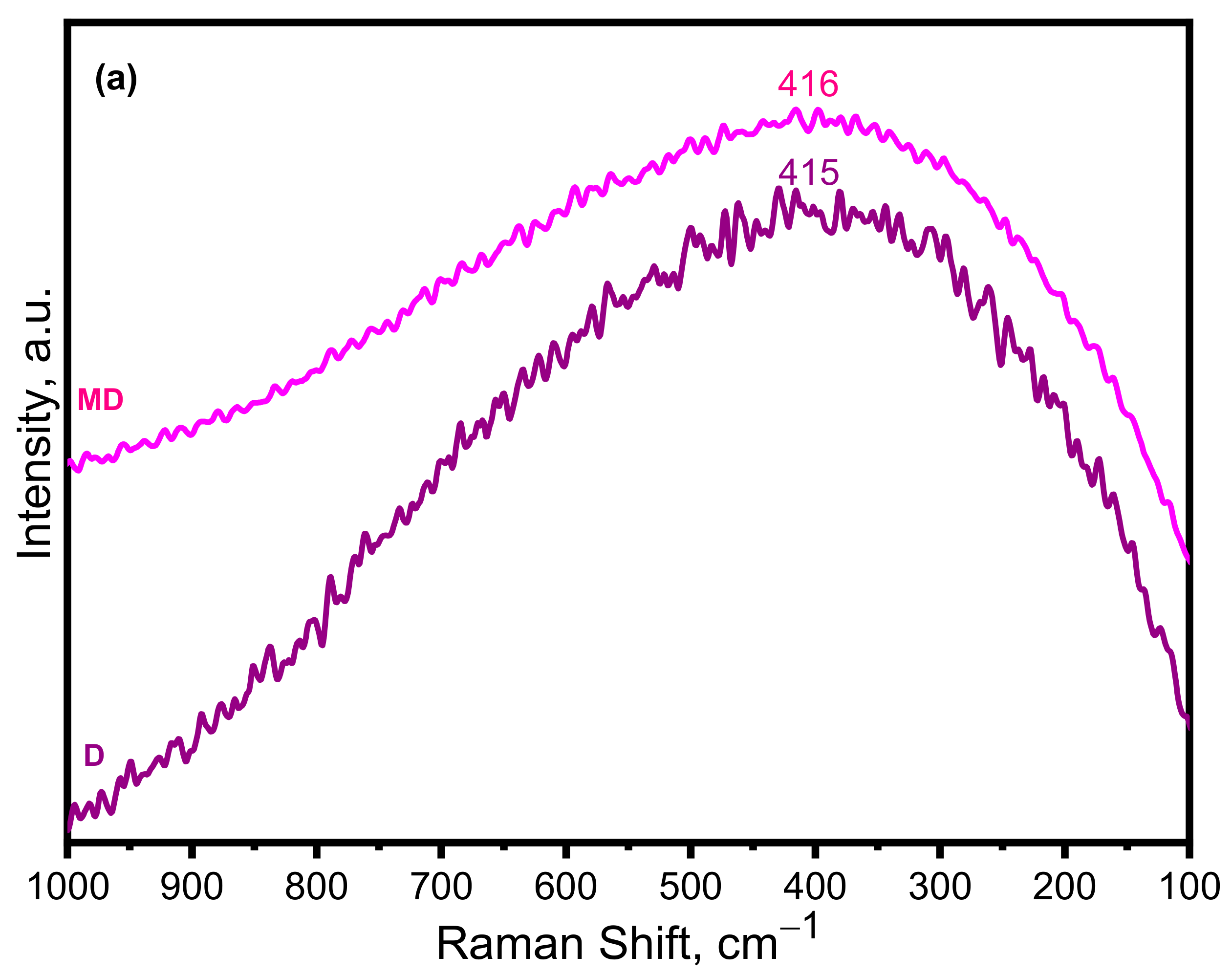
3.1.2. Raman Spectroscopy
3.1.3. XRD Analysis
3.1.4. ESEM Analysis
3.1.5. BET Analysis
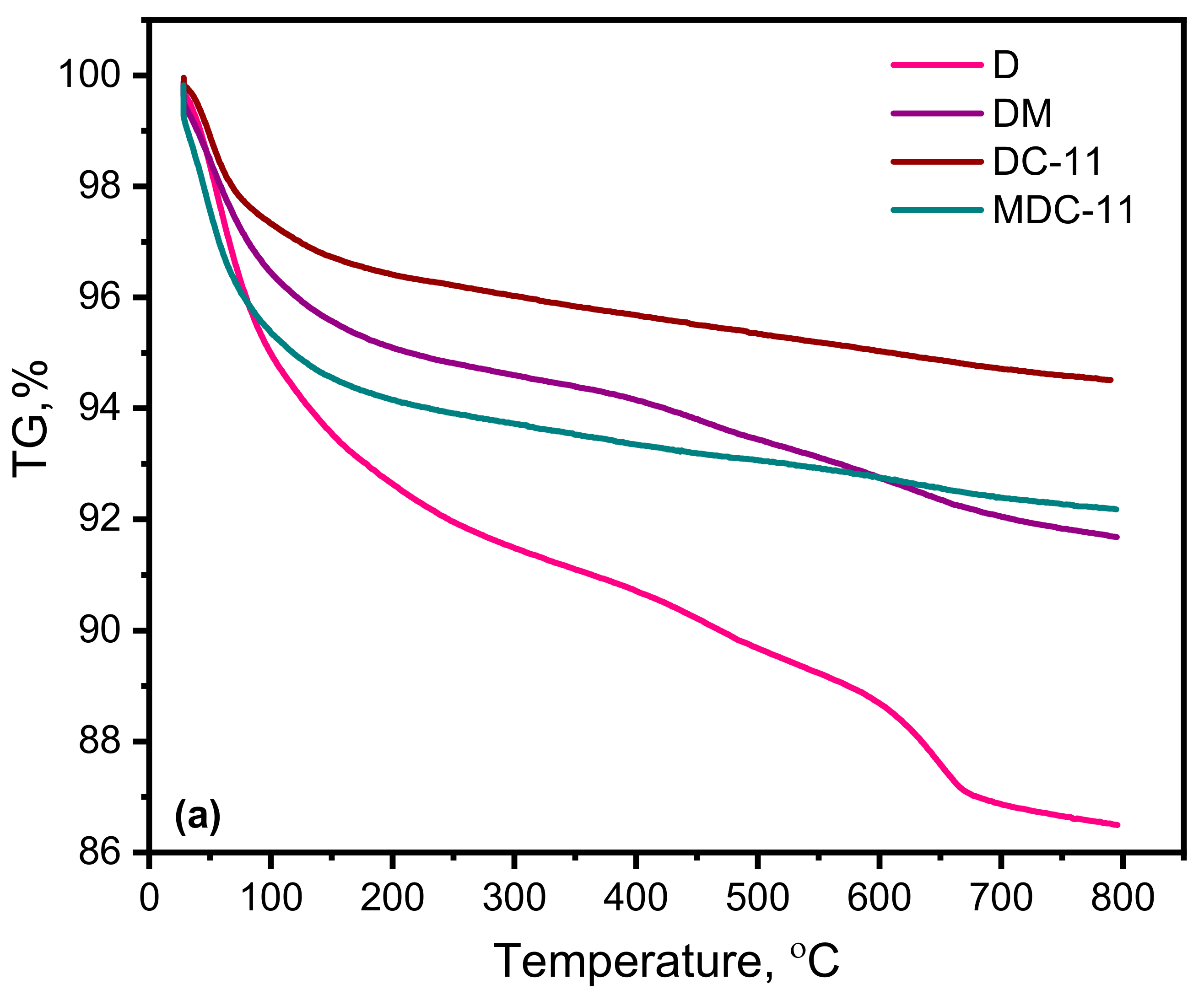
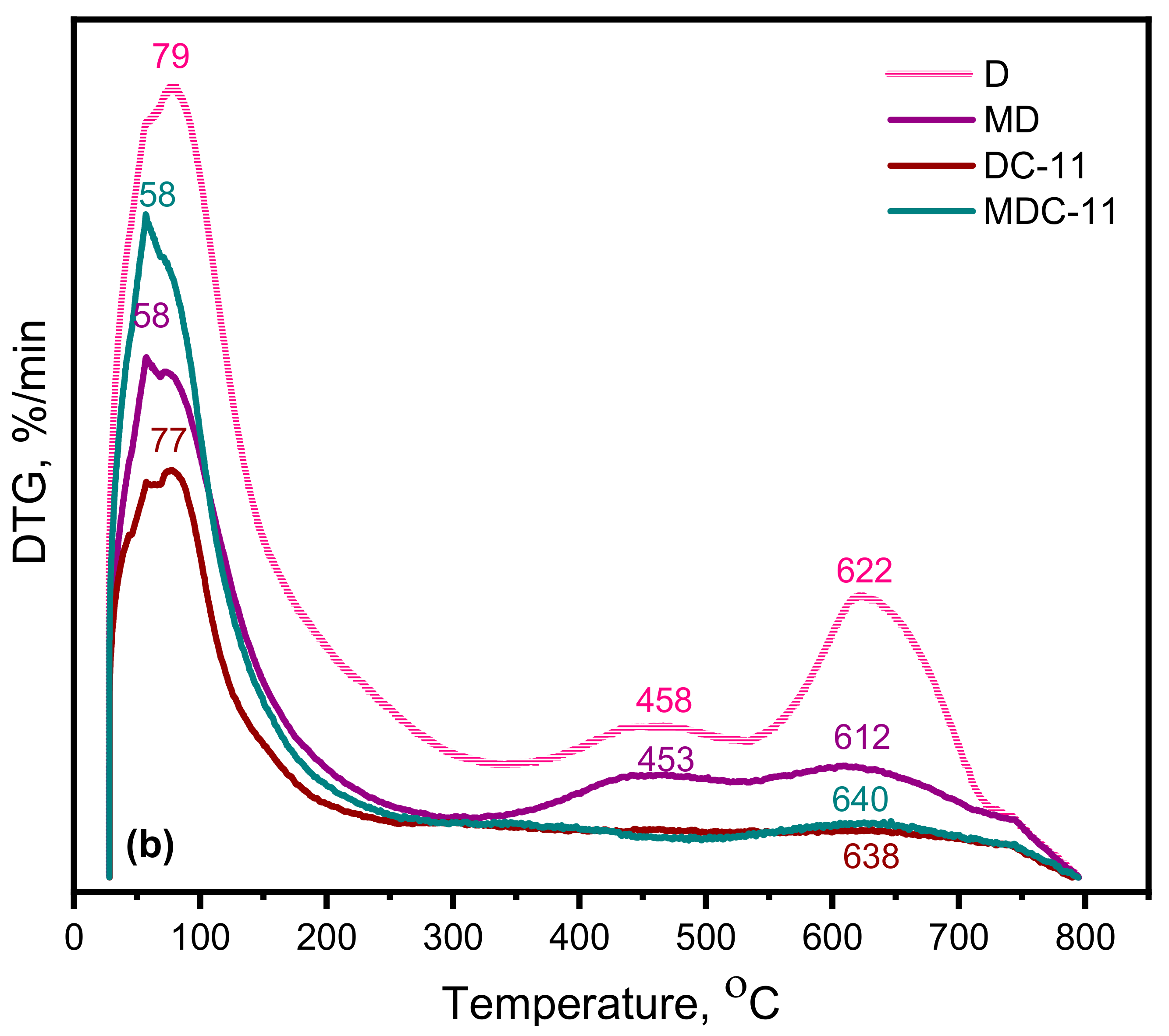
3.1.6. Thermal Analysis
3.1.7. PL Analysis
3.1.8. Zeta Potential Analysis
3.2. Assessment of Photocatalytic Activity
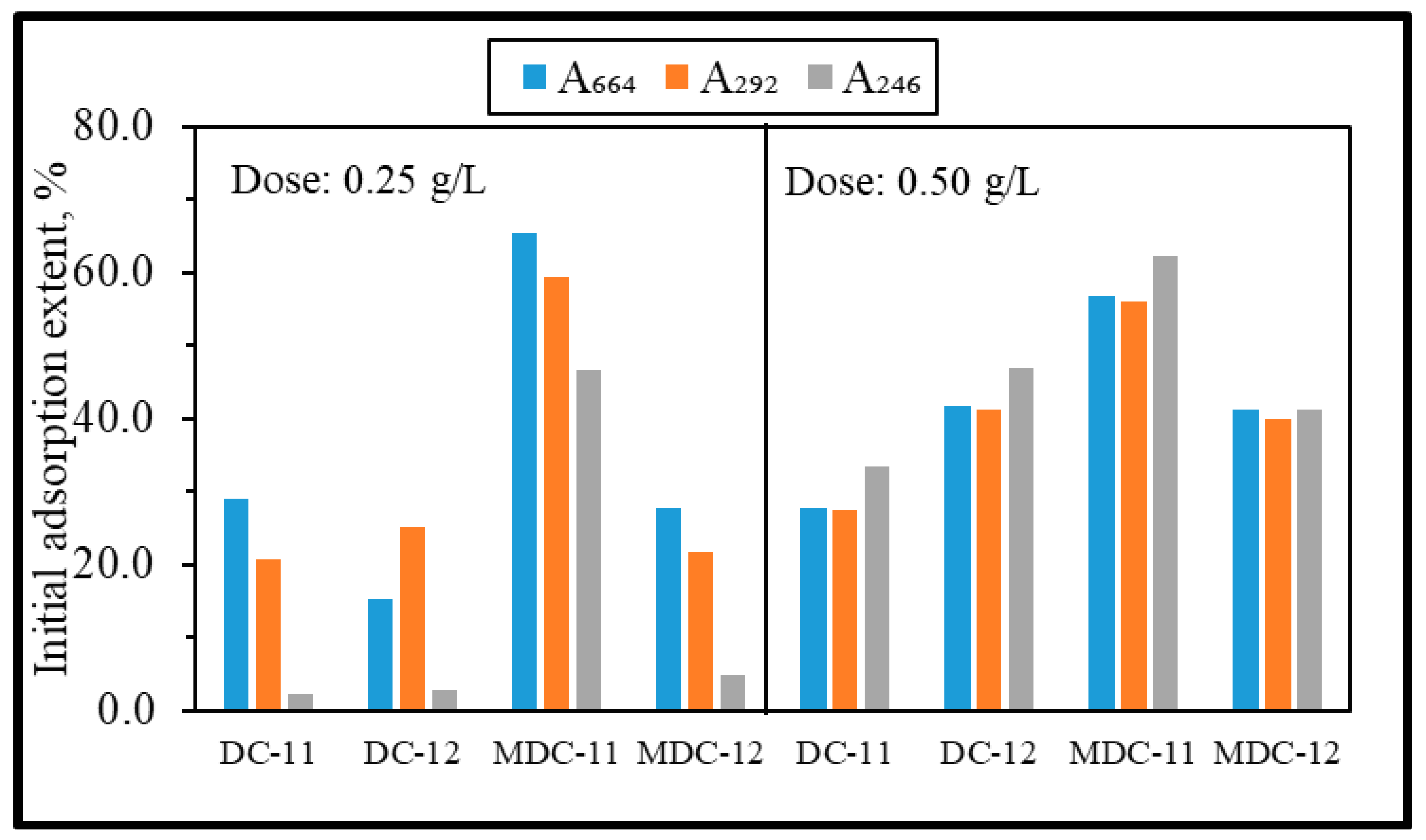
3.2.1. Preliminary Experiments
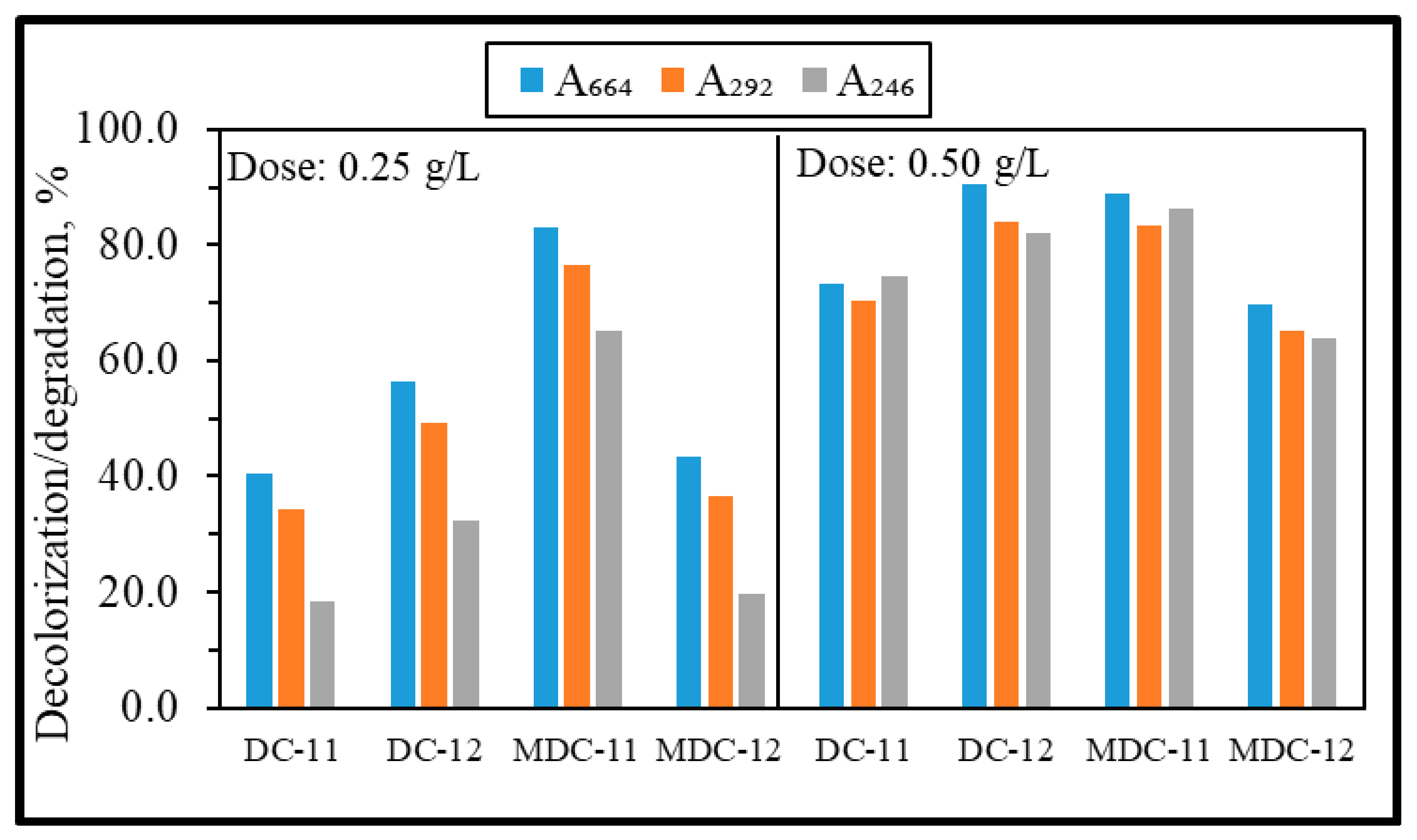
3.2.2. Photocatalytic Degradation of MB using CeO2-Diatomite Composites
4. Conclusions
Supplementary Materials
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Waghchaure, R.H.; Adole, V.A.; Jagdale, B.S. Photocatalytic degradation of methylene blue, rhodamine B, methyl orange and Eriochrome black T dyes by modified ZnO nanocatalysts: A concise review. Inorg. Chem. Commun. 2022, 143, 109764. [Google Scholar] [CrossRef]
- Hamad, H.N.; Idrus, S. Recent developments in the application of bio-waste-derived adsorbents for the removal of methylene blue from wastewater: A review. Polymers 2022, 14, 783. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Zekker, I.; Zhang, B.; Hendi, A.H.; Ahmad, A.; Ahmad, S.; Zada, N.; Ahmad, H.; Shah, L.A.; et al. Review on methylene blue: Its properties, uses, toxicity and photodegradation. Water 2022, 14, 242. [Google Scholar] [CrossRef]
- Rafatullah, M.; Sulaiman, O.; Hashim, R.; Ahmad, A. Adsorption of methylene blue on low-cost adsorbents: A review. J. Hazard. Mater. 2010, 177, 70–80. [Google Scholar] [CrossRef]
- Kuang, Y.; Zhang, X.; Zhou, S. Adsorption of methylene blue in water onto activated carbon by surfactant modification. Water 2020, 12, 587. [Google Scholar] [CrossRef]
- Labena, A.; Abdelhamid, A.E.; Amin, A.S.; Husien, S.; Hamid, L.; Safwat, G.; Diab, A.; Gobouri, A.A.; Azab, E. Removal of methylene blue and congo red using adsorptive membrane impregnated with dried Ulva fasciata and Sargassum dentifolium. Plants 2021, 10, 384. [Google Scholar] [CrossRef]
- Verma, A.K.; Dash, R.R.; Bhunia, P. A review on chemical coagulation/flocculation technologies for removal of colour from textile wastewaters. J. Environ. Manage. 2012, 93, 154–168. [Google Scholar] [CrossRef] [PubMed]
- Gomes, J.; Lincho, J.; Domingues, E.; Quinta-Ferreira, R.M.; Martins, R.C. N–TiO2 Photocatalysts: A Review of Their Characteristics and Capacity for Emerging Contaminants Removal. Water 2019, 11, 373. [Google Scholar] [CrossRef]
- Kumar, M.S.; Sonawane, S.H.; Pandit, A.B. Degradation of methylene blue dye in aqueous solution using hydrodynamic cavitation based hybrid advanced oxidation processes. Chem. Eng. Process. Process Intensif. 2017, 122, 288–295. [Google Scholar] [CrossRef]
- Kurian, M. Advanced oxidation processes and nanomaterials—A review. Cleaner Eng. Technol. 2021, 2, 100090. [Google Scholar] [CrossRef]
- Modi, S.; Yadav, V.K.; Gacem, A.; Ali, I.H.; Dave, D.; Khan, S.H.; Yadav, K.K.; Rather, S.-u.; Ahn, Y.; Son, C.T.; et al. Recent and Emerging Trends in Remediation of Methylene Blue Dye from Wastewater by Using Zinc Oxide Nanoparticles. Water 2022, 14, 1749. [Google Scholar] [CrossRef]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemann, D.W. Environmental Applications of Semiconductor Photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Turchi, C.S.; Ollis, D.F. Photocatalytic degradation of organic water contaminants: Mechanisms involving hydroxyl radical attack. J. Catal. 1990, 122, 178–192. [Google Scholar] [CrossRef]
- Gharbani, P.; Mehrizad, A.; Mosavi, S.A. Optimization, kinetics and thermodynamics studies for photocatalytic degradation of Methylene Blue using cadmium selenide nanoparticles. npJ. Clean Water 2022, 5, 34. [Google Scholar] [CrossRef]
- George, A.; Magimai Antoni Raj, D.; Venci, X.; Dhayal Raj, A.; Albert Irudayaraj, A.; Josephine, R.L.; John Sundaram, S.; Al-Mohaimeed, A.M.; Al Farraj, D.A.; Chen, T.-W.; et al. Photocatalytic effect of CuO nanoparticles flower-like 3D nanostructures under visible light irradiation with the degradation of methylene blue (MB) dye for environmental application. Environ. Res. 2022, 203, 111880. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Khan, M.; Khan, S.; Luque, R.; Almutairi, T.M.; Karami, A.M. Bio-construction of MgO nanoparticles using Texas sage plant extract for catalytical degradation of methylene blue via photocatalysis. Int. J. Environ. Sci. Technol. 2022, 1–12. [Google Scholar] [CrossRef]
- Perumal, V.; Inmozhi, C.; Uthrakumar, R.; Robert, R.; Chandrasekar, M.; Mohamed, S.B.; Honey, S.; Raja, A.; Al-Mekhlafi, F.A.; Kaviyarasu, K. Enhancing the photocatalytic performance of surface—Treated SnO2 hierarchical nanorods against methylene blue dye under solar irradiation and biological degradation. Environ. Res. 2022, 209, 112821. [Google Scholar] [CrossRef]
- Abbas, N.; Rubab, N.; Sadiq, N.; Manzoor, S.; Khan, M.I.; Fernandez Garcia, J.; Barbosa Aragao, I.; Tariq, M.; Akhtar, Z.; Yasmin, G. Aluminum-Doped Cobalt Ferrite as an Efficient Photocatalyst for the Abatement of Methylene Blue. Water 2020, 12, 2285. [Google Scholar] [CrossRef]
- Turkten, N.; Karatas, Y.; Bekbolet, M. Preparation of PANI Modified ZnO Composites via Different Methods: Structural, Morphological and Photocatalytic Properties. Water 2021, 13, 1025. [Google Scholar] [CrossRef]
- Basumatary, B.; Basumatary, R.; Ramchiary, A.; Konwar, D. Evaluation of Ag@TiO2/WO3 heterojunction photocatalyst for enhanced photocatalytic activity towards methylene blue degradation. Chemosphere 2022, 286, 131848. [Google Scholar] [CrossRef] [PubMed]
- Birben, N.C.; Paganini, M.C.; Calza, P.; Bekbolet, M. Photocatalytic degradation of humic acid using a novel photocatalyst: Ce-doped ZnO. Photochem. Photobiol. Sci. 2017, 16, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Cao, Y.; Jia, D. Preparation and photocatalytic property of CeO2 lamellar. Appl. Surf. Sci. 2011, 257, 9226–9231. [Google Scholar] [CrossRef]
- Phanichphant, S.; Nakaruk, A.; Channei, D. Photocatalytic activity of the binary composite CeO2/SiO2 for degradation of dye. Appl. Surf. Sci. 2016, 387, 214–220. [Google Scholar] [CrossRef]
- Litter, M.I. Heterogeneous photocatalysis: Transition metal ions in photocatalytic systems. Appl. Catal. B 1999, 23, 89–114. [Google Scholar] [CrossRef]
- Cheng, Z.; Luo, S.; Liu, Z.; Zhang, Y.; Liao, Y.; Guo, M.; Nguyen, T.T. Visible-light-driven hierarchical porous CeO2 derived from wood for effective photocatalytic degradation of methylene blue. Opt. Mater. 2022, 129, 112429. [Google Scholar] [CrossRef]
- Jasim, S.A.; Machek, P.; Abdelbasset, W.K.; Jarosova, M.; Majdi, H.S.; Khalaji, A.D. Solution combustion synthesis of CeO2 nanoparticles for excellent photocatalytic degradation of methylene blue. Appl. Phys. A 2022, 128, 475. [Google Scholar] [CrossRef]
- Majumder, D.; Chakraborty, I.; Mandal, K.; Roy, S. Facet-Dependent Photodegradation of Methylene Blue Using Pristine CeO2 Nanostructures. ACS Omega 2019, 4, 4243–4251. [Google Scholar] [CrossRef]
- Pouretedal, H.R.; Kadkhodaie, A. Synthetic CeO2 Nanoparticle Catalysis of Methylene Blue Photodegradation: Kinetics and Mechanism. Chinese J. Catal. 2010, 31, 1328–1334. [Google Scholar] [CrossRef]
- Reddy Yadav, L.S.; Lingaraju, K.; Daruka Prasad, B.; Kavitha, C.; Banuprakash, G.; Nagaraju, G. Synthesis of CeO2 nanoparticles: Photocatalytic and antibacterial activities. Eur. Phys. J. Plus 2017, 132, 239. [Google Scholar] [CrossRef]
- Saadoon, S.J.; Jarosova, M.; Machek, P.; Kadhim, M.M.; Ali, M.H.; Khalaji, A.D. Methylene blue photodegradation using as-synthesized CeO2 nanoparticles. J. Chin. Chem. Soc. 2022, 69, 280–288. [Google Scholar] [CrossRef]
- Christy, E.J.S.; Alagar, R.; Dhanu, M.; Pius, A. Porous nonhierarchical CeO2/SiO2 monolith for effective degradation of organic pollutants. Environ. Nanotechnol. Monit. Manag. 2020, 14, 100365. [Google Scholar] [CrossRef]
- Dhmees, A.S.; Rashad, A.M.; Eliwa, A.A.; Zawrah, M.F. Preparation and characterization of nano SiO2@CeO2 extracted from blast furnace slag and uranium extraction waste for wastewater treatment. Ceram. Int. 2019, 45, 7309–7317. [Google Scholar] [CrossRef]
- Farrukh, M.A.; Butt, K.M.; Chong, K.-K.; Chang, W.S. Photoluminescence emission behavior on the reduced band gap of Fe doping in CeO2-SiO2 nanocomposite and photophysical properties. J. Saudi Chem. Soc. 2019, 23, 561–575. [Google Scholar] [CrossRef]
- Mohamed, R.M.; Aazam, E.S. Synthesis and Characterization of CeO2-SiO2 Nanoparticles by Microwave-Assisted Irradiation Method for Photocatalytic Oxidation of Methylene Blue Dye. Int. J. Photoenergy 2012, 2012, 928760. [Google Scholar] [CrossRef][Green Version]
- Rani, N.; Ahlawat, R.; Goswami, B. Annealing effect on bandgap energy and photocatalytic properties of CeO2-SiO2 nanocomposite prepared by sol-gel technique. Mater. Chem. Phys. 2020, 241, 122401. [Google Scholar] [CrossRef]
- Li, X.; Simon, U.; Bekheet, M.F.; Gurlo, A. Mineral-Supported Photocatalysts: A Review of Materials, Mechanisms and Environmental Applications. Energies 2022, 15, 5607. [Google Scholar] [CrossRef]
- Mishra, A.; Mehta, A.; Basu, S. Clay supported TiO2 nanoparticles for photocatalytic degradation of environmental pollutants: A review. J. Environ. Chem. Eng. 2018, 6, 6088–6107. [Google Scholar] [CrossRef]
- Tang, X.; Tang, R.; Xiong, S.; Zheng, J.; Li, L.; Zhou, Z.; Gong, D.; Deng, Y.; Su, L.; Liao, C. Application of natural minerals in photocatalytic degradation of organic pollutants: A review. Sci. Total Environ. 2022, 812, 152434. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, R.; Wang, D.; Hong, X.; Yang, S. SnO2/Diatomite Composite Prepared by Solvothermal Reaction for Low-Cost Photocatalysts. Catalysts 2019, 9, 1060. [Google Scholar] [CrossRef]
- Xiong, C.; Ren, Q.; Chen, S.; Liu, X.; Jin, Z.; Ding, Y. A multifunctional Ag3PO4/Fe3O4/Diatomite composites: Photocatalysis, adsorption and sterilization. Mater. Today Commun. 2021, 28, 102695. [Google Scholar] [CrossRef]
- Zhang, G.; Cai, D.; Wang, M.; Zhang, C.; Zhang, J.; Wu, Z. Microstructural modification of diatomite by acid treatment, high-speed shear, and ultrasound. Microporous Mesoporous Mater. 2013, 165, 106–112. [Google Scholar] [CrossRef]
- Cherrak, R.; Hadjel, M.; Benderdouche, N.; Bellayer, S.; Traisnel, M. Treatment of recalcitrant organic pollutants in water by heterogeneous catalysis using a mixed material (TiO2-diatomite of algeria). Desalination Water Treat. 2016, 57, 17139–17148. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, B.; Sun, Z.; Zheng, S.; Liu, S. A comparative study of different diatomite-supported TiO2 composites and their photocatalytic performance for dye degradation. Desalination Water Treat. 2016, 57, 17512–17522. [Google Scholar] [CrossRef]
- Tanniratt, P.; Wasanapiarnpong, T.; Mongkolkachit, C.; Sujaridworakun, P. Utilization of industrial wastes for preparation of high performance ZnO/diatomite hybrid photocatalyst. Ceram. Int. 2016, 42, 17605–17609. [Google Scholar] [CrossRef]
- Tajmiri, S.; Hosseini, M.R.; Azimi, E. Combined photocatalytic-adsorptive removal of water contaminants using a biologically prepared CdS-diatomite nanocomposite. Mater. Chem. Phys. 2021, 258, 123913. [Google Scholar] [CrossRef]
- Yang, B.; Ma, Z.; Wang, Q.; Yang, J. Synthesis and Photoelectrocatalytic Applications of TiO2/ZnO/Diatomite Composites. Catalysts 2022, 12, 268. [Google Scholar] [CrossRef]
- Zhou, Y.; Xi, W.; Xie, Z.; You, Z.; Jiang, X.; Han, B.; Lang, R.; Wu, C. High-Loading Pt Single-Atom Catalyst on CeO2-Modified Diatomite Support. Chem. Asian J. 2021, 16, 2622–2625. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Wang, H.; Li, M.; Qu, Y.; Zhu, Y. Tribological behavior and characterization analysis of modified nano-CeO2 filled oily diatomite/PVDF composites. Tribol. Int. 2019, 130, 299–307. [Google Scholar] [CrossRef]
- Seeharaj, P.; Kongmun, P.; Paiplod, P.; Prakobmit, S.; Sriwong, C.; Kim-Lohsoontorn, P.; Vittayakorn, N. Ultrasonically-assisted surface modified TiO2/rGO/CeO2 heterojunction photocatalysts for conversion of CO2 to methanol and ethanol. Ultrason. Sonochem. 2019, 58, 104657. [Google Scholar] [CrossRef]
- Hatchard, C.G.; Parker, C.A.; Bowen, E.J. A new sensitive chemical actinometer—II. Potassium ferrioxalate as a standard chemical actinometer. Proc. R. Soc. Lond. A 1956, 235, 518–536. [Google Scholar] [CrossRef]
- Dai, X.; Zeng, H.; Jin, C.; Rao, J.; Liu, X.; Li, K.; Zhang, Y.; Yu, Y.; Zhang, Y. 2D–3D graphene-coated diatomite as a support toward growing ZnO for advanced photocatalytic degradation of methylene blue. RSC Adv. 2021, 11, 38505–38514. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Xu, H.; Xie, Q.; Chen, N.; Yu, Q.; Li, C. Mechanism of As(V) adsorption from aqueous solution by chitosan-modified diatomite adsorbent. J. Dispersion Sci. Technol. 2021, 43, 1512–1524. [Google Scholar] [CrossRef]
- Pu, X.; Dang, Q.; Liu, C.; Xu, Q.; Li, B.; Ji, X.; Liu, H.; Ma, Y.; Zhang, B.; Cha, D. Selective capture of mercury(II) in aqueous media using nanoporous diatomite modified by allyl thiourea. J. Mater. Sci. 2022, 57, 9246–9264. [Google Scholar] [CrossRef]
- Nguyen, Q.-B.; Vahabi, H.; Rios de Anda, A.; Versace, D.-L.; Langlois, V.; Perrot, C.; Nguyen, V.-H.; Naili, S.; Renard, E. Dual UV-Thermal Curing of Biobased Resorcinol Epoxy Resin-Diatomite Composites with Improved Acoustic Performance and Attractive Flame Retardancy Behavior. Sustain. Chem. 2021, 2, 24–48. [Google Scholar] [CrossRef]
- Othman, A.; Vargo, P.; Andreescu, S. Recyclable Adsorbents Based on Ceria Nanostructures on Mesoporous Silica Beads for the Removal and Recovery of Phosphate from Eutrophic Waters. ACS Appl. Nano Mater. 2019, 2, 7008–7018. [Google Scholar] [CrossRef]
- Xie, L.; Ren, Z.; Zhu, P.; Xu, J.; Luo, D.; Lin, J. A novel CeO2–TiO2/PANI/NiFe2O4 magnetic photocatalyst: Preparation, characterization and photodegradation of tetracycline hydrochloride under visible light. J. Solid State Chem. 2021, 300, 122208. [Google Scholar] [CrossRef]
- Laha, S.C.; Mukherjee, P.; Sainkar, S.R.; Kumar, R. Cerium Containing MCM-41-Type Mesoporous Materials and their Acidic and Redox Catalytic Properties. J. Catal. 2002, 207, 213–223. [Google Scholar] [CrossRef]
- Yao, G.; Lei, J.; Zhang, X.; Sun, Z.; Zheng, S.; Komarneni, S. Mechanism of zeolite X crystallization from diatomite. Mater. Res. Bull. 2018, 107, 132–138. [Google Scholar] [CrossRef]
- Reddy, B.M.; Khan, A.; Yamada, Y.; Kobayashi, T.; Loridant, S.; Volta, J.-C. Surface Characterization of CeO2/SiO2 and V2O5/CeO2/SiO2 Catalysts by Raman, XPS, and Other Techniques. J. Phys. Chem. B 2002, 106, 10964–10972. [Google Scholar] [CrossRef]
- Mamontov, G.V.; Grabchenko, M.V.; Sobolev, V.I.; Zaikovskii, V.I.; Vodyankina, O.V. Ethanol dehydrogenation over Ag-CeO2/SiO2 catalyst: Role of Ag-CeO2 interface. Appl. Catal. A 2016, 528, 161–167. [Google Scholar] [CrossRef]
- Weber, W.H.; Hass, K.C.; McBride, J.R. Raman study of CeO2: Second-order scattering, lattice dynamics, and particle-size effects. Phys. Rev. B 1993, 48, 178–185. [Google Scholar] [CrossRef]
- Grabchenko, M.V.; Mamontov, G.V.; Zaikovskii, V.I.; La Parola, V.; Liotta, L.F.; Vodyankina, O.V. Design of Ag-CeO2/SiO2 catalyst for oxidative dehydrogenation of ethanol: Control of Ag-CeO2 interfacial interaction. Catal. Today 2019, 333, 2–9. [Google Scholar] [CrossRef]
- Humbert, B.; Burneau, A.; Gallas, J.P.; Lavalley, J.C. Origin of the Raman bands, D1 and D2, in high surface area and vitreous silicas. J. Non-Cryst. Solids 1992, 143, 75–83. [Google Scholar] [CrossRef]
- Cerrato, E.; Calza, P.; Cristina Paganini, M. Photocatalytic reductive and oxidative ability study of pristine ZnO and CeO2-ZnO heterojunction impregnated with Cu2O. J. Photochem. Photobiol. A 2022, 427, 113775. [Google Scholar] [CrossRef]
- Scherrer, P. Estimation of the size and internal structure of colloidal particles by means of röntgen. Nachr. Ges. Wiss. Göttingen 1918, 2, 96–100. [Google Scholar]
- Gao, L.; Luo, Y.; Kang, Y.; Gao, M.; Abdulhafidh, O. Experimental Study on Physical Mechanical Properties and Microstructure of Diatomite Soil in Zhejiang Province, China. Appl. Sci. 2022, 12, 387. [Google Scholar] [CrossRef]
- Zuo, X.; Wang, L.; He, J.; Li, Z.; Yu, S. SEM-EDX studies of SiO2/PVDF membranes fouling in electrodialysis of polymer-flooding produced wastewater: Diatomite, APAM and crude oil. Desalination 2014, 347, 43–51. [Google Scholar] [CrossRef]
- Sing, K.S.W. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Rouquerol, F.; Rouquerol, J.; Sing, K.S.W.; Llewellyn, P.; Maurin, G. Adsorption by Powders and Porous Solids, 2nd ed.; Academic Press: Cambridge, MA, USA, 2014. [Google Scholar] [CrossRef]
- Yurdakal, S.; Garlisi, C.; Özcan, L.; Bellardita, M.; Palmisano, G. Chapter 4—(Photo)catalyst Characterization Techniques: Adsorption Isotherms and BET, SEM, FTIR, UV–Vis, Photoluminescence, and Electrochemical Characterizations. In Heterogeneous Photocatalysis; Marcì, G., Palmisano, L., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 87–152. [Google Scholar] [CrossRef]
- Azimi Pirsaraei, S.R.; Asilian Mahabadi, H.; Jonidi Jafari, A. Airborne toluene degradation by using manganese oxide supported on a modified natural diatomite. J. Porous Mater. 2016, 23, 1015–1024. [Google Scholar] [CrossRef]
- Benkacem, T.; Hamdi, B.; Chamayou, A.; Balard, H.; Calvet, R. Physicochemical characterization of a diatomaceous upon an acid treatment: A focus on surface properties by inverse gas chromatography. Powder Technol. 2016, 294, 498–507. [Google Scholar] [CrossRef]
- Inchaurrondo, N.; Ramos, C.P.; Žerjav, G.; Font, J.; Pintar, A.; Haure, P. Modified diatomites for Fenton-like oxidation of phenol. Microporous Mesoporous Mater. 2017, 239, 396–408. [Google Scholar] [CrossRef]
- Mazidi, M.; Behbahani, R.M.; Fazeli, A. Screening of treated diatomaceous earth to apply as V2O5 catalyst support. Mater. Res. Innovations 2017, 21, 269–278. [Google Scholar] [CrossRef]
- Xia, Y.; Li, F.; Jiang, Y.; Xia, M.; Xue, B.; Li, Y. Interface actions between TiO2 and porous diatomite on the structure and photocatalytic activity of TiO2-diatomite. Appl. Surf. Sci. 2014, 303, 290–296. [Google Scholar] [CrossRef]
- Sprynskyy, M.; Kowalkowski, T.; Tutu, H.; Cukrowska, E.M.; Buszewski, B. Ionic liquid modified diatomite as a new effective adsorbent for uranium ions removal from aqueous solution. Colloids Surf. A Physicochem. Eng. Asp. 2015, 465, 159–167. [Google Scholar] [CrossRef]
- Caliskan, N.; Kul, A.R.; Alkan, S.; Sogut, E.G.; Alacabey, İ. Adsorption of Zinc (II) on diatomite and manganese-oxide-modified diatomite: A kinetic and equilibrium study. J. Hazard. Mater. 2011, 193, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Ilia, I.; Stamatakis, M.; Perraki, T. Mineralogy and technical properties of clayey diatomites from north and central Greece. Open Geosci. 2009, 1, 393–403. [Google Scholar] [CrossRef]
- Kong, X.; Chong, X.; Squire, K.; Wang, A.X. Microfluidic diatomite analytical devices for illicit drug sensing with ppb-Level sensitivity. Sens. Actuators B Chem. 2018, 259, 587–595. [Google Scholar] [CrossRef]
- Rea, I.; De Stefano, L. Recent Advances on Diatom-Based Biosensors. Sensors 2019, 19, 5208. [Google Scholar] [CrossRef] [PubMed]
- Viji, S.; Anbazhagi, M.; Ponpandian, N.; Mangalaraj, D.; Jeyanthi, S.; Santhanam, P.; Devi, A.S.; Viswanathan, C. Diatom-Based Label-Free Optical Biosensor for Biomolecules. Appl. Biochem. Biotechnol. 2014, 174, 1166–1173. [Google Scholar] [CrossRef] [PubMed]
- Rea, I.; Martucci, N.M.; De Stefano, L.; Ruggiero, I.; Terracciano, M.; Dardano, P.; Migliaccio, N.; Arcari, P.; Taté, R.; Rendina, I.; et al. Diatomite biosilica nanocarriers for siRNA transport inside cancer cells. Biochim. Biophys. Acta Gen. Subj. 2014, 1840, 3393–3403. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, S.; Sahu, K.; Bisht, A.; Singhal, R.; Mohapatra, S. Template-free and surfactant-free synthesis of CeO2 nanodiscs with enhanced photocatalytic activity. Appl. Surf. Sci. 2020, 503, 144102. [Google Scholar] [CrossRef]
- Kaviyarasu, K.; Fuku, X.; Mola, G.T.; Manikandan, E.; Kennedy, J.; Maaza, M. Photoluminescence of well-aligned ZnO doped CeO2 nanoplatelets by a solvothermal route. Mater. Lett. 2016, 183, 351–354. [Google Scholar] [CrossRef]
- Wang, G.; Mu, Q.; Chen, T.; Wang, Y. Synthesis, characterization and photoluminescence of CeO2 nanoparticles by a facile method at room temperature. J. Alloys Compd. 2010, 493, 202–207. [Google Scholar] [CrossRef]
- Parks, G.A. Aqueous Surface Chemistry of Oxides and Complex Oxide Minerals. In Equilibrium Concepts in Natural Water Systems; Advances in Chemistry; American Chemical Society: Washington, DC, USA, 1967; Volume 67, pp. 121–160. [Google Scholar]
- Nosrati, A.; Larsson, M.; Lindén, J.B.; Zihao, Z.; Addai-Mensah, J.; Nydén, M. Polyethyleneimine functionalized mesoporous diatomite particles for selective copper recovery from aqueous media. Int. J. Miner. Process. 2017, 166, 29–36. [Google Scholar] [CrossRef]
- Sondi, I.; Bišćan, J.; Pravdić, V. Electrokinetics of Pure Clay Minerals Revisited. J. Colloid Interface Sci. 1996, 178, 514–522. [Google Scholar] [CrossRef]
- Ye, X.; Kang, S.; Wang, H.; Li, H.; Zhang, Y.; Wang, G.; Zhao, H. Modified natural diatomite and its enhanced immobilization of lead, copper and cadmium in simulated contaminated soils. J. Hazard. Mater. 2015, 289, 210–218. [Google Scholar] [CrossRef]
- Gao, B.; Jiang, P.; An, F.; Zhao, S.; Ge, Z. Studies on the surface modification of diatomite with polyethyleneimine and trapping effect of the modified diatomite for phenol. Appl. Surf. Sci. 2005, 250, 273–279. [Google Scholar] [CrossRef]
- Sun, Z.; Yang, X.; Zhang, G.; Zheng, S.; Frost, R.L. A novel method for purification of low grade diatomite powders in centrifugal fields. Int. J. Miner. Process. 2013, 125, 18–26. [Google Scholar] [CrossRef]
- Houas, A.; Lachheb, H.; Ksibi, M.; Elaloui, E.; Guillard, C.; Herrmann, J.M. Photocatalytic degradation pathway of methylene blue in water. Appl. Catal. B 2001, 31, 145–157. [Google Scholar] [CrossRef]
- Al-Qodah, Z.; Lafi, W.K.; Al-Anber, Z.; Al-Shannag, M.; Harahsheh, A. Adsorption of methylene blue by acid and heat treated diatomaceous silica. Desalination 2007, 217, 212–224. [Google Scholar] [CrossRef]
- Zhang, T.; Oyama, T.; Aoshima, A.; Hidaka, H.; Zhao, J.; Serpone, N. Photooxidative N-demethylation of methylene blue in aqueous TiO2 dispersions under UV irradiation. J. Photochem. Photobiol. A 2001, 140, 163–172. [Google Scholar] [CrossRef]
- Zhang, T.; Oyama, T.k.; Horikoshi, S.; Hidaka, H.; Zhao, J.; Serpone, N. Photocatalyzed N-demethylation and degradation of methylene blue in titania dispersions exposed to concentrated sunlight. Sol. Energy Mater. Sol. Cells 2002, 73, 287–303. [Google Scholar] [CrossRef]
- Lin, J.; Luo, Z.; Liu, J.; Li, P. Photocatalytic degradation of methylene blue in aqueous solution by using ZnO-SnO2 nanocomposites. Mater. Sci. Semicond. Process. 2018, 87, 24–31. [Google Scholar] [CrossRef]
- Hao, C.; Li, J.; Zhang, Z.; Ji, Y.; Zhan, H.; Xiao, F.; Wang, D.; Liu, B.; Su, F. Enhancement of photocatalytic properties of TiO2 nanoparticles doped with CeO2 and supported on SiO2 for phenol degradation. Appl. Surf. Sci. 2015, 331, 17–26. [Google Scholar] [CrossRef]
- Melcher, J.; Barth, N.; Schilde, C.; Kwade, A.; Bahnemann, D. Influence of TiO2 agglomerate and aggregate sizes on photocatalytic activity. J. Mater. Sci. 2016, 52, 1047–1056. [Google Scholar] [CrossRef]






| Specimens | SBET *, m2/g | Vp **, cm3/g | D ***, nm |
|---|---|---|---|
| D | 109 | 0.3082 | 4.55 |
| MD | 118 | 0.3478 | 5.05 |
| DC-11 | 76 | 0.0954 | 2.29 |
| DC-12 | 84 | 0.1578 | 1.73 |
| MDC-11 | 127 | 0.2032 | 3.83 |
| MDC-12 | 126 | 0.1792 | 3.49 |
| First-Order Kinetic Parameters Dose: 0.25 g/L | First-Order Kinetic Parameters Dose: 0.50 g/L | |||||
|---|---|---|---|---|---|---|
| A664 | k × 10−3, min−1 | t1/2, min | R × 10−3, cm−1 min−1 | k × 10−2, min−1 | t1/2, min | R × 10−3, cm−1 min−1 |
| DC-11 | 2.24 | 309 | 2.15 | 5.91 | 117 | 5.68 |
| DC-12 | 3.54 | 196 | 3.40 | 8.98 | 77 | 8.63 |
| MDC-11 | 10.2 | 68 | 9.84 | 9.00 | 77 | 8.65 |
| MDC-12 | 3.05 | 227 | 2.93 | 5.36 | 129 | 5.15 |
| A292 | ||||||
| DC-11 | 1.93 | 359 | 1.05 | 5.00 | 139 | 2.72 |
| DC-12 | 2.76 | 251 | 1.50 | 6.56 | 106 | 3.57 |
| MDC-11 | 2.76 | 251 | 1.50 | 6.11 | 113 | 3.32 |
| MDC-12 | 2.49 | 278 | 1.35 | 4.33 | 160 | 2.35 |
| A246 | ||||||
| DC-11 | 1.42 | 488 | 0.344 | 6.19 | 112 | 1.50 |
| DC-12 | 1.83 | 379 | 0.443 | 7.41 | 94 | 1.80 |
| MDC-11 | 3.77 | 184 | 0.913 | 8.58 | 81 | 2.07 |
| MDC-12 | 1.64 | 423 | 0.397 | 4.22 | 164 | 1.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turkten, N. A Novel Low-Cost Photocatalyst: Preparation, Characterization, and Photocatalytic Properties of CeO2-Diatomite Composites. Water 2022, 14, 3373. https://doi.org/10.3390/w14213373
Turkten N. A Novel Low-Cost Photocatalyst: Preparation, Characterization, and Photocatalytic Properties of CeO2-Diatomite Composites. Water. 2022; 14(21):3373. https://doi.org/10.3390/w14213373
Chicago/Turabian StyleTurkten, Nazli. 2022. "A Novel Low-Cost Photocatalyst: Preparation, Characterization, and Photocatalytic Properties of CeO2-Diatomite Composites" Water 14, no. 21: 3373. https://doi.org/10.3390/w14213373
APA StyleTurkten, N. (2022). A Novel Low-Cost Photocatalyst: Preparation, Characterization, and Photocatalytic Properties of CeO2-Diatomite Composites. Water, 14(21), 3373. https://doi.org/10.3390/w14213373